Siberian Pine and Larch Response to Warming-Drying Climate in the Southern Boundary of Their Range
Abstract
:1. Introduction
- What are the Siberian pine and larch radial growth dynamics within the treeline, mixewood forest, and forest-steppe ecotone?
- How do larch and Siberian pine growth depend on the air temperature, moisture variables (precipitation, VPD, soil moisture), and wind speed along the elevation gradient?
- What are the differences between larch and Siberian pine’s responses to the changing climate?
2. Materials and Methods
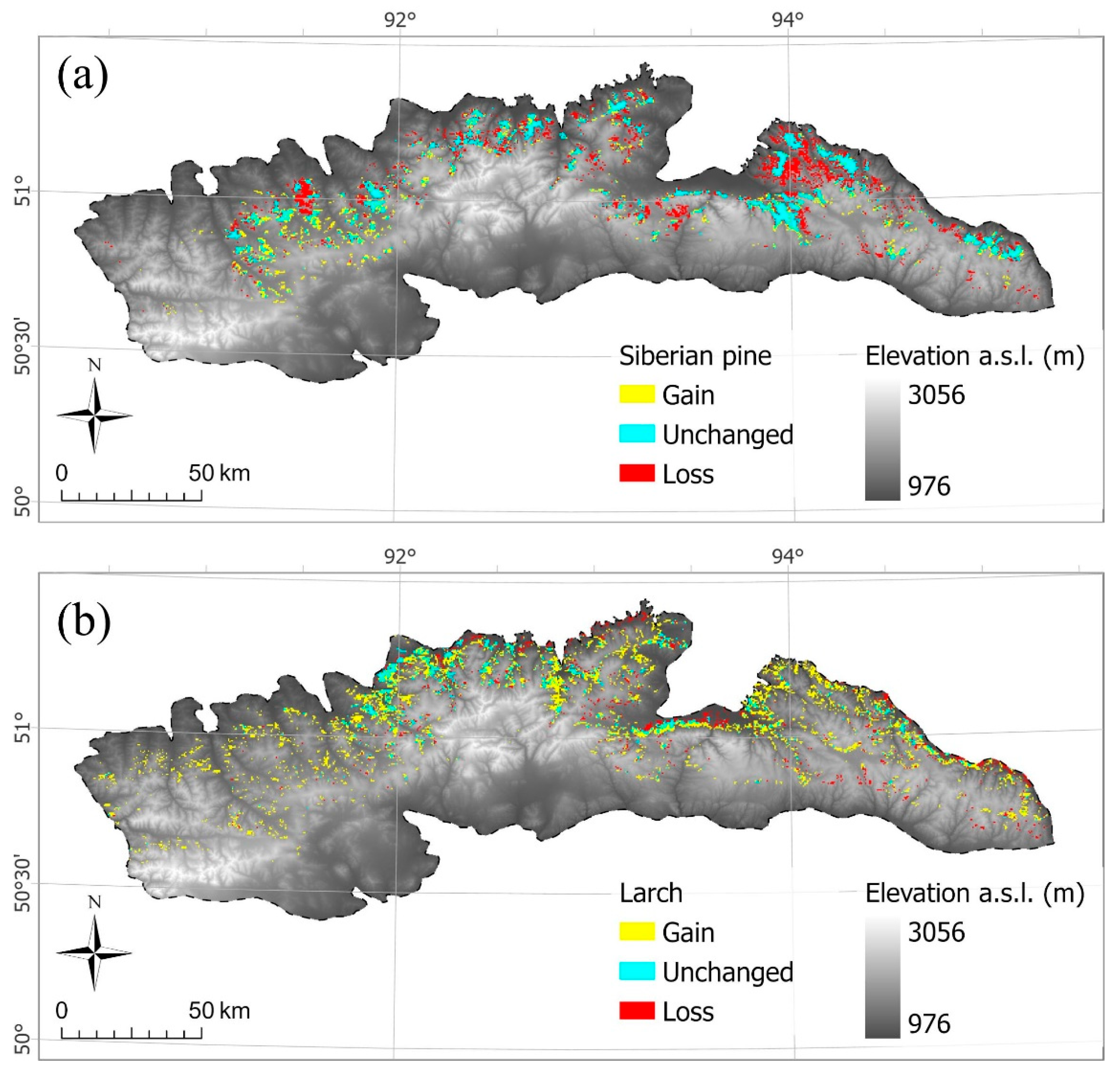
2.1. Study Area
2.2. On-Ground Studies
2.3. Climate Variables
2.4. Dendrochronological Analysis
2.5. Satellite Data Analysis
2.5.1. Landsat-Based Analysis along the Elevation Transect
2.5.2. MODIS-Based Analysis of the Land Cover Classes in the Tannu-Ola Ridge
2.6. Statistical Analysis
3. Results
3.1. Climate Variables’ Dynamic
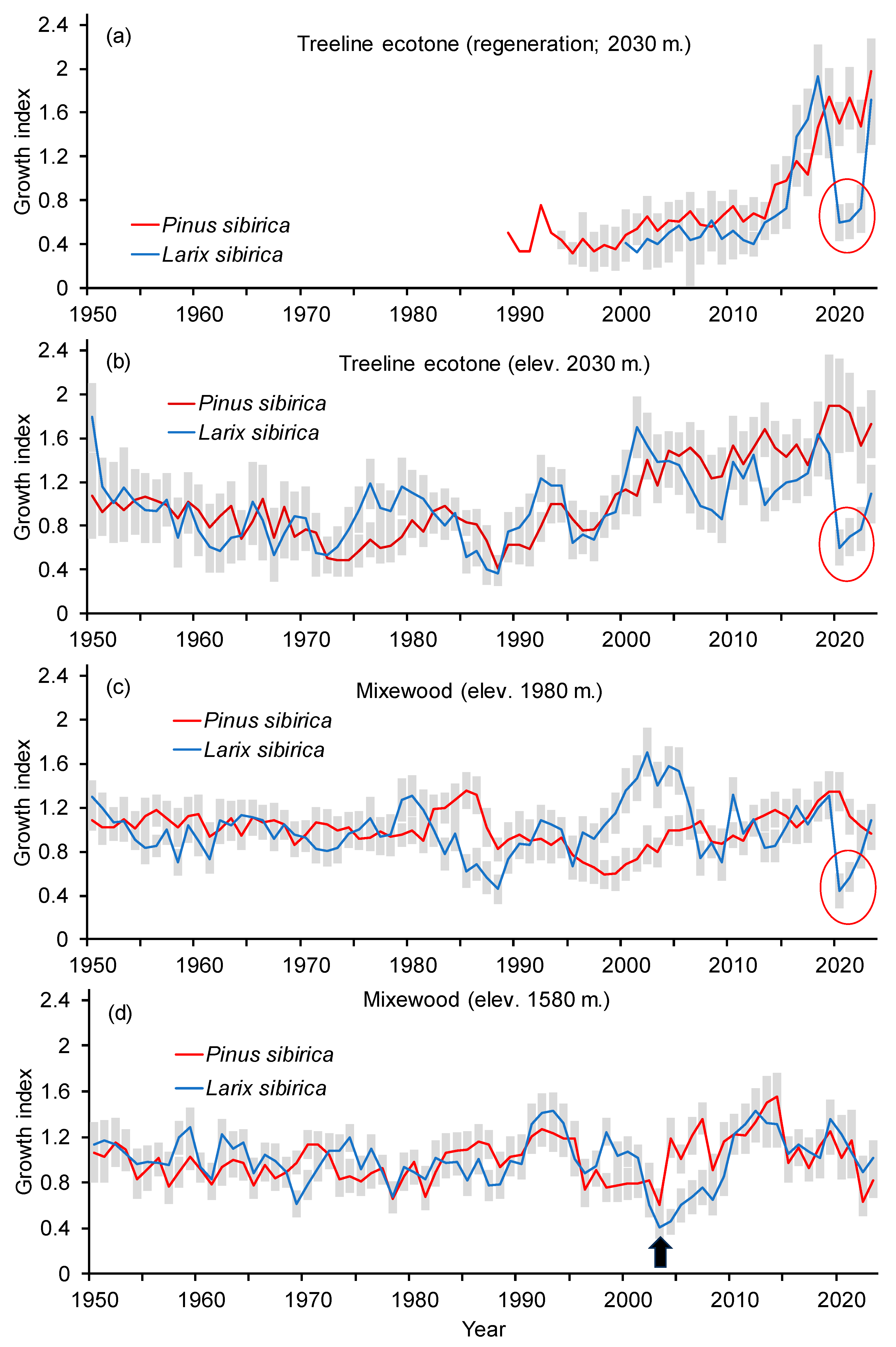
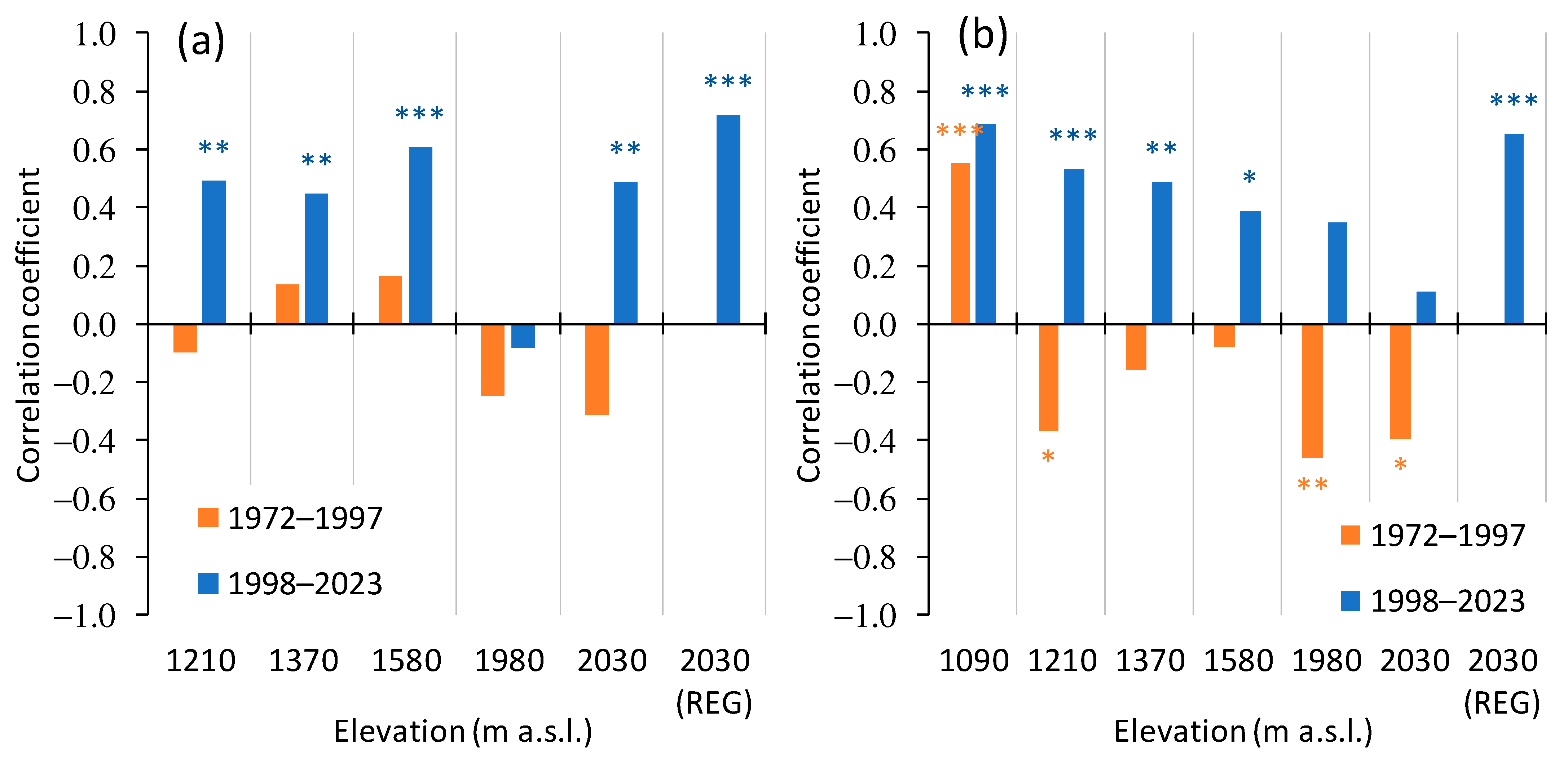
3.2. Trees Growth Dynamics
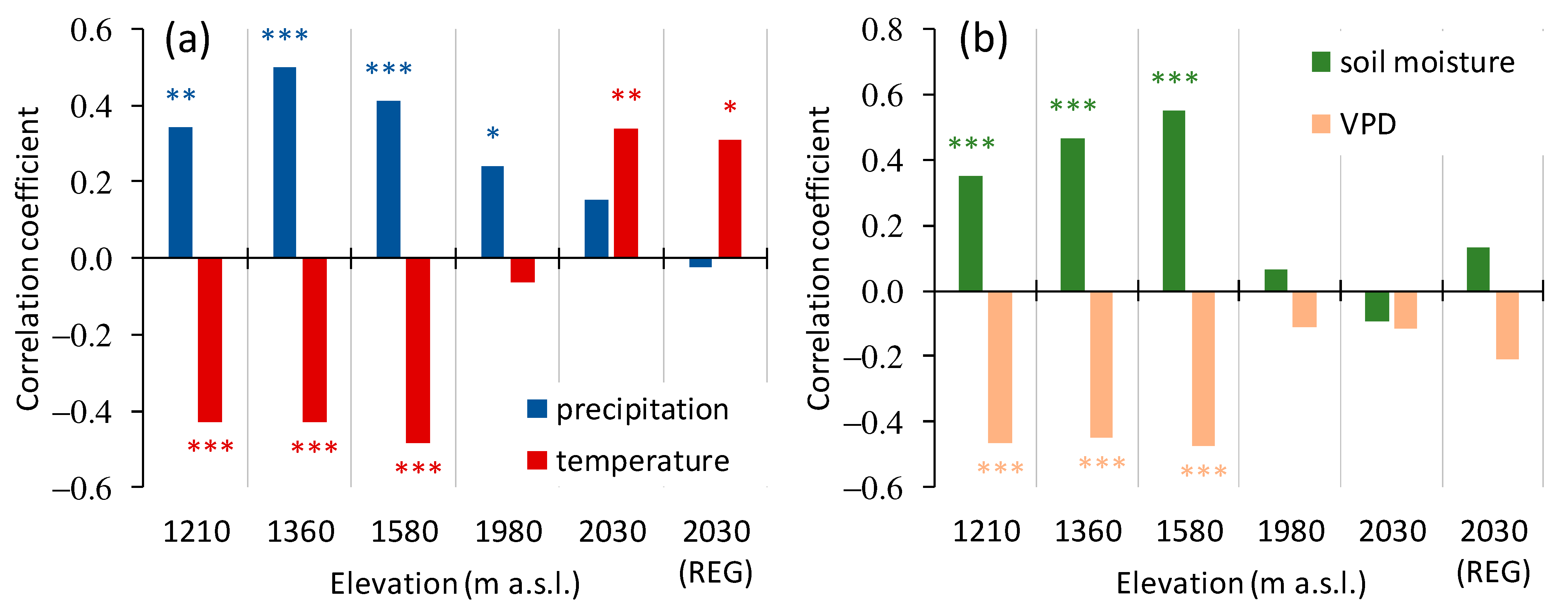
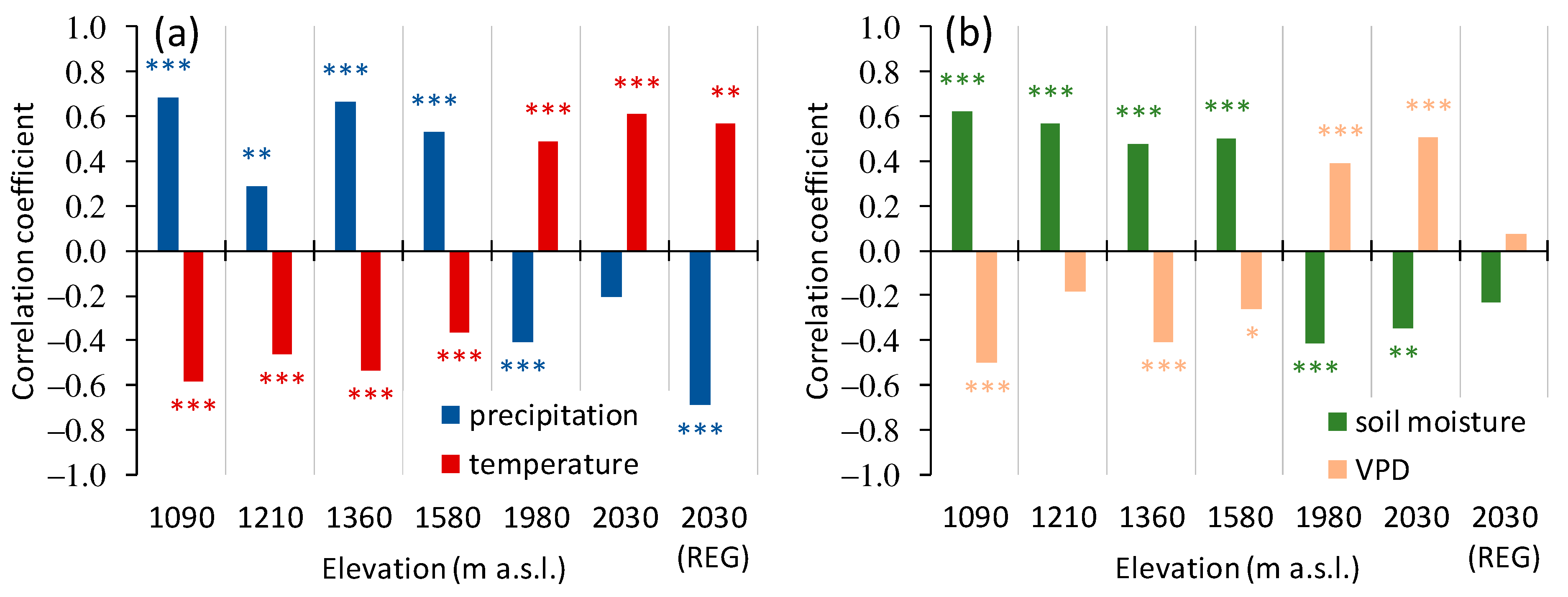
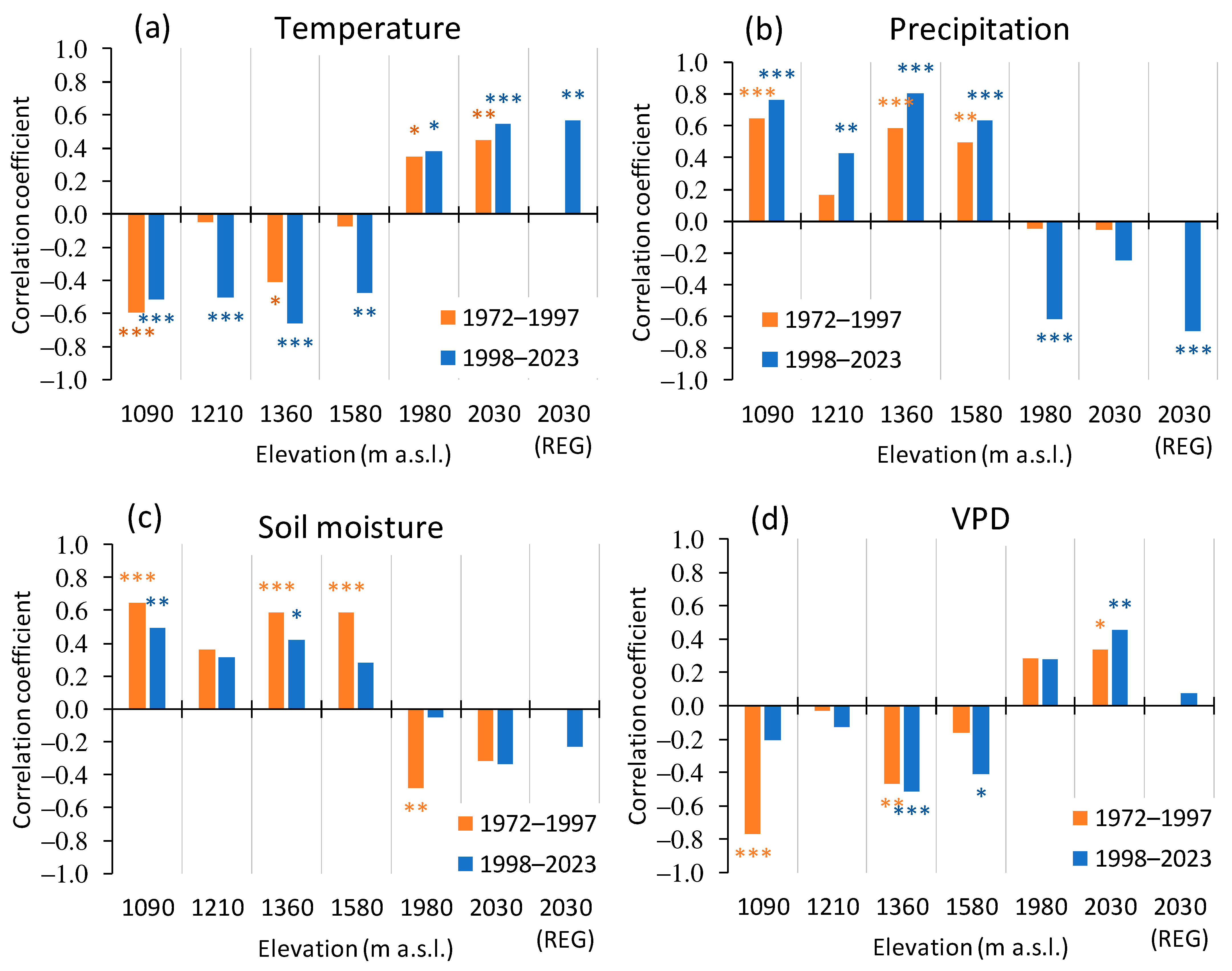
3.3. Trees Growth Dependence on the Climate Variables
3.3.1. A General Pattern of Siberian Pine and Larch Growth along the Elevation Gradient
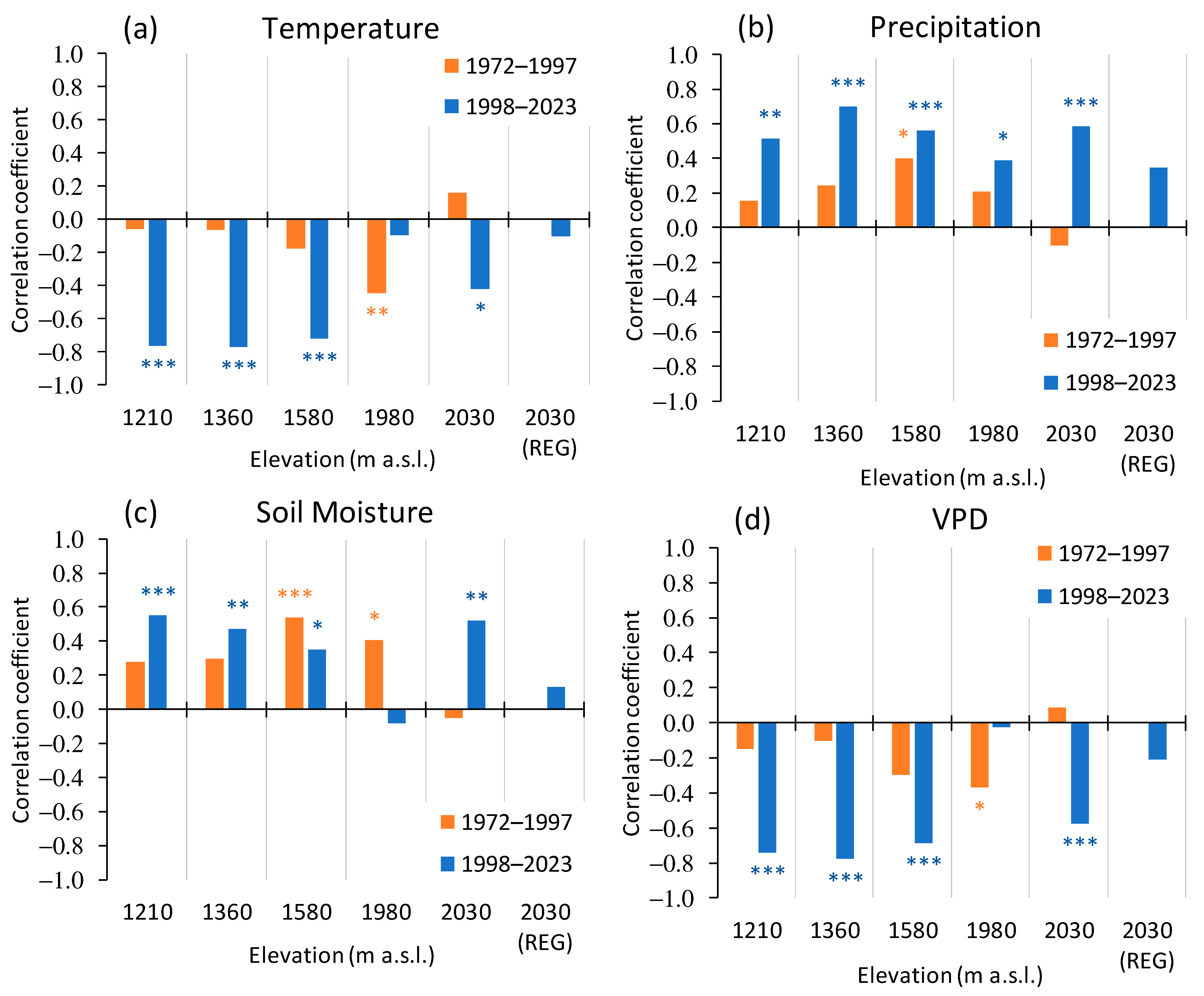
3.3.2. Siberian Pine and Larch Growth since the Warming Restart
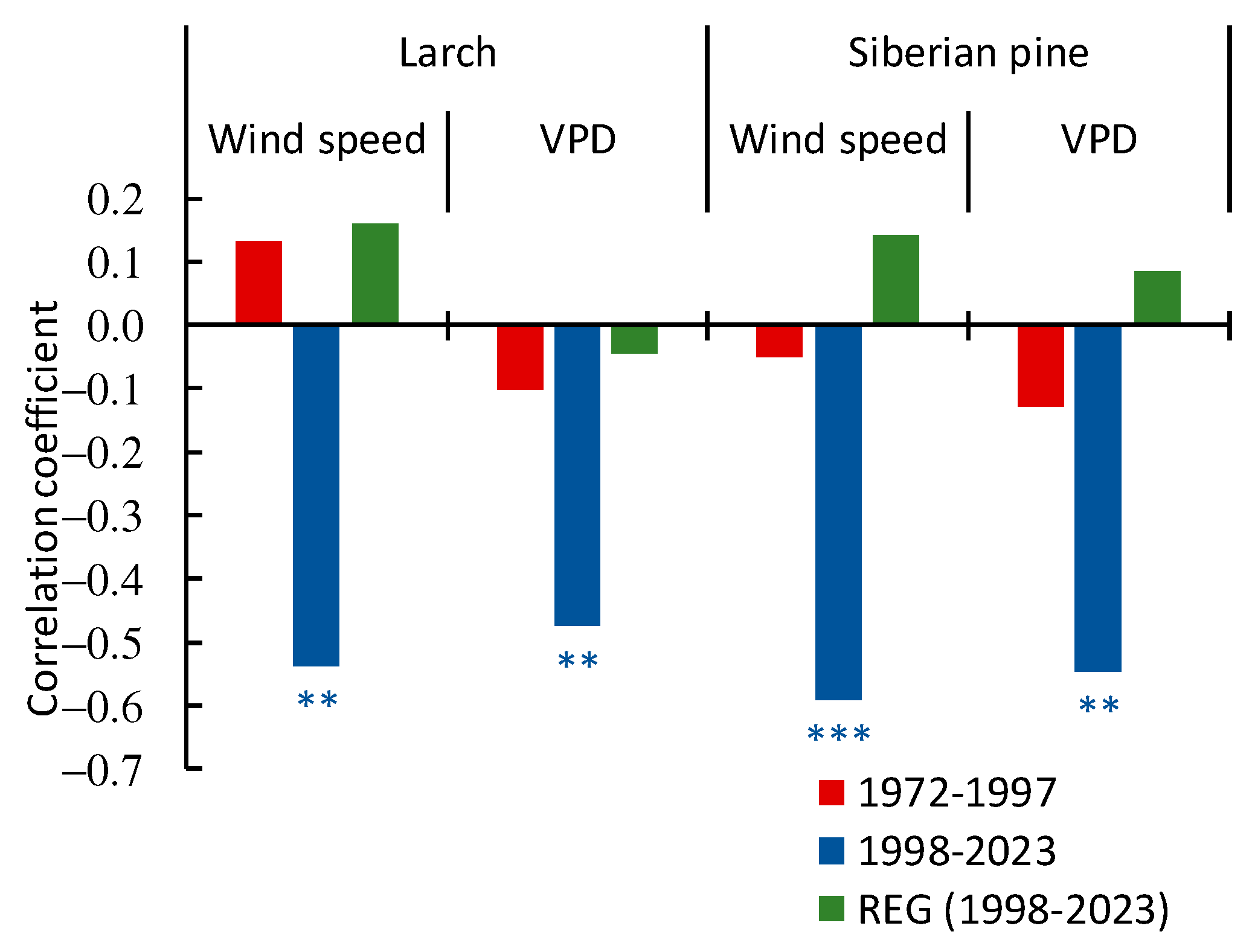
3.3.3. Winter Winds and VPD Influence on the Tree’s Growth
3.4. The Satellite Data Analysis
3.4.1. The Landsat-Based Analysis along the On-Ground Transect
3.4.2. The MODIS-Based Analysis of Land Cover Changes in the Tannu-Ola Ridge
4. Discussion
4.1. General Pattern of Trees Growth along the Elevation Gradient
4.2. Siberian Pine and Larch Growth since the Warming Restart
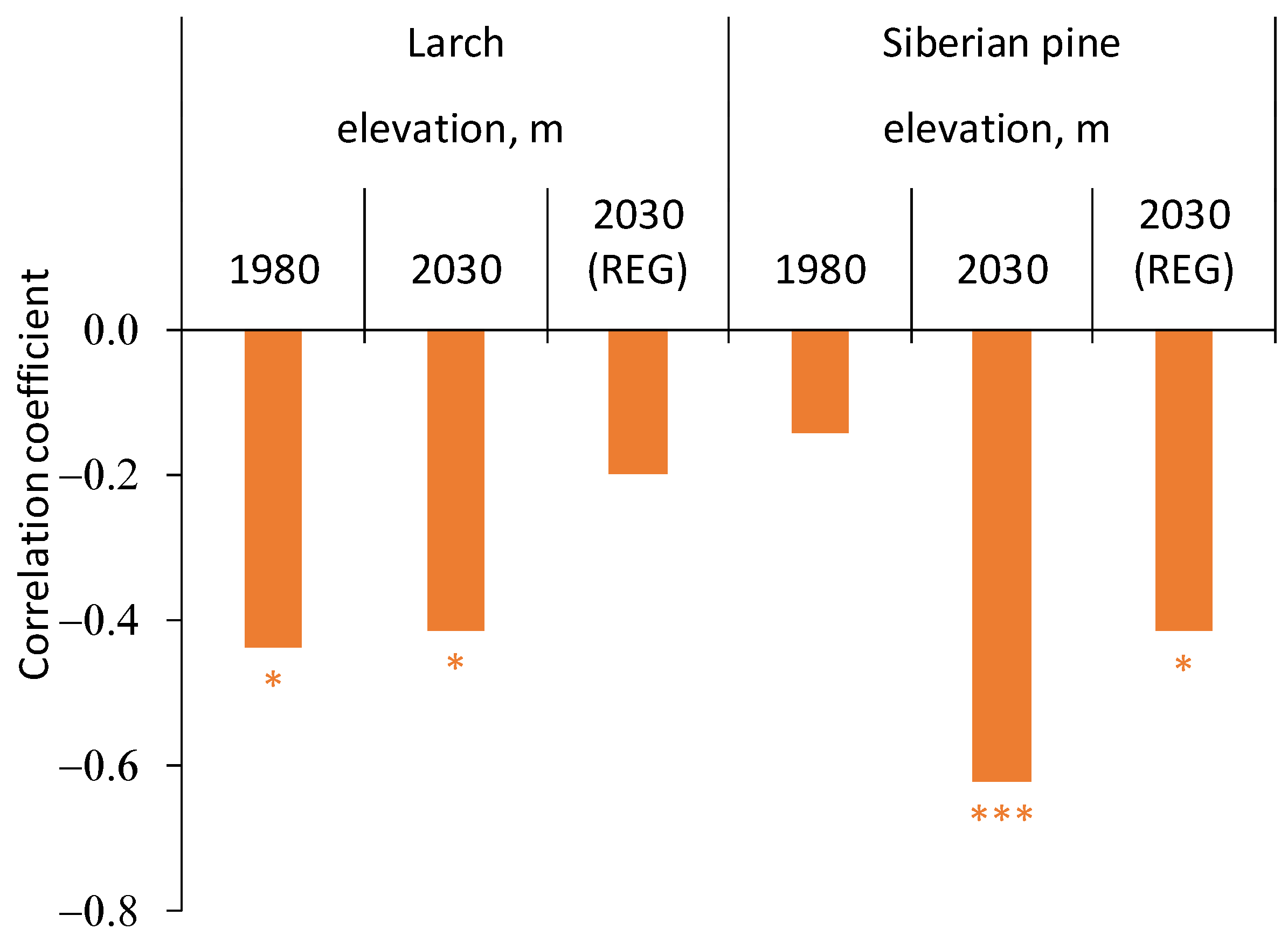
4.2.1. Siberian Pine and Larch Regeneration
4.2.2. Winter Winds and VPD Influence on the Trees and Regeneration
4.2.3. Early Springs Warming Influence on the Tree’s Growth
4.3. Comparative Dynamic of Land Cover Classes in the Tannu-Ola Ridge
