Monitoring Changes in Composition and Diversity of Forest Vegetation Layers after the Cessation of Management for Renaturalization
Abstract
:1. Introduction
2. Materials and Methods
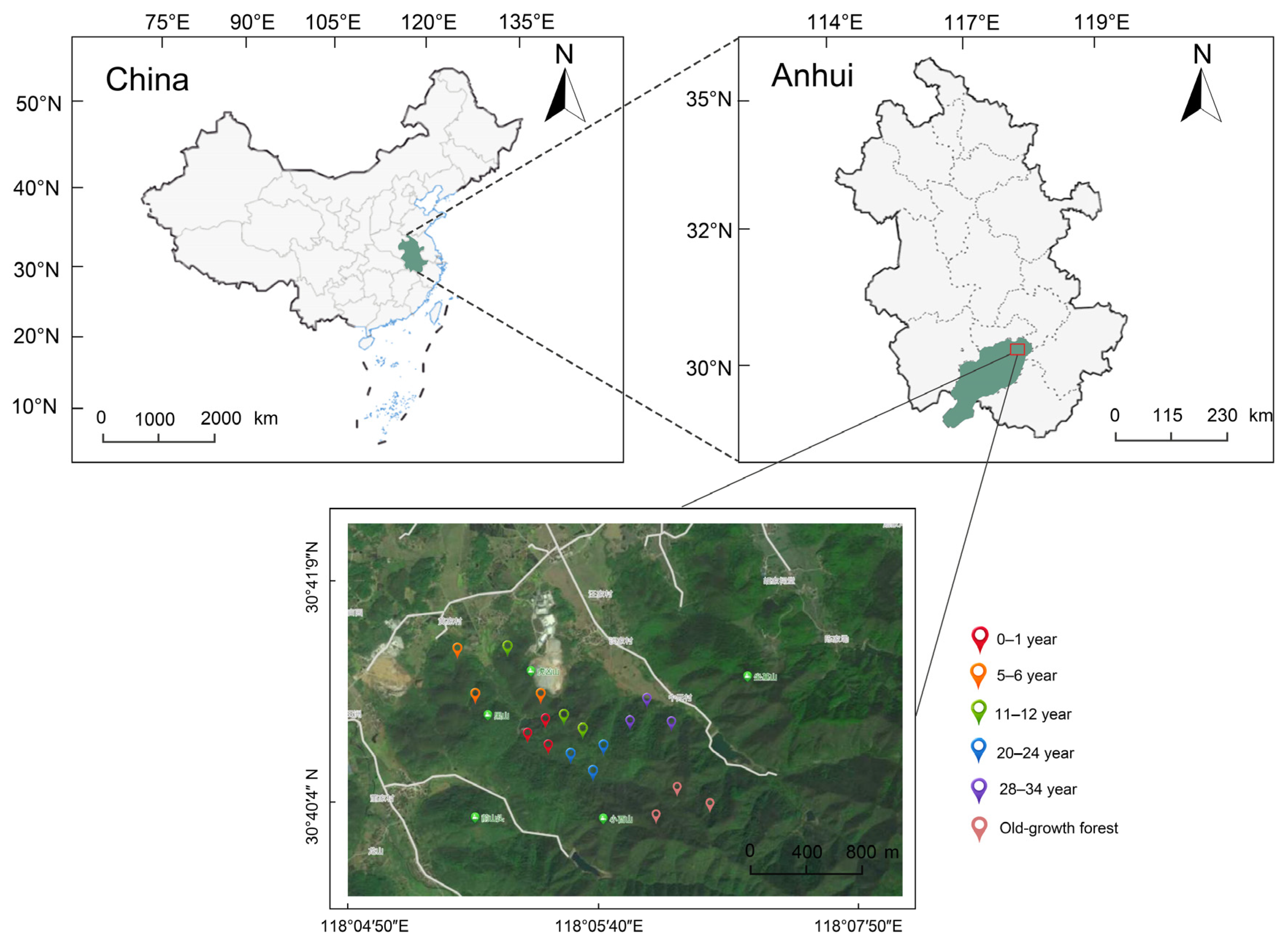
2.1. Study Area
2.2. Sampling Design
2.3. Field Measurements
2.4. Data Analysis
3. Results
3.1. Overstory Stem Basal Area and Understory Vegetation Cover
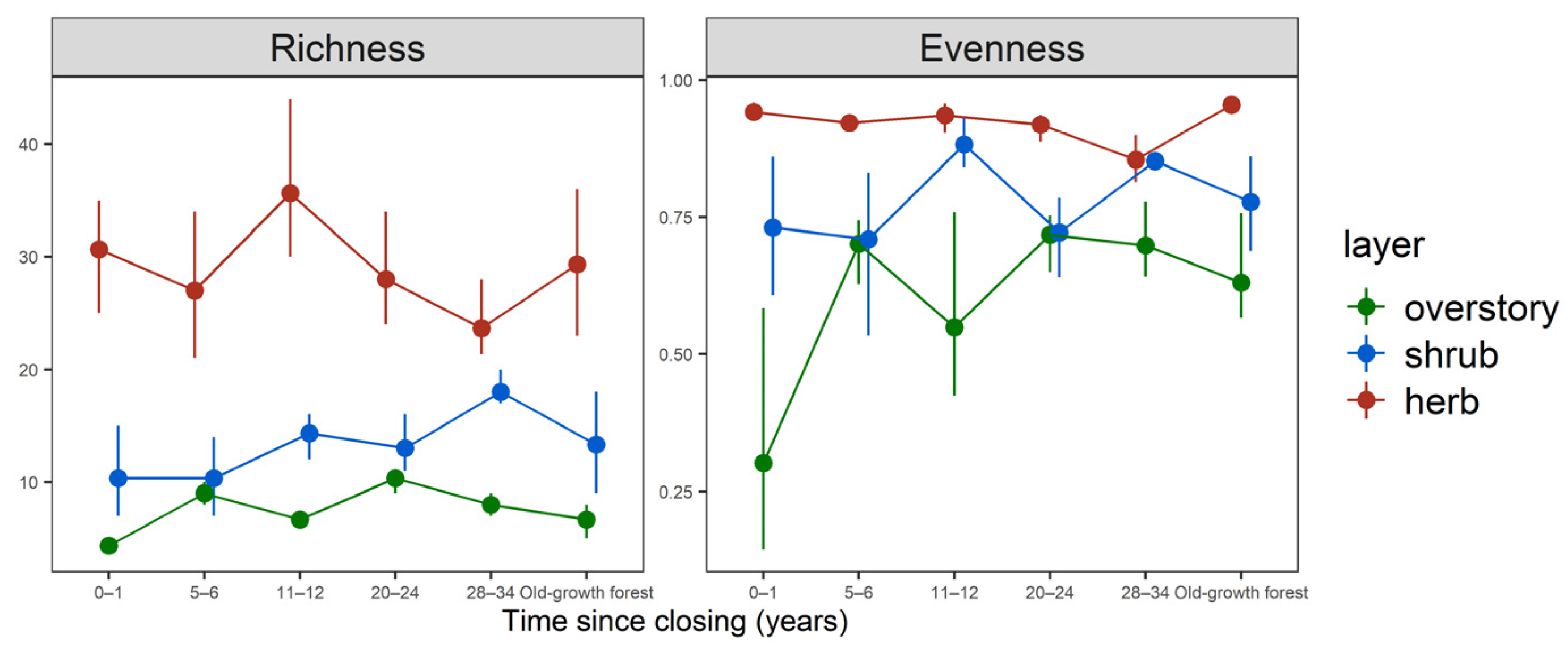
3.2. Species Richness and Evenness
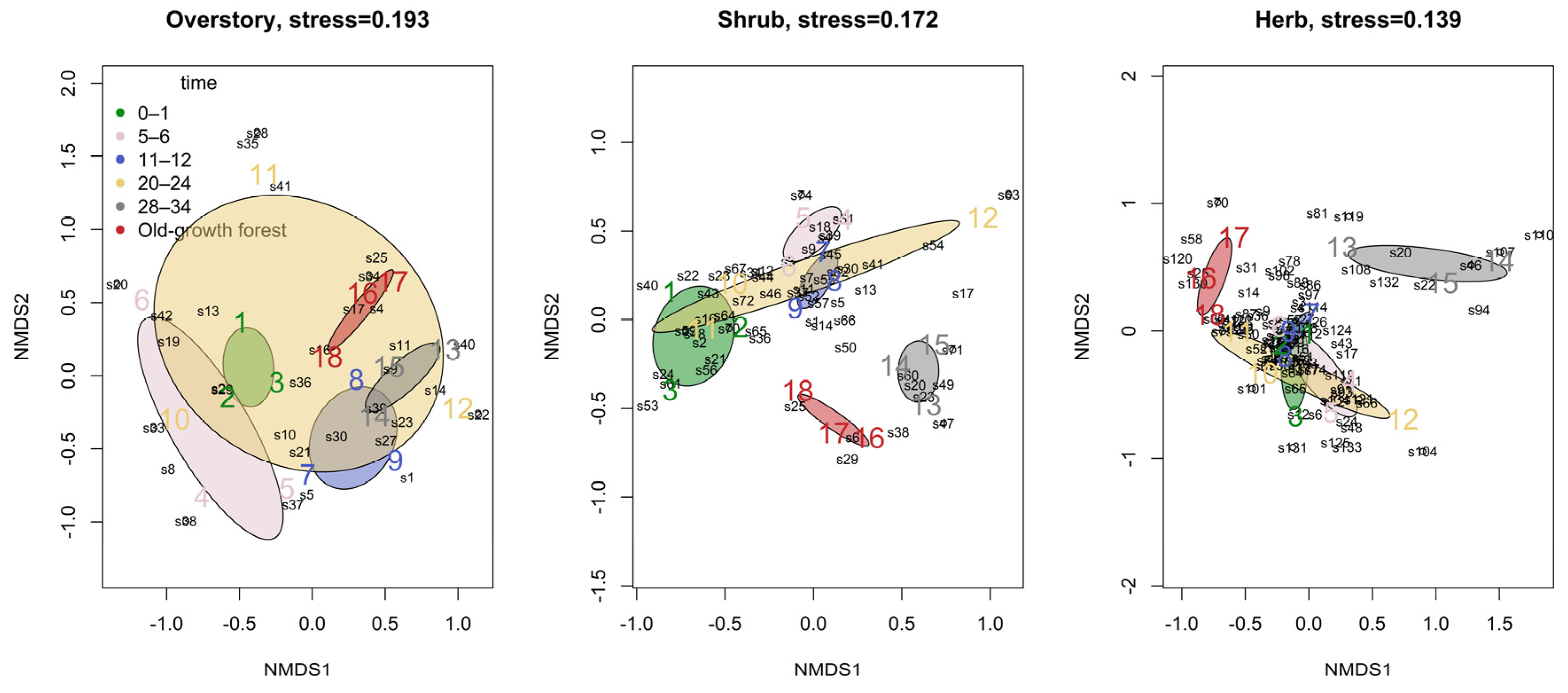
3.3. Species Composition
3.4. Linking Resource Availability and Heterogeneity to Herb Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Pott, R. Tropical rainforests and forest degradation: The global loss of biodiversity. In Proceedings of the International Symposium on Rehabilitation of Tropical Rainforest Ecosystems 2011, Kuala Lumpur, Malaysia, 24–25 October 2011; Baraplas Kreatif Enterprise: Seri Kembangan, Malaysia, 2011. [Google Scholar]
- Baccini, A.; Walker, W.; Carvalho, L.; Farina, M.; Sulla-Menashe, D.; Houghton, R.A. Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science 2017, 358, 230–234. [Google Scholar] [CrossRef]
- Giam, X. Global biodiversity loss from tropical deforestation. Proc. Natl. Acad. Sci. USA 2017, 114, 5775. [Google Scholar] [CrossRef] [PubMed]
- Menz, M.H.; Dixon, K.W.; Hobbs, R.J. Hurdles and opportunities for landscape-scale restoration. Science 2013, 339, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Chandra, L.R.; Gupta, S.; Pande, V.; Singh, N. Impact of forest vegetation on soil characteristics: A correlation between soil biological and physico-chemical properties. 3 Biotech 2016, 6, 188. [Google Scholar] [CrossRef]
- Mori, A.S.; Lertzman, K.P.; Gustafsson, L. Biodiversity and ecosystem services in forest ecosystems. J. Appl. Ecol. 2017, 54, 12–27. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Biswas, S.R.; Sobey, T.M.; Brassard, B.W.; Bartels, S.F. Reclamation strategies for mined forest soils and overstory drive understorey vegetation. J. Appl. Ecol. 2018, 55, 926–936. [Google Scholar] [CrossRef]
- Fakhry, A.M.; Khazzan, M.M.; Aljedaani, G.S. Impact of disturbance on species diversity and composition of Cyperus conglomeratus plant community in southern Jeddah, Saudi Arabia. J. King Saud Univ.-Sci. 2020, 32, 600–605. [Google Scholar] [CrossRef]
