The Effects of Different Vegetation Restoration Models on Soil Quality in Karst Areas of Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Soil Sampling and Analysis
2.3. Evaluation of Soil Quality Index (SQI)
2.4. Statistical Analyses
3. Results
3.1. Soil Physical and Chemical Properties in Models
3.2. Soil Microbial Biomass in Models
3.3. Soil Enzyme Activities in Restoration Models
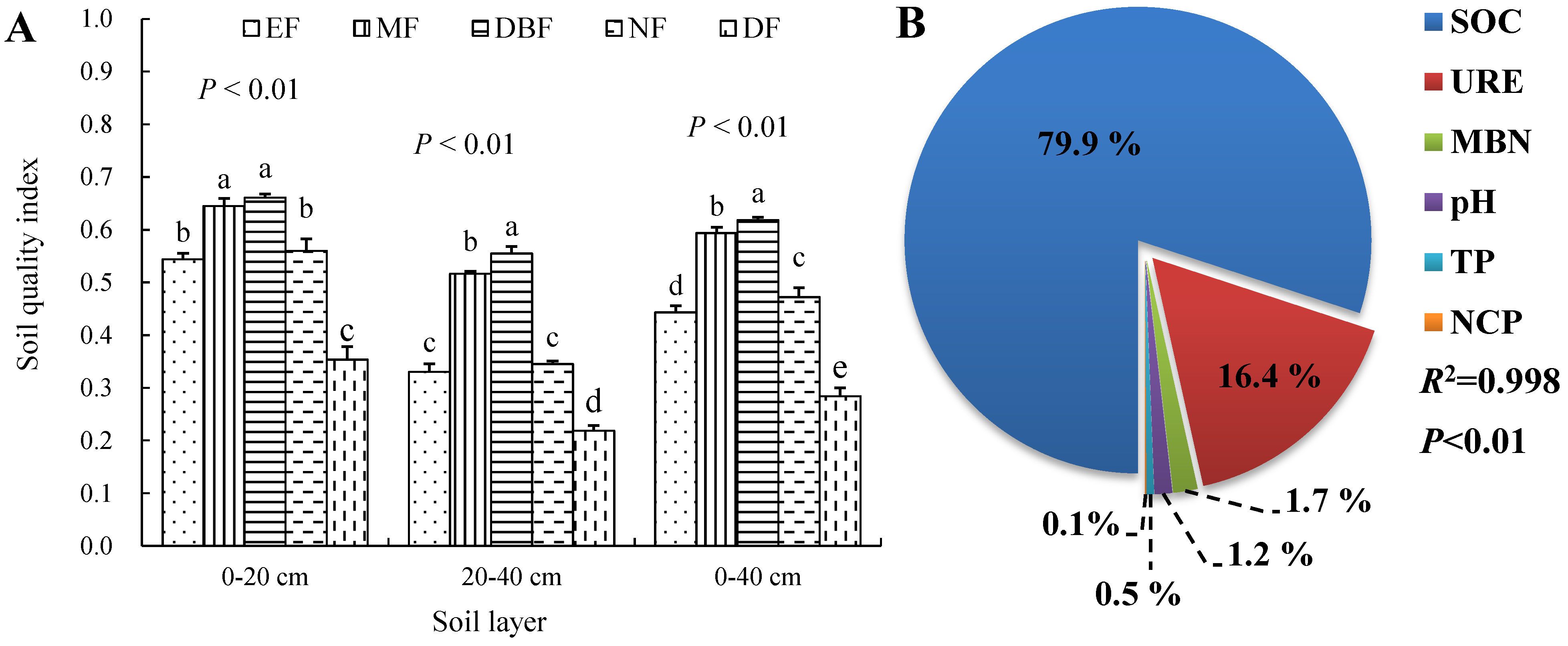
3.4. SQI Evaluation
4. Discussion
4.1. Soil Properties in Different Models
4.2. The Effects of Vegetation Type on Soil Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, M.M.; Yi, W.M.; Liao, L.Y.; Martens, R.; Insam, H. Effect of afforestation on microbial biomass and activity in soils of tropical China. Soil Biol. Biochem. 1992, 24, 865–872. [Google Scholar] [CrossRef]
- Badiane, N.N.Y.; Chotte, J.L.; Pate, E.; Masse, D.; Rouland, C. Use of soil enzymes to monitor soil quality in natural and improved fallows in semi-arid tropical regions. Appl. Soil Ecol. 2001, 18, 229–238. [Google Scholar] [CrossRef]
- Karlen, D.L.; Ditzler, C.A.; Andrews, S.S. Soil quality: Why and how? Geoderma 2003, 114, 145–156. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Li, Z.; Zhang, J.; Song, H.Y.; Liang, Q.H.; Tao, J.P.; Cornelissen, J.H.C.; Liu, J.C. Do shallow soil, low water availability, or their combination increase the competition between grasses with different root systems in karst soil? Environ. Sci. Pollut. R. 2017, 24, 10640–10651. [Google Scholar] [CrossRef]
- Hu, N.; Li, H.; Tang, Z.; Li, Z.F.; Li, G.C.; Jiang, Y.; Hu, X.M.; Lou, Y.L. Community size, activity and C: N stoichiometry of soil microorganisms following reforestation in a Karst region. Eur. J. Soil Biol. 2016, 73, 77–83. [Google Scholar] [CrossRef]
- Pedraza, R.A.; Williams-Linera, G. Evaluation of native tree species for the rehabilitation of deforested areas in a Mexican cloud forest. New For. 2003, 26, 83–99. [Google Scholar] [CrossRef]
- Diemont, S.A.W.; Martin, J.F.; Levy-Tacher, S.I.; Nigh, R.B.; Lopez, P.R.; Golicher, J.D. Lacandon Maya forest management: Restoration of soil fertility using native tree species. Ecol. Eng. 2006, 28, 205–212. [Google Scholar] [CrossRef]
- Deng, L.; Liu, G.B.; Shangguan, Z.P. Land-use conversion and changing soil carbon stocks in China’s ‘Grain-for-Green’ program: A synthesis. Glob. Change Biol. 2015, 20, 3544–3556. [Google Scholar] [CrossRef]
- Homolák, M.; Kriaková, E.; Pichler, V.; Gömöryová, E.; Bebej, J. Isolating the soil type effect on the organic carbon content in a Rendzic Leptosol and an Andosol on a limestone plateau with andesite protrusions. Geoderma 2017, 302, 1–5. [Google Scholar] [CrossRef]
