Woody Plant Structural Diversity Changes across an Inverse Elevation-Dependent Warming Gradient in a Subtropical Mountain Forest
Abstract
:1. Introduction
2. Materials and Methods
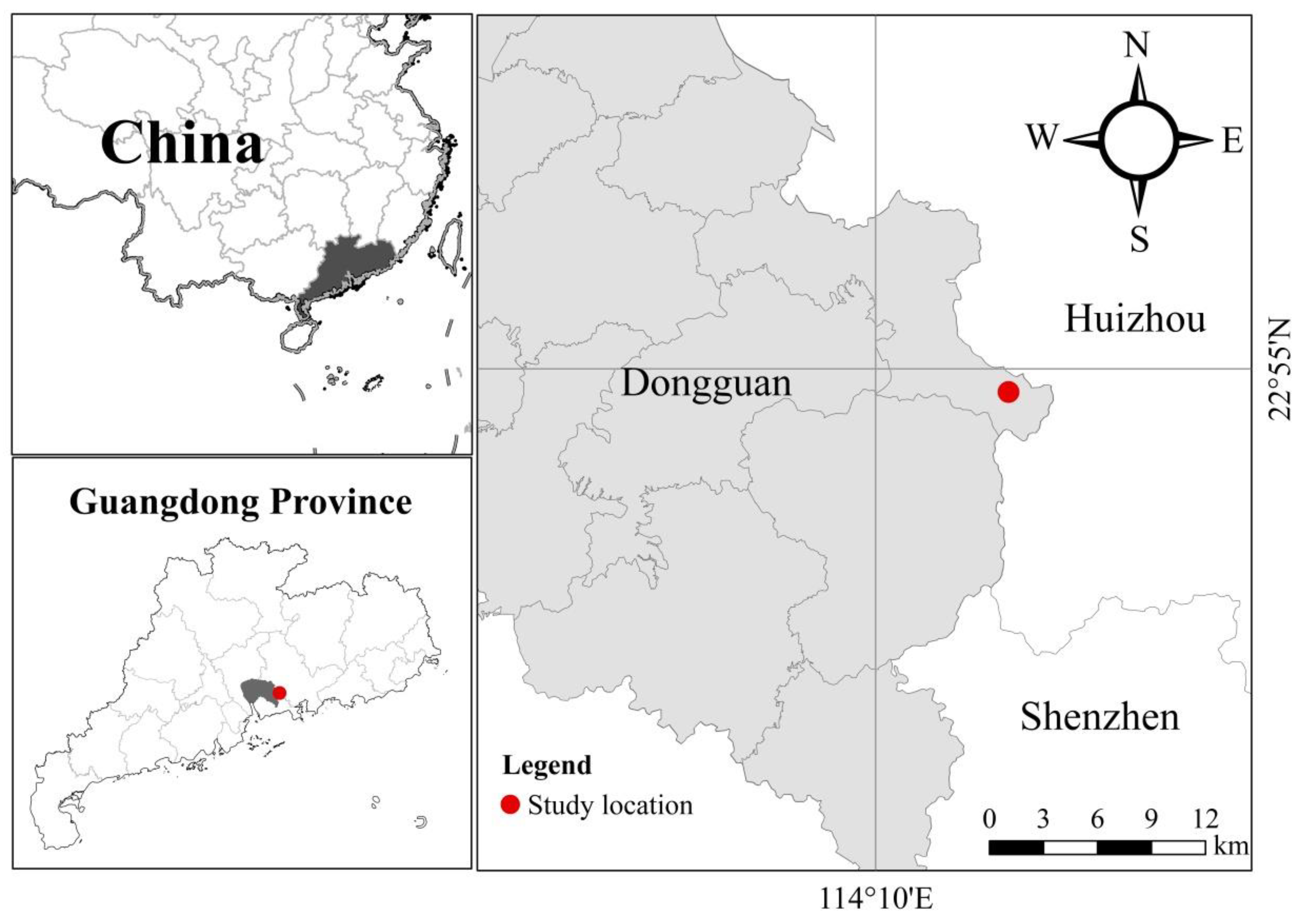
2.1. Study Sites
2.2. Sampling Design and Data Collection
2.3. Statistical Analysis
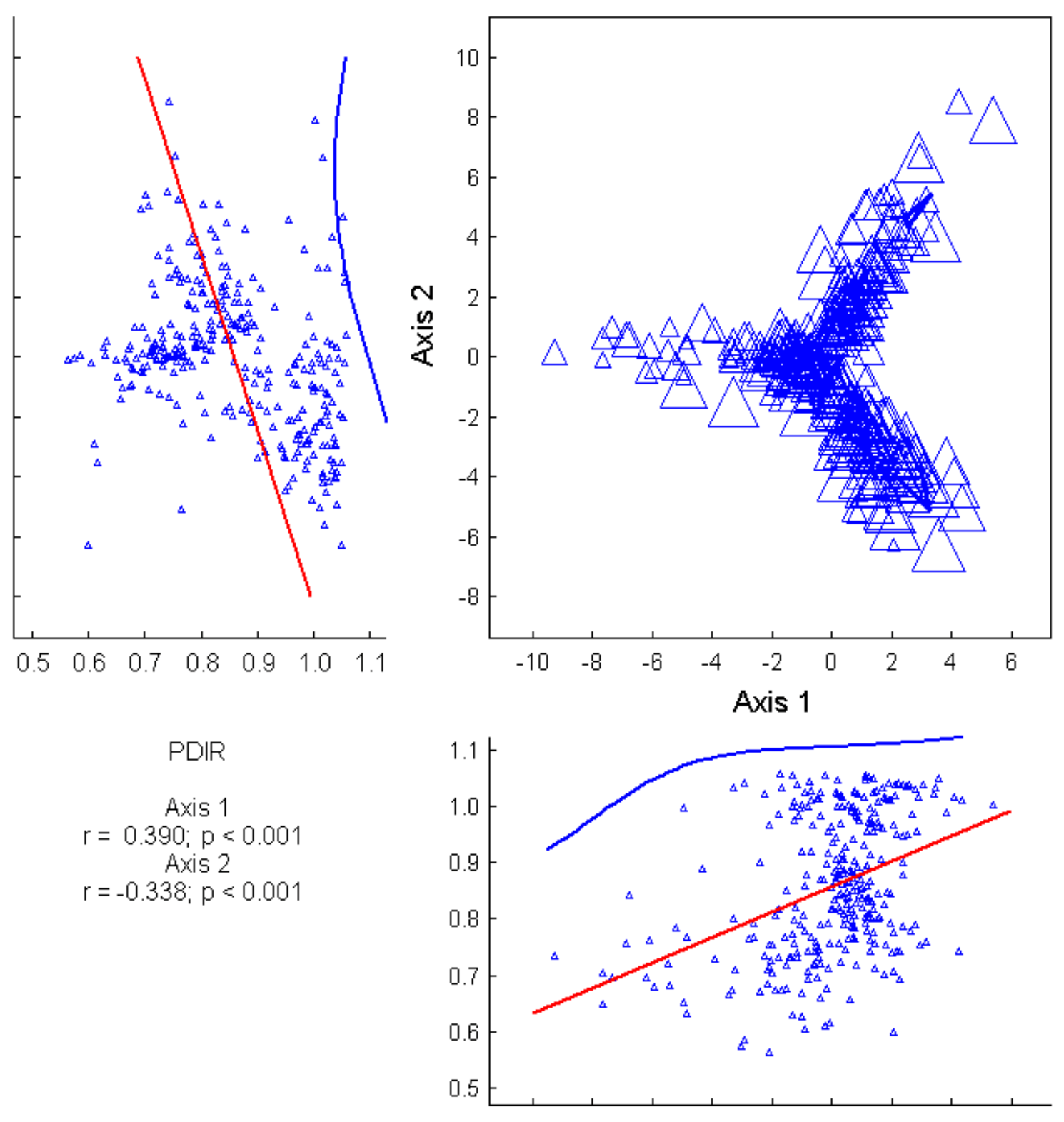
3. Results
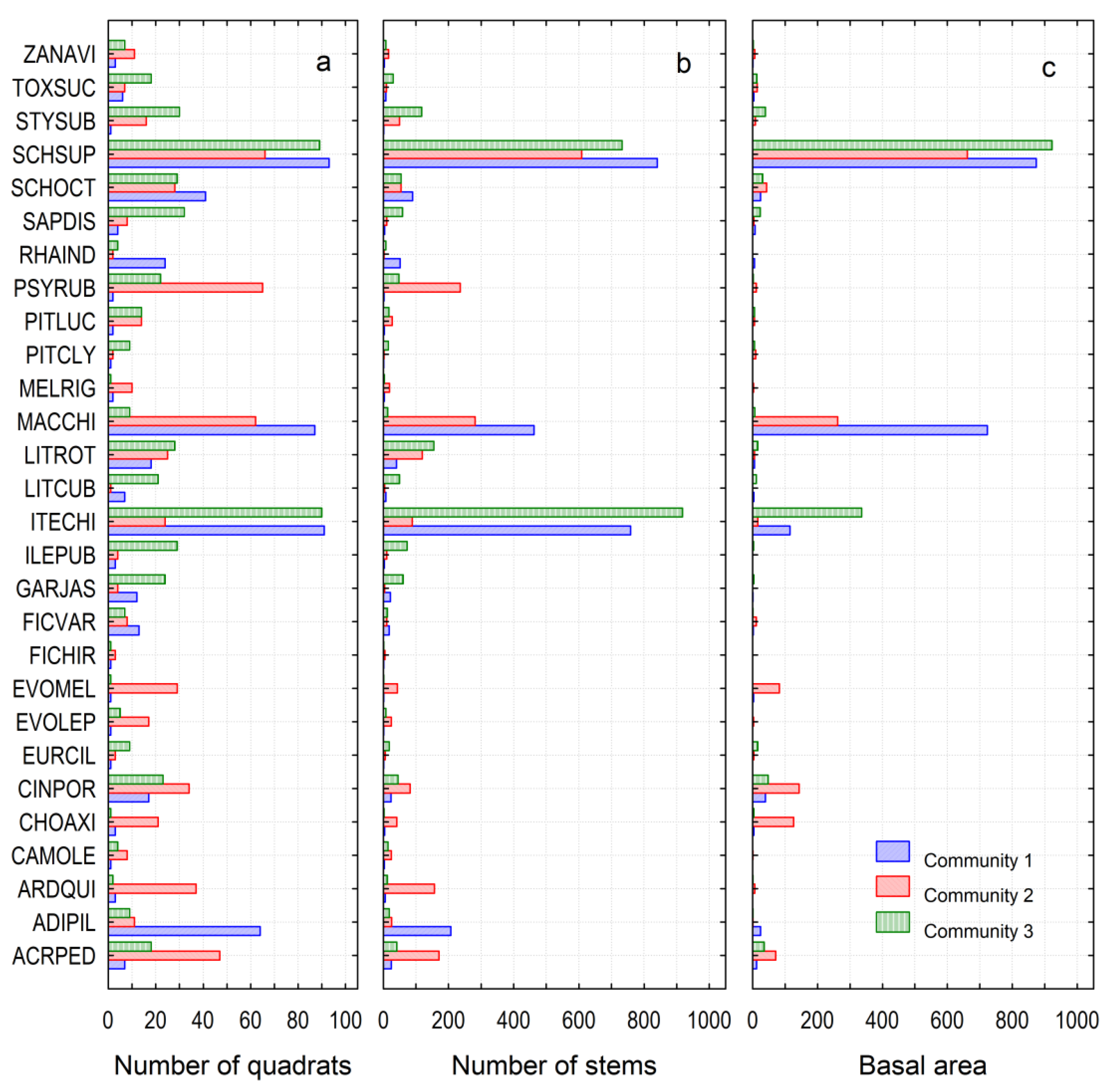
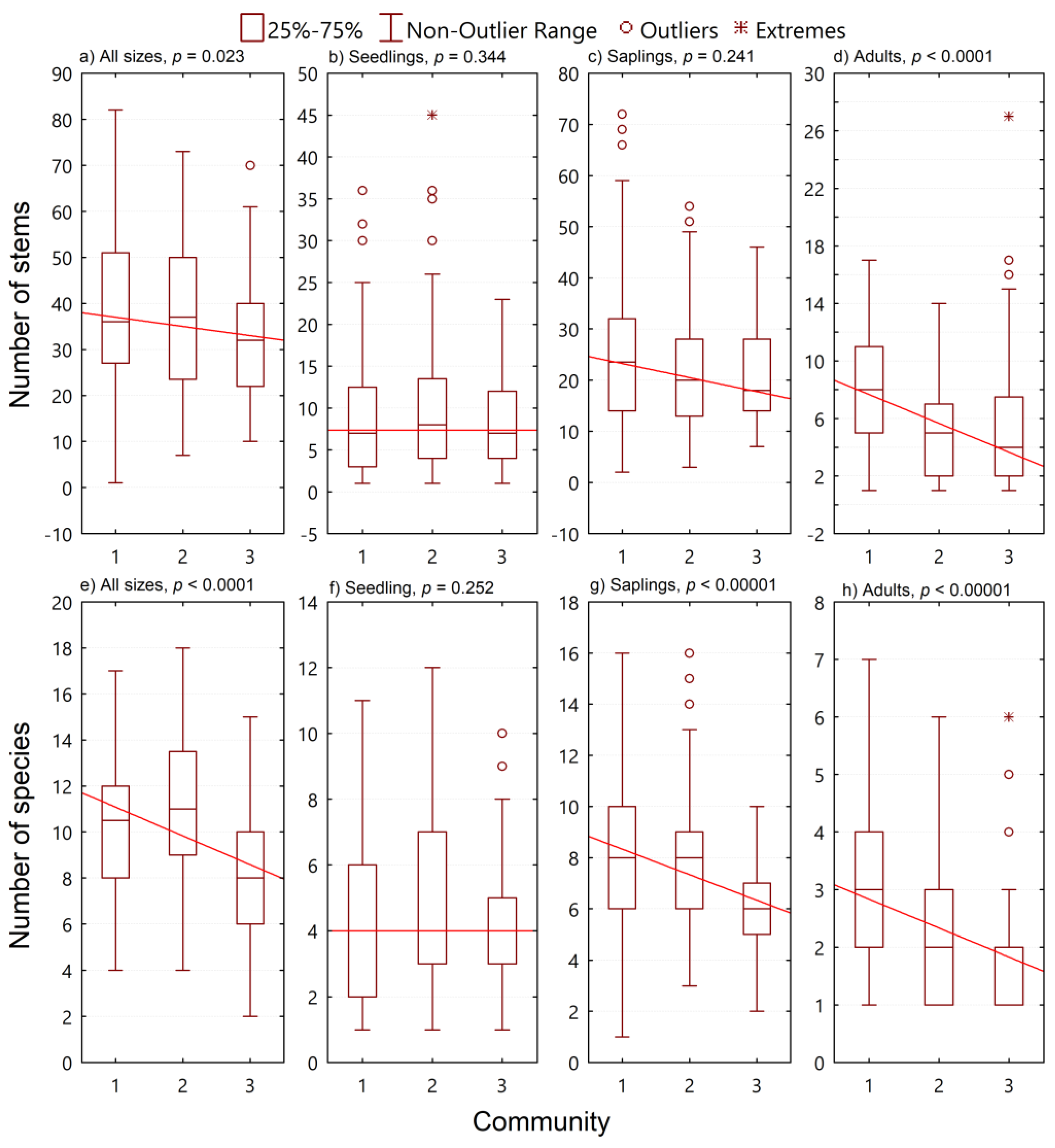
3.1. Community Structure
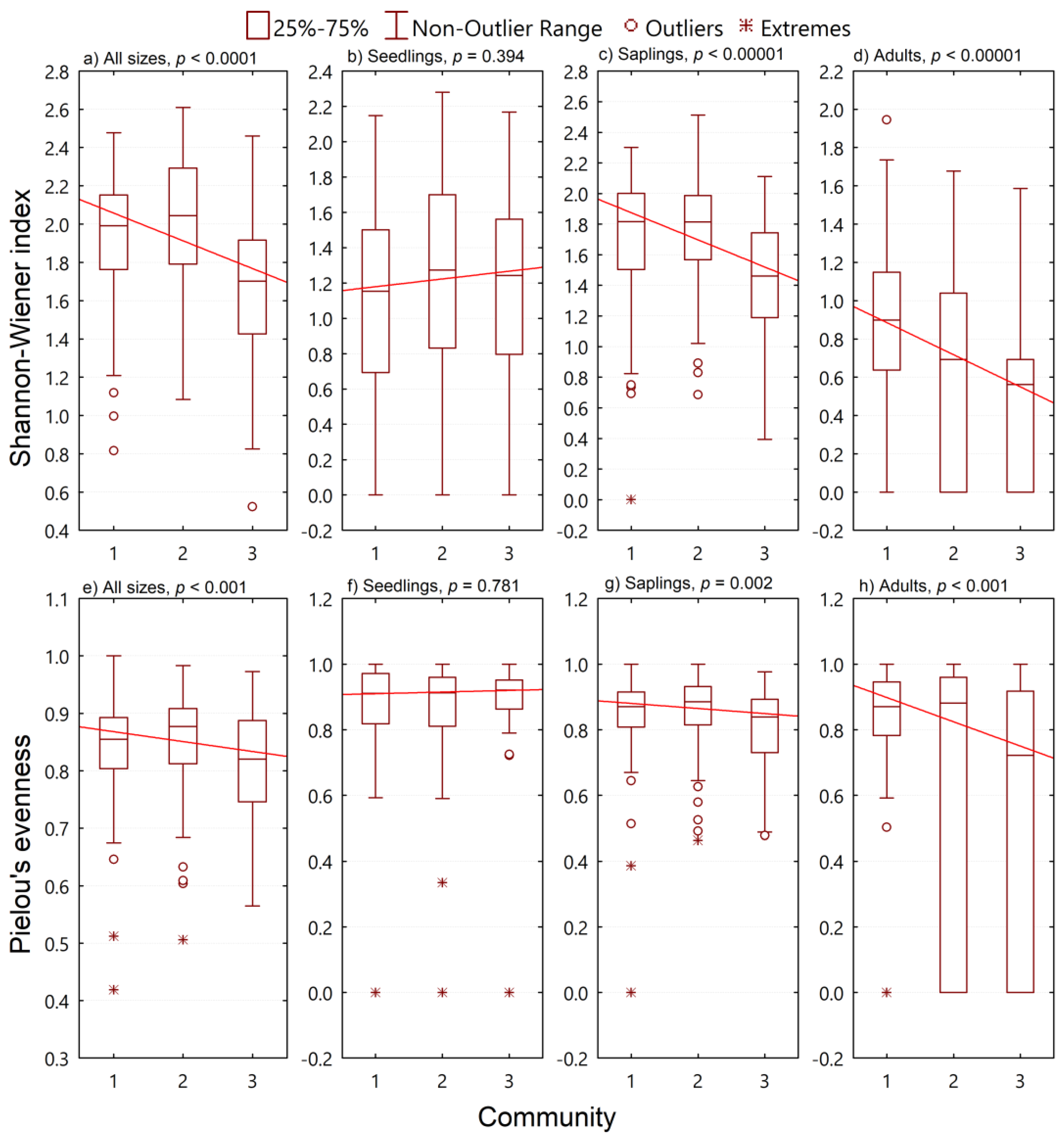
3.2. Species Diversity
3.3. Size Diversity
3.4. Species Composition
3.5. Indicator Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haider, S.; Kueffer, C.; Bruelheide, H.; Seipel, T.; Alexander, J.M.; Rew, L.J.; Arévalo, J.R.; Cavieres, L.A.; McDougall, K.L.; Milbau, A. Mountain roads and non-native species modify elevational patterns of plant diversity. Global Ecol. Biogeogr. 2018, 27, 667–678. [Google Scholar] [CrossRef]
- Karger, D.N.; Kluge, J.; Krömer, T.; Hemp, A.; Lehnert, M.; Kessler, M. The effect of area on local and regional elevational patterns of species richness. J. Biogeogr. 2011, 38, 1177–1185. [Google Scholar] [CrossRef]
- Toivonen, J.M.; Gonzales-Inca, C.A.; Bader, M.Y.; Ruokolainen, K.; Kessler, M. Elevational shifts in the topographic position of Polylepis forest stands in the Andes of southern Peru. Forests 2018, 9, 7. [Google Scholar] [CrossRef]
- Haq, S.M.; Calixto, E.S.; Rashid, I.; Srivastava, G.; Khuroo, A.A. Tree diversity, distribution and regeneration in major forest types along an extensive elevational gradient in Indian Himalaya: Implications for sustainable forest management. Forest Ecol. Manag. 2022, 506, 119968. [Google Scholar] [CrossRef]