- Harrison, S.; Spasojevic, M.J.; Li, D. Climate and plant community diversity in space and time. Proc. Natl. Acad. Sci. USA 2020, 117, 4464. [Google Scholar] [CrossRef]
- Azeria, E.T.; Bouchard, M.; Pothier, D.; Fortin, D.; Hébert, C. Using biodiversity deconstruction to disentangle assembly and diversity dynamics of understoreyunderstory plants along post-fire succession in boreal forest. Glob. Ecol. Biogeogr. 2011, 20, 119–133. [Google Scholar] [CrossRef]
- Rembold, K.; Mangopo, H.; Tjitrosoedirdjo, S.S.; Kreft, H. Plant diversity, forest dependency, and alien plant invasions in tropical agricultural landscapes. Biol. Conserv. 2017, 213, 234–242. [Google Scholar] [CrossRef]
- Cano-Ortiz, A.; Piñar Fuentes, J.C.; Pinto-Gomes, C.; Musarella, C.M.; Cano, E. Expansion of the Juniperus genus due to anthropic activity. In Old-Growth Forests and Coniferous Forests: Ecology, Habitat and Conservation; Nova Science Publishers, Inc.: New York, NY, USA, 2015. [Google Scholar]
- Rivas-Martínez, S.; Sánchez-Mata, D.; Costa, M. North American new phytosociological classes. Itinera Geobot. 1999, 13, 349–352. [Google Scholar]
- Rivas Martinez, S.; Aguiar, C.; Aguilella, A.; Alonso, R.; Alvarez, M.; Amich, F.; Arnaiz, C.; Baccheta, G.; Barbero, M.; Barbour, M.G.; et al. Map of series, geoseries and geopermaseries of vegetation in Spain [Memory of map of potential vegetation of Spain, 2011] Part II. Itinera Geobot. 2011, 18, 1–764. [Google Scholar]
- Corlett, R.T. What is secondary forest? J. Trop. Ecol. 1994, 10, 445–447. [Google Scholar] [CrossRef]
- Chokkalingam, U.; De Jong, W. Secondary forest: A working definition and typology. Int. For. Rev. 2001, 3, 19–26. [Google Scholar]
- Norden, N.; Chazdon, R.L.; Chao, A.; Jiang, Y.H.; Vílchez-Alvarado, B. Resilience of tropical rain forests: Tree community reassembly in secondary forests. Ecol. Lett. 2009, 12, 385–394. [Google Scholar] [CrossRef]
- Brown, K.A.; Gurevitch, J. Long-term impacts of logging on forest diversity in Madagascar. Proc. Natl. Acad. Sci. USA 2004, 101, 6045. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Scatena, F.N.; Gurevitch, J. Effects of an invasive tree on community structure and diversity in a tropical forest in Puerto Rico. For. Ecol. Manag. 2006, 226, 145–152. [Google Scholar] [CrossRef]
