Phosphorous Fractions in Soils of Natural Shrub-Grass Communities and Leucaena leucocephala Plantations in a Dry-Hot Valley
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Phosphorus Fractionation
2.3. Acid Phosphatase Activity and Soil Microbial Biomass Phosphorus
2.4. DNA Extraction, and Quantitative Polymerase Chain Reaction
2.5. Statistical Analysis
3. Results
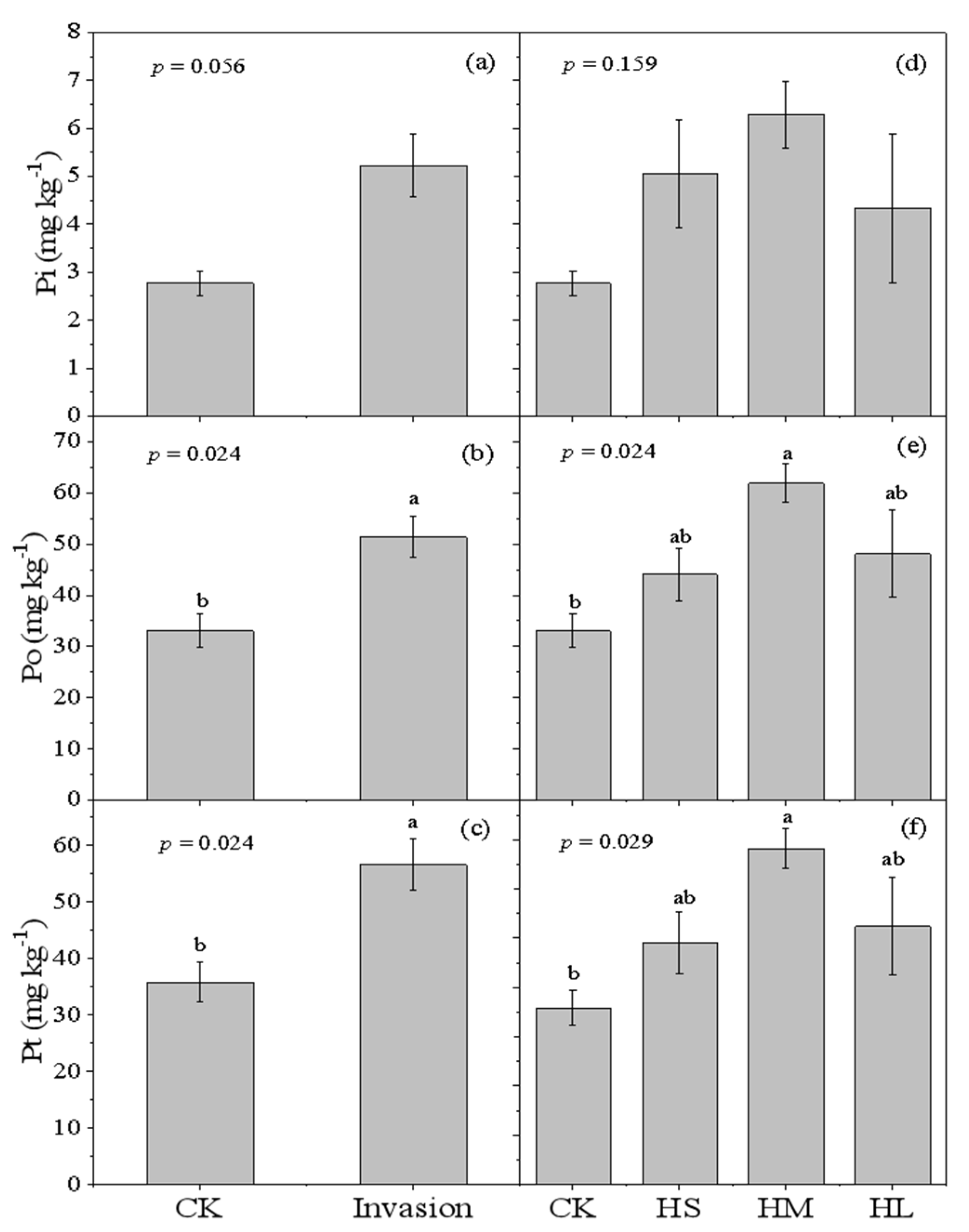
3.1. Soil Pt, Po and Pi Concentrations
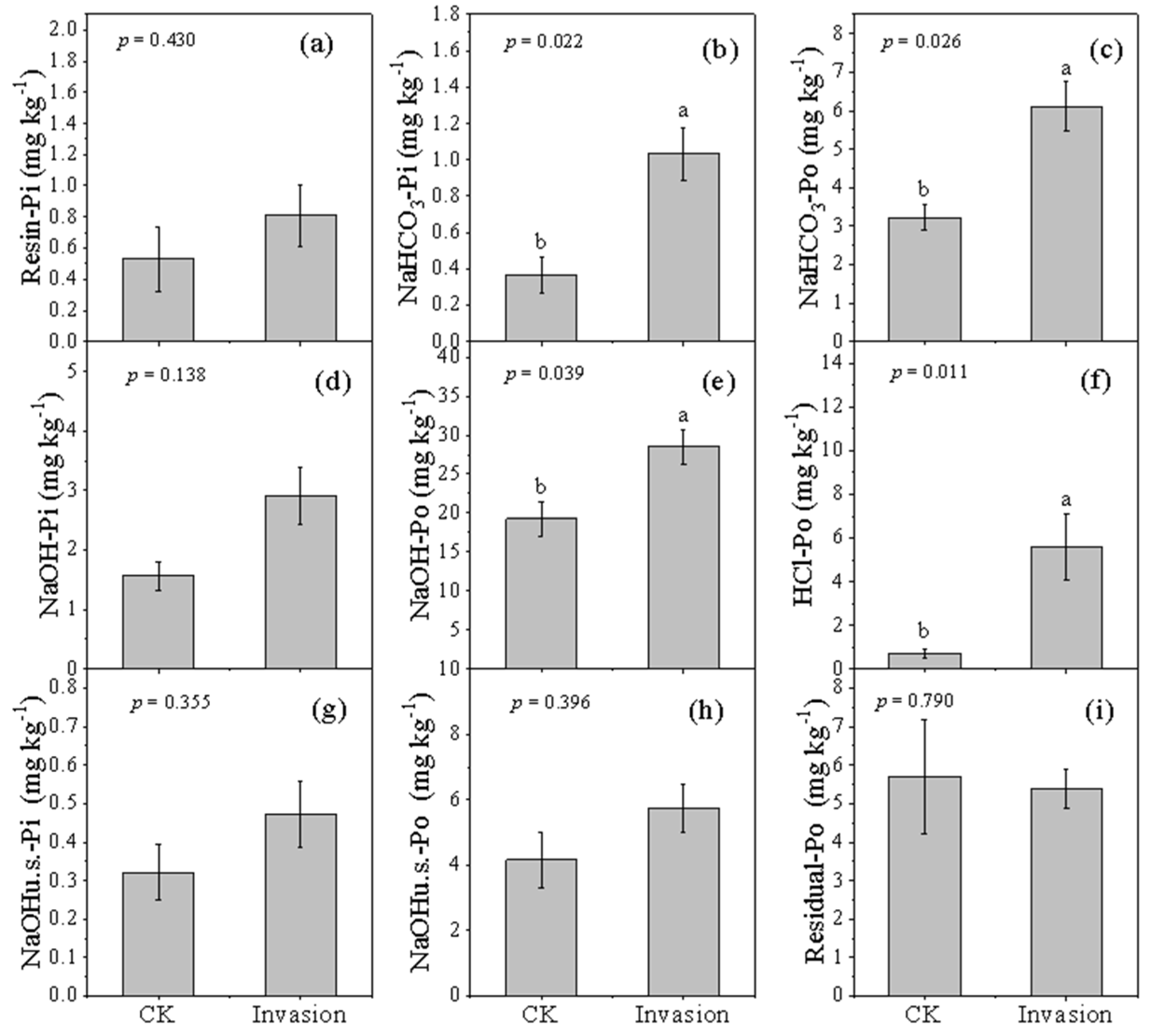
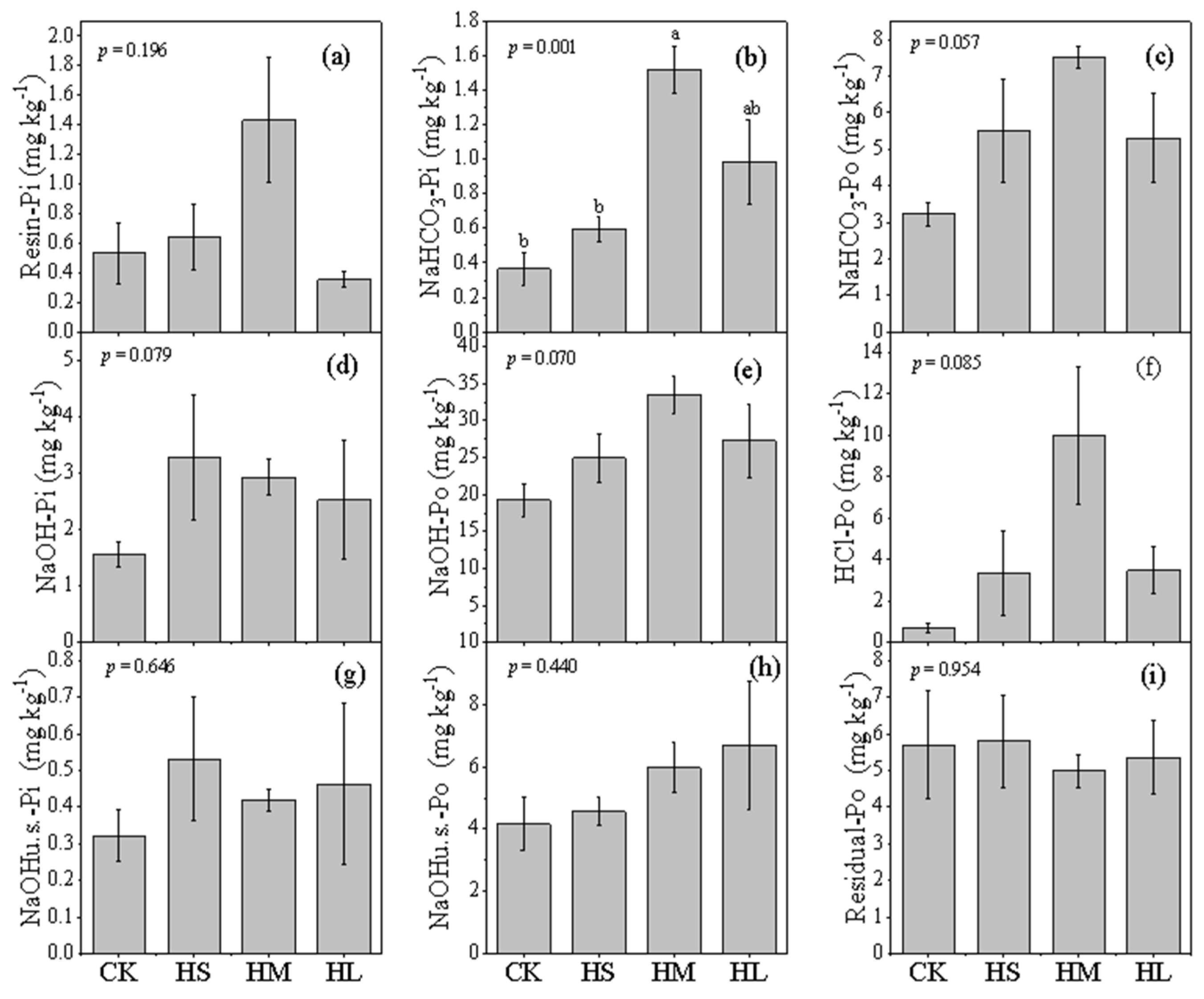
3.2. Soil P Fractions Concentration
3.3. Soil ACP Activities, SMBP, and Abundance of Soil Microbe
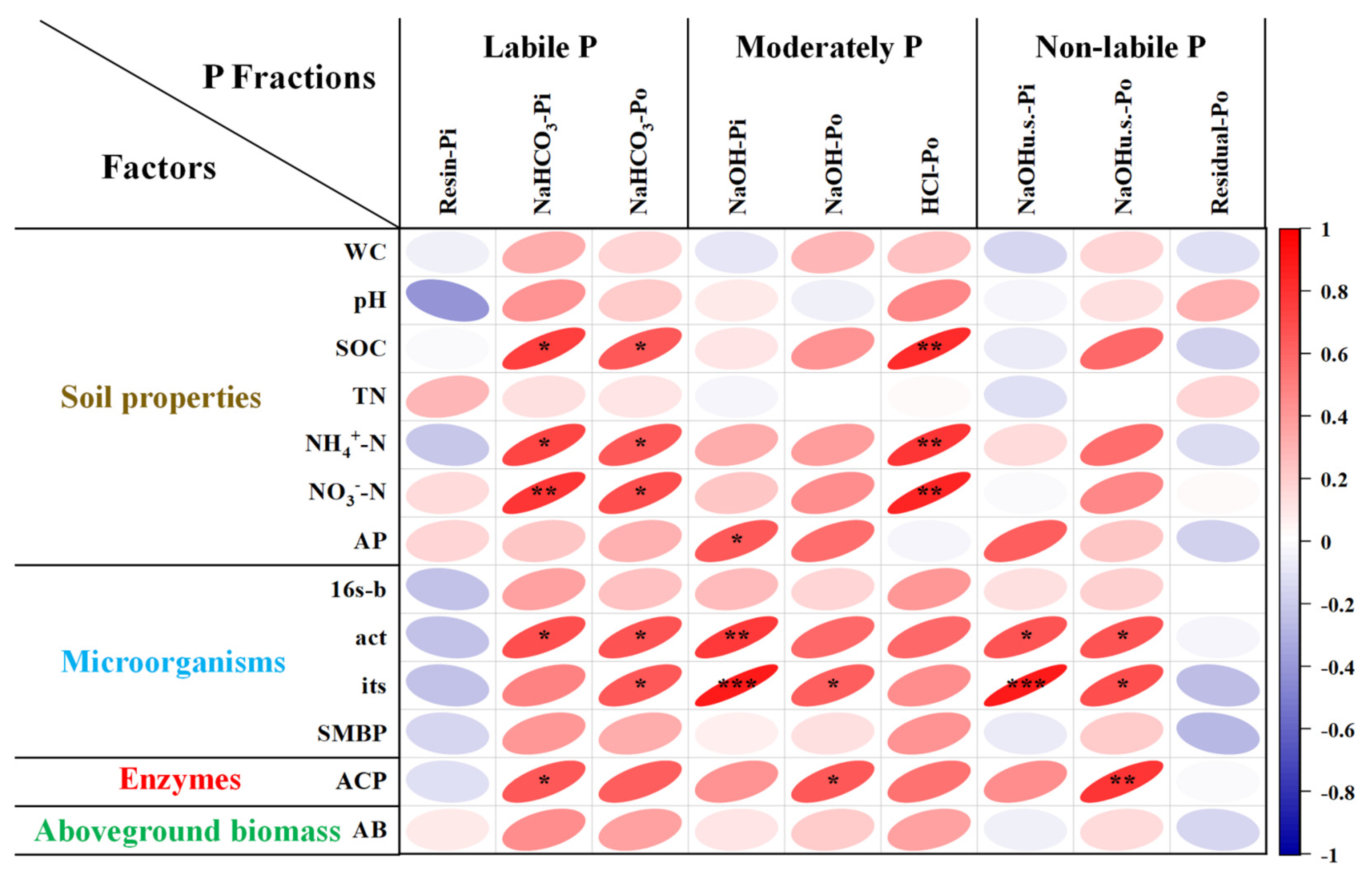
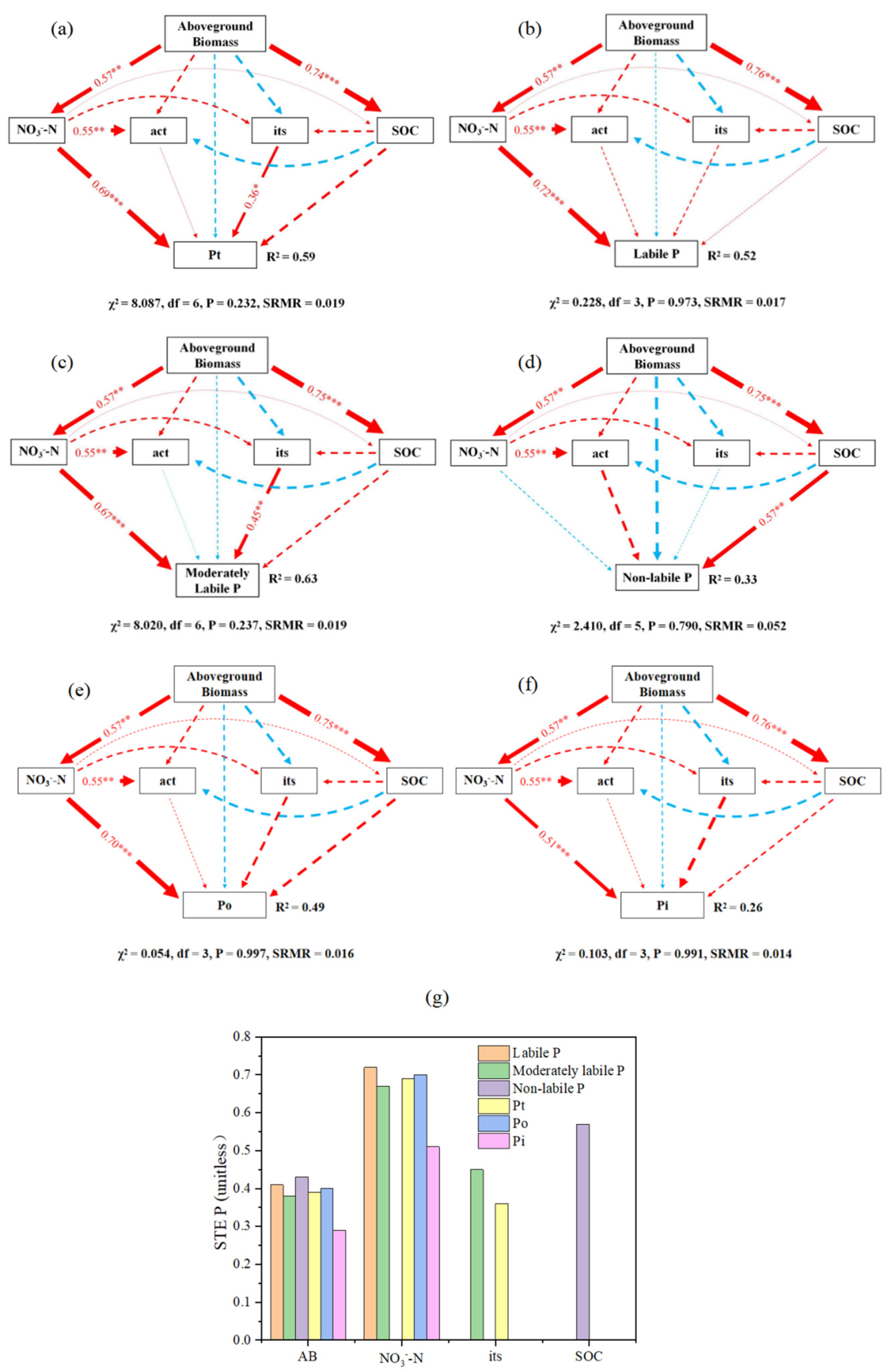
3.4. Correlation between P Fractions and Aboveground Biomass, Soil Biochemical Properties
4. Discussion
4.1. Dynamics of Soil P fractions under Afforestation
4.2. Factors Driving Soil P Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spohn, M. Phosphorus and carbon in soil particle size fractions: A synthesis. Biogeochemistry 2020, 147, 225–242. [Google Scholar] [CrossRef]
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Wen, D. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- Lajtha, K.; Schlesinger, W.H. The Biogeochemistry of Phosphorus Cycling and Phosphorus Availability Along a Desert Soil Chronosequence. Ecology 1988, 69, 24–39. [Google Scholar] [CrossRef]
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Phosphorus Changes and Sorption Characteristics in a Calcareous Soil Under Long-Term Fertilization—ScienceDirect. Pedosphere 2008, 18, 248–256. [Google Scholar]
- Condron, L.M.; Newman, S. Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J. Soils Sediments 2011, 11, 830–840. [Google Scholar] [CrossRef]
- Harrison, A.F. Soil Organic Phosphorus A Review of World Literature; CAB International: Wallingford, UK, 1987. [Google Scholar]