- Pepin, N.; Bradley, R.S.; Diaz, H.; Baraër, M.; Caceres, E.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.; Liu, X. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Change 2015, 5, 424–430. [Google Scholar]
- Rangwala, I.; Miller, J.R. Climate change in mountains: A review of elevation-dependent warming and its possible causes. Clim. Change 2012, 114, 527–547. [Google Scholar] [CrossRef]
- Tudoroiu, M.; Eccel, E.; Gioli, B.; Gianelle, D.; Schume, H.; Genesio, L.; Miglietta, F. Negative elevation-dependent warming trend in the Eastern Alps. Environ. Res. Lett. 2016, 11, 044021. [Google Scholar] [CrossRef]
- Thayyen, R.J.; Dimri, A.P. Modeling slope environmental lapse rate (SELR) of temperature in the monsoon glacio-hydrological regime of the Himalaya. Cryosphere Discuss. 2016, 1–35. [Google Scholar] [CrossRef]
- Singh, P.; Bengtsson, L. Hydrological sensitivity of a large Himalayan basin to climate change. Hydrol. Process 2004, 18, 2363–2385. [Google Scholar] [CrossRef]
- Su, Y.; Wu, Z.; Xie, P.; Zhang, L.; Chen, H. Warming effects on topsoil organic carbon and C: N: P stoichiometry in a subtropical forested landscape. Forests 2020, 11, 66. [Google Scholar] [CrossRef]
- Lee, C.; Chun, J.; Cho, H. Elevational patterns and determinants of plant diversity in the Baekdudaegan Mountains, South Korea: Species vs. functional diversity. Chin. Sci. Bull. 2013, 58, 3747–3759. [Google Scholar] [CrossRef]
- Kromer, T.; Acebey, A.; Kluge, J.; Kessler, M. Effects of altitude and climate in determining elevational plant species richness patterns: A case study from Los Tuxtlas, Mexico. Flora 2013, 208, 197–210. [Google Scholar] [CrossRef]