- Chen, L.F.; He, Z.B.; Zhu, X.; Du, J.; Yang, J.J.; Li, J. Impacts of afforestation on plant diversity, soil properties, and soil organic carbon storage in a semi-arid grassland of northwestern China. Catena 2016, 147, 300–307. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A. Above- and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agric. For. Meteorol. 2006, 140, 51–63. [Google Scholar] [CrossRef]
- Li, X.; Aguila, L.C.R.; Wu, D.H.; Lie, Z.Y.; Xu, W.F.; Tang, X.L.; Liu, J.X. Carbon sequestration and storage capacity of Chinese fir at different stand ages. Sci. Total Environ. 2023, 904, 166962. [Google Scholar] [CrossRef] [PubMed]
- Klopf, R.P.; Baer, S.G.; Bach, E.M.; Six, J. Restoration and management for plant diversity enhances the rate of belowground ecosystem recovery. Ecol. Appl. 2017, 27, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tong, S.; Li, Y.; Qi, Q.; Zhang, D.; Lyu, X.; Guo, Y.; Liu, Y. Plant diversity performance after natural restoration in reclaimed Deyeuxia angustifolia Wetland. Chin. Geogr. Sci. 2019, 29, 437–445. [Google Scholar] [CrossRef]
- Su, X.; Li, S.; Wan, X.; Huang, Z.; Liu, B.; Fu, S.L.; Kumar, P.; Chen, H.Y.H. Understory vegetation dynamics of Chinese fir plantations and natural secondary forests in subtropical China. For. Ecol. Manag. 2021, 483, 118750. [Google Scholar] [CrossRef]
- Su, X.; Wang, M.; Huang, Z.; Fu, S.L.; Chen, H.Y.H. Forest understoreyunderstory vegetation: Colonization and the availability and heterogeneity of resources. Forests 2019, 10, 944. [Google Scholar] [CrossRef]
- Tavora, A.C.; Carvalho-Silva, M.; Amaral, E.D.; Cupertino-Eisenlohr, M.A.; Nogueira, E.; Munhoz, C.B.R. Revealing the permanent and transient plant understory in gallery forests in the Cerrado of Central Brazil. For. Sci. 2023, 69, 254–264. [Google Scholar] [CrossRef]
- Wang, T.; Dong, L.; Liu, Z. Climate strengthens the positive effects of stand structure on understory plant diversity in Chinese temperate forests. Forests 2023, 14, 2138. [Google Scholar] [CrossRef]