- Chang, J.; Zhu, J.; Xu, L.; Su, H.; Gao, Y.; Cai, X.; Peng, T.; Wen, X.; Zhang, J.; He, N. Rational land-use types in the karst regions of China: Insights from soil organic matter composition and stability. Catena 2018, 160, 345–353. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Green, S.M.; Dungait, J.A.J.; Wen, X.; Tang, Y.; Guo, Z.; Yang, Y.; Sun, X.; Quine, T.A. Nitrogen functional gene activity in soil profiles under pro-gressive vegetative recovery after abandonment of agriculture at the Puding Karst Critical Zone Observatory, SW China. Soil Biol. Biochem. 2018, 125, 93–102. [Google Scholar] [CrossRef]
- Lan, J.; Hu, N.; Fu, W. Soil carbon–nitrogen coupled accumulation following the natural vegetation restoration of abandoned farmlands in a karst rocky desertification region. Ecol. Eng. 2020, 158, 106033. [Google Scholar] [CrossRef]
- Garousi, F.; Shan, Z.; Ni, K.; Yang, H.; Shan, J.; Cao, J.; Jiang, Z.; Yang, J.; Zhu, T.; Müller, C. Decreased inorganic N supply capacity and turnover in calcareous soil under degraded rubber plantation in the tropical karst region. Geoderma 2021, 381, 114754. [Google Scholar] [CrossRef]
- Raiesi, F.; Beheshti, A. Soil C turnover, microbial biomass and respiration, and enzymatic activities following rangeland conversion to wheat–alfalfa cropping in a semi-arid climate. Environ. Earth Sci. 2014, 72, 5073–5088. [Google Scholar] [CrossRef]
- Fu, T.; Chen, H.; Zhang, W.; Nie, Y.; Gao, P.; Wang, K. Spatial variability of surface soil saturated hydraulic conductivity in a small karst catchment of southwest China. Environ. Earth Sci. 2015, 74, 2381–2391. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, W.; Liu, S.; Li, D.; Wang, K. Leaf N:P stoichiometry across plant functional groups in the karst region of southwestern China. Trees 2015, 29, 883–892. [Google Scholar] [CrossRef]
- Knáb, M.; Szili-Kovács, T.; Kiss, K.; Palatinszky, M.; Márialigeti, K.; Móga, J.; Borsodi, A. Comparison of soil microbial communities from two distinct karst areas in Hungary. Acta Microbiol. Imm. H. 2012, 59, 91–105. [Google Scholar] [CrossRef]
- Zhao, C.; Long, J.; Liao, H.; Zheng, C.; Li, J.; Liu, L.; Zhang, M. Dynamics of soil microbial communities following vegetation succession in a karst mountain ecosystem, Southwest China. Sci. Rep. 2019, 9, 2160. [Google Scholar] [CrossRef]
- Hu, P.; Xiao, J.; Zhang, W.; Xiao, L.; Yang, R.; Xiao, D.; Zhao, J.; Wang, K. Response of soil microbial communities to natural and managed vegetation restoration in a subtropical karst region. Catena 2020, 195, 104849. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Li, Z.; Liu, M.; Xu, C.; Zhang, R.; Luo, W. Effects of vegetation restoration on soil quality in degraded karst landscapes of southwest China. Sci. Total Environ. 2019, 650, 2657–2665. [Google Scholar] [CrossRef]
- Guan, H.; Fan, J. Effects of vegetation restoration on soil quality in fragile karst ecosystems of southwest China. PeerJ 2020, 8, e9456. [Google Scholar] [CrossRef]
- Pang, D.; Cao, J.; Dan, X.; Guan, Y.; Peng, X.; Cui, M.; Wu, X.; Zhou, J. Recovery approach affects soil quality in fragile karst ecosystems of southwest China: Implications for vegetation restoration. Ecol. Eng. 2018, 123, 151–160. [Google Scholar] [CrossRef]
