Radial Growth of Dahurian Larch (Larix gmelinii) Responses to Climate and Competition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Field Sampling and Measurements
2.3. Data Processing and Analysis
2.3.1. Chronology Establishment and Determination of Statistical Parameters
2.3.2. Competition Indices
2.3.3. Data Analysis
3. Results
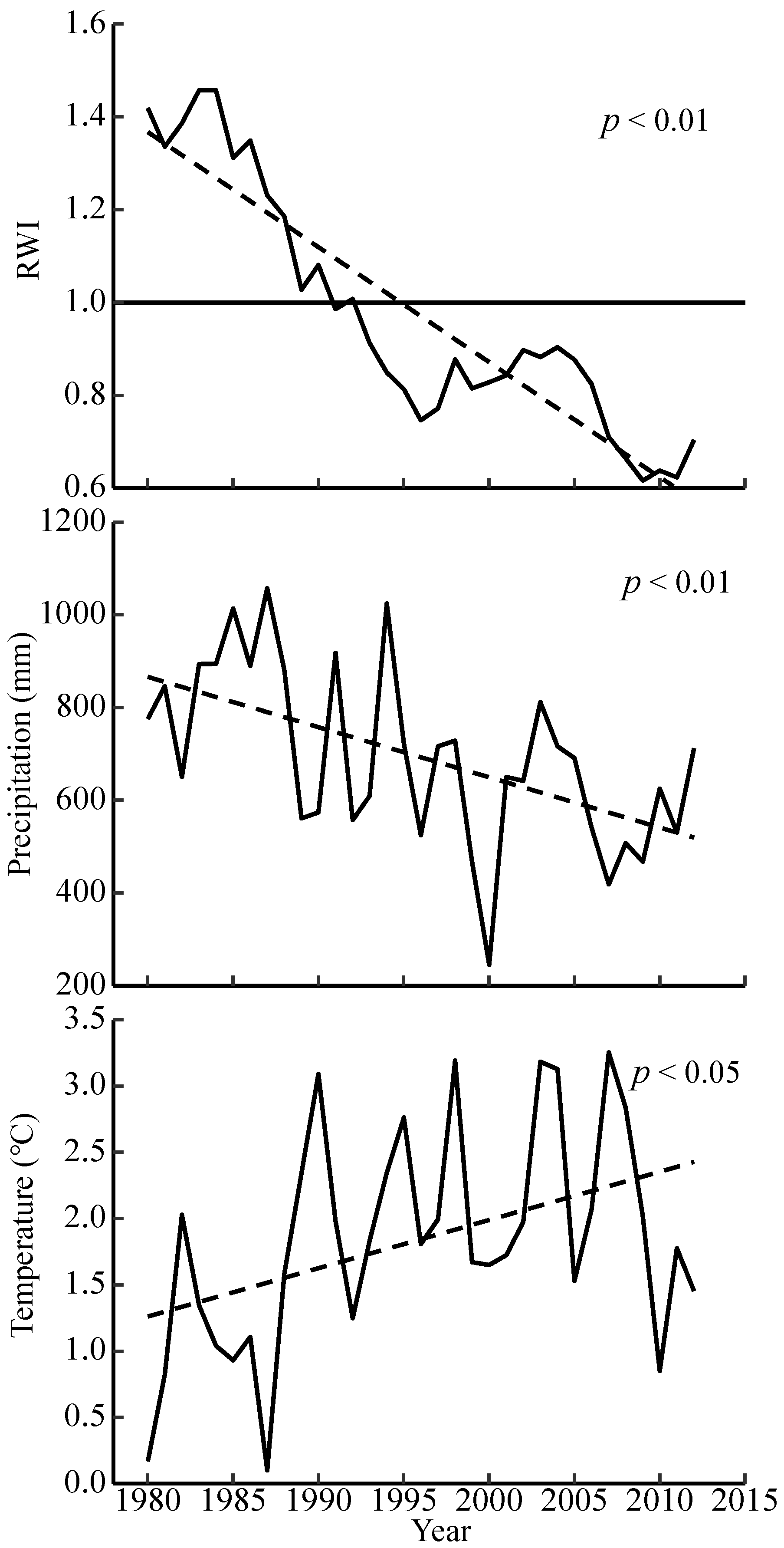
3.1. Interannual Trends in Climate and Chronology Statistics
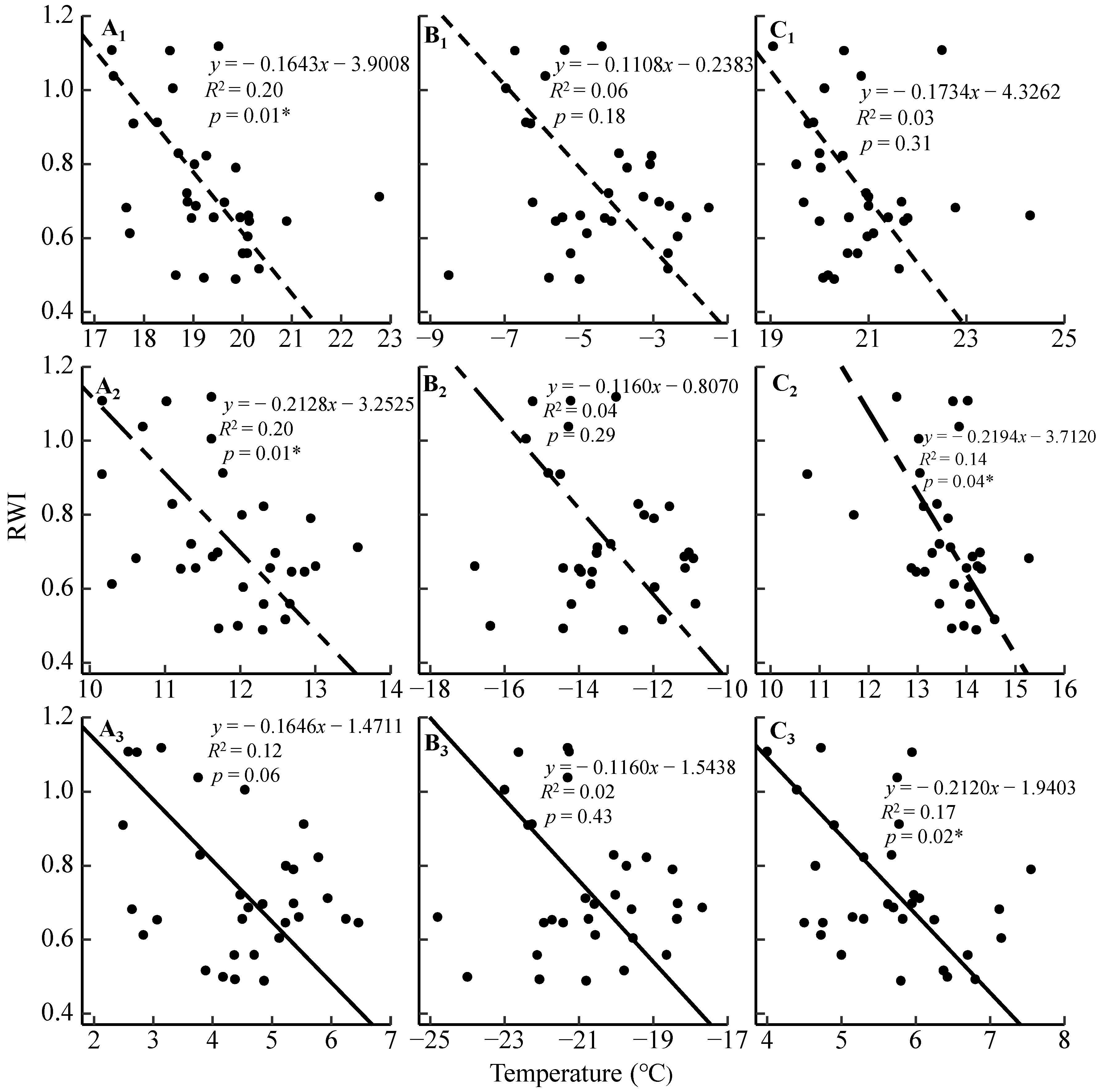
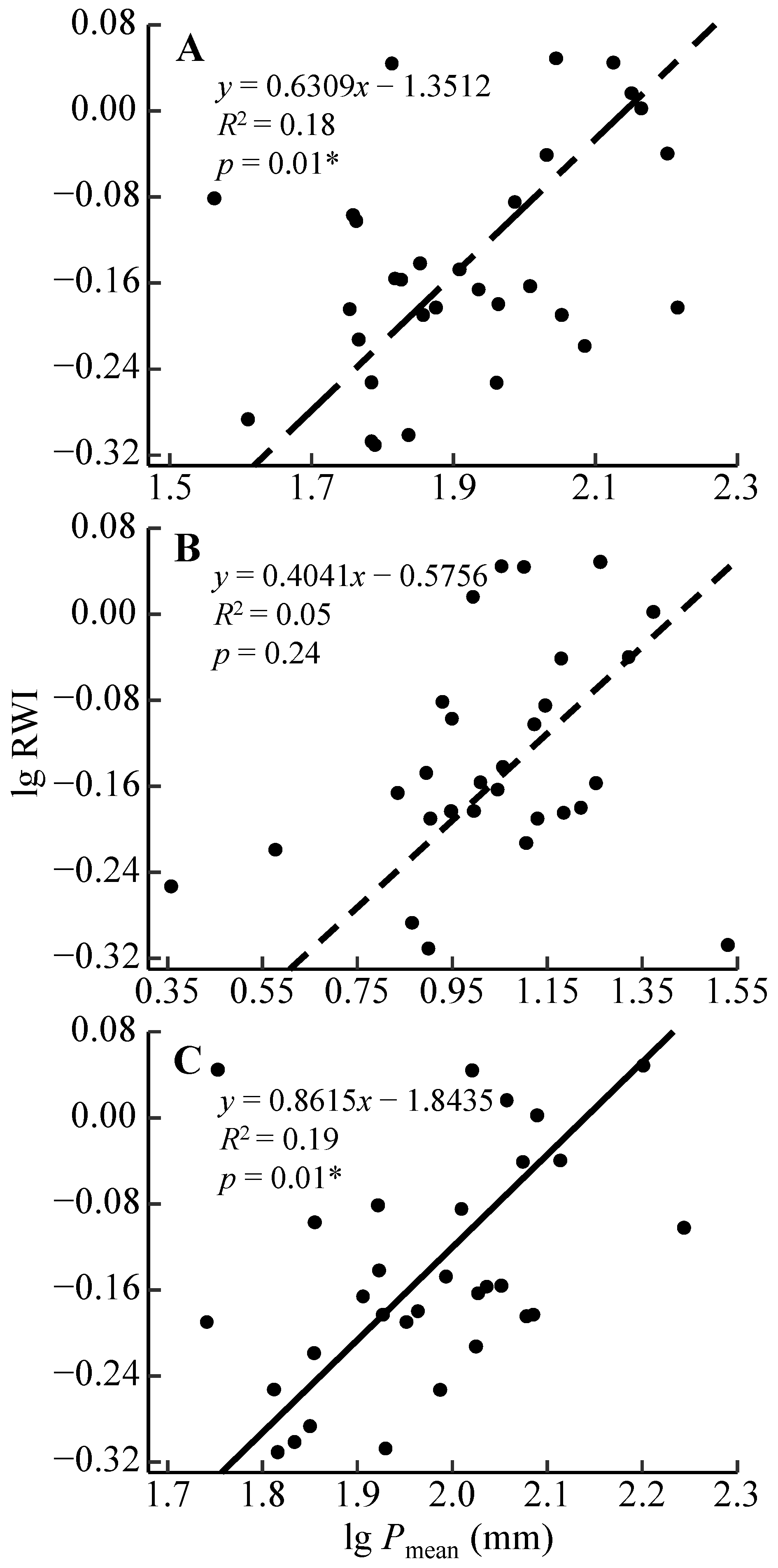
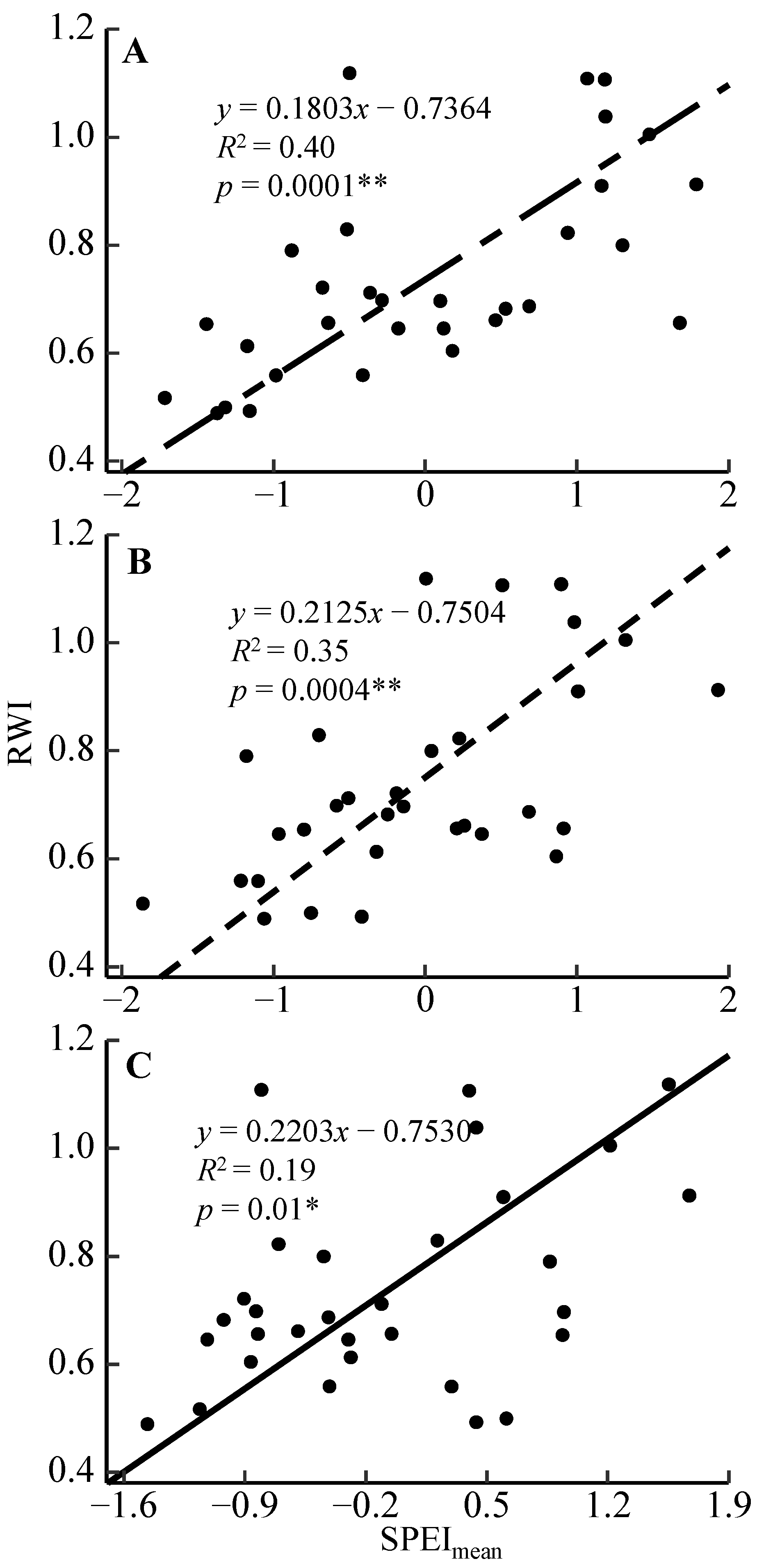
3.2. Radial Growth–Climate Associations
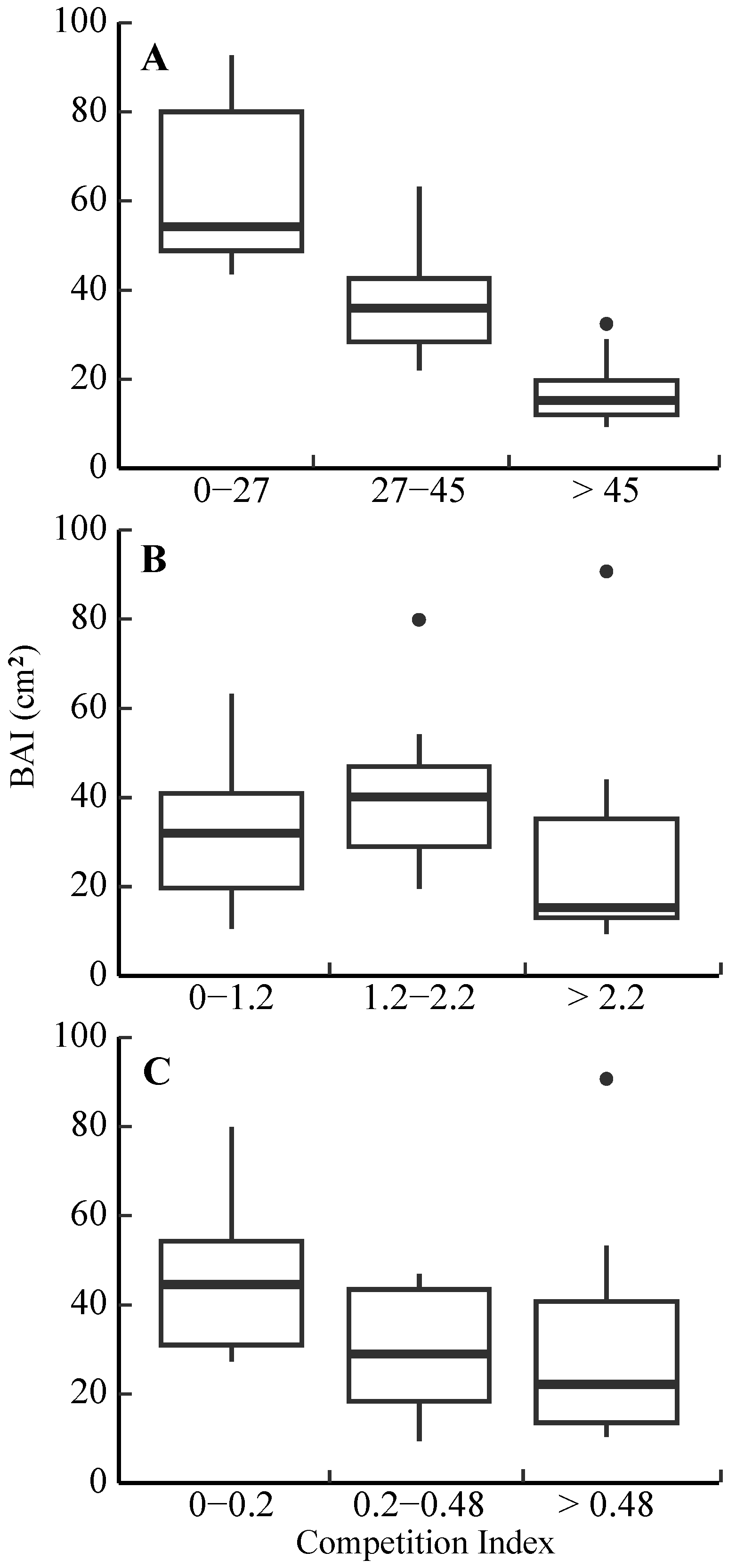
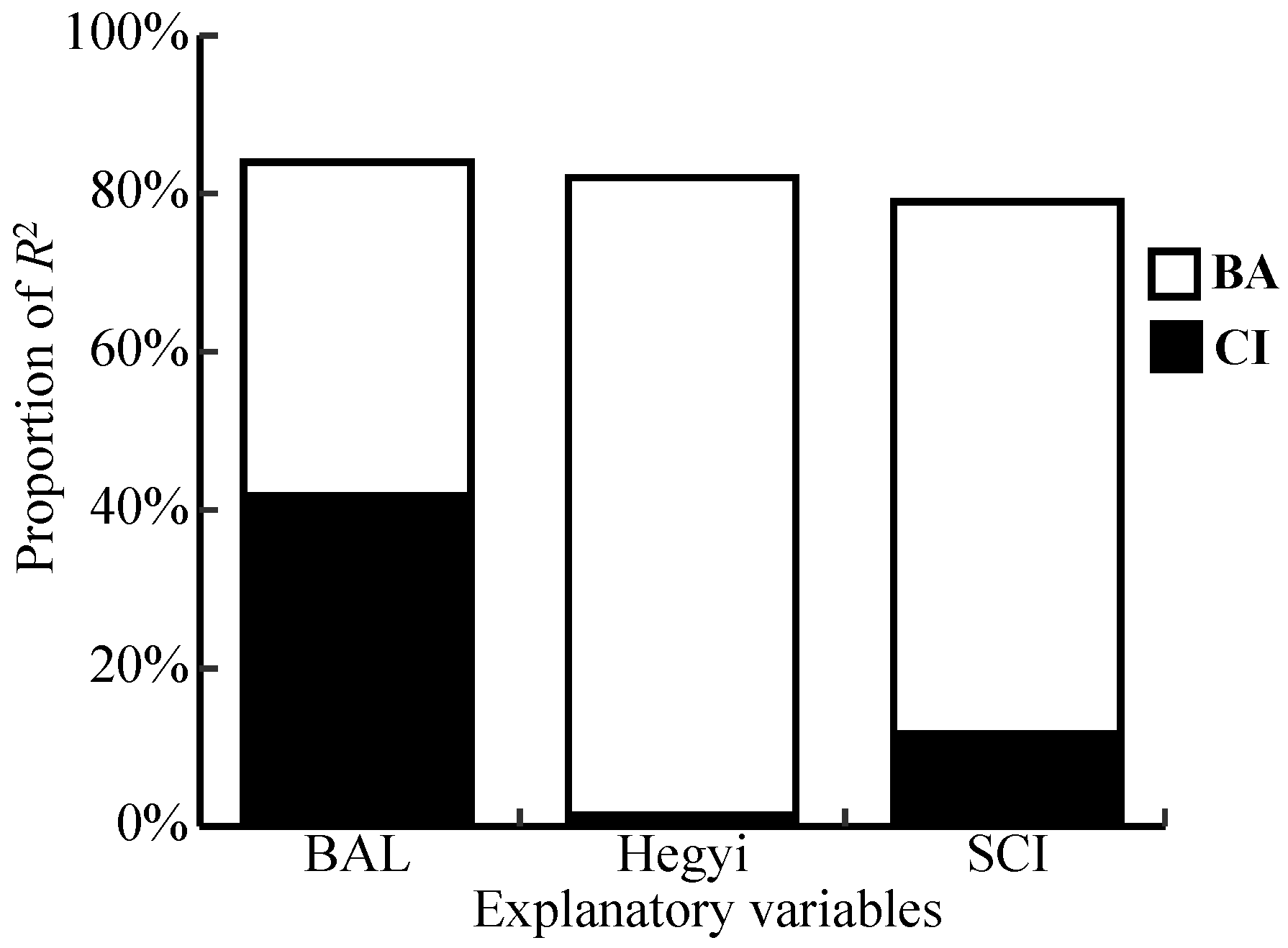
3.3. Competitive Effect on Radial Growth
4. Discussion
4.1. Radial Growth Responses to the Regional Temperature
4.2. Radial Growth Responses to the Moisture Condition
4.3. Radial Growth Responses to Competition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Olsson, P.-O.; Jonsson, A.M.; Eklundh, L. A new invasive insect in Sweden Physokermes inopinatus: Tracing forest damage with satellite based remote sensing. For. Ecol. Manag. 2012, 285, 29–37. [Google Scholar] [CrossRef]
- Johnson, C.; Chhin, S.; Zhang, J. Effects of climate on competitive dynamics in mixed conifer forests of the Sierra Nevada. For. Ecol. Manag. 2017, 394, 1–12. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liang, H.; Jiang, S.; Zhu, H.; Zhou, P.; Huang, J. Effects of competition and climate on tree radial growth of Pinus sibirica in Altai Mountains, Xinjiang, China. J. Plant Ecol. 2020, 44, 1195–1202. (In Chinese) [Google Scholar]
- Han, Y.; Gai, X.; Qiu, S.; Zhang, Y.; Wang, S.; Zhou, L.; Yu, D. Spatial and temporal variations of the responses of radial growth of Larix gmelinii to climate in the Daxing’anling Mountains of Northeast China. J. Appl. Ecol. 2021, 32, 3397–3404. (In Chinese) [Google Scholar]