- Oishi, Y. Factors that shape the elevational patterns of plant diversity in the Yatsugatake Mountains, Japan. Ecol. Evol. 2021, 11, 4887–4897. [Google Scholar] [CrossRef]
- Wu, G.P.; Su, Y.G.; Wang, J.J.; Lin, S.N.; Huang, Z.Y.; Huang, G. Elevational patterns of microbial carbon use efficiency in a subtropical mountain forest. Biol. Fert. Soils 2023, 60, 5–15. [Google Scholar] [CrossRef]
- Su, Y.; Jia, X.; Zhang, L.; Chen, H. Size-dependent associations of woody plant structural diversity with soil C:N:P stoichiometry in a subtropical forest. Front. Environ. Sci. 2022, 10, 990387. [Google Scholar] [CrossRef]
- He, S.; Zhong, Y.; Sun, Y.; Su, Z.; Jia, X.; Hu, Y.; Zhou, Q. Topography-associated thermal gradient predicts warming effects on woody plant structural diversity in a subtropical forest. Sci. Rep. 2017, 7, 40387. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Jia, X.; Xue, Y. Application of several diversity indexes in forest community analysis. Ecol. Sci. 2017, 36, 132–138. [Google Scholar]
- Zhou, Y.; Su, Y.; Zhong, Y.; Xie, P.; Xu, M.; Su, Z. Community attributes predict the relationship between habitat invasibility and land use types in an agricultural and forest landscape. Forests 2019, 10, 867. [Google Scholar] [CrossRef]
- Lai, J.; Mi, X.; Ren, H.; Ma, K.J.J.o.V.S. Species-habitat associations change in a subtropical forest of China. J. Veg. Sci. 2009, 20, 415–423. [Google Scholar] [CrossRef]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Ye, H.G.; Peng, S.L. Plant Diversity Inventory of Guangdong; Guangdong World Publishing Corporation: Guangzhou, China, 2006. [Google Scholar]