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2009, 259, 660–684. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Wu, C.; Acil, N.; Carvalhais, N.; Pugh, T.A.M.; Sadler, J.P.; Seidl, R. A climate risk analysis of Earth’s forests in the 21st century. Science 2022, 377, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Boonman, C.C.F.; Serra-Diaz, J.M.; Hoeks, S.; Guo, W.-Y.; Enquist, B.J.; Maitner, B.; Malhi, Y.; Merow, C.; Buitenwerf, R.; Svenning, J.-C. More than 17,000 tree species are at risk from rapid global change. Nat. Commun. 2024, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.A.; Régnière, J.; Powell, J.A. Assessing the impacts of global warming on forest pest dynamics. Front. Ecol. Environ. 2003, 1, 130–137. [Google Scholar] [CrossRef]
- Guarin, A.; Taylor, A.H. Drought triggered tree mortality in mixed conifer forests in Yosemite National Park, California, USA. For. Ecol. Manag. 2005, 218, 229–244. [Google Scholar] [CrossRef]
- Redmond, M.D.; Barger, N.N. Tree regeneration following drought- and insect-induced mortality in piñon–juniper woodlands. New Phytol. 2013, 200, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Lloret, F.; Breshears, D.D. Drought-induced forest decline: Causes, scope and implications. Biol. Lett. 2012, 8, 689–691. [Google Scholar] [CrossRef]
- Neumann, M.; Mues, V.; Moreno, A.; Hasenauer, H.; Seidl, R. Climate variability drives recent tree mortality in Europe. Glob. Change Biol. 2017, 23, 4788–4797. [Google Scholar] [CrossRef]
- Yousefpour, R.; Hanewinkel, M.; Le Moguédec, G. Evaluating the suitability of management strategies of pure Norway spruce forests in the black forest area of Southwest Germany for adaptation to or mitigation of climate change. Environ. Manag. 2010, 45, 387–402. [Google Scholar] [CrossRef]
- Hlásny, T.; Barka, I.; Kulla, L.; Bucha, T.; Sedmák, R.; Trombik, J. Sustainable forest management in a mountain region in the Central Western Carpathians, northeastern Slovakia: The role of climate change. Reg. Environ. Change 2017, 17, 65–77. [Google Scholar] [CrossRef]
- Zamolodchikov, D.G. Evaluation of climate-induced changes in diversity of tree species according to forest fund data records. Biol. Bull. Rev. 2011, 131, 382–392. (In Russian) [Google Scholar]