- Benitez-Nelson, C.R.; Buesseler, K.O. Variability of inorganic and organic phosphorus turnover rates in the coastal ocean. Nature 1999, 398, 502–505. [Google Scholar] [CrossRef]
- Qing-Hui, H.; Dong-Hong, W.; Mei, M.A.; Chun-Xia, W.; Zi-Jian, W. New Method to Assess Phosphorus Bioavailability in the Sediments and Soils. Environ. Sci. 2005, 26, 206–208. [Google Scholar]
- Andrade dos Santos, E.D.; Yue Phin Tng, D.; Guimaraes Apgaua, D.M.; Assuncao, R.R.; Manzi, A.O. Soil phosphorus fractions and their relation to leaf litterfall in a central Amazonian terra firme rainforest. Acta Amaz. 2022, 52, 104–113. [Google Scholar] [CrossRef]
- Viana, T.d.O.; Gama-Rodrigues, A.C.; Gama-Rodrigues, E.F.; Aleixo, S.; de Souza Moreira, R.V.; da Silva Sales, M.V.; Bonadie Marques, J.R. Phosphorus transformations in alfisols and ultisols under different land uses in the atlantic forest region of Brazil. Geoderma Reg. 2018, 14, e00184. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Liu, C.; Tang, J.; Wang, Q.; Xu, M. Phosphorous fractions in soils of rubber-based agroforestry systems: Influence of season, management and stand age. Sci. Total Environ. 2018, 616, 1576–1588. [Google Scholar] [CrossRef]
- Xu, G.; Shao, H.-B.; Sun, J.-N.; Chang, S.X. Phosphorus fractions and profile distribution in newly formed wetland soils along a salinity gradient in the Yellow River Delta in China. J. Plant Nutr. Soil Sci. 2012, 175, 721–728. [Google Scholar] [CrossRef]
- Cui, H.; Ou, Y.; Wang, L.; Wu, H.; Yan, B.; Han, L.; Li, Y. Identification of environmental factors controlling phosphorus fractions and mobility in restored wetlands by multivariate statistics. Environ. Sci. Pollut. Res. 2019, 26, 16014–16025. [Google Scholar] [CrossRef] [PubMed]
- Herlihy, M.; McCarthy, J. Association of soil-test phosphorus with phosphorus fractions and adsorption characteristics. Nutr. Cycl. Agroecosystems 2006, 75, 79–90. [Google Scholar] [CrossRef]