- Britton, T.G.; Richards, S.A.; Hovenden, M.J. Quantifying neighbour effects on tree growth: Are common ‘competition’indices biased? J. Ecol. 2023, 111, 1270–1280. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.-G.; Stadt, K.J.; Comeau, P.G.; Chen, H.Y.H. Spatial climate-dependent growth response of boreal mixedwood forest in western Canada. Glob. Planet. Change 2016, 139, 141–150. [Google Scholar] [CrossRef]
- Fichtner, A.; Forrester, D.I.; Haerdtle, W.; Sturm, K.; von Oheimb, G. Facilitative-Competitive Interactions in an Old-Growth Forest: The Importance of Large-Diameter Trees as Benefactors and Stimulators for Forest Community Assembly. PLoS ONE 2015, 10, 335–352. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, L.; Li, L.; Chen, X.; Yan, X. Divergent responses of radial growth of Larix sibirica to climate change in Altay Mountains of Xinjiang, China. J. Plant Ecol. 2019, 43, 320–330. (In Chinese) [Google Scholar] [CrossRef]
- Han, X.; Gao, L.; Qing, L.; Pang, R.; Liu, Q.; Zhu, Y.; Tian, Y.; Zhang, J. Effect of stand density on radial growth-climate relationship of Larix gmelinii. J. Nanjing For. Univ. Nat. Sci. Ed. 2023, 48, 1–13. (In Chinese) [Google Scholar]
- Kozlowski, T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Chapin III, F.S.; Schulze, E.-D.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Yasmeen, S.; Wang, X.; Zhao, H.; Zhu, L.; Yuan, D.; Li, Z.; Zhang, Y.; Ahmad, S.; Han, S. Contrasting climate-growth relationship between Larix gmelinii and Pinus sylvestris var. mongolica along a latitudinal gradient in Daxing’an Mountains, China. Dendrochronologia 2019, 58, 645–657. [Google Scholar] [CrossRef]