- Rivas-Martinez, S. Mapa de series, geoseries y geopermaseries de vegetación de España: [Memory of the potential vegetation map of Spain]. Part I. [Salvador Rivas Martínez and collaborators]. Itinera Geobot. 2007, 17, 5–436. [Google Scholar]
- Feng, C.; Ma, Y.; Fu, S.L.; Chen, H.Y.H. Soil carbon and nutrient dynamics following cessation of anthropogenic disturbances in degraded subtropical forests. Land Degrad. Dev. 2017, 28, 2457–2467. [Google Scholar] [CrossRef]
- Ma, Y.H.; Feng, C.; Wang, Z.C.; Huang, C.; Huang, X.Z.; Wang, W.J.; Yang, S.B.; Fu, S.L.; Chen, H.Y.H. Restoration in degraded subtropical broadleaved forests induces changes in soil bacterial communities. Glob. Ecol. Conserv. 2021, 30, e01775. [Google Scholar] [CrossRef]
- Ouyang, Z.; Zheng, H.; Xiao, Y.; Polasky, S.; Liu, J.; Xu, W.; Wang, Q.; Zhang, L.; Xiao, Y.; Rao, E.; et al. Improvements in ecosystem services from investments in natural capital. Science 2016, 352, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ramos, A.L.C.; Luo, J.C.; Liu, Y.; Wu, T.; Lie, Z.Y.; Liu, X.J.; Cheng, Y.; Jiang, F.; Liu, J.X. Carbon storage capacity of Castanopsis hystrix plantations at different stand-ages in South China. Sci. Total Environ. 2023, 894, 164974. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wen, D.; Zhu, J.; Tang, X.; Xu, L.; Zhang, L.; Hu, H.; Huang, M.; Yu, G. Vegetation carbon sequestration in Chinese forests from 2010 to 2050. Glob. Chang. Biol. 2017, 23, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.R.; Wardle, D.A.; Bardgett, R.D.; Clarkson, B.D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef]
- Su, X.P.; Zheng, G.C.; Chen, H.Y.H. Understory diversity are driven by resource availability rather than resource heterogeneity in subtropical forests. For. Ecol. Manag. 2022, 483, 118750. [Google Scholar] [CrossRef]
- Avery, T.E.; Burkhart, H.E. Forest Measurements; McGraw Hill: New York, NY, USA, 2002. [Google Scholar]
- Pielou, E.C.; Grassle, J.F.; Patil, G.P.; Smith, W.; Taillie, C. Ecological diversity in theory and practice. Biometrics 1979, 36, 742. [Google Scholar] [CrossRef]