- Cao, Z.; Kuehn, P.; He, J.-S.; Bauhus, J.; Guan, Z.-H.; Scholten, T. Calibration of Near-Infrared Spectra for Phosphorus Fractions in Grassland Soils on the Tibetan Plateau. Agronomy 2022, 12, 783. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Franco, A.L.C.; Cerri, C.E.P.; Karlen, D.L.; Pavinato, P.S.; Rodrigues, M.; Davies, C.A.; Cerri, C.C. Phosphorus pools responses to land-use change for sugarcane expansion in weathered Brazilian soils. Geoderma 2016, 265, 27–38. [Google Scholar] [CrossRef]
- Jian, Z.; Ni, Y.; Lei, L.; Xu, J.; Xiao, W.; Zeng, L. Phosphorus is the key soil indicator controlling productivity in planted Masson pine forests across subtropical China. Sci. Total Environ. 2022, 822, 153525. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [PubMed]
- Garay, M.; Amiotti, N.; Zalba, P. Response of phosphorus pools in Mollisols to afforestation with Pinus radiata D. Don in the Argentinean Pampa. For. Ecol. Manag. 2018, 422, 33–40. [Google Scholar] [CrossRef]
- Bai, J.; Chen, R.; Men, X.; Cheng, X. Divergent linkages of soil phosphorus fractions to edaphic properties following afforestation in the riparian zone of the upper Yangtze river, China. Chemosphere 2023, 313, 137452. [Google Scholar] [CrossRef]
- Finzi, A.C.; Austin, A.T.; Cleland, E.E.; Frey, S.D.; Houlton, B.Z.; Wallenstein, M.D. Responses and feedbacks of coupled biogeochemical cycles to climate change: Examples from terrestrial ecosystems. Front. Ecol. Environ. 2011, 9, 61–67. [Google Scholar] [CrossRef]
- Yue, K.; Fornara, D.A.; Yang, W.; Peng, Y.; Li, Z.; Wu, F.; Peng, C. Effects of three global change drivers on terrestrial C:N:P stoichiometry: A global synthesis. Glob. Change Biol. 2017, 23, 2450–2463. [Google Scholar] [CrossRef]