- Sperling, O.; Earles, J.M.; Secchi, F.; Godfrey, J.; Zwieniecki, M.A. Frost induces respiration and accelerates carbon depletion in trees. PLoS ONE 2015, 10, e0144124. [Google Scholar] [CrossRef] [PubMed]
- Charrier, G.; Lacointe, A.; Améglio, T. Dynamic modeling of carbon metabolism during the dormant period accurately predicts the changes in frost hardiness in walnut trees Juglans regia L. Front. Plant Sci. 2018, 9, 1746–1759. [Google Scholar] [CrossRef]
- Mei, L.; Xiong, Y.; Gu, J.; Wang, Z.; Guo, D. Whole-tree dynamics of non-structural carbohydrate and nitrogen pools across different seasons and in response to girdling in two temperate trees. Oecologia 2015, 177, 333–344. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Y.; Dong, M.; Du, E.; Wu, F.; Zhao, S.; Xu, H. Species-specific growth-climate responses of Dahurian larch (Larix gmelinii) and Mongolian pine (Pinus sylvestris var. mongolica) in the Greater Khingan Range, northeast China. Dendrochronologia 2021, 65, 125803. [Google Scholar] [CrossRef]
- Wang, C.K. Biomass allometric equations for 10 co-occurring tree species in Chinese temperate forests. For. Ecol. Manag. 2006, 222, 9–16. [Google Scholar] [CrossRef]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Wigley, T.M.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Hegyi, F. A simulation model for managing jack-pine standssimulation. RoyalColl. For. Res. Notes 1974, 30, 74–90. [Google Scholar]
- Wykoff, W. User’s Guide to the Stand Prognosis Model; US Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1982. [Google Scholar]
- Hui, G.; Wang, Y.; Zhang, G.; Zhao, Z.; Bai, C.; Liu, W. A novel approach for assessing the neighborhood competition in two different aged forests. For. Ecol. Manag. 2018, 422, 49–58. [Google Scholar] [CrossRef]
