Stand Structure and Functional Traits Determine Productivity of Larix principis-rupprechtii Forests
Abstract
:1. Introduction
2. Materials and Methods
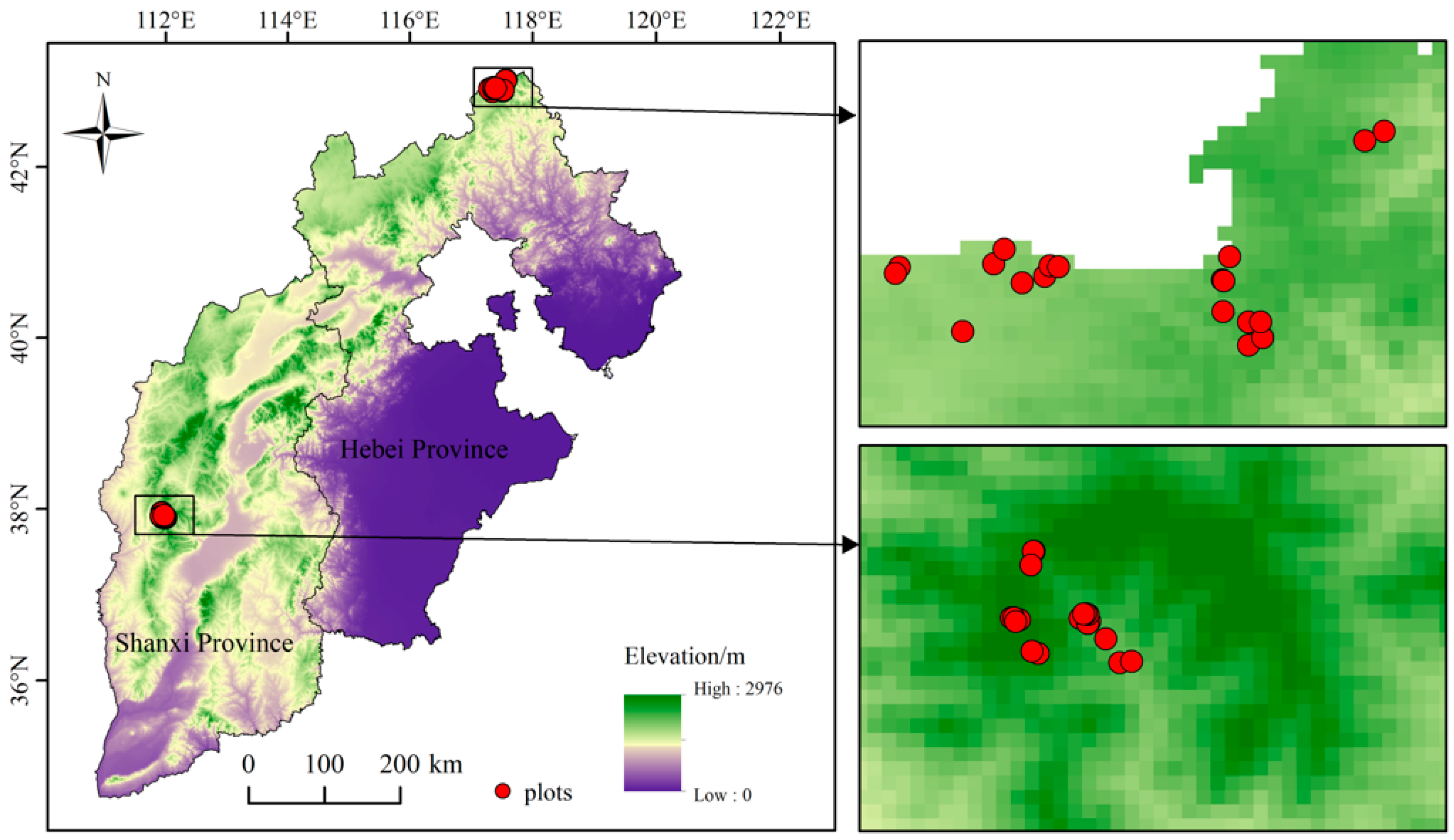
2.1. Study Area
2.2. Data Collection
2.3. Calculation of Productivity
2.4. Stand Structure
2.5. Functional Traits and Tree Species Diversity
2.6. Environmental Variables
2.7. Statistical Analysis
3. Results
3.1. Effect of Stand Structure on the BAI
3.2. Relative Importance of the Effects of SD and Functional Traits on the BAI
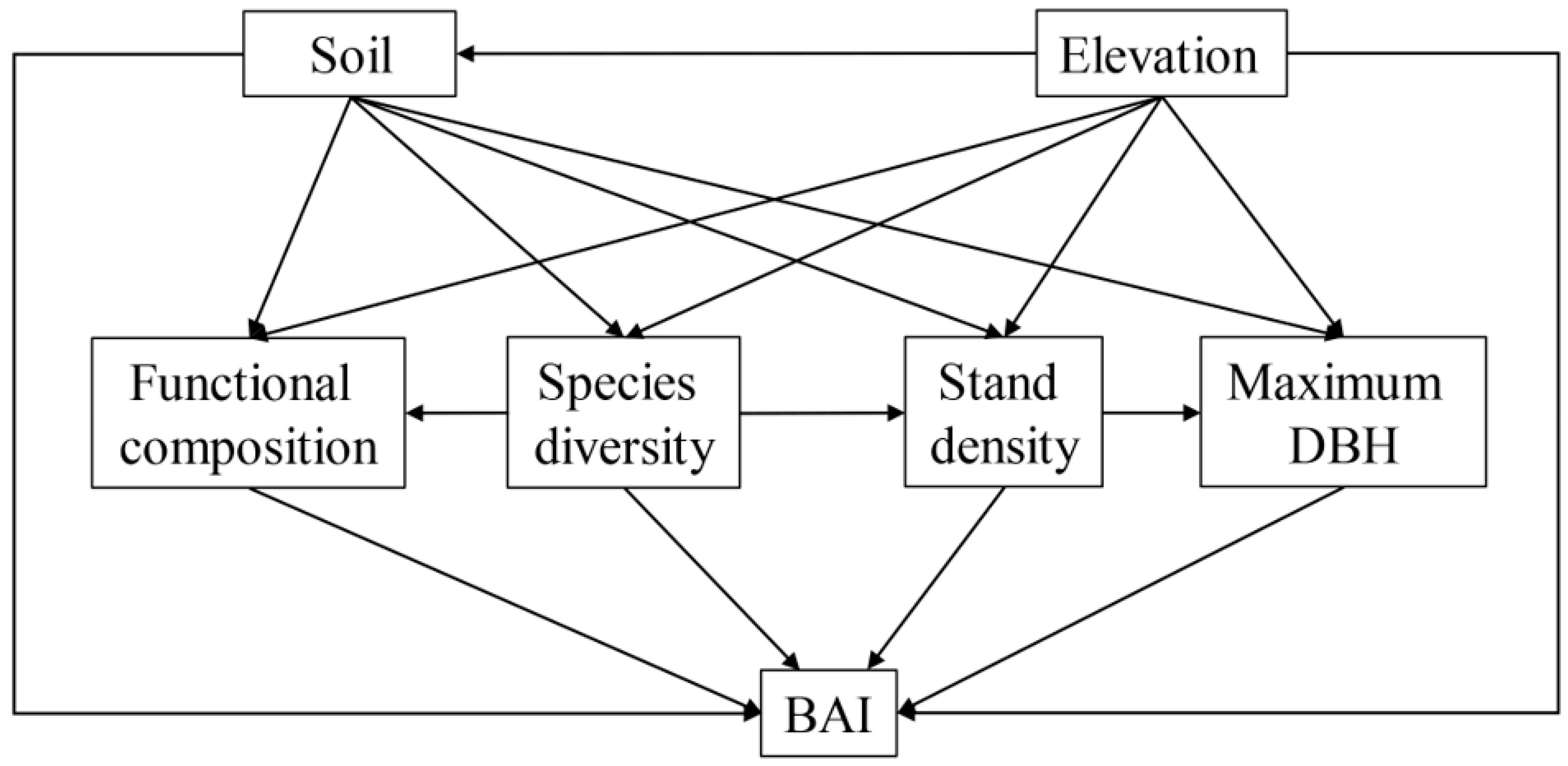
3.3. Impact Factors Directly and Indirectly Affect BAI
4. Discussion
4.1. Stand Structure Plays an Important Role in Explaining the Variation in Larch FP
4.2. The Biomass Ratio Hypothesis Is More Important Than the Niche Complementarity Hypothesis to Explain Variation in Larch Productivity
4.3. Relationship between Environmental Factors and Larch Productivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, J.J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Q.; Ali, A.; Wang, S.P.; Gazol, A.; Freckleton, R.; Wang, X.G.; Lin, F.; Ye, J.; Zhou, L.; Hao, Z.Q. Abiotic and biotic determinants of coarse woody productivity in temperate mixed forests. Sci. Total Environ. 2018, 630, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Xiang, W.H.; Wang, X.P.; Xiao, W.F.; Chen, L.; Li, S.G.; Sun, H.; Deng, X.W.; Forrester, D.I.; Zeng, L.X. Effects of stand age, richness and density on productivity in subtropical forests in China. J. Ecol. 2019, 107, 2266–2277. [Google Scholar] [CrossRef]
- Zeller, L.; Pretzsch, H. Effect of forest structure on stand productivity in Central European forests depends on developmental stage and tree species diversity. For. Ecol. Manag. 2019, 434, 193–204. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T.; Thompson, J.; Arets, E.J.; Alarcón, A.; Álvarez-Sánchez, J.; Ascarrunz, N.; Balvanera, P.; Barajas-Guzmán, G.; Boit, A.; et al. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Brunner, A.; Forrester, D.I. Tree species mixture effects on stem growth vary with stand density–An analysis based on individual tree responses. For. Ecol. Manag. 2020, 473, 118334. [Google Scholar] [CrossRef]
- Jucker, T.; Bouriaud, O.; Coomes, D.A. Crown plasticity enables trees to optimize canopy packing in mixed-species forests. Funct. Ecol. 2015, 29, 1078–1086. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; de Bello, F.; Quétier, F.; Grigulis, K.; Robson, T.M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Lin, S.L.; He, J.K.; Kong, F.M.; Yu, J.H.; Jiang, H.S. Climate and soils determine aboveground biomass indirectly via species diversity and stand structural complexity in tropical forests. For. Ecol. Manag. 2019, 432, 823–831. [Google Scholar] [CrossRef]