- Goulden, M.L.; Bales, R.C. California forest die-off linked to multi-year deep soil drying in 2012–2015 drought. Nat. Geosci. 2019, 12, 632–637. [Google Scholar] [CrossRef]
- Harvey, J.E.; Batllori, E.; Lloret, F.; Aakala, T.; Anderegg, W.R.L.; Aynekulu, E.; Bendixsen, D.P.; Bentouati, A.; Bigler, C.; Burk, C.J.; et al. Forest and woodland replacement patterns following drought-related mortality. Proc. Natl. Acad. Sci. USA 2020, 117, 29720–29729. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, D.; de Boer, W.F.; Henkens, R.J.H.G.; Sass-Klaassena, U.G.W. Water availability as driver of birch mortality in Hustai National Park, Mongolia. Dendrochronologia 2018, 49, 127–133. [Google Scholar] [CrossRef]
- Boyd, M.A.; Berner, L.T.; Doak, P.; Goetz, S.J.; Rogers, B.M.; Wagner, D.; Walker, X.J.; Mack, M.C. Impacts of climate and insect herbivory on productivity and physiology of trembling aspen (Populus tremuloides) in Alaskan boreal forests. Environ. Res. Lett. 2019, 14, 085010. [Google Scholar] [CrossRef]
- Etzold, S.; Ziemińska, K.; Rohner, B.; Bottero, A.; Bose, A.K.; Ruehr, N.K.; Zingg, A.; Rigling, A. One century of forest monitoring data in Switzerland reveals species—And site-specific trends of climate-induced tree mortality. Front. Plan. Sci. 2019, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Neycken, A.; Scheggia, M.; Bigler, C.; L’evesque, M. Long-term growth decline precedes sudden crown dieback of European beech. Agric. For. Meteorol. 2022, 324, 109103. [Google Scholar] [CrossRef]
- Mamet, S.D.; Carissa, D.B.; Andrew, J.T.; Colin, P.L. Shifting global Larix distributions: Northern expansion and southern retraction as species respond to changing climate. J. Biogeogr. 2018, 46, 30–44. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Petrov, I.A.; Krivobokov, L.V.; Golyukov, A.S.; Dvinskaya, M.L.; Im, S.T.; Shushpanov, A.S.; Smith, K.T. Larch response to warming in northern Siberia. Reg. Environ. Change 2023, 23, 17. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Petrov, I.A.; Golyukov, A.S.; Dvinskaya, M.L.; Im, S.T.; Shushpanov, A.S. Larch growth across thermal and moisture gradients in the Siberian Mountains. J. Mt. Sci. 2023, 20, 101–114. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Im, S.T.; Petrov, I.A.; Dvinskaya, M.L.; Shushpanov, A.S.; Golyukov, A.S. Climate-driven conifer mortality in Siberia. Glob. Ecol. Biog. 2021, 30, 543–556. [Google Scholar] [CrossRef]