- Kumar, P.; Chen, H.Y.H.; Thomas, S.C.; Shahi, C. Linking resource availability and heterogeneity to understoreyunderstory species diversity through succession in boreal forest of Canada. J. Ecol. 2018, 106, 1266–1276. [Google Scholar] [CrossRef]
- Muller-Landau, H.C.; Condit, R.S.; Chave, J.; Thomas, S.C.; Bohlman, S.A.; Bunyavejchewin, S.; Davies, S.; Foster, R.; Gunatilleke, S.; Gunatilleke, N.; et al. Testing metabolic ecology theory for allometric scaling of tree size, growth and mortality in tropical forests. Ecol. Lett. 2006, 9, 575–588. [Google Scholar] [CrossRef]
- Larson, A.J.; Franklin, J.F. The tree mortality regime in temperate old-growth coniferous forests: The role of physical damage. Can. J. For. Res. 2010, 40, 2091–2103. [Google Scholar] [CrossRef]
- Jordan, S.M.R.; Barraclough, T.G.; Rosindell, J. Quantifying the effects of the break up of Pangaea on global terrestrial diversification with neutral theory. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2016, 371, 20150221. [Google Scholar] [CrossRef]
- Canham, C.D.; LePage, P.T.; Coates, K.D. A neighborhood analysis of canopy tree competition: Effects of shading versus crowding. Can. J. For. Res. 2004, 34, 778–787. [Google Scholar] [CrossRef]
- Coates, K.D.; Canham, C.D.; LePage, P.T. Above- versus below-ground competitive effects and responses of a guild of temperate tree species. J. Ecol. 2009, 97, 118–130. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved-A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.H.; Taylor, A.R. Positive species diversity and above-ground biomass relationships are ubiquitous across forest strata despite interference from overstory trees. Funct. Ecol. 2017, 31, 419–426. [Google Scholar] [CrossRef]
- Chelli, S.; Bricca, A.; Tsakalos, J.L.; Andreetta, A.; Bonari, G.; Campetella, G.; Carnicelli, S.; Cervellini, M.; Puletti, N.; Wellstein, C.; et al. Multiple drivers of functional diversity in temperate forest understories: Climate, soil, and forest structure effects. Sci. Total Environ. 2024, 961, 170258. [Google Scholar] [CrossRef]
- Wyckoff, P.H.; Clark, J.S. The relationship between growth and mortality for seven co-occurring tree species in the Southern Appalachian Mountains. J. Ecol. 2002, 90, 604–615. [Google Scholar] [CrossRef]