- Stegen, J.C.; Swenson, N.G.; Enquist, B.J.; White, E.P.; Phillips, O.L.; Jørgensen, P.M.; Weiser, M.D.; Mendoza, A.M.; Vargas, P.N. Variation in above-ground forest biomass across broad climatic gradients. Global. Ecol. Biogeogr. 2010, 20, 744–754. [Google Scholar] [CrossRef]
- Wu, Z.F.; Fan, C.Y.; Zhang, C.Y.; Zhao, X.H.; von Gadow, K. Effects of biotic and abiotic drivers on the growth rates of individual trees in temperate natural forests. For. Ecol. Manag. 2022, 503, 119769. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.M.; Cheng, R.M.; Ge, Z.X.; Zhang, Z.D. Effects of neighborhood competition and stand structure on the productivity of pure and mixed Larix principis-rupprechtii forests. Forests 2022, 13, 1318. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J. A review of processes behind diversity—Productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef]
- Drössler, L.; Agestam, E.; Bielak, K.; Dudzinska, M.; Koricheva, J.; Liziniewicz, M.; Löf, M.; Mason, B.; Pretzsch, H.; Valkonen, S. Over-and underyielding in time and space in experiments with mixed stands of scots pine and Norway Spruce. Forests 2018, 9, 495. [Google Scholar] [CrossRef]
- Ding, Y.; Zang, R.G. Determinants of aboveground biomass in forests across three climatic zones in China. For. Ecol. Manag. 2021, 482, 118805. [Google Scholar] [CrossRef]
- Fan, C.Y.; Tan, L.Z.; Zhang, C.Y.; Zhao, X.H.; Gao, L.S.; von Gadow, K. Scale-dependent effects of neighborhood biodiversity on individual tree productivity in a coniferous and broad-leaved mixed forest in China. Ecol. Evol. 2020, 10, 8225–8234. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef]
- Grime, J. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Pretzsch, H.; Bielak, K.; Block, J.; Bruchwald, A.; Dieler, J.; Ehrhart, H.P.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zasada, M. Productivity of mixed versus pure stands of oak (Quercus petraea (Matt.) Liebl.; Quercus robur L.) and European beech (Fagus sylvatica L.) along an ecological gradient. Eur. J. Forest. Res. 2013, 132, 263–280. [Google Scholar] [CrossRef]
- Kweon, D.; Comeau, P.G. Relationships between tree survival, stand structure and age in trembling aspen dominated stands. For. Ecol. Manag. 2019, 438, 114–122. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5, 4967. [Google Scholar] [CrossRef]