- Chaer, G.M.; Resende, A.S.; Carneiro Campello, E.F.; de Faria, S.M.; Boddey, R.M. Nitrogen-fixing legume tree species for the reclamation of severely degraded lands in Brazil. Tree Physiol. 2011, 31, 139–149. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Zhu, X.; Liang, X.; Lei, Y.; He, C. N-fixing trees in wetland restoration plantings: Effects on nitrogensupply and soil microbial communities. Environ. Sci. Pollut. Res. 2016, 23, 24749–24757. [Google Scholar] [CrossRef]
- Hussain, A.; Chen, L.; Jamil, M.A.; Abid, K.; Khan, K.; Duan, W.; Li, C.; Khan, A. Changes in Soil-Phosphorus Fractions by Nitrogen and Phosphorus Fertilization in Korean Pine Plantation and Its Natural Forest. Forests 2022, 13, 527. [Google Scholar] [CrossRef]
- Zarif, N.; Khan, A.; Wang, Q. Linking Soil Acidity to P Fractions and Exchangeable Base Cations under Increased N and P Fertilization of Mono and Mixed Plantations in Northeast China. Forests 2020, 11, 1274. [Google Scholar] [CrossRef]
- Kunito, T.; Hiruta, N.; Miyagishi, Y.; Sumi, H.; Moro, H. Changes in phosphorus fractions caused by increased microbial activity in forest soil in a short-term incubation study. Chem. Speciat. Bioavailab. 2018, 30, 9–13. [Google Scholar]
- Yang, L.; Yang, Z.; Peng, Y.; Lin, Y.; Xiong, D.; Li, Y.; Yang, Y. Evaluating P availability influenced by warming and N deposition in a subtropical forest soil: A bioassay mesocosm experiment. Plant Soil 2019, 444, 87–99. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Z.; Zhong, X.; Xu, C.; Lin, Y.; Fan, Y.; Wang, M.; Chen, G.; Yang, Y. Decreases in soil P availability are associated with soil organic P declines following forest conversion in subtropical China. Catena 2021, 205, 105459. [Google Scholar] [CrossRef]
- Cai, X.; Lin, Z.; Penttinen, P.; Li, Y.; Li, Y.; Luo, Y.; Yue, T.; Jiang, P.; Fu, W. Effects of conversion from a natural evergreen broadleaf forest to a Moso bamboo plantation on the soil nutrient pools, microbial biomass and enzyme activities in a subtropical area. For. Ecol. Manag. 2018, 422, 161–171. [Google Scholar] [CrossRef]
