Prediction of the Global Distribution of Arhopalus rusticus under Future Climate Change Scenarios of the CMIP6
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence Data Collection
2.2. Acquisition and Selection of Bioclimate Variables
2.3. Model Calculation and Evaluation
2.4. Model Prediction and Visual Analytics
3. Results
3.1. Comparison of the Contribution of Key Environmental Variables to Influencing the Distribution of A. rusticus
3.2. Comparison of the Accuracy of the Prediction Results of Different Models
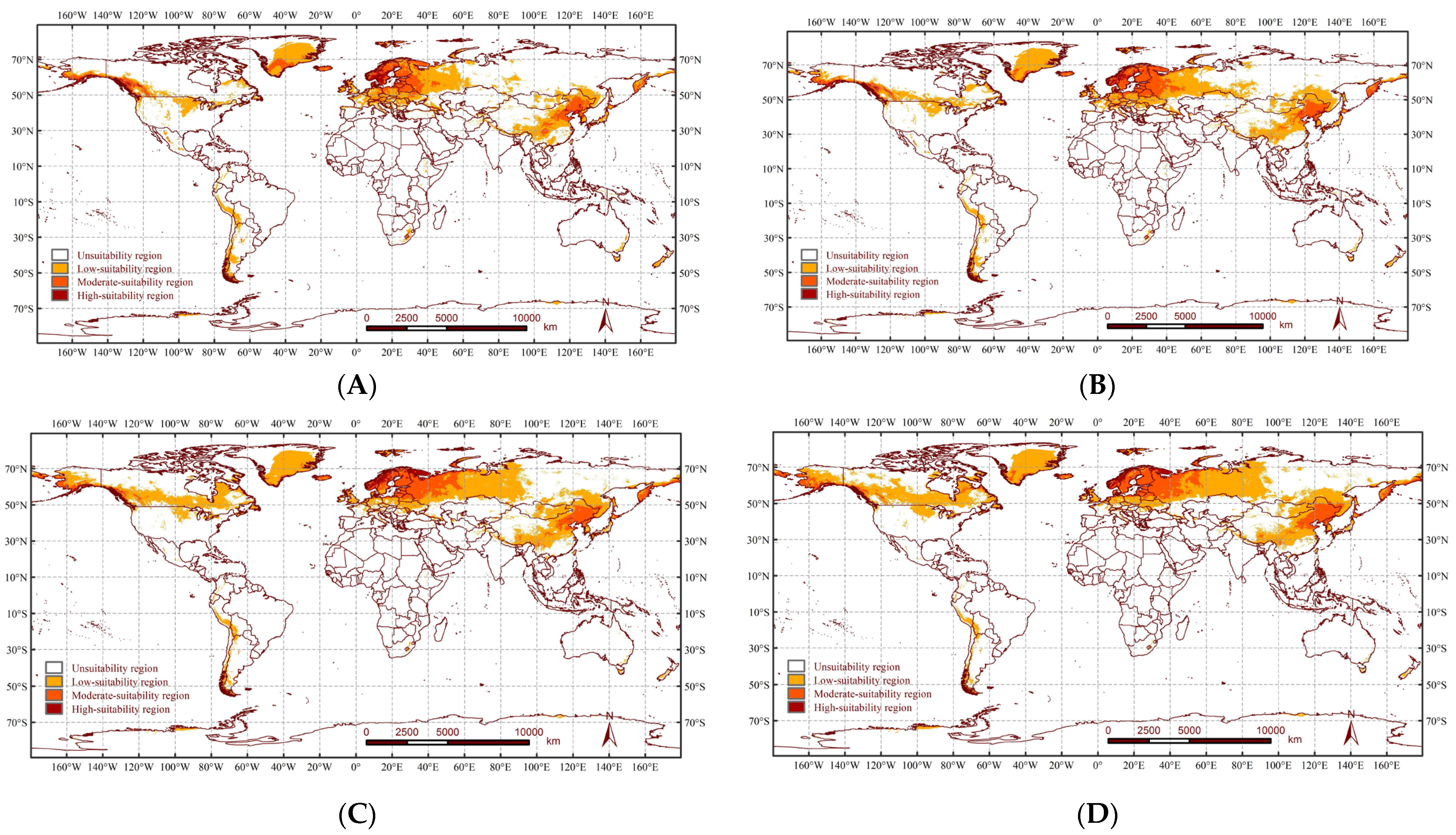
3.3. Potential Distribution Changes of A. rusticus under Historical Climatic Conditions
3.4. Comparison of the Potential Distribution of A. rusticus under Future Climate Scenarios
4. Discussion
4.1. Reliability of RF Model Prediction Results
4.2. Climatic Variables That Dominate the Distribution of A. rusticus
4.3. Potential Distribution Range of A. rusticus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.C.; Chen, G.F.; Zhang, X.D.; Chi, Z.Q.; Wang, C.Z.; Xu, J.; Dai, Q.H.; Zhang, X.F.; Liu, R.M. The occurrence, current damage status, and control strategies of Arhopalus rusticus in China. J. Shandong For. Sci. Technol. 2021, 51, 96–100. [Google Scholar]