- Hui, G.; Albert, M.; Gadow, K. The diameter dominance as a parameter for simulating forest structure. Forstwiss. Cent. 1998, 117, 258–266. [Google Scholar] [CrossRef]
- Hu, Y.; Hui, G. How to describe the crowding degree of trees based on the relationship of neighboring trees. J. Beijing Forest. Univ. 2015, 37, 1–8. (In Chinese) [Google Scholar] [CrossRef]
- Gadow, K.; Huy, G.; Albert, M. The uniform angle index—A structural parameter for describing tree distribution in forest stands. Cent. Ges. Forstwes. 1998, 115, 1–10. [Google Scholar]
- MacFarlane, D.W.; Kobe, R.K. Selecting models for capturing tree-size effects on growth-resource relationships. Can. J. For. Res. 2006, 36, 1695–1704. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C. China Ecosystem Positioning Observation and Research Data Set: 1979–2017. In Forest Ecosystem Volume Heilongjiang Maoershan Station; China Agriculture Press: Beijing, China, 2022. [Google Scholar]
- ShangGuan, H.; Liu, H.; Hu, G.; Guo, W. Seasonal Patterns and Their Determinants of Non-structural Carbohydrates in Different Tree Species at Xeric Timberline. Acta Sci. Nat. Univ. Pekin. 2019, 55, 553–560. (In Chinese) [Google Scholar] [CrossRef]
- Dow, C.; Kim, A.Y.; D’Orangeville, L.; Gonzalez-Akre, E.B.; Helcoski, R.; Herrmann, V.; Harley, G.L.; Maxwell, J.T.; McGregor, I.R.; McShea, W.J.; et al. Warm springs alter timing but not total growth of temperate deciduous trees. Nature 2022, 608, 552–557. [Google Scholar] [CrossRef]
- Richardson, A.D.; Carbone, M.S.; Keenan, T.F.; Czimczik, C.I.; Hollinger, D.Y.; Murakami, P.; Schaberg, P.G.; Xu, X.M. Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol. 2013, 197, 850–861. [Google Scholar] [CrossRef]
- Team, R.c. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. smatr 3—An R package for estimation and inference about allometric lines. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- Grömping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2007, 17, 1–27. [Google Scholar] [CrossRef]