- Campetella, G.; Botta-Dukát, Z.; Wellstein, C.; Canullo, R.; Gatto, S.; Chelli, S.; Mucina, L.; Bartha, S. Patterns of plant trait–environment relationships along a forest succession chronosequence. Agric. Ecosyst. Environ. 2011, 145, 38–48. [Google Scholar] [CrossRef]
- Barry, K.E.; Mommer, L.; van Ruijven, J.; Wirth, C.; Wright, A.J.; Bai, Y.; Connolly, J.; De Deyn, G.B.; De Kroon, H.; Isbell, F. The future of complementarity: Disentangling causes from consequences. Trends. Ecol. Evol. 2019, 34, 167–180. [Google Scholar] [CrossRef]
- Forrester, D.I.; Ammer, C.; Annighöfer, P.J.; Barbeito, I.; Bielak, K.; Bravo-Oviedo, A.; Coll, L.; del Río, M.; Drössler, L.; Heym, M. Effects of crown architecture and stand structure on light absorption in mixed and monospecific Fagus sylvatica and Pinus sylvestris forests along a productivity and climate gradient through Europe. J. Ecol. 2018, 106, 746–760. [Google Scholar] [CrossRef]
- Amoroso, M.; Turnblom, E. Comparing productivity of pure and mixed Douglas-fir and western hemlock plantations in the Pacific Northwest. Can. J. Forest. Res. 2006, 36, 1484–1496. [Google Scholar] [CrossRef]
- Bauhus, J.; Van Winden, A.P.; Nicotra, A.B. Aboveground interactions and productivity in mixed-species plantations of Acacia mearnsii and Eucalyptus globulus. Can. J. Forest. Res. 2004, 34, 686–694. [Google Scholar] [CrossRef]
- Bourdier, T.; Cordonnier, T.; Kunstler, G.; Piedallu, C.; Lagarrigues, G.; Courbaud, B. Tree size inequality reduces forest productivity: An analysis combining inventory data for ten European species and a light competition model. PLoS ONE 2016, 11, e0151852. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y. Individual size inequality links forest diversity and above-ground biomass. J. Ecol. 2015, 103, 1245–1252. [Google Scholar] [CrossRef]
- Long, J.N.; Shaw, J.D. The influence of compositional and structural diversity on forest productivity. Forestry 2010, 83, 121–128. [Google Scholar] [CrossRef]
- Soares, A.A.; Leite, H.G.; Souza, A.L.; Silva, S.R.; Lourenço, H.M.; Forrester, D.I. Increasing stand structural heterogeneity reduces productivity in Brazilian Eucalyptus monoclonal stands. For. Ecol. Manag. 2016, 373, 26–32. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.R. The forest strata-dependent relationship between biodiversity and aboveground biomass within a subtropical forest. For. Ecol. Manag. 2017, 401, 125–134. [Google Scholar] [CrossRef]