- Wang, Z.-R.; Niu, D.-C.; Hu, Y.-G.; Wang, Y.-S.; Huang, L.; Liu, Y.-B. Changes in soil phosphorus fractions associated with altered vegetation and edaphic conditions across a chronosequence of revegetated dunes in a desert area. Geoderma 2022, 424, 115995. [Google Scholar] [CrossRef]
- Yan, Z.; Wu, L.; Lv, T.; Tong, C.; Gao, Z.; Liu, Y.; Xing, B.; Chao, C.; Li, Y.; Wang, L.; et al. Response of spatio-temporal changes in sediment phosphorus fractions to vegetation restoration in the degraded river-lake ecotone. Environ. Pollut. 2022, 308, 119650. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ye, H.Y.; Zhang, D.H.; Gu, J.D.; Deng, O.P. The dynamics of phosphorus fractions and the factors driving phosphorus cycle in Zoige Plateau peatland soil. Chemosphere 2021, 278, 130501. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Condron, L.M.; Dunfield, K.E.; Wakelin, S.A.; Chen, L.J. Impact of grassland afforestation with contrasting tree species on soil phosphorus fractions and alkaline phosphatase gene communities. Soil Biol. Biochem. 2021, 159, 108274. [Google Scholar] [CrossRef]
- Horodecki, P.; Jagodzinski, A.M. Tree species effects on litter decomposition in pure stands on afforested post-mining sites. For. Ecol. Manag. 2017, 406, 1–11. [Google Scholar] [CrossRef]
- Zhang, J. Human influence on land desertification in yuanmou dry-hot valley. J. Mt. Res. 1997, 1, 53–56. [Google Scholar]
- Ma, J.; Li, K. Effects of Plantation on Soil Nutrient and Evaluation in Yuanmou Dry-hot Valley. For. Res. 2006, 19, 467–471. [Google Scholar]