- Magurran, A.E.; McGill, B.J. Biological Diversity: Frontiers in Measurement and Assessment; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- McCune, B. Improved estimates of incident radiation and heat load using non-parametric regression against topographic variables. J. Veg. Sci. 2007, 18, 751–754. [Google Scholar]
- Santos, R.M.; Oliveira, A.T.; Eisenlohr, P.V.; Queiroz, L.P.; Cardoso, D.B.O.S.; Rodal, M.J.N. Identity and relationships of the Arboreal Caatinga among other floristic units of seasonally dry tropical forests (SDTFs) of north-eastern and Central Brazil. Ecol. Evol. 2012, 2, 409–428. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Lee, C.B.; Chun, J.H. Patterns and determinants of plant richness by elevation in a mountain ecosystem in South Korea: Area, mid-domain effect, climate and productivity. J. For. Res. 2015, 26, 905–917. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.Y.; Zhang, D.J.; Tian, W.; Huang, H.; Ma, A. Altitudinal patterns of plant species richness in the Honghe region of China. Pak. J. Bot. 2017, 49, 1039–1048. [Google Scholar]
- Gheyret, G.; Guo, Y.P.; Fang, J.Y.; Tang, Z.Y. Latitudinal and elevational patterns of phylogenetic structure in forest communities in China’s mountains. Sci. China Life Sci. 2020, 63, 1895–1904. [Google Scholar] [CrossRef]
- Zhao, M.F.; Wang, Y.H.; Xue, F.; Zuo, W.Y.; Xing, K.X.; Wang, G.Y.; Kang, M.Y.; Jiang, Y. Elevational patterns and ecological determinants of mean family age of angiosperm assemblages in temperate forests within Mount Taibai, China. J. Plant Ecol. 2018, 11, 919–927. [Google Scholar] [CrossRef]