- Forrester, D.I. Linking forest growth with stand structure: Tree size inequality, tree growth or resource partitioning and the asymmetry of competition. For. Ecol. Manag. 2019, 447, 139–157. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T.; Arets, E.J.; Ascarrunz, N.; Enquist, B.J.; Finegan, B.; Licona, J.C.; Martínez-Ramos, M.; Mazzei, L.; Meave, J.A. Biodiversity and climate determine the functioning of Neotropical forests. Global. Ecol. Biogeogr. 2017, 26, 1423–1434. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Sanders, N.J.; Wardle, D.A. Community and ecosystem responses to elevational gradients: Processes, mechanisms, and insights for global change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 261–280. [Google Scholar] [CrossRef]
- Xu, A.Y.; Wang, D.Z.; Liu, Q.; Zhang, D.Y.; Zhang, Z.D.; Huang, X.R. Incorporating stand density effects and regression techniques for stem taper modeling of a Larix principis-rupprechtii plantation. Front. Plant. Sci. 2022, 13, 902325. [Google Scholar] [CrossRef]
- Duchesne, L.; Houle, D.; Ouimet, R.; Caldwell, L.; Gloor, M.; Brienen, R. Large apparent growth increases in boreal forests inferred from tree-rings are an artefact of sampling biases. Sci. Rep. 2019, 9, 6832. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 51–67. [Google Scholar]
- Finegan, B.; Peña-Claros, M.; de Oliveira, A.; Ascarrunz, N.; Bret-Harte, M.S.; Carreño-Rocabado, G.; Casanoves, F.; Díaz, S.; Eguiguren Velepucha, P.; Fernandez, F. Does functional trait diversity predict above-ground biomass and productivity of tropical forests? Testing three alternative hypotheses. J. Ecol. 2015, 103, 191–201. [Google Scholar] [CrossRef]
- Tobner, C.M.; Paquette, A.; Gravel, D.; Reich, P.B.; Williams, L.J.; Messier, C. Functional identity is the main driver of diversity effects in young tree communities. Ecol. Lett. 2016, 19, 638–647. [Google Scholar] [CrossRef]
- van der Sande, M.T.; Peña-Claros, M.; Ascarrunz, N.; Arets, E.J.; Licona, J.C.; Toledo, M.; Poorter, L. Abiotic and biotic drivers of biomass change in a Neotropical forest. J. Ecol. 2017, 105, 1223–1234. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.; Cornwell, W.; Craine, J.; Gurvich, D. New handbook for standardised measurement of plant functional traits worldwide. Aust. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Luo, Y.; Li, D. Flora of China. J. Plant. Classif. Resour. 2013, 35, 742. [Google Scholar]
- Chen, L.; Xiang, W.H.; Ouyang, S.; Wu, H.L.; Xia, Q.; Ma, J.N.; Zeng, Y.L.; Lei, P.F.; Xiao, W.F.; Li, S.G. Tight coupling of fungal community composition with soil quality in a Chinese fir plantation chronosequence. Land Degrad. Dev. 2021, 32, 1164–1178. [Google Scholar] [CrossRef]
- Jiang, Y.; Zang, R.G.; Lu, X.H.; Huang, Y.F.; Ding, Y.; Liu, W.D.; Long, W.X.; Zhang, J.Y.; Zhang, Z.D. Effects of soil and microclimatic conditions on the community-level plant functional traits across different tropical forest types. Plant Soil 2015, 390, 351–367. [Google Scholar] [CrossRef]