- Lu, Z.X.; Wang, P.; Ou, H.B.; Wei, S.X.; Wu, L.C.; Jiang, Y. Effects of different vegetation restoration on soil nutrients, enzyme activities, and microbial communities in degraded karst landscapes in southwest China. For. Ecol. Manag. 2022, 508, 120002. [Google Scholar] [CrossRef]
- Liu, G. Soil Physical and Chemical Analysis & Description of Soil Profiles; Standards Press of China: Beijing, China, 1996; pp. 5–19. [Google Scholar]
- Lu, R.K. Analysis Methods of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 1999; pp. 474–490. [Google Scholar]
- Huang, C.; Zeng, Y.; Wang, L.; Wang, S. Responses of soil nutrients to vegetation restoration in China. Reg. Environ. Change 2020, 20, 82. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Iqbal, J.; Hu, R.; Feng, M.; Lin, S.; Malghani, S.; Ali, I.M. Microbial biomass, and dissolved organic carbon and nitrogen strongly affect soil respiration in different land uses: A case study at Three Gorges Reservoir Area, South China. Agr. Ecosyst. Environ. 2010, 137, 294–307. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Notario del Pino, J.; Capowiez, Y.; Mazzia, C.; Rault, M. Soil enzyme dynamics in chlorpyrifos-treated soils under the influence of earthworms. Sci. Total Environ. 2018, 612, 1407–1416. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The soil management assessment framework: A quantitative soil quality evaluation method. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Danise, T.; Andriuzzi, W.S.; Battipaglia, G.; Certini, G.; Guggenberger, G.; Innangi, M.; Mastrolonardo, G.; Niccoli, F.; Pelleri, F.; Fioretto, A. Mixed-species plantation effects on soil biological and chemical quality and tree growth of a former agricultural land. Forests 2021, 12, 842. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, Z.S.; Huang, L.; Zhao, Y.; Hu, Y.G.; Zhang, P.; Zhang, D.H.; Zhang, H. Co-variation of fine-root distribution with vegetation and soil properties along a revegetation chronosequence in a desert area in northwestern China. Catena 2017, 151, 16–25. [Google Scholar] [CrossRef]
- Ström, L.; Owen, A.; Godbold, D.; Jones, D. Organic acid behaviour in a calcareous soil: Sorption reactions and biodegradation rates. Soil Biol. Biochem. 2001, 33, 2125–2133. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U.; Rosling, A. Organic acid induced release of nutrients from metal-stabilized soil organic matter—The unbutton model. Soil Biol. Biochem. 2015, 84, 168–176. [Google Scholar] [CrossRef]
- Zhang, K.; Dang, H.; Tan, S.; Wang, Z.; Zhang, Q. Vegetation community and soil characteristics of abandoned agricultural land and pine plantation in the Qinling Mountains, China. For. Ecol. Manag. 2010, 259, 2036–2047. [Google Scholar] [CrossRef]
- Friedel, J.K.; Munch, J.C.; Fischer, W.R. Soil microbial properties and the assessment of available soil organic matter in a haplic Luvisol after several years of different cultivation and crop rotation. Soil Biol. Biochem. 1996, 28, 479–488. [Google Scholar] [CrossRef]