- Shiyatov, S.G.; Terent’ev, M.M.; Fomin, V.V.; Zimmermann, N.E. Altitudinal and horizontal shifts of the upper boundaries of open and closed forests in the Polar Urals in the 20th century. Russ. J Ecol. 2007, 38, 223–227. [Google Scholar] [CrossRef]
- Dial, R.J.; Maher, C.T.; Hewitt, R.E.; Sullivan, P.F. Sufficient conditions for rapid range expansion of a boreal conifer. Nature 2022, 608, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Penuelas, J.; Camarero, J.J.; Zeng, X.; Liu, X.; Zhao, L.; Xu, G.; Wang, L. Recent shift from dominant nitrogen to CO2 fertilization control on the growth of mature Qinghai spruce in China’s Qilian Mountains. Agric. For. Meteorol. 2023, 343, 109779. [Google Scholar] [CrossRef]
- Kloeppel, B.D.; Treichel, I.W.; Kharuk, S.; Gower, S.T. Foliar carbon isotope discrimination in Larix species and sympatric evergreen conifers: A global comparison. Oecologia 1998, 114, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kirdyanov, A.V.; Prokushkin, A.S.; Tabakova, M.A. Tree-ring growth of Gmelin larch under contrasting local conditions in the north of Central Siberia. Dendrochronologia 2013, 31, 114–119. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Kolb, T.E.; Fettig, C.J.; Ayres, M.P.; Bentz, B.J.; Hicke, J.A.; Mathiasen, R.; Stewart, J.E.; Weed, A.S. Observed and anticipated impacts of drought on forest insects and diseases in the United States. For. Ecol. Manag. 2016, 380, 321–324. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Petrov, I.A.; Golyukov, A.S.; Im, S.T.; Shushpanov, A.S. Mountain Taiga in a Warming Climate: Contrast of Siberian Pine Growth along an Elevation Gradient. Forests 2024, 15, 50. [Google Scholar] [CrossRef]
- Makunina, N.I. Main types of plant communities of the steppe belt of the southern macroslope of the Tannu-Ola ranges. Flora Veg. Asian Russ. 2010, 1, 49–57. (In Russian) [Google Scholar]