- Marqués, L.; Camarero, J.J.; Zavala, M.A.; Stoffel, M.; Ballesteros-Cánovas, J.A.; Sancho-García, C.; Madrigal-González, J. Evaluating tree-to-tree competition during stand development in a relict Scots pine forest: How much does climate matter? Trees 2021, 35, 1207–1219. [Google Scholar] [CrossRef]
- Weiner, J.; Solbrig, O.T. The meaning and measurement of size hierarchies in plant populations. Oecologia 1984, 61, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Binkley, D.; Kashian, D.M.; Boyden, S.; Kaye, M.W.; Bradford, J.B.; Arthur, M.A.; Fornwalt, P.J.; Ryan, M.G. Patterns of growth dominance in forests of the Rocky Mountains, USA. For. Ecol. Manag. 2006, 236, 193–201. [Google Scholar] [CrossRef]
- Lexerød, N.L.; Eid, T. An evaluation of different diameter diversity indices based on criteria related to forest management planning. For. Ecol. Manage. 2006, 222, 17–28. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Van der Plas, F.; Schröder-Georgi, T.; Weigelt, A.; Barry, K.; Meyer, S.; Alzate, A.; Barnard, R.L.; Buchmann, N.; De Kroon, H.; Ebeling, A. Plant traits alone are poor predictors of ecosystem properties and long-term ecosystem functioning. Nat. Ecol. Evol. 2020, 4, 1602–1611. [Google Scholar] [CrossRef]
- Bongers, F.J.; Schmid, B.; Bruelheide, H.; Bongers, F.; Li, S.; von Oheimb, G.; Li, Y.; Cheng, A.; Ma, K.; Liu, X. Functional diversity effects on productivity increase with age in a forest biodiversity experiment. Nat. Ecol. Evol. 2021, 5, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.L.; Wang, G.Y.; Innes, J.L.; Seely, B.; Chen, B.Z. ClimateAP: An application for dynamic local downscaling of historical and future climate data in Asia Pacific. Front. Agric. Sci. Eng. 2017, 4, 448–458. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods. Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Egbert, J.; Plonsky, L. Bootstrapping techniques. A Practical Handbook of Corpus Linguistics; Springer International Publishing: Cham, Switzerland, 2021; pp. 593–610. [Google Scholar]
- Jucker, T.; Sanchez, A.C.; Lindsell, J.A.; Allen, H.D.; Amable, G.S.; Coomes, D.A. Drivers of aboveground wood production in a lowland tropical forest of West Africa: Teasing apart the roles of tree density, tree diversity, soil phosphorus, and historical logging. Ecol. Evol. 2016, 6, 4004–4017. [Google Scholar] [CrossRef] [PubMed]