- Pignataro, A.; Moscatelli, M.C.; Mocali, S.; Grego, S.; Benedetti, A. Assessment of soil microbial functional diversity in a coppiced forest system. Appl. Soil Ecol. 2012, 62, 115–123. [Google Scholar] [CrossRef]
- Xu, X.; Yin, L.; Duan, C.; Jing, Y. Effect of N addition, moisture, and temperature on soil microbial respiration and microbial biomass in forest soil at different stages of litter decomposition. J. Soil Sediment. 2016, 16, 1421–1439. [Google Scholar] [CrossRef]
- Song, P.; Ren, H.; Jia, Q.; Guo, J.; Zhang, N.; Ma, K. Effects of historical logging on soil microbial communities in a subtropical forest in southern China. Plant Soil 2015, 397, 115–126. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Kremer, R.J.; Garrett, H.E.; Anderson, S.H. Soil enzyme activities and physical properties in a watershed managed under agroforestry and row-crop systems. Agric. Ecosyst. Environ. 2009, 131, 98–104. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Cruz, L.; Sotomayor-Ramírez, D.; Pérez-Alegría, L. Enzyme activities as affected by soil properties and land use in a tropical watershed. Appl. Soil Ecol. 2007, 35, 35–45. [Google Scholar] [CrossRef]
- Hao, Y.; Chang, Q.; Li, L.; Wei, X. Impacts of landform, land use and soil type on soil chemical properties and enzymatic activities in a Loessial Gully watershed. Soil Res. 2014, 52, 453. [Google Scholar] [CrossRef]
- Marzaioli, R.; D’Ascoli, R.; Pascale, R.A.D.; Rutigliano, F.A. Soil quality in a Mediterra-nean area of southern Italy as related to different land use types. Appl. Soil Ecol. 2010, 44, 205–212. [Google Scholar] [CrossRef]
- Guo, S.; Han, X.; Li, H.; Wang, T.; Tong, X.; Ren, G.; Feng, Y.; Yang, G. Evaluation of soil quality along two revegetation chronosequences on the Loess Hilly Region of China. Sci. Total Environ. 2018, 633, 808–815. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, X.; Qian, S.; Li, J. Spatial variability of soil nitrogen and phosphorus of a mixed forest ecosystem in Beijing, China. Environ. Earth Sci. 2009, 60, 1783–1792. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Masto, R.E.; Yadav, A.; George, J.; Ram, L.C.; Shukla, S.P. Soil quality index for evaluation of reclaimed coal mine spoil. Sci. Total Environ. 2016, 542, 540–550. [Google Scholar] [CrossRef]
- Chen, H.S.; Wang, K.L. Characteristics of karst drought and its countermeasures. Res. Agric. Mod. 2004, 25, 70–73. (In Chinese) [Google Scholar]
- Chen, H.S.; Zhang, W.; Wang, K.L.; Hou, Y. Soil organic carbon and total nitrogen as affected by land use types in karst and non-karst areas of northwest Guangxi, China. J. Sci. Food Agric. 2012, 92, 1086–1093. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhang, W.; Wang, K.L.; Pan, F.; Yang, S.; Shu, S. Factors controlling accumulation of soil organic carbon along vegetation succession in a typical karst region in Southwest China. Sci. Total Environ. 2015, 521, 52–58. [Google Scholar] [CrossRef]