- Yu, D.; Wang, S.; Tang, L.; Dai, L.; Wang, Q.; Wang, S. Relationship between tree-ring chronology of Larix olgensis in Changbai Mountains and the climae change. J. Appl. Ecol. 2005, 16, 14–20. (In Chinese) [Google Scholar]
- Brauning, A. Dendroclimatological potential of drought-sensitive tree stands in southern Tibet for the reconstruction of monsoonal activity. LAWA J. 1999, 20, 325–338. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, Z.; Zhang, X.; Bai, X.; Zhao, X.; Li, J.; Lu, X. Responses of radial growth to temperature in Larix gmelinii of the Da Hinggan Ling under climate warming. J. Plant Ecol. 2017, 41, 279–289. (In Chinese) [Google Scholar]
- Jiang, Y.; Zhang, J.; Han, S.; Chen, Z.; Setala, H.; Yu, J.; Zheng, X.; Guo, Y.; Gu, Y. Radial Growth Response of Larix gmelinii to Climate along a Latitudinal Gradient in the Greater Khingan Mountains, Northeastern China. Forests 2016, 7, 295. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Wilson, R.; Liepert, B.; Cherubini, P. On the ‘Divergence Problem’ in Northern Forests: A review of the tree-ring evidence and possible causes. Glob. Planet. Chang. 2008, 60, 289–305. [Google Scholar] [CrossRef]
- Żywiec, M.; Muter, E.; Zielonka, T.; Delibes, M.; Calvo, G.; Fedriani, J.M. Long-term effect of temperature and precipitation on radial growth in a threatened thermo-Mediterranean tree population. Trees 2017, 31, 491–501. [Google Scholar] [CrossRef]