- Huang, P. Three major forestry pests in the Arhopalus. Plant Quar. 2008, 22, 234–235. [Google Scholar]
- Wang, Q.; Leschen, R.A.B. Identification and distribution of Arhopalus species (Coleoptera: Cerambycidae: Aseminae) in Australia and New Zealand. New Zealand Entomol. 2003, 26, 53–59. [Google Scholar] [CrossRef]
- Grilli, M.P.; Fachinetti, R. The role of sex and mating status in the expansion process of Arhopalus rusticus (coleoptera: Cerambycidae)—An exotic cerambycid in Argentina. Environ. Entomol. 2017, 46, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.K.; Griffiths, H.M.; Thomas, C.D. Climate change and evolutionary adaptations at species’ range margins. Annu. Rev. Entomol. 2011, 56, 143–159. [Google Scholar] [CrossRef]
- Barton, M.G.; Terblanche, J.S. Predicting performance and survival across topographically heterogeneous landscapes: The global pest insect Helicoverpa armigera (Hübner, 1808) (Lepidoptera: Noctuidae). Austral Entomol. 2014, 53, 249–258. [Google Scholar] [CrossRef]
- Adhikari, P.; Kim, B.J.; Hong, S.H.; Lee, D.H. Climate change induced habitat expansion of nutria (Myocastor coypus) in South Korea. Sci. Rep. 2022, 12, 3300. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.K.; Li, Y.D.; Yang, W.; Yang, Y. Potential distribution of Monochamus alternatus Hope (Coleoptera: Cerambycidae) in China based on MaxEnt model and its response to climate change. J. Sichuan For. Sci. Technol. 2024, 45, 78–87. [Google Scholar]