- Peng, S.; Chen, A.; Fang, H.; Wu, J.; Liu, G. Effects of vegetation restoration types on soil quality in Yuanmou dry-hot valley, China. Soil Sci. Plant Nutr. 2013, 59, 347–360. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, A.; Yan, S.; Rafay, L.; Du, K.; Wang, D.; Ge, Y.; Li, J. Available soil nutrients and water content affect leaf nutrient concentrations and stoichiometry at different ages of Leucaena leucocephala forests in dry-hot valley. J. Soils Sediments 2019, 19, 511–521. [Google Scholar] [CrossRef]
- Gong, Z.; Tang, Y.; Xu, W.; Mou, Z. Rapid Sequestration of Ecosystem Carbon in 30-year Reforestation with Mixed Species in Dry Hot Valley of the Jinsha River. Int. J. Environ. Res. Public Health 2019, 16, 1937. [Google Scholar] [CrossRef]
- Gong, Z.; Li, Y.; Liu, L.; Deng, S. Great Facilitation of Thirty Years of Reforestation with Mixed Species to Ecosystem Nitrogen Accumulation in Dry-Hot Valley in the Jinsha River. Int. J. Environ. Res. Public Health 2022, 19, 12660. [Google Scholar] [CrossRef]
- Liu, F.Y.; Gao, C.J.; Chen, M.; Li, K. Above- and below-ground biomass relationships of Leucaena leucocephala (Lam.) de Wit in different plant stands. PLoS ONE 2018, 13, e0207059. [Google Scholar] [CrossRef]
- Lou, M.; Mao, J.; Shi, R.; Li, W.; Hu, B.; Feng, D. Characteristics of organic carbon and nitrogen in the particulate state of soils of new silver acacia plantation forests in dry and hot river valleys. For. Sci. Res. 2022, 35, 40–47. [Google Scholar]
- Kovar, J.L.; Pierzynski, G.M. Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters second edition. In Methods of Phosphorus Analysis for Soils; North Carolina State University: Raleigh, NC, USA, 2009. [Google Scholar]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Chen, G.C.; He, Z.L. Determination of soil microbial biomass phosphorus in acid red soils from southern China. Biol. Fertil. Soils 2004, 39, 446–451. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, S.-Y.; Ding, W.; Kang, H. Impact of elevated CO2 and N addition on bacteria, fungi, and archaea in a marsh ecosystem with various types of plants. Appl. Microbiol. Biotechnol. 2015, 99, 5295–5305. [Google Scholar] [CrossRef]