- Bhogal, A.; Nicholson, F.A.; Chambers, B.J. Organic carbon additions: Effects on soil bio-physical and physico-chemical properties. Eur. J. Soil Sci. 2009, 60, 276–286. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Lu, X.; Yan, Y.; Fan, J. Soil organic carbon and nutrients along an alpine grassland transect across Northern Tibet. J. Mt. Sci. 2013, 10, 564–573. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Zhu, S. Vertical distribution characteristics of soil organic carbon content in Caohai wetland ecosystem of Guizhou plateau, China. J. For. Res. 2015, 7, 551–556. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Z.; Lu, X. Changes of soil labile organic carbon fractions and their relation to soil microbial characteristics in four typical wetlands of Sanjiang Plain, Northeast China. Ecol. Eng. 2015, 82, 381–389. [Google Scholar] [CrossRef]
- Yan, Y.; Dai, Q.; Wang, X.; Jin, L.; Mei, L. Response of shallow karst fissure soil quality to secondary succession in a degraded karst area of southwestern China. Geoderma 2019, 348, 76–85. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Modrzynski, J.; Mrozinski, P.; Hobbie, S.E.; Eissenstat, D.M.; Chorover, J.; Chadwick, O.A.; Hale, C.M.; Joelker, M.G. Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol. Lett. 2005, 8, 811–818. [Google Scholar] [CrossRef]
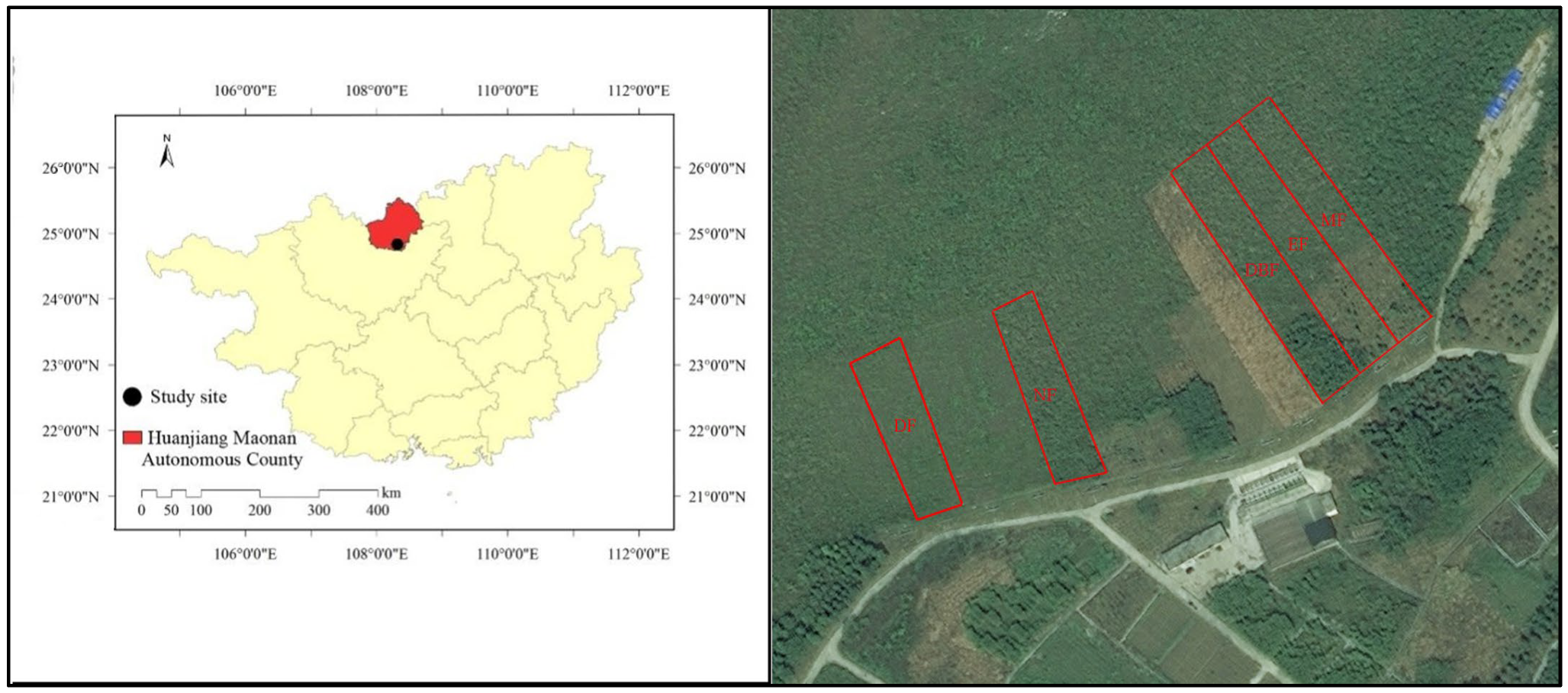

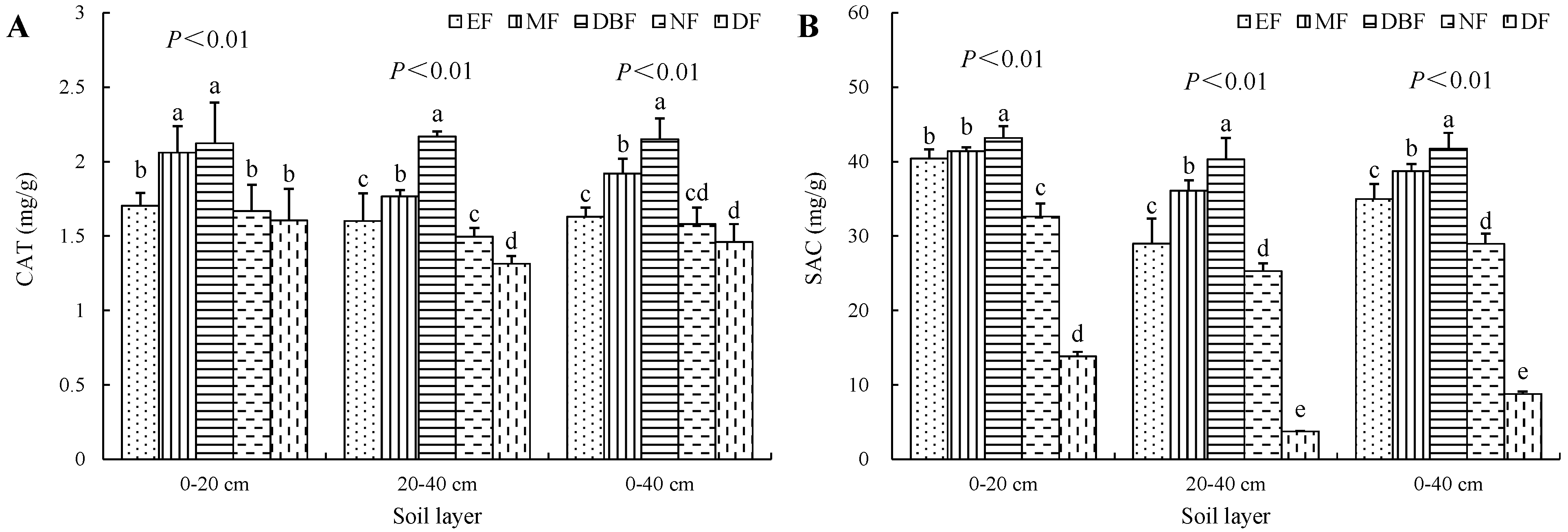
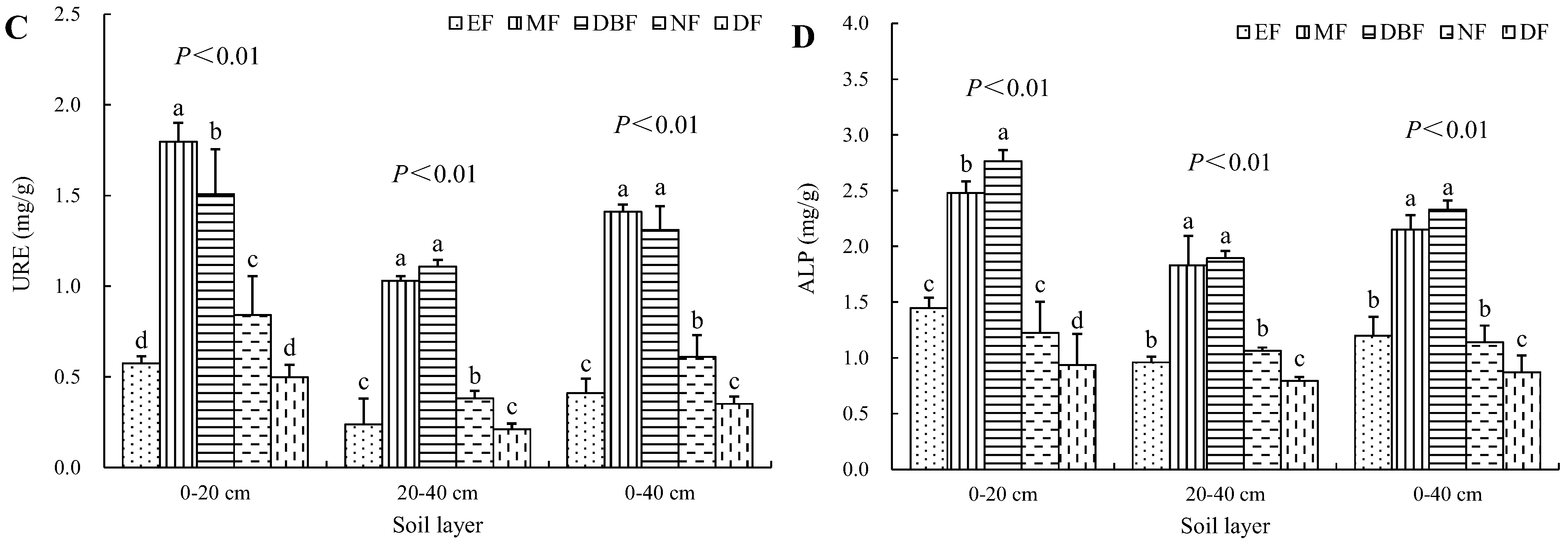
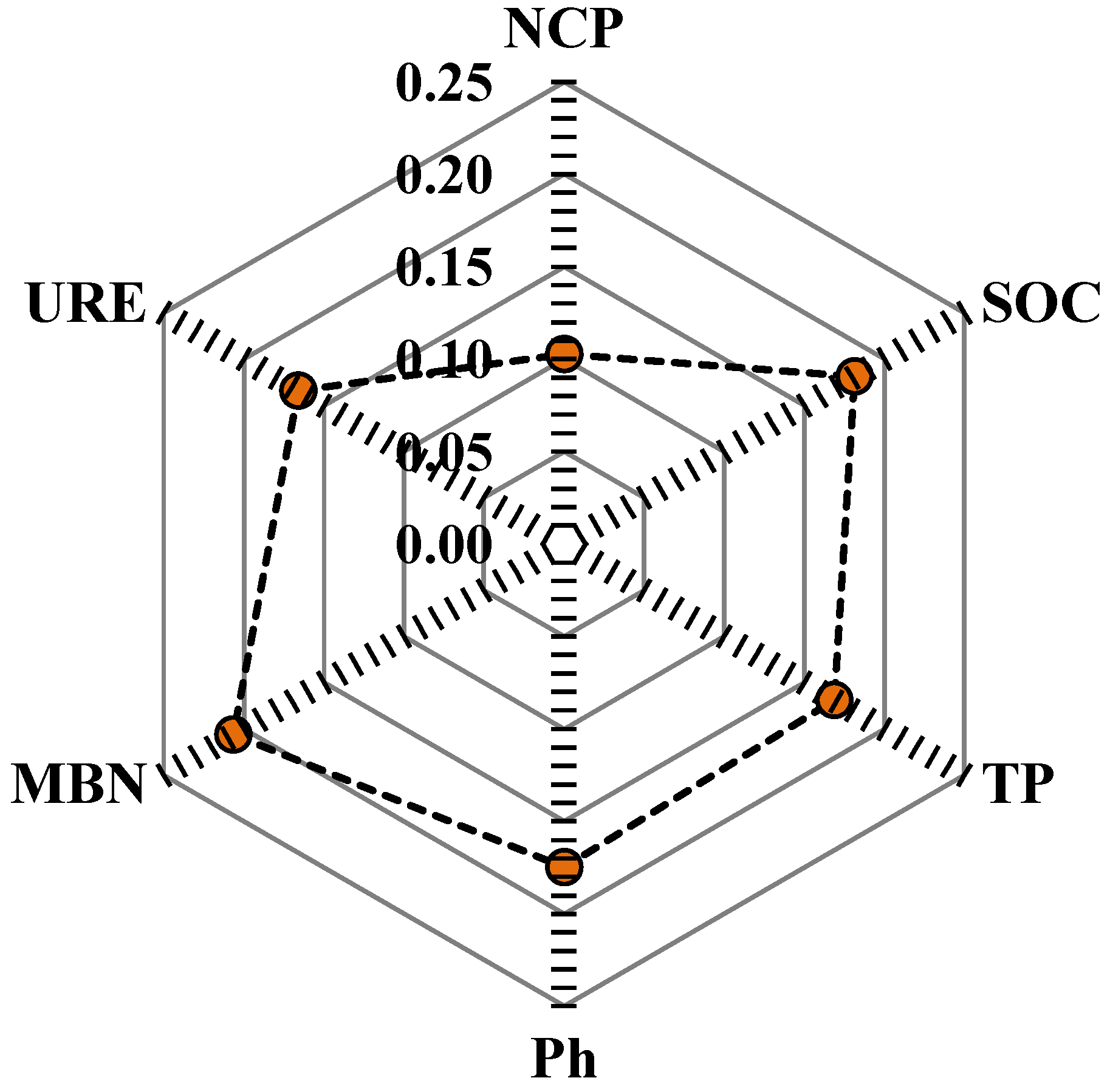
| Site Characteristics | Evergreen Broad-Leaved Forest (EF) | Mixed Forest (MF) | Deciduous Broad-Leaved Forest (DBF) | Natural Closed Forest (NF) | Disturbed Forest (DF) |
|---|---|---|---|---|---|
| Test area (hm2) | 0.28 | 0.29 | 0.23 | 0.14 | 0.15 |
| Elevation (m) | 286–337 | 288–337 | 287–337 | 291–339 | 294–340 |
| Slope (°) | 24 | 24 | 24 | 33 | 35 |
| Aspect | Southeast | Southeast | Southeast | Southeast | Southeast |
| Soil type | Calcareous | Calcareous | Calcareous | Calcareous | Calcareous |
| Treatment | Cut down a small part of the original vegetation for planting evergreen trees | Cut down a small part of the original vegetation for planting evergreen and deciduous trees | Cut down a small part of the original vegetation for planting deciduous trees | Preserve the original vegetation | All shrubs and herbs were regularly cut down every year without removing underground roots |
| Recovery time (year) | 13 | 13 | 13 | 13 | 13 |
| Mean tree height (m) | 9.1 | 9.2 | 12.1 | 3.5 | — |
| Mean diameter at BREAST Height (cm) | 8.4 | 8.9 | 13.2 | 3.2 | — |
| Vegetation cover (%) | 60 | 70 | 60 | 85 | — |
| Mean density (Plant/hm2) | 913 | 1150 | 1450 | — | — |
| Thickness of humus layer/cm | 1–3 | 3–4 | 5–6 | 4–5 | 0–1 |
| Soil thickness/cm | 40–60 | 40–60 | 40–60 | 50–70 | 40–60 |