- Ge, X.Z.; Zong, S.X.; He, S.Y.; Liu, Y.T.; Kong, X.Q. Areas of China predicted to have a suitable climate for Anoplophora chinensis under a climate-warming scenario. Entomol. Exp. Appl. 2014, 153, 256–265. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.G.; Lei, Y.H. Predicting Distribution of the Asian Longhorned Beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae) and Its Natural Enemies in China. Insects 2022, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.X.; Du, W.W.; Li, M.L. Global Warming and Adaptive Changes of Forest Pests. J. Northwest For. Univ. 2011, 26, 124–128. [Google Scholar]
- Wan, N. Research on the impact of climate change on forest tree pests and diseases. Rural Econ. Sci. 2022, 33, 42–44. [Google Scholar]
- Pearson, R.G. Species’ Distribution Modeling for Conservation Educators and Practitioners. Lessons Conserv. 2010, 3, 54–89. [Google Scholar]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Fachinetti, R.; Grilli, M.P. Biological traits and field distribution of introduced Arhopalus species in Central Argentina. Int. J. Pest Manag. 2022, 68, 274–284. [Google Scholar] [CrossRef]
- Zhang, L. Preliminary Study on the Biology and Attractants of Arhopalus rusticus. Master’s Thesis, Beijing Forestry University, Beijing, China, 2016. [Google Scholar]
- Wang, H.T.; Yu, N.; Wang, D.F.; Hou, R.; Liu, Z.Y.; Lu, X.P. Control Efficiency of Several Pollution-free Techniques against Arhopalus rusticus (L.) (Coleoptera: Cerambycidae). Chin. J. Biol. Control 2016, 32, 666–671. [Google Scholar]
- Ze, S.Z.; Zhao, N.; Wang, D.W.; Ji, M.; Yang, B. The Effectiveness of trans-2-Hexenal as an Attractant for Adult Arhopalus rusticus. For. Pest China 2013, 32, 46. [Google Scholar]
- Pan, J.L.; Chen, G.F.; Wang, C.Z.; Xu, J.; Cui, D.Y. Evaluation and Application of Aggregation-sex Pheromone to Trap Arhopalus rusticus (Coleoptera: Cerambycidae). Chin. J. Biol. Control 2022, 38, 1043–1047. [Google Scholar]
- Yu, N. Researches on Biological Characteristics and Adult Emergence Period Prediction of Arhopalus rusticus. Master’s Thesis, Shandong Agricultural University, Taian, China, 2017. [Google Scholar]
- Sillero, N. What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecol. Model. 2011, 222, 1343–1346. [Google Scholar] [CrossRef]