- Galván, J.D.; Büntgen, U.; Ginzler, C.; Grudd, H.; Gutiérrez, E.; Labuhn, I.; Camarero, J.J. Drought-induced weakening of growth–temperature associations in high-elevation Iberian pines. Glob. Planet. Chang. 2015, 124, 95–106. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Tixier, A.; Gambetta, G.A.; Godfrey, J.; Orozco, J.; Zwieniecki, M.A. Non-structural Carbohydrates in Dormant Woody Perennials; The Tale of Winter Survival and Spring Arrival. Front. For. Glob. Chang. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Manzanedo, R.D.; D’Orangeville, L.; Rademacher, T.T.; Li, J.; Bai, X.; Hou, M.; Chen, Z.; Zou, F.; Song, F.; et al. Snowmelt and early to mid-growing season water availability augment tree growth during rapid warming in southern Asian boreal forests. Glob. Chang. Biol. 2019, 25, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Liu, X.H.; Zhang, Q.L.; Zeng, X.M.; Xu, G.B.; Wu, G.J.; Wang, W.Z. Species-specific tree growth and intrinsic water-use efficiency of Dahurian larch (Larbc gmelinii) and Mongolian pine (Pinus sylvestris var. mongolica) growing in a boreal permafrost region of the Greater Hinggan Mountains, Northeastern China. Agric. For. Meteorol. 2018, 248, 145–155. [Google Scholar] [CrossRef]
- Yocom, L.; Ogle, K.; Peltier, D.; Szejner, P.; Liu, Y.; Monson, R.K. Tree growth sensitivity to climate varies across a seasonal precipitation gradient. Oecologia 2022, 198, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.A.; Affleck, D.; Chung, W. Evaluating tree competition indices as predictors of basal area increment in western Montana forests. For. Ecol. Manag. 2011, 262, 1939–1949. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Y.; Pan, L.; Sun, Y. Hegyi competition index decomposition to improve estimation accuracy of Larix olgensis crown radius. Ecol. Indic. 2022, 143, 109322. [Google Scholar] [CrossRef]
- Rivas, J.J.C.; González, J.G.A.; Aguirre, O.; Hernández, F. The effect of competition on individual tree basal area growth in mature stands of Pinus cooperi Blanco in Durango (Mexico). Eur. J. For. Res. 2005, 124, 133–142. [Google Scholar] [CrossRef]