- Bartels, S.F.; Chen, H.Y.H. Interactions between overstory and understoreyunderstory vegetation along an overstory compositional gradient. J. Veg. Sci. 2013, 24, 543–552. [Google Scholar] [CrossRef]
- Domec, J.C.; Lachenbruch, B.; Meinzer, F.C.; Woodruff, D.R.; Warren, J.M.; McCulloh, K.A. Maximum height in a conifer is associated with conflicting requirements for xylem design. Proc. Natl. Acad. Sci. USA 2008, 105, 12069–12074. [Google Scholar] [CrossRef] [PubMed]
- Lugo, A.E.; Scatena, F.N. Background and catastrophic tree mortality in tropical moist, wet, and rain forests. Biotropica 1996, 28, 585–599. [Google Scholar] [CrossRef]
- Koorem, K.; Moora, M. Positive association between understory species richness and a dominant shrub species (Corylus avellana) in a boreonemoral spruce forest. For. Ecol. Manag. 2010, 260, 1407–1413. [Google Scholar] [CrossRef]
- Evy, A.; Federico, S.; Harald, A.; Lander, B.; Sigrid, B.; Elisa, C.; Andrea, C.; Mariangela, F.; Kalliopi, R.; Nurlaila, S.N.; et al. Driving mechanisms of overstory-understorey diversity relationships in European forests. Perspect. Plant Ecol. Evol. Syst. 2016, 19, 21–29. [Google Scholar] [CrossRef]
- Reich, P.B.; Frelich, L.E.; Voldseth, R.A.; Bakken, P.; Adair, E.C. Understorey diversity in southern boreal forests is regulated by productivity and its indirect impacts on resource availability and heterogeneity. J. Ecol. 2012, 100, 539–545. [Google Scholar] [CrossRef]
- Zhang, S.T.; Yang, X.; Li, D.B.; Li, S.C.; Chen, Z.; Wu, J.P. A meta-analysis of understory plant removal impacts on soil properties in forest ecosystems. Geoderma 2022, 426, 116116. [Google Scholar] [CrossRef]


| Time Since Closing (Years) | 0–1 | 5–6 | 11–12 | 20–24 | 28–34 | Old-Growth Forest |
|---|---|---|---|---|---|---|
| Mean tree DBH (cm) | 8.4 ± 1.6 b | 6.7 ± 2.5 b | 7.4 ± 2.0 b | 7.7 ± 1.2 b | 9.7 ± 0.2 b | 15.9 ± 0.7 a |
| Mean tree height (m) | 8.1 ± 1.2 ab | 7.1 ± 0.8 ab | 8.7 ± 3 ab | 6.9 ± 0.8 b | 8.2 ± 0.8 ab | 9.8 ± 1.1 a |
| Stand density (trees ha−1) | 200.0 ± 25.0 b | 1358.3 ± 639.0 ab | 1167.0 ± 503.0 ab | 1433.0 ± 479.0 a | 1283.0 ± 414.0 ab | 1975.0 ± 1267.0 a |
| Stem basal area (m2 ha−1) | 2.0 ± 0.7 c | 8.6 ± 5.4 bc | 8.9 ± 5.7 bc | 9.7 ± 2.7 bc | 16.6 ± 5.9 b | 42.9 ± 7.1 a |