- Yachi, S.; Loreau, M. Does complementary resource use enhance ecosystem functioning? A model of light competition in plant communities. Ecol. Lett. 2007, 10, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.T.; Zhang, J.; Zhang, Z.D.; Huang, X.R. Effects of tree species interaction, stand density, and tree size on the productivity of Larix principis-rupprechtii. Chin. J. Appl. Ecol. 2021, 32, 2722–2728. [Google Scholar]
- Garber, S.M.; Maguire, D.A. Stand productivity and development in two mixed-species spacing trials in the central Oregon cascades. Forest Sci. 2004, 50, 92–105. [Google Scholar] [CrossRef]
- Bordin, K.M.; Esquivel-Muelbert, A.; Bergamin, R.S.; Klipel, J.; Picolotto, R.C.; Frangipani, M.A.; Zanini, K.J.; Cianciaruso, M.V.; Jarenkow, J.A.; Jurinitz, C.F. Climate and large-sized trees, but not diversity, drive above-ground biomass in subtropical forests. For. Ecol. Manag. 2021, 490, 119126. [Google Scholar] [CrossRef]
- Silva Pedro, M.; Rammer, W.; Seidl, R. Disentangling the effects of compositional and structural diversity on forest productivity. J. Veg. Sci. 2017, 28, 649–658. [Google Scholar] [CrossRef]
- Ligot, G.; Gourlet-Fleury, S.; Ouédraogo, D.Y.; Morin, X.; Bauwens, S.; Baya, F.; Brostaux, Y.; Doucet, J.L.; Fayolle, A. The limited contribution of large trees to annual biomass production in an old-growth tropical forest. Ecol. Appl. 2018, 28, 1273–1281. [Google Scholar] [CrossRef]
- Li, J.J.; Chen, D.S.; Yang, X.; Fan, N.Q.; Wang, Y.W.; Zhang, Z.D. Effects of stand density, age, and drought on the size–growth relationship in Larix principis-rupprechtii forests. Forests 2024, 15, 413. [Google Scholar] [CrossRef]
- Michel, L.; Andy, H. Partitioning selection and complementarity in biodiversity experiments. Nature 2001, 412, 72–76. [Google Scholar]
- Conti, G.; Díaz, S. Plant functional diversity and carbon storage–an empirical test in semi-arid forest ecosystems. J. Ecol. 2013, 101, 18–28. [Google Scholar] [CrossRef]
- Su, L.; Heydari, M.; Omidipour, R.; Soheili, F.; Cheraghi, J.; Villa, P.M.; Prévosto, B. Stand structural diversity and elevation rather than functional diversity drive aboveground biomass in historically disturbed semiarid oak forests. For. Ecol. Manag. 2023, 543, 121139. [Google Scholar] [CrossRef]
- Larsary, M.K.; Pourbabaei, H.; Salehi, A.; Yousefpour, R.; Ali, A. Few functionally acquisitive big-sized trees restrict but soil nutrients promote soil organic carbon storage in temperate deciduous forests. For. Ecol. Manag. 2023, 541, 121059. [Google Scholar] [CrossRef]
- Chun, J.H.; Ali, A.; Lee, C.B. Topography and forest diversity facets regulate overstory and understory aboveground biomass in a temperate forest of South Korea. Sci. Total Environ. 2020, 744, 140783. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.F.; Ren, H. Productivity as related to diversity and age in planted versus natural forests. Glob. Ecol. Biogeogr. 2014, 23, 1461–1471. [Google Scholar] [CrossRef]