- Ali, A.; Lin, S.L.; He, J.K.; Kong, F.M.; Yu, J.H.; Jiang, H.S. Climatic water availability is the main limiting factor of biotic attributes across large-scale elevational gradients in tropical forests. Sci. Total Environ. 2019, 647, 1211–1221. [Google Scholar] [CrossRef]
- Randin, C.F.; Paulsen, J.; Vitasse, Y.; Kollas, C.; Wohlgemuth, T.; Zimmermann, N.E.; Korner, C. Do the elevational limits of deciduous tree species match their thermal latitudinal limits? Global Ecol. Biogeogr. 2013, 22, 913–923. [Google Scholar] [CrossRef]
- Halbritter, A.H.; Alexander, J.M.; Edwards, P.J.; Billeter, R. How comparable are species distributions along elevational and latitudinal climate gradients? Global Ecol. Biogeogr. 2013, 22, 1228–1237. [Google Scholar] [CrossRef]
- Zlatanov, T.; Schleppi, P.; Velichkov, I.; Hinkov, G.; Georgieva, M.; Eggertsson, O.; Zlatanova, M.; Vacik, H. Structural diversity of abandoned chestnut (Castanea sativa Mill.) dominated forests: Implications for forest management. Forest Ecol. Manag. 2013, 291, 326–335. [Google Scholar] [CrossRef]
- Xu, M.H.; Zhang, S.X.; Wen, J.; Yang, X.Y. Multiscale spatial patterns of species diversity and biomass together with their correlations along geographical gradients in subalpine meadows. PLoS ONE 2019, 14, e0211560. [Google Scholar] [CrossRef]
- Maharjan, S.K.; Sterck, F.J.; Raes, N.; Poorter, L. Temperature and soils predict the distribution of plant species along the Himalayan elevational gradient. J. Trop. Ecol. 2022, 38, 58–70. [Google Scholar] [CrossRef]
- Monge-Gonzalez, M.L.; Craven, D.; Kromer, T.; Castillo-Campos, G.; Hernandez-Sanchez, A.; Guzman-Jacob, V.; Guerrero-Ramirez, N.; Kreft, H. Response of tree diversity and community composition to forest use intensity along a tropical elevational gradient. Appl. Veg. Sci. 2020, 23, 69–79. [Google Scholar] [CrossRef]