| Tree species richness | 4.3 ± 0.6 d | 9.0 ± 1.0 ab | 6.7 ± 0.6 c | 10.3 ± 1.2 a | 8.0 ± 1.0 bc | 6.7 ± 1.5 c |
| Overstory composition (% of stem basal area) | ||||||
| Alangium chinense | 0.11 ± 0.15 a | 0.06 ± 0.1 a | 0 | 0.21 ± 0.36 a | 0 | 0 |
| Liquidambar formosana | 1.8 ± 0.8 c | 2.0 ± 0.7 c | 5.3 ± 2.6 bc | 3.0 ± 1.8 c | 8.2 ± 3.8 b | 24.6 ± 5.1 a |
| Cornus kousa subsp. chinensis | 0 | 0 | 0 | 0.2 ± 0.4 c | 5.5 ± 2.9 b | 16.0 ± 4.6 a |
| Other broad leaves | 98.1 ± 0.7 a | 97.9 ± 0.8 a | 94.7 ± 2.6 a | 96.6 ± 1.8 a | 86.3 ± 3.1 b | 59.4 ± 7.5 c |
| Understory shrub layer cover (%) | 50.6 ± 44.5 a | 40.3 ± 10.7 a | 36.0 ± 19.1 a | 29.7 ± 3.9 a | 34.2 ± 12.1 a | 19.6 ± 8.8 a |
| Understory herb layer cover (%) | 107.2 ± 32.3 ab | 108.3 ± 38.6 ab | 116.5 ± 19.4 a | 82.2 ± 11.8 ab | 78.2 ± 21.8 ab | 70.5 ± 12.1 b |
| Light available | 15.7 ± 3.2 a | 7.1 ± 0.3 bc | 8.7 ± 1.3 b | 6.1 ± 1.2 bc | 5.6 ± 0.7 c | 6.6 ± 2.0 bc |
| Light variability | 4.1 ± 0.6 a | 1.0 ± 0.2 b | 1.6 ± 0.2 b | 1.3 ± 0.1 b | 1.7 ± 0.6 b | 1.5 ± 0.2 b |
| Substrate diversity | 1.1 ± 0.1 a | 0.8 ± 0.1 b | 0.8 ± 0.0 b | 0.8 ± 0.2 b | 0.7 ± 0.1 b | 1.1 ± 0.1 a |
| Soil C (g kg−1) | 13.5 ± 2.0 c | 20.0 ± 2.0 bc | 22.7 ± 1.5 bc | 23.6 ± 3.6 b | 36.6 ± 7.2 a | 41.1 ± 9.3 a |
| Soil N (g kg−1) | 1.7 ± 0.1 c | 2.2 ± 0.1 bc | 2.4 ± 0.2 bc | 2.5 ± 0.5 b | 3.5 ± 0.7 a | 2.2 ± 0.1 bc |
| Soil pH | 5.9 ± 0.2 a | 5.4 ± 0.6 ab | 5.0 ± 0.5 b | 4.7 ± 0.3 b | 4.7 ± 0.5 b | 4.8 ± 0.3 b |
| Soil water content (%) | 21.5 ± 4.5 c | 23.8 ± 3.7 bc | 28.0 ± 3.7 ab | 27.6 ± 3.1 abc | 28.7 ± 3.7 ab | 32.0 ± 3.0 a |
| Attribute | Df | Overstory Layer | Shrub Layer | Herb Layer | |||
|---|---|---|---|---|---|---|---|
| Deviance or Variance Explained (%) | p | Deviance or Variance Explained (%) | p | Deviance or Variance Explained (%) | p | ||
| Vegetative cover | 5 | 20.68 | 0.000 | 4.28 | 0.018 | 12.13 | 0.000 |
| Stem basal area | |||||||
| Species richness | 5 | 16.87 | 0.000 | 3.46 | 0.036 | 1.97 | 0.156 |
| Species evenness | 5 | 3.75 | 0.028 | 2.02 | 0.148 | 5.16 | 0.009 |
| Shannon index | 5 | 11.08 | 0.000 | 2.50 | 0.090 | 3.47 | 0.036 |
| Species composition | 5 | 3.56 | 0.001 | 2.79 | 0.001 | 2.73 | 0.001 |