- Canham, C.D.; Papaik, M.J.; Uriarte, M.; McWilliams, W.H.; Jenkins, J.C.; Twery, M.J. Neighborhood analyses of canopy tree competition along environmental gradients in new England forests. Ecol. Appl. 2006, 16, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Coates, K.D.; Canham, C.D.; LePage, P.T. Above- versus below-ground competitive effects and responses of a guild of temperate tree species. J. Ecol. 2009, 97, 118–130. [Google Scholar] [CrossRef]
- Das, A. The effect of size and competition on tree growth rate in old-growth coniferous forests. Can. J. For. Res. 2012, 42, 1983–1995. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, S.; Meng, S.X.; Trincado, G.; VanderSchaaf, C.L. A multilevel individual tree basal area increment model for aspen in boreal mixedwood stands. Can. J. For. Res. 2009, 39, 2203–2214. [Google Scholar] [CrossRef]
- Martins, F.B.; Soares, C.P.B.; Leite, H.G.; de Souza, A.L.; Castro, R.V.O. Competition indexes for individual eucalyptus trees. Pesquisa Agropecuária Brasileira 2011, 46, 1089–1098. [Google Scholar] [CrossRef]
- King, D.A.; Davies, S.J.; Noor, N.S.M. Growth and mortality are related to adult tree size in a Malaysian mixed dipterocarp forest. For. Ecol. Manag. 2006, 223, 152–158. [Google Scholar] [CrossRef]
- Coomes, D.A.; Allen, R.B. Effects of size, competition and altitude on tree growth. J. Ecol. 2007, 95, 1084–1097. [Google Scholar] [CrossRef]
- Matsushita, M.; Takata, K.; Hitsuma, G.; Yagihashi, T.; Noguchi, M.; Shibata, M.; Masaki, T. A novel growth model evaluating age–size effect on long-term trends in tree growth. Funct. Ecol. 2015, 29, 1250–1259. [Google Scholar] [CrossRef]