- Makunina, N.I. Botanical and geographical characteristics of the forest-steppe of the Altai-Sayan Mountain region. Contemp. Probl. Ecol. 2016, 9, 342–348. [Google Scholar] [CrossRef]
- Rinn, F. TSAP V 3.6 Reference Manual: Computer Program for Tree-Ring Analysis and Presentation; Frank Rinn Distribution: Heidelberg, Germany, 1996. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 44, 69–75. [Google Scholar]
- Cook, E.R.; Holmes, R.L. Chronology Development, Statistical Analysis. Guide for Computer Program ARSTAN; Laboratory of Tree-Ring Research, The University of Arizona: Tucson, AZ, USA, 1986; pp. 50–65. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar]
- Riano, D.; Chuvieco, E.; Salas, J.; Aguado, I. Assessment of different topographic corrections in Landsat-TM data for mapping vegetation types. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1056–1061. [Google Scholar] [CrossRef]
- Tyukavina, A.; Potapov, P.; Hansen, M.C.; Pickens, A.H.; Stehman, S.V.; Turubanova, S.; Parker, D.; Zalles, V.; Lima, A.; Kommareddy, I.; et al. Global Trends of Forest Loss Due to Fire from 2001 to 2019. Front. Remote Sens. 2022, 3, 825190. [Google Scholar] [CrossRef]
- Congalton, R.; Green, K. Assessing the Accuracy of Remotely Sensed Data: Principles and Practices, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019; p. 346. [Google Scholar] [CrossRef]
- Sulla-Menashe, D.; Gray, J.M.; Abercrombie, S.P.; Friedl, M.A. Hierarchical mapping of annual global land cover 2001 to present: The MODIS Collection 6 Land Cover product. Remote Sens. Environ. 2019, 222, 183–194. [Google Scholar] [CrossRef]
- Stephenson, N.L.; Das, A.J.; Ampersee, N.J.; Bulaon, B.M.; Yee, J.L. Which trees die during drought? The key role of insect host-tree selection. J. Ecol. 2019, 107, 2383–2401. [Google Scholar] [CrossRef]
- Vorontsov, A.I. Forest Entomology; Higher School: Moscow, Russia, 1982; 384p. (In Russian) [Google Scholar]
- Kharuk, V.I.; Petrov, I.A.; Im, S.T.; Golyukov, A.S.; Dvinskaya, M.L.; Shushpanov, A.S. Tree Clusters Migration into Alpine Tundra, Siberia. J. Mt. Sci. 2022, 19, 3426–3440. [Google Scholar] [CrossRef]
- Shiyatov, S.G.; Terent’ev, M.M.; Fomin, V.V. Spatiotemporal dynamics of forest-tundra communities in the Polar Urals. Russ. J. Ecol. 2005, 36, 69–75. [Google Scholar] [CrossRef]