- Vainio, E.J.; Hantula, J. Direct analysis of wood-inhabiting fungi using denaturing gradient gel electrophoresis of amplified ribosomal DNA. Mycol. Res. 2000, 104, 927–936. [Google Scholar] [CrossRef]
- De Gregoris, T.B.; Aldred, N.; Clare, A.S.; Burgess, J.G. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J. Microbiol. Methods 2011, 86, 351–356. [Google Scholar] [CrossRef]
- George, T.S.; Giles, C.D.; Menezes-Blackburn, D.; Condron, L.M.; Gama-Rodrigues, A.C.; Jaisi, D.; Lang, F.; Neal, A.L.; Stutter, M.I.; Almeida, D.S.; et al. Organic phosphorus in the terrestrial environment: A perspective on the state of the art and future priorities. Plant Soil 2018, 427, 191–208. [Google Scholar] [CrossRef]
- Schreeg, L.A.; Mack, M.C.; Turner, B.L. Leaf litter inputs decrease phosphate sorption in a strongly weathered tropical soil over two time scales. Biogeochemistry 2013, 113, 507–524. [Google Scholar] [CrossRef]
- Harrison, A.F. Labile organic phosphorus mineralization in relationship to soil properties. Soil Biol. Biochem. 1982, 14, 343–351. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, R.; Liu, Q.; Qiang, W.; Liang, J.; Hou, E.; Zhao, C.; Pang, X. Linking soil phosphorus fractions to abiotic factors and the microbial community during subalpine secondary succession: Implications for soil phosphorus availability. Catena 2023, 233, 107501. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ge, F.; Zhang, D.Y.; Deng, S.Q.; Liu, X.W. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, J.P.; Zhao, Z.W. Arbuscular mycorrhizas in a valley-type savanna in southwest China. Mycorrhiza 2004, 14, 323–327. [Google Scholar]