- Ramos, C.S.; Picca, P.; Pocco, M.E.; Filloy, J. Disentangling the role of environment in cross-taxon congruence of species richness along elevational gradients. Sci. Rep. 2021, 11, 4711. [Google Scholar] [CrossRef]
- Knight, K.S.; Oleksyn, J.; Jagodzinski, A.M.; Reich, P.B.; Kasprowicz, M. Overstorey tree species regulate colonization by native and exotic plants: A source of positive relationships between understorey diversity and invasibility. Divers. Distrib. 2008, 14, 666–675. [Google Scholar] [CrossRef]
- Urza, A.K.; Weisberg, P.J.; Chambers, J.C.; Sullivan, B.W. Shrub facilitation of tree establishment varies with ontogenetic stage across environmental gradients. New Phytol. 2019, 223, 1795–1808. [Google Scholar] [CrossRef]
- Zhong, Y.; Sun, Y.; Xu, M.; Zhang, Y.; Wang, Y.; Su, Z. Spatially destabilising effect of woody plant diversity on forest productivity in a subtropical mountain forest. Sci. Rep. 2017, 7, 9551. [Google Scholar] [CrossRef]
- Sharma, C.M.; Mishra, A.K.; Tiwari, O.P.; Krishan, R.; Rana, Y.S. Regeneration patterns of tree species along an elevational gradient in the Garhwal Himalaya. Mt. Res. Dev. 2018, 38, 211–219. [Google Scholar] [CrossRef]
- Ke, X.; Su, Z.; Hu, Y.; Zhou, Y.; Xu, M. Measuring species diversity in a subtropical forest across a tree size gradient: A comparison of diversity indices. Pak. J. Bot. 2017, 49, 1373–1379. [Google Scholar]
- He, J.; Chen, W. A review of gradient changes in species diversity of land plant communities. Acta Ecol. Sin. 1997, 17, 91–99. [Google Scholar]