- Sánchez-Montes, G.; Recuero, E.; Barbosa, A.M.; Martínez-Solano, I. Complementing the Pleistocene biogeography of European amphibians: Testimony from a southern Atlantic species. J. Biogeogr. 2019, 46, 568–583. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Hastie, T.J. Generalized additive models. In Statistical Models in S; Routledge: London, UK, 2017; pp. 249–307. [Google Scholar]
- Choe, H.; Thorne, J.H.; Seo, C. Mapping national plant biodiversity patterns in South Korea with the MARS species distribution model. PLoS ONE 2016, 11, e0149511. [Google Scholar] [CrossRef] [PubMed]
- Ripley, B.D. Pattern Recognition and Neural Networks. Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Breiman, L. Classification and Regression Trees; Routledge: London, UK, 2017. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. blockCV: An r package for generating spatially or environmentally separated folds for k-fold cross-validation of species distribution models. Biorxiv 2018, 10, 357798. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Xu, C.Q.; Liu, Y.H.; Hu, J.; Li, Y.; Ma, R.Y. Potential distribution prediction of apple-grass aphid Rhopalosiphum oxyacanthae in China based on MaxEnt model. J. Plant Prot. 2020, 47, 528–536. [Google Scholar]
- Zou, Y.T.; Ge, X.Z.; Zou, Y.; Guo, S.W.; Wang, T.; Zong, S.X. Prediction of the potential global distribution of the Asian longhorned beetle Anoplophora glabripennis under climate change. Agric. For. Entomol. 2021, 23, 557–568. [Google Scholar] [CrossRef]
- Lin, Y.W.; Chen, Y.; Weng, R.Q. An important forest pest-Arhopalus rusticus captured in Fuzhou Port. Fujian J. For. Sci. Technol. 2007, 3, 214–215. [Google Scholar]
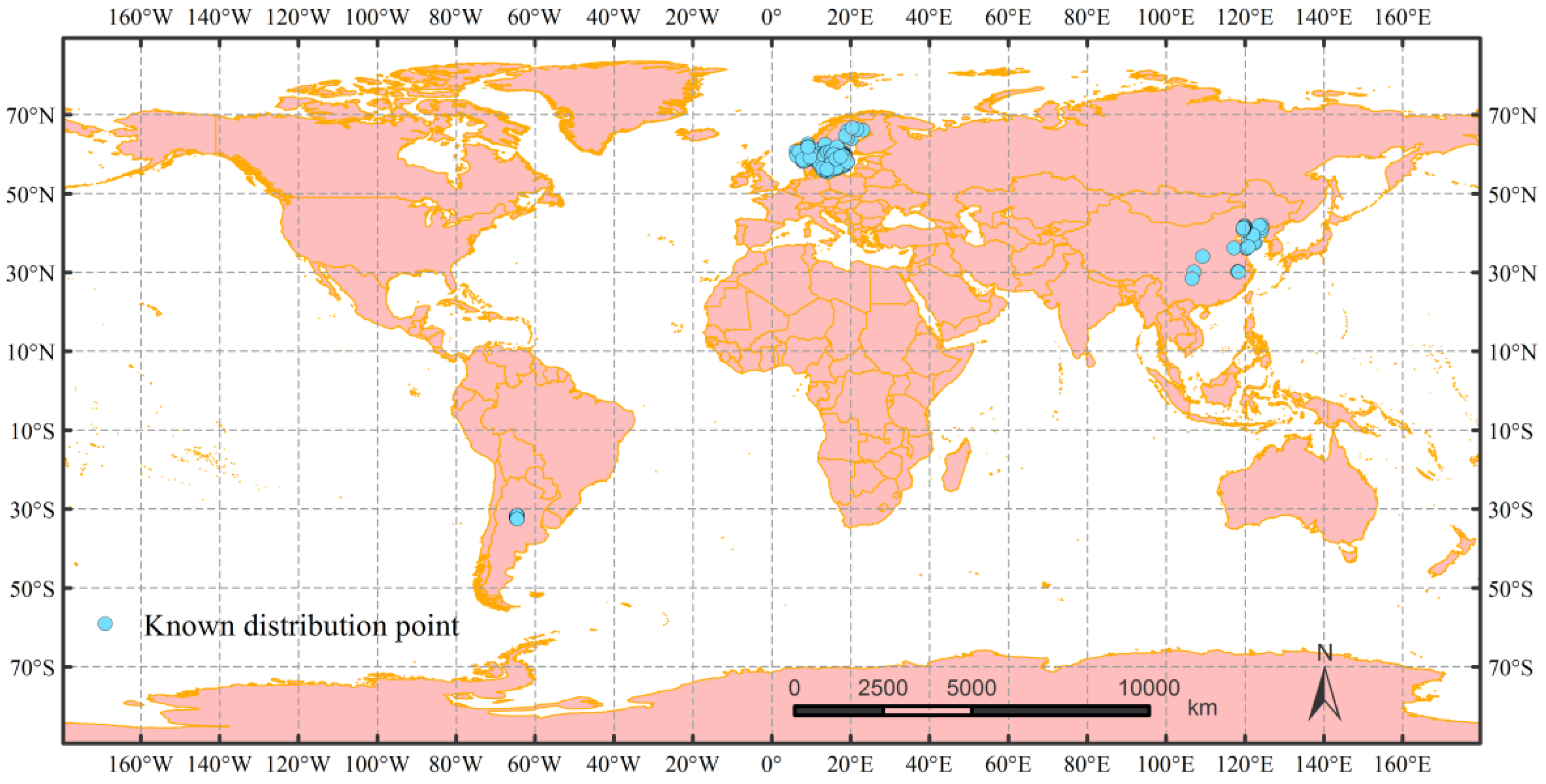
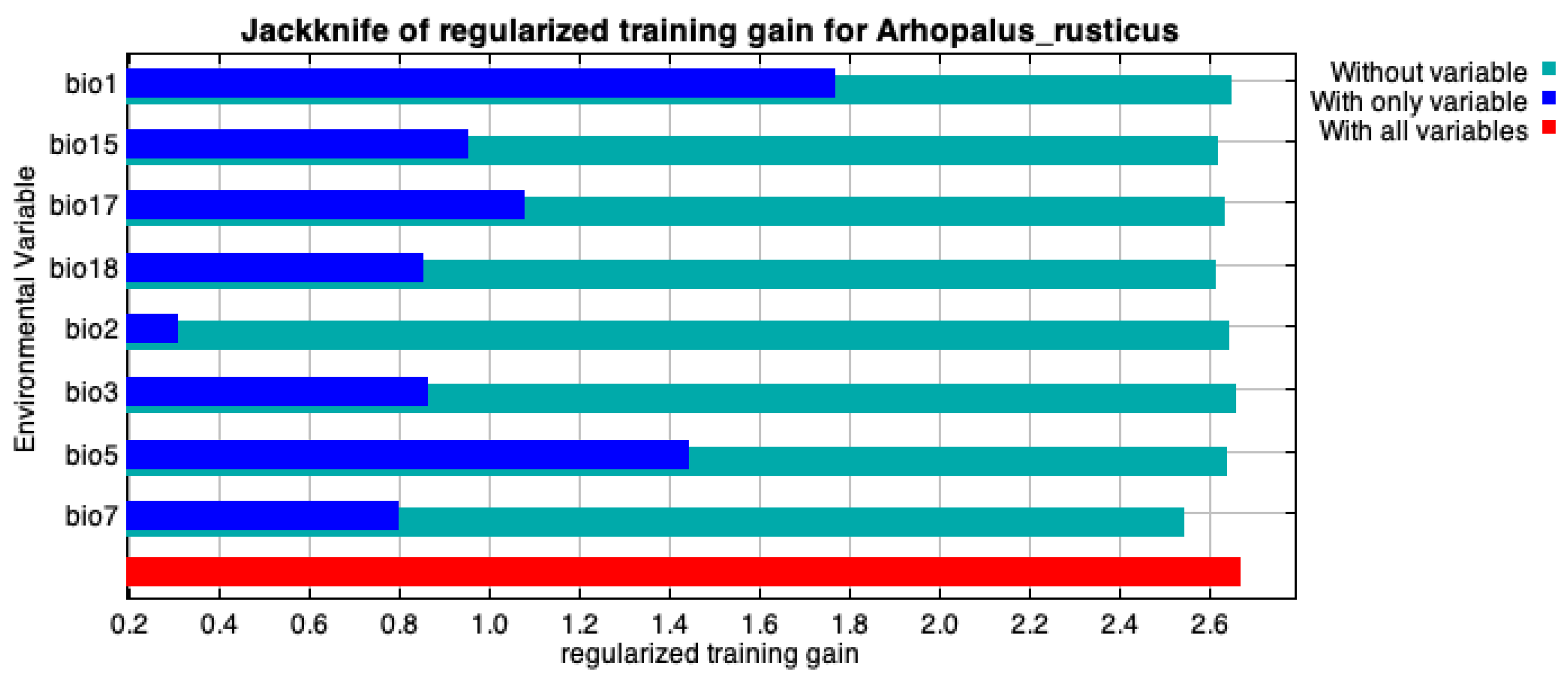

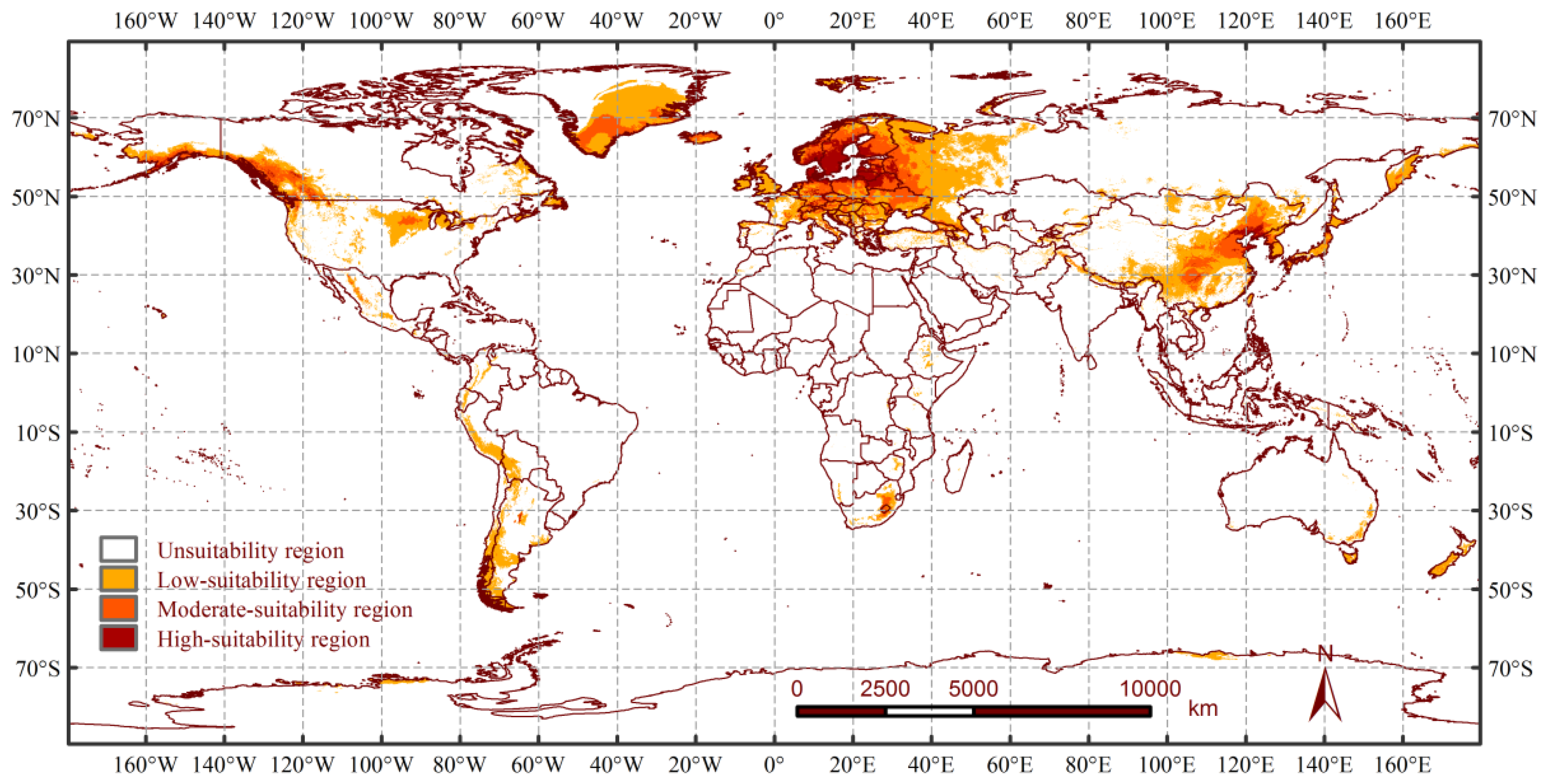
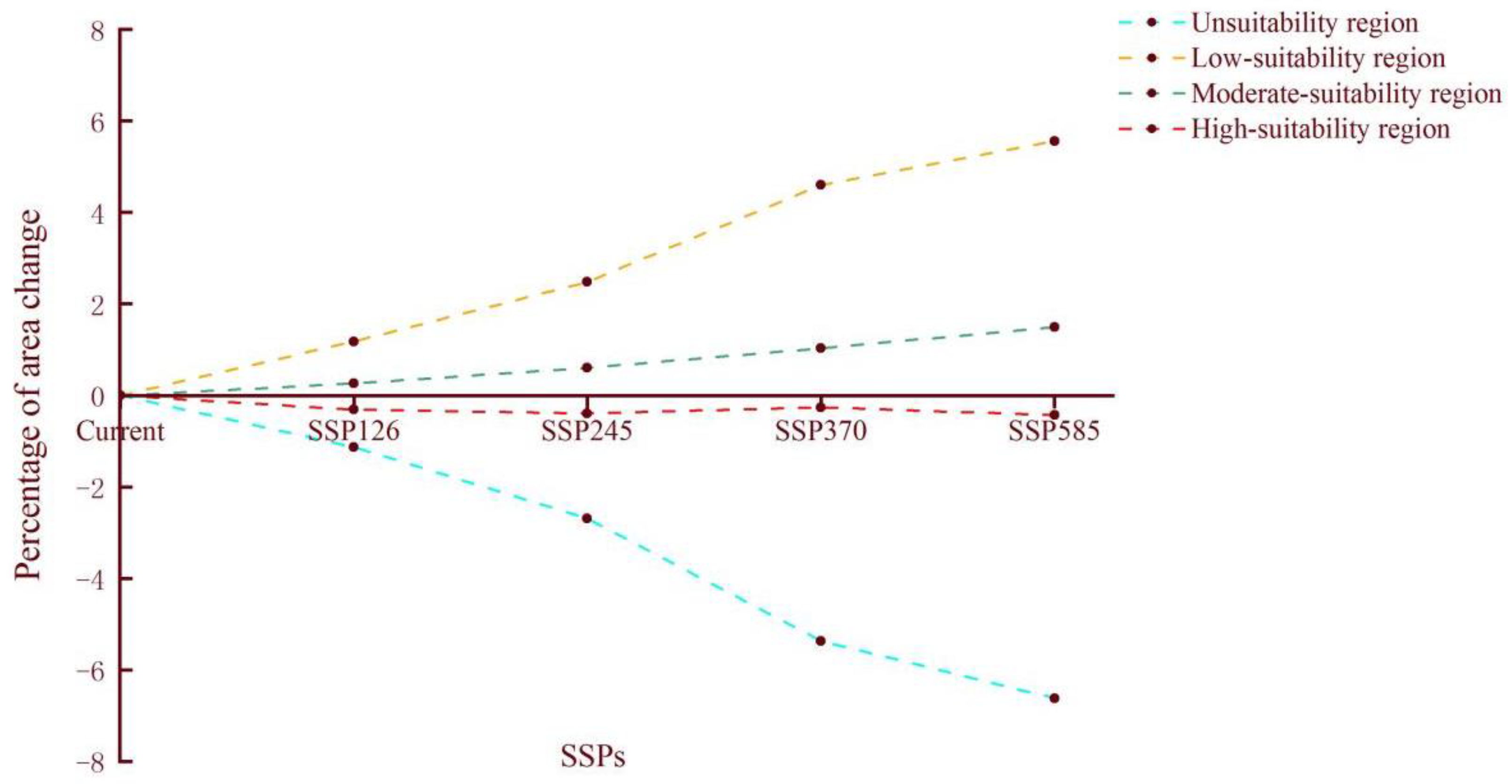
| Bioclimate Variable | Contribution Rate/% | Suitable Range |
|---|---|---|
| Annual mean temperature (Bio1)/°C | 27.1 | 5~8 |
| Temperature annual range (Bio7)/°C | 23.7 | 23~29 |
| Precipitation of the warmest quarter (Bio18)/mm | 17.2 | 100~250 |
| Precipitation of the driest quarter (Bio17)/mm | 17 | 90~190 |
| Max. temperature of the warmest month (Bio5)/°C | 5.9 | 20~23 |
| Precipitation seasonality (Bio15) | 4 | 20~30 |
| Mean diurnal range (Bio2)/°C | 2.6 | 6~9 |
| Isothermality (Bio3) | 2.5 | 26~31 |
| Year and SSP Value | Percentage of Different Areas | |||
|---|---|---|---|---|
| Unsuitable Region | Low-Suitability Region | Moderate-Suitability Region | High-Suitability Region | |
| Current | 86.39 | 9.43 | 3.31 | 0.87 |