- Zhang, Z.; Papaik, M.J.; Wang, X.; Hao, Z.; Ye, J.; Lin, F.; Yuan, Z. The effect of tree size, neighborhood competition and environment on tree growth in an old-growth temperate forest. J. Plant Ecol. 2017, 10, 970–980. [Google Scholar] [CrossRef]
- McDonald, E.; Kruger, E.; Riemenschneider, D.; Isebrands, J. Competitive status influences tree-growth responses to elevated CO2 and O3 in aggrading aspen stands. Funct. Ecol. 2002, 16, 792–801. [Google Scholar] [CrossRef]
- Wyckoff, P.H.; Clark, J.S. Tree growth prediction using size and exposed crown area. Can. J. For. Res. 2005, 35, 13–20. [Google Scholar] [CrossRef]

| Model | Explanation Variables | Adjust R2 | F-Value | p-Value | AIC |
|---|---|---|---|---|---|
| 1 | Intercept | 77.6% | 49.6 | <0.001 | 19.77 |
| BA | <0.001 | ||||
| SCI | 0.998 | ||||
| 2 | Intercept | 81.9% | 79.7 | <0.001 | 18.94 |
| BA | <0.001 | ||||
| Hegyi | 0.659 | ||||
| 3 | Intercept | 83.2% | 105.1 | <0.001 | 16.85 |
| BA | <0.001 | ||||
| BAL | 0.0543 | ||||
| 4 | Intercept | 82.0% | 192.5 | <0.001 | 18.88 |
| BA | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, Q.; Wei, Y.; Zhao, K. Radial Growth of Dahurian Larch (Larix gmelinii) Responses to Climate and Competition. Forests 2024, 15, 1084. https://doi.org/10.3390/f15071084
Li Z, Zhang Q, Wei Y, Zhao K. Radial Growth of Dahurian Larch (Larix gmelinii) Responses to Climate and Competition. Forests. 2024; 15(7):1084. https://doi.org/10.3390/f15071084
Chicago/Turabian StyleLi, Ziyu, Quanzhi Zhang, Yongkang Wei, and Kunyi Zhao. 2024. "Radial Growth of Dahurian Larch (Larix gmelinii) Responses to Climate and Competition" Forests 15, no. 7: 1084. https://doi.org/10.3390/f15071084
APA StyleLi, Z., Zhang, Q., Wei, Y., & Zhao, K. (2024). Radial Growth of Dahurian Larch (Larix gmelinii) Responses to Climate and Competition. Forests, 15(7), 1084. https://doi.org/10.3390/f15071084