| Attribute | Community | γ-Diversity | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Mean elevation (m) | 576.9 | 433.2 | 239.5 | |
| Number of stems | 4177 | 3683 | 3289 | 11,149 |
| Number of species | 85 | 89 | 66 | 152 |
| Number of genera | 59 | 72 | 53 | 105 |
| Number of families | 36 | 43 | 29 | 54 |
| Total basal area (m2/ha) | 30.389 | 21.183 | 24.537 | |
| Average basal area (cm2/tree) | 72.75 | 57.52 | 74.60 | |
| Dominant species | Schima superba, Itea chinensis Machilus chinensis | Schima superba Aporosa dioica Machilus chinensis | Itea chinensis Schima superba Castanopsis fissa | |
| Communities Compared | T | A | p |
|---|---|---|---|
| Overall comparison | −119.0 | 0.12 | <10−7 |
| Pairwise comparison | |||
| Community 1 vs. Community 2 | −91.47 | 0.10 | <10−7 |
| Community 1 vs. Community 3 | −72.64 | 0.08 | <10−7 |
| Community 2 vs. Community 3 | −80.15 | 0.09 | <10−7 |
| Indicator Species | Indicated Community | Observed Indicator Value | Indicator Value from Randomized Groups | p-Value | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| Adina pilulifera (Lam.) Franch. ex Drake | 1 | 53 | 12.4 | 1.91 | 0.001 |
| Dendropanax proteus (Harmp.) Benth. | 1 | 44 | 7.5 | 1.67 | 0.001 |
| Diospyros morrisiana Hance | 1 | 59 | 9.3 | 1.72 | 0.001 |
| Enkianthus quinqueflorus Lour. | 1 | 29 | 5.4 | 1.36 | 0.001 |
| Machilus chinensis (Benth.) Hemsl. | 1 | 53.2 | 21.1 | 2.24 | 0.001 |
| Rapanea neriifolia (S. et Z.) Mez | 1 | 55 | 9.1 | 1.99 | 0.001 |
| Rhodoleia championii Benth. | 1 | 77 | 11.6 | 2.0 | 0.001 |
| Schima superba Gardner et Champ. | 1 | 35.8 | 30.6 | 2.01 | 0.006 |
| Symplocos crassilimba Merr. | 1 | 35 | 6.3 | 1.5 | 0.001 |