| Time Since Closing (Years) | Indicator Species | Life Forms | Specificity | Sensitivity | Indicator Value | p |
|---|---|---|---|---|---|---|
| Overstory layer | ||||||
| 5–6 | Pistacia chinensis | Tree | 0.954 | 1 | 0.977 | 0.018 |
| 11–12 | Quercus serrata | Tree | 1 | 1 | 1 | 0.012 |
| 28–34 | Castanea mollissima | Tree | 0.742 | 1 | 0.861 | 0.026 |
| 28–34 | Litsea cubeba | Tree | 0.53 | 1 | 0.728 | 0.036 |
| Old-growth forest | Liquidambar formosana | Tree | 0.542 | 1 | 0.739 | 0.004 |
| Old-growth forest | Cornus kousa subsp. chinensis | Tree | 0.886 | 1 | 0.941 | 0.004 |
| Old-growth forest | Dead wood | Tree | 0.897 | 1 | 0.947 | 0.004 |
| Shrub layer | ||||||
| 0–1 | Alangium chinense | Shrub | 0.882 | 1 | 0.939 | 0.008 |
| 5–6 | Nandina domestica | Shrub | 0.77 | 1 | 0.877 | 0.008 |
| 11–12 | Celtis biondii | Shrub | 0.688 | 1 | 0.829 | 0.021 |
| 28–34 | Spiraea salicifolia | Shrub | 1 | 1 | 1 | 0.007 |
| Old-growth forest | Cornus kousa subsp. chinensis | Tree seedling | 0.787 | 1 | 0.887 | 0.005 |
| Herb layer | ||||||
| 0–1 | Oplismenus undulatifolius | Gramineae | 0.645 | 1 | 0.809 | 0.031 |
| 5–6 | Nandina domestica | Shrub seedling | 0.772 | 1 | 0.879 | 0.013 |
| 28–34 | Dryopteris uniformis Makino | Fern | 0.787 | 1 | 0.887 | 0.026 |
| 28–34 | Selaginella tamariscina | Fern | 0.653 | 1 | 0.808 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wei, J.; Wang, W.; Huang, C.; Feng, C.; Xu, D.; Haider, F.U.; Li, X. Monitoring Changes in Composition and Diversity of Forest Vegetation Layers after the Cessation of Management for Renaturalization. Forests 2024, 15, 907. https://doi.org/10.3390/f15060907
Ma Y, Wei J, Wang W, Huang C, Feng C, Xu D, Haider FU, Li X. Monitoring Changes in Composition and Diversity of Forest Vegetation Layers after the Cessation of Management for Renaturalization. Forests. 2024; 15(6):907. https://doi.org/10.3390/f15060907
Chicago/Turabian StyleMa, Yuhua, Jingya Wei, Wenjing Wang, Cheng Huang, Chun Feng, Duanyang Xu, Fasih Ullah Haider, and Xu Li. 2024. "Monitoring Changes in Composition and Diversity of Forest Vegetation Layers after the Cessation of Management for Renaturalization" Forests 15, no. 6: 907. https://doi.org/10.3390/f15060907