- Kullman, L. Tree line population monitoring of Pinus sylvestris in the Swedish Scandes, 1973–2005: Implications for tree line theory and climate change ecology. J. Ecol. 2007, 95, 41–52. [Google Scholar] [CrossRef]
- Franke, A.; Feilhauer, H.; Bräuning, A.; Rautio, P.; Braun, M. Remotely sensed estimation of vegetation shifts in the polar and alpine treeline ecotone in Finnish Lapland during the last three decades. For. Ecol. Manag. 2019, 454, 117668. [Google Scholar] [CrossRef]
- Lu, X.; Liang, E.; Wang, Y.; Babst, F.; Camarero, J.J. Mountain treelines climb slowly despite rapid climate warming. Glob. Ecol. Biogeogr. 2021, 30, 305–315. [Google Scholar] [CrossRef]
- Holtmeier, F.K.; Broll, G. Wind as an ecological agent at treelines in North America, the Alps, and the European subarctic. Phys. Geogr. 2010, 31, 203–233. [Google Scholar] [CrossRef]
- Holtmeier, F.K. Mountain Timberlines: Ecology, Patchiness, and Dynamics; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2009; pp. 1–437. [Google Scholar] [CrossRef]
- Sakai, A. Temperature fluctuations in wintering trees. Physiol. Plant 1966, 19, 105–114. [Google Scholar] [CrossRef]
- Sindewald, L.; Tomback, D. Recruitment at treeline in the Central Rocky Mountains shifts in favor of a drought-tolerant species as climate water deficit increases. In Proceedings of the EGU General Assembly 2024, Vienna, Austria, 14–19 April 2024. EGU24-14114. [Google Scholar] [CrossRef]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.M.; Ruthrof, K.X.; et al. Climate change risks to global forest health: Emergence of unexpected events of elevated tree mortality worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef]






| Test Plot | Elevation, m | Coordinates | Canopy Closure | Species Composition | Tree Species | Tree Density, n/ha | Mean Tree’s Height, m | Mean Tree’s DBH, cm | Mean Tree’s Age, Years | Regeneration, n/ha |
|---|---|---|---|---|---|---|---|---|---|---|
| Treeline ecotone | ||||||||||
| 1 | 2030 | 50°59′ N 94°23′ E | <0.3 | 7P3L | Larix sibirica | 100 | 4 | 15 | 80 | 1300 |
| Pinus sibirica | 150 | 5 | 25 | 80 | 1100 | |||||