| Acronychia pedunculata (L.) Miq. | 2 | 34.1 | 10.9 | 1.94 | 0.001 |
| Adenanthera pavonina L. | 2 | 53.6 | 8.9 | 1.72 | 0.001 |
| Aporosa dioica (Roxb.) Muell.-Arg. | 2 | 58.3 | 15.2 | 2.15 | 0.001 |
| Ardisia quinquegona Blume | 2 | 33.2 | 7.3 | 1.79 | 0.001 |
| Evodia meliaefolia Benth. | 2 | 27.7 | 5.5 | 1.42 | 0.001 |
| Psychotria rubra (Lour.) Poir. | 2 | 53.6 | 13.2 | 2.07 | 0.001 |
| Scolopia saeva (Hance) Hance | 2 | 33 | 6 | 1.39 | 0.001 |
| Syzygium jambos (L.) Alston | 2 | 35 | 6.2 | 1.56 | 0.001 |
| Syzygium rehderianum Merr. et Perry | 2 | 61 | 9.4 | 1.69 | 0.001 |
| Viburnum odoratissimum Ker. | 2 | 29 | 5.3 | 1.37 | 0.001 |
| Castanopsis fissa Rehder and E.H.Wilson | 3 | 46 | 7.7 | 1.69 | 0.001 |
| Itea chinensis Hook. et Arn. | 3 | 46.8 | 26.1 | 2.14 | 0.001 |
| Sapium discolor (Champ.) Muell.-Arg. | 3 | 25.9 | 7.3 | 1.55 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Gan, X.; Zhang, W.; Wu, G.; Huang, F. Woody Plant Structural Diversity Changes across an Inverse Elevation-Dependent Warming Gradient in a Subtropical Mountain Forest. Forests 2024, 15, 1051. https://doi.org/10.3390/f15061051
Su Y, Gan X, Zhang W, Wu G, Huang F. Woody Plant Structural Diversity Changes across an Inverse Elevation-Dependent Warming Gradient in a Subtropical Mountain Forest. Forests. 2024; 15(6):1051. https://doi.org/10.3390/f15061051
Chicago/Turabian StyleSu, Yuqiao, Xianhua Gan, Weiqiang Zhang, Guozhang Wu, and Fangfang Huang. 2024. "Woody Plant Structural Diversity Changes across an Inverse Elevation-Dependent Warming Gradient in a Subtropical Mountain Forest" Forests 15, no. 6: 1051. https://doi.org/10.3390/f15061051