| Main species | C. glauca, Rhus chinensis Mill, Jasminum nervosum Lour., Ficus tikoua Bur., Microstegium fasciculatum (L.) Henrard, Cyclosorus parasiticus (L.) Farw. | C. axillaris, Delavaya toxocarpa Franch, Z. insignis, C. glauca, Vitex neguwndo Linn., Mallotus barbatus, J. nervosum, C. Parasiticus, M. fasciculatum | Z. insignis, C. axillaris, J. nervosum, Leucaena leucocephala (Lam.) de Wit, M. fasciculatum, Miscanthus floridulus (Lab.) Warb. ex Schum. et Laut. | V. negundo, Mallotus barbatus (Wall.) Muell. Arg., M. fasciculatum, Oplismenus undulatifolius (Ard.) Roem. & Schult. | V. negundo, Dalbergia balansae Prain, M. fasciculatum, Imperata cylindrica (L.) Raeusch. |
| Index | Soil layer | EF | MF | DBF | NF | DF | F |
|---|---|---|---|---|---|---|---|
| MC (%) | 0–20 cm | 34.24 ± 2.11a | 36.18 ± 3.43a | 26.35 ± 1.27b | 22.37 ± 2.86c | 20.43 ± 1.59c | 43.291 ** |
| 20–40 cm | 25.86 ± 2.01ab | 28.17 ± 1.35a | 25.10 ± 2.18b | 19.95 ± 0.20c | 19.17 ± 3.53c | 16.517 ** | |
| 0–40 cm | 30.05 ± 1.49a | 32.18 ± 1.36a | 25.72 ± 1.56b | 21.15 ± 1.45c | 19.80 ± 2.15c | 54.959 ** | |
| BD (g/cm3) | 0–20 cm | 0.88 ± 0.19b | 0.81 ± 0.06b | 0.85 ± 0.18b | 1.10 ± 0.04a | 1.15 ± 0.03a | 8.388 ** |
| 20–40 cm | 1.01 ± 0.07bc | 0.91 ± 0.25c | 0.97 ± 0.04c | 1.24 ± 0.16c | 1.21 ± 0.17ab | 4.472 ** | |
| 0–40 cm | 0.95 ± 0.13b | 0.86 ± 0.15b | 0.92 ± 0.10b | 1.17 ± 0.10a | 1.18 ± 0.07a | 8.848 ** | |
| NCP (%) | 0–20 cm | 5.41 ± 0.35cd | 5.25 ± 0.41d | 5.81 ± 0.14c | 8.90 ± 0.58a | 8.09 ± 0.26b | 98.364 ** |
| 20–40 cm | 6.82 ± 0.10b | 7.11 ± 0.35b | 3.72 ± 0.14d | 7.89 ± 0.34a | 6.42 ± 0.19c | 209.944 ** | |
| 0–40 cm | 6.09 ± 0.19c | 6.18 ± 0.19c | 4.77 ± 0.13d | 8.40 ± 0.43a | 7.26 ± 0.22b | 143.891 ** | |
| CP (%) | 0–20 cm | 53.12 ± 1.36b | 57.81 ± 0.68a | 41.02 ± 0.97c | 35.08 ± 0.61d | 25.03 ± 0.75e | 1057.997 ** |
| 20–40 cm | 42.79 ± 0.72b | 47.09 ± 1.04a | 40.48 ± 0.57c | 33.91 ± 2.14d | 20.02 ± 0.44e | 414.490 ** | |
| 0–40 cm | 48.47 ± 1.16b | 52.45 ± 0.76a | 40.75 ± 0.55c | 34.49 ± 1.21d | 22.53 ± 0.53e | 882.855 ** | |
| TPO (%) | 0–20 cm | 58.53 ± 1.18b | 63.06 ± 1.22a | 46.84 ± 1.00c | 43.98 ± 1.06d | 33.12 ± 1.63e | 465.011 ** |
| 20–40 cm | 49.61 ± 1.05b | 54.20 ± 1.18a | 44.20 ± 0.34c | 41.80 ± 1.35d | 26.44 ± 0.90e | 532.092 ** | |
| 0–40 cm | 54.36 ± 1.02b | 58.63 ± 0.58a | 45.52 ± 0.48c | 42.89 ± 0.84d | 29.78 ± 1.11e | 885.410 ** |
| Index | Soil Layer | EF | MF | DBF | NF | DF | F |
|---|---|---|---|---|---|---|---|
| SOC (g/kg) | 0–20 cm | 88.11 ± 2.84a | 89.94 ± 2.78a | 90.39 ± 0.12a | 88.29 ± 2.46a | 73.68 ± 2.33b | 45.006 ** |
| 20–40 cm | 69.06 ± 1.78bc | 74.16 ± 2.57a | 71.04 ± 1.13b | 67.74 ± 0.83c | 39.09 ± 2.37d | 292.465 ** | |
| 0–40 cm | 76.69 ± 3.27b | 82.05 ± 1.84a | 80.72 ± 0.59a | 78.02 ± 1.51b | 56.39 ± 1.86c | 136.373 ** | |
| TN (g/kg) | 0–20 cm | 5.10 ± 0.06a | 5.11 ± 0.11a | 5.26 ± 0.09a | 4.56 ± 0.28b | 4.34 ± 0.28b | 22.600 ** |
| 20–40 cm | 3.42 ± 0.07b | 3.57 ± 0.20ab | 3.81 ± 0.28a | 3.39 ± 0.22b | 1.14 ± 0.03c | 171.715 ** | |
| 0–40 cm | 4.22 ± 0.09b | 4.34 ± 0.11b | 4.54 ± 0.12a | 3.98 ± 0.17c | 2.75 ± 0.13d | 154.910 ** | |