| 2081–2100 SSP126 | 85.26 (−1.13) | 10.61 (1.18) | 3.57 (0.26) | 0.56 (−0.31) |
| 2081–2100 SSP245 | 83.7 (−2.69) | 11.91 (2.48) | 3.91 (0.6) | 0.48 (−0.39) |
| 2081–2100 SSP370 | 81.02 (−5.37) | 14.03 (4.6) | 4.34 (1.03) | 0.61 (−0.26) |
| 2081–2100 SSP585 | 79.77 (−6.62) | 14.99 (5.56) | 4.8 (1.49) | 0.44 (−0.43) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Zhang, X.; Zhou, Y.; Zong, S. Prediction of the Global Distribution of Arhopalus rusticus under Future Climate Change Scenarios of the CMIP6. Forests 2024, 15, 955. https://doi.org/10.3390/f15060955
Fan Y, Zhang X, Zhou Y, Zong S. Prediction of the Global Distribution of Arhopalus rusticus under Future Climate Change Scenarios of the CMIP6. Forests. 2024; 15(6):955. https://doi.org/10.3390/f15060955
Chicago/Turabian StyleFan, Yuhang, Xuemei Zhang, Yuting Zhou, and Shixiang Zong. 2024. "Prediction of the Global Distribution of Arhopalus rusticus under Future Climate Change Scenarios of the CMIP6" Forests 15, no. 6: 955. https://doi.org/10.3390/f15060955
APA StyleFan, Y., Zhang, X., Zhou, Y., & Zong, S. (2024). Prediction of the Global Distribution of Arhopalus rusticus under Future Climate Change Scenarios of the CMIP6. Forests, 15(6), 955. https://doi.org/10.3390/f15060955