| Mixewood | ||||||||||
| 2 | 1980 | 51°00′ N 94°23′ E | 0.7 | 6P4L | Larix sibirica | 500 | 18.5 | 35 | 150 | 0 |
| Pinus sibirica | 400 | 17.5 | 35 | 85 | 700 | |||||
| 3 | 1580 | 51°00′ N 94°26′ E | 0.4 | 6P4L | Larix sibirica | 250 | 16.5 | 27 | 110 | 0 |
| Pinus sibirica | 400 | 14.5 | 30 | 75 | 800 | |||||
| 4 | 1360 | 51°01′ N 94°29′ E | 0.4 | 6L4P | Larix sibirica | 500 | 22.5 | 35 | 130 | 0 |
| Pinus sibirica | 400 | 17.5 | 30 | 80 | 1050 | |||||
| 5 | 1210 | 51°01′ N 94°31′ E | 0.6 | 6Ls4P | Larix sibirica | 330 | 17.5 | 25 | 180 | 0 |
| Pinus sibirica | 250 | 17.5 | 28 | 120 | 1125 | |||||
| Forest-steppe ecotone | ||||||||||
| 6 | 1090 | 51°04′ N 94°36′ E | 0.5 | 10L | Larix sibirica | 500 | 15 | 30 | 55 | 22,000 |
| Test Plot | Elevation, m | Tree Species | Mean Age, Years | Total Length of Chronology | Sample Depth | EPS | First Order Autocorrelation | Mean Sensitivity |
|---|---|---|---|---|---|---|---|---|
| 1 | 2030 (REG) | Larix sibirica | 14 | 2000–2023 | 26 | 0.93 | 0.63 | 0.26 |
| Pinus sibirica | 21 | 1989–2023 | 25 | 0.93 | 0.80 | 0.20 | ||
| 2 | 2030 | Larix sibirica | 80 | 1798–2023 | 23 | 0.97 | 0.75 | 0.24 |
| Pinus sibirica | 77 | 1894–2023 | 18 | 0.95 | 0.91 | 0.17 | ||
| 3 | 1980 | Larix sibirica | 136 | 1720–2023 | 20 | 0.97 | 0.61 | 0.19 |
| Pinus sibirica | 85 | 1888–2023 | 17 | 0.93 | 0.62 | 0.12 | ||
| 4 | 1580 | Larix sibirica | 111 | 1850–2023 | 24 | 0.97 | 0.59 | 0.19 |
| Pinus sibirica | 73 | 1932–2023 | 20 | 0.97 | 0.63 | 0.15 | ||
| 5 | 1360 | Larix sibirica | 130 | 1793–2023 | 24 | 0.98 | 0.60 | 0.27 |
| Pinus sibirica | 81 | 1886–2023 | 23 | 0.96 | 0.72 | 0.18 | ||
| 6 | 1210 | Larix sibirica | 178 | 1789–2023 | 21 | 0.97 | 0.78 | 0.22 |
| Pinus sibirica | 117 | 1812–2023 | 20 | 0.96 | 0.79 | 0.19 | ||
| 7 | 1090 | Larix sibirica | 55 | 1946–2023 | 36 | 0.99 | 0.55 | 0.37 |
| Class Name | MODIS MOD12Q1 IGBP Class Name | Description | |
|---|---|---|---|
| 1 | Siberian pine stands | Evergreen needleleaf forests | Dominated by Siberian pine (tree cover > 60 %) |
| 2 | Larch stands | Deciduous needleleaf forests | Dominated by larch (tree cover > 60 %) |
| 3 | Birch stands | Deciduous broadleaf forests | Dominated by birch trees (tree cover > 60 %) |