| TK (g/kg) | 0–20 cm | 4.23 ± 0.08c | 5.79 ± 0.16b | 6.35 ± 0.11a | 5.71 ± 0.14b | 3.96 ± 0.17d | 289.145 ** |
| 20–40 cm | 2.62 ± 0.10c | 3.67 ± 0.09b | 4.23 ± 0.09a | 3.85 ± 0.11b | 2.33 ± 0.27d | 154.723 ** | |
| 0–40 cm | 3.40 ± 0.08c | 4.73 ± 0.13b | 5.29 ± 0.10a | 4.78 ± 0.06b | 3.15 ± 0.14d | 423.276 ** | |
| TP (g/kg) | 0–20 cm | 1.13 ± 0.15b | 1.57 ± 0.26a | 1.15 ± 0.02b | 1.01 ± 0.20b | 1.08 ± 0.14b | 8.313 ** |
| 20–40 cm | 0.71 ± 0.03c | 0.90 ± 0.05a | 0.84 ± 0.17ab | 0.74 ± 0.04bc | 0.67 ± 0.05c | 6.659 ** | |
| 0–40 cm | 0.98 ± 0.12b | 1.24 ± 0.14a | 0.99 ± 0.09b | 0.88 ± 0.10b | 0.87 ± 0.07b | 10.052 ** | |
| AN (mg/kg) | 0–20 cm | 256.33 ± 3.61d | 274.13 ± 1.43b | 354.53 ± 1.82a | 263.20 ± 2.22c | 252.90 ± 6.43d | 692.814 ** |
| 20–40 cm | 160.27 ± 1.59d | 180.20 ± 1.4b | 188.53 ± 3.65a | 168.8 ± 3.45c | 154.10 ± 2.41e | 140.017 ** | |
| 0–40 cm | 208.30 ± 1.66d | 227.17 ± 0.37b | 271.53 ± 2.20a | 216.00 ± 2.72c | 203.50 ± 4.28e | 558.075 ** | |
| AP (mg/kg) | 0–20 cm | 4.00 ± 0.31c | 9.13 ± 0.26a | 6.79 ± 0.17b | 3.36 ± 0.12d | 2.32 ± 0.25e | 721.685 ** |
| 20–40 cm | 2.17 ± 0.19b | 4.51 ± 0.32a | 4.31 ± 0.10a | 1.84 ± 0.06c | 1.59 ± 0.07d | 317.125 ** | |
| 0–40 cm | 2.90 ± 0.47c | 6.83 ± 0.16a | 5.55 ± 0.11b | 2.60 ± 0.07c | 1.95 ± 0.14d | 388.917 ** | |
| AK (mg/kg) | 0–20 cm | 153.74 ± 1.83d | 160.75 ± 0.68c | 243.33 ± 1.93a | 167.48 ± 1.13b | 135.04 ± 2.61e | 2773.203 ** |
| 20–40 cm | 108.27 ± 2.46c | 112.79 ± 1.7b | 139.49 ± 3.85a | 109.05 ± 3.16c | 89.08 ± 1.36d | 228.543 ** | |
| 0–40 cm | 129.52 ± 2.37c | 136.77 ± 1.03b | 191.42 ± 2.55a | 138.27 ± 2.03b | 112.06 ± 1.94d | 1046.522 ** | |
| pH | 0–20 cm | 6.59 ± 0.07b | 6.66 ± 0.13b | 7.57 ± 0.18a | 7.72 ± 0.07a | 7.60 ± 0.20a | 2773.203 ** |
| 20–40 cm | 7.08 ± 0.11b | 6.94 ± 0.15b | 7.63 ± 0.14a | 7.74 ± 0.13a | 7.73 ± 0.08a | 48.939 ** | |
| 0–40 cm | 6.84 ± 0.06b | 6.80 ± 0.09b | 7.60 ± 0.15a | 7.73 ± 0.09a | 7.67 ± 0.14a | 89.008 ** |
| Principal Component | PC-1 | PC-2 | PC-3 | PC-4 |
|---|---|---|---|---|
| Eigenvalues | 12.071 | 3.583 | 1.44 | 1.044 |
| Variance (%) | 63.25 | 18.86 | 7.58 | 5.49 |
| Cumulative (%) | 63.25 | 82.11 | 89.69 | 95.18 |
| MC | 0.062 | 0.900 | 0.289 | 0.207 |
| BD | −0.188 | −0.606 | −0.088 | −0.572 |
| NCP | −0.143 | −0.302 | −0.203 | −0.896 |
| CP | 0.324 | 0.911 | 0.191 | 0.133 |
| TPO | 0.326 | 0.922 | 0.184 | 0.031 |
| SOC | 0.785 | 0.877 | 0.162 | 0.044 |
| TN | 0.748 | 0.588 | 0.118 | 0.222 |
| TK | 0.879 | −0.027 | 0.444 | 0.089 |
| TP | 0.000 | 0.587 | 0.731 | 0.018 |
| AN | 0.668 | −0.116 | 0.358 | 0.631 |
| AP | 0.375 | 0.432 | 0.712 | 0.343 |
| AK | 0.780 | −0.088 | 0.189 | 0.568 |
| pH | 0.227 | −0.944 | −0.103 | −0.098 |
| MBC | 0.780 | 0.083 | 0.569 | 0.193 |
| MBN | 0.968 | 0.144 | 0.054 | 0.155 |
| CAT | 0.541 | 0.192 | 0.505 | 0.567 |
| SAC | 0.716 | 0.602 | 0.151 | 0.303 |
| URE | 0.500 | 0.223 | 0.739 | 0.325 |
| ALP | 0.527 | 0.262 | 0.642 | 0.475 |