- Zheng, L.L.; Zhao, Q.; Lin, G.G.; Hong, X.; Zeng, D.H. Nitrogen addition impacts on soil phosphorus transformations depending upon its influences on soil organic carbon and microbial biomass in temperate larch forests across northern China. Catena 2023, 230, 107252. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Oberson, A.; Liebisch, F.; Keller, F.; Annaheim, K.E.; Huguenin-Elie, O.; Frossard, E. Rapid microbial phosphorus immobilization dominates gross phosphorus fluxes in a grassland soil with low inorganic phosphorus availability. Soil Biol. Biochem. 2012, 51, 84–95. [Google Scholar] [CrossRef]
- Ying-Ping, W.; Huang, Y.; Augusto, L.; Goll, D.S.; Helfenstein, J.; Hou, E. Toward a Global Model for Soil Inorganic Phosphorus Dynamics: Dependence of Exchange Kinetics and Soil Bioavailability on Soil Physicochemical Properties. Glob. Biogeochem. Cycles 2022, 36, e2021GB007061. [Google Scholar]
- Spohn, M. Increasing the organic carbon stocks in mineral soils sequesters large amounts of phosphorus. Glob. Change Biol. 2020, 26, 4169–4177. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Luo, Y.; Liu, C.; Zhang, J.; Gao, M.; Yuan, L.; Hu, B.; Feng, D.; Li, W. Phosphorous Fractions in Soils of Natural Shrub-Grass Communities and Leucaena leucocephala Plantations in a Dry-Hot Valley. Forests 2024, 15, 974. https://doi.org/10.3390/f15060974
Jin J, Luo Y, Liu C, Zhang J, Gao M, Yuan L, Hu B, Feng D, Li W. Phosphorous Fractions in Soils of Natural Shrub-Grass Communities and Leucaena leucocephala Plantations in a Dry-Hot Valley. Forests. 2024; 15(6):974. https://doi.org/10.3390/f15060974
Chicago/Turabian StyleJin, Jun, Yiyun Luo, Chengyu Liu, Jiajia Zhang, Mengxi Gao, Lingchen Yuan, Bin Hu, Defeng Feng, and Wei Li. 2024. "Phosphorous Fractions in Soils of Natural Shrub-Grass Communities and Leucaena leucocephala Plantations in a Dry-Hot Valley" Forests 15, no. 6: 974. https://doi.org/10.3390/f15060974