- Clark, D.B.; Clark, D.A. Landscape-scale variation in forest structure and biomass in a tropical rain forest. For. Ecol. Manag. 2000, 137, 185–198. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Talbot, J.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Begne, S.K.; Chave, J.; Cuni-Sanchez, A.; Hubau, W.; Lopez-Gonzalez, G. Diversity and carbon storage across the tropical forest biome. Sci. Rep. 2017, 7, 39102. [Google Scholar] [CrossRef]
- Khadanga, S.S.; Jayakumar, S. Tree biomass and carbon stock: Understanding the role of species richness, elevation, and disturbance. Trop. Ecol. 2020, 61, 128–141. [Google Scholar] [CrossRef]




| Variable | Pure Larch Forests | Mixed Larch Forests | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | |
| Age (year) | 10.00 | 76.00 | 32.07 | 19.23 | 32.00 | 88.00 | 53.33 | 16.88 |
| DBH (cm) | 8.53 | 33.98 | 17.82 | 8.03 | 12.78 | 38.76 | 20.39 | 5.46 |
| dq (cm) | 8.80 | 32.50 | 18.15 | 7.83 | 13.60 | 31.50 | 22.23 | 4.69 |
| Elevation (m) | 1482 | 2282 | 1736.73 | 253.70 | 1491 | 2224 | 1797.25 | 238.68 |
| BAI (m2/ha/year) | 0.51 | 1.60 | 0.99 | 0.36 | 0.21 | 1.13 | 0.72 | 0.22 |
| Larch proportion | 0.20 | 0.80 | 0.48 | 0.20 | ||||
| SR | 2.00 | 5.00 | 2.96 | 0.81 | ||||
| No. plots | 16 | 23 | ||||||
| Variable | Estimate | SE | t-Value | p-Value | Sig. |
|---|---|---|---|---|---|
| Intercept | 3.91 | 0.02 | 267.99 | <0.001 | *** |
| Elevation | –0.03 | 0.02 | –1.29 | 0.21 | |
| TN | 0.02 | 0.02 | 1.36 | 0.19 | |
| Richness | –0.02 | 0.02 | –1.19 | 0.24 | |
| Density (trees/ha) | 0.13 | 0.02 | –1.19 | <0.001 | *** |
| Maximum DBH | 0.05 | 0.03 | 2.09 | 0.04 | * |
| CWM_LCC | 0.04 | 0.02 | 1.98 | 0.06 | |
| CWM_LKC | 0.05 | 0.02 | 3.51 | <0.01 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, M.; Cheng, R.; Ge, Z.; Liu, C.; Zhang, Z. Stand Structure and Functional Traits Determine Productivity of Larix principis-rupprechtii Forests. Forests 2024, 15, 924. https://doi.org/10.3390/f15060924
Zhang J, Li M, Cheng R, Ge Z, Liu C, Zhang Z. Stand Structure and Functional Traits Determine Productivity of Larix principis-rupprechtii Forests. Forests. 2024; 15(6):924. https://doi.org/10.3390/f15060924
Chicago/Turabian StyleZhang, Jing, Ming Li, Ruiming Cheng, Zhaoxuan Ge, Chong Liu, and Zhidong Zhang. 2024. "Stand Structure and Functional Traits Determine Productivity of Larix principis-rupprechtii Forests" Forests 15, no. 6: 924. https://doi.org/10.3390/f15060924