| Index | MC | BD | NCP | CP | TPO | SOC | TN | TK | TP | AN | AP | AK | pH | MBC | MBN | CAT | SAC | URE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BD | −0.65 ** | 1.00 | ||||||||||||||||
| NCP | −0.53 ** | 0.65 ** | 1.00 | |||||||||||||||
| CP | 0.92 ** | −0.68 ** | −0.49 | 1.00 | ||||||||||||||
| TPO | 0.91 ** | −0.64 ** | −0.40 | 0.99 ** | 1.00 | |||||||||||||
| SOC | 0.62 ** | −0.54 ** | −0.36 | 0.81 ** | 0.82 ** | 1.00 | ||||||||||||
| TN | 0.67 ** | −0.59 ** | −0.53 ** | 0.83 ** | 0.81 * | 0.95 ** | 1.00 | |||||||||||
| TK | 0.16 | −0.30 | −0.27 | 0.35 | 0.34 | 0.75 ** | 0.70 ** | 1.00 | ||||||||||
| TP | 0.76 ** | −0.36 | −0.37 | 0.68 ** | 0.67 ** | 0.44 | 0.47 | 0.29 | 1.00 | |||||||||
| AN | 0.19 | −0.41 | −0.70 ** | 0.26 | 0.20 | 0.54 ** | 0.62 ** | 0.80 ** | 0.22 | 1.00 | ||||||||
| AP | 0.67 ** | −0.65 ** | −0.62 ** | 0.70 ** | 0.66 ** | 0.68 ** | 0.68 ** | 0.68 ** | 0.72 ** | 0.66 ** | 1.00 | |||||||
| AK | 0.16 | −0.38 | −0.65 ** | 0.29 | 0.23 | 0.61 ** | 0.70 ** | 0.81 ** | 0.14 | 0.97 ** | 0.57 ** | 1.00 | ||||||
| pH | −0.87 ** | 0.59 ** | 0.36 | −0.82 ** | −0.82 ** | −0.39 | −0.40 | 0.16 | −0.61 ** | 0.16 | −0.44 | 0.19 | 1.00 | |||||
| MBC | 0.31 | −0.42 | −0.41 | 0.46 | 0.44 | 0.76 ** | 0.73 ** | 0.97 ** | 0.41 | 0.83 ** | 0.82 ** | 0.80 ** | 0.01 | 1.00 | ||||
| MBN | 0.24 | −0.33 | −0.34 | 0.48 | 0.46 | 0.85 ** | 0.85 ** | 0.88 ** | 0.16 | 0.76 ** | 0.50 * | 0.85 ** | 0.06 | 0.82 ** | 1.00 | |||
| CAT | 0.48 | −0.53 ** | −0.75 ** | 0.52 ** | 0.46 | 0.65 ** | 0.70 ** | 0.74 ** | 0.52 ** | 0.89 ** | 0.80 ** | 0.82 ** | −0.18 | 0.81 ** | 0.68 ** | 1.00 | ||
| SAC | 0.70 ** | −0.67 ** | −0.59 ** | 0.85 ** | 0.83 ** | 0.94 ** | 0.97 ** | 0.71 ** | 0.47 | 0.66 ** | 0.73 ** | 0.71 ** | −0.45 | 0.75 ** | 0.84 ** | 0.76 ** | 1.00 | |
| URE | 0.50 * | −0.52 ** | −0.57 ** | 0.55 ** | 0.52 ** | 0.66 ** | 0.65 ** | 0.80 ** | 0.52 ** | 0.77 ** | 0.95 ** | 0.67 ** | −0.21 | 0.91 ** | 0.59 ** | 0.84 ** | 0.70 ** | 1.00 |
| ALP | 0.58 ** | −0.59 ** | −0.72 ** | 0.61 ** | 0.56 ** | 0.71 ** | 0.74 ** | 0.77 ** | 0.62 ** | 0.85 ** | 0.93 ** | 0.78 ** | −0.25 | 0.87 ** | 0.65 ** | 0.92 ** | 0.78 ** | 0.95 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, H.-B.; Liu, X.-S.; Wei, S.-X.; Jiang, Y.; Gao, F.; Wang, Z.-H.; Fu, W.; Du, H. The Effects of Different Vegetation Restoration Models on Soil Quality in Karst Areas of Southwest China. Forests 2024, 15, 1061. https://doi.org/10.3390/f15061061
Ou H-B, Liu X-S, Wei S-X, Jiang Y, Gao F, Wang Z-H, Fu W, Du H. The Effects of Different Vegetation Restoration Models on Soil Quality in Karst Areas of Southwest China. Forests. 2024; 15(6):1061. https://doi.org/10.3390/f15061061
Chicago/Turabian StyleOu, Han-Biao, Xiong-Sheng Liu, Shuo-Xing Wei, Yi Jiang, Feng Gao, Zhi-Hui Wang, Wei Fu, and Hu Du. 2024. "The Effects of Different Vegetation Restoration Models on Soil Quality in Karst Areas of Southwest China" Forests 15, no. 6: 1061. https://doi.org/10.3390/f15061061