| 4 | Mixed stands | Mixed forests + woody savannas | Dominated by neither deciduous nor evergreen (40–60% of each) tree types (tree cover > 60 %) |
| 5 | Shrubs and grasses | Closed and open shrublands + grasslands + permanent wetlands | Dominated by shrublands and grasslands |
| 6 | Background | Barren + water bodies | No vegetation |
| Class Name | Area in 2001–2003 (sq. km) | Area in 2020–2022 (sq. km) | Δ (sq. km) | Δ (% of 2001–2003) | |
|---|---|---|---|---|---|
| 1 | Siberian pine stands | 1235.8 | 1093.7 | −142.1 | −12 |
| 2 | Larch stands | 547.3 | 1352.9 | +805.6 | +147 |
| 3 | Birch stands | 21.7 | 43.5 | +21.9 | +101 |
| 4 | Mixed stands | 5211.7 | 6012.2 | +800.5 | +15 |
| 5 | Shrubs and grasses | 11,135.4 | 9646.6 | −1488.8 | −13 |
| 6 | Background | 3.4 | 6.4 | +3.0 | +88 |
| 2001–2003 | Land Cover Area (sq. km) | |||||
|---|---|---|---|---|---|---|
| 2020–2022 | ||||||
| Siberian Pine Stands | Larch Stands | Birch Stands | Mixed Stands | Shrubs and Grasses | Background | |
| Siberian pine stands | 713.6 | 60.2 | 0.0 | 428.5 | 33.4 | 0.0 |
| Larch stands | 14.4 | 316.2 | 10.9 | 195.3 | 10.5 | 0.0 |
| Birch stands | 0.0 | 1.5 | 9.9 | 9.2 | 1.1 | 0.0 |
| Mixed stands | 359.7 | 940.7 | 21.4 | 3730.2 | 159.7 | 0.0 |
| Shrubs and grasses | 6.0 | 34.3 | 1.3 | 1648.9 | 9441.5 | 3.4 |
| Background | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, I.A.; Kharuk, V.I.; Golyukov, A.S.; Im, S.T.; Ondar, S.O.; Shushpanov, A.S. Siberian Pine and Larch Response to Warming-Drying Climate in the Southern Boundary of Their Range. Forests 2024, 15, 1054. https://doi.org/10.3390/f15061054
Petrov IA, Kharuk VI, Golyukov AS, Im ST, Ondar SO, Shushpanov AS. Siberian Pine and Larch Response to Warming-Drying Climate in the Southern Boundary of Their Range. Forests. 2024; 15(6):1054. https://doi.org/10.3390/f15061054
Chicago/Turabian StylePetrov, Ilya A., Viacheslav I. Kharuk, Alexey S. Golyukov, Sergei T. Im, Sergei O. Ondar, and Alexander S. Shushpanov. 2024. "Siberian Pine and Larch Response to Warming-Drying Climate in the Southern Boundary of Their Range" Forests 15, no. 6: 1054. https://doi.org/10.3390/f15061054





