DNA Barcodes for Wood Identification of Anatomically Similar Species of Genus Chamaecyparis
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement of Nucleotide Diversity
2.2. Gene Alignment and Phylogenetic Analysis
3. Results and Discussion
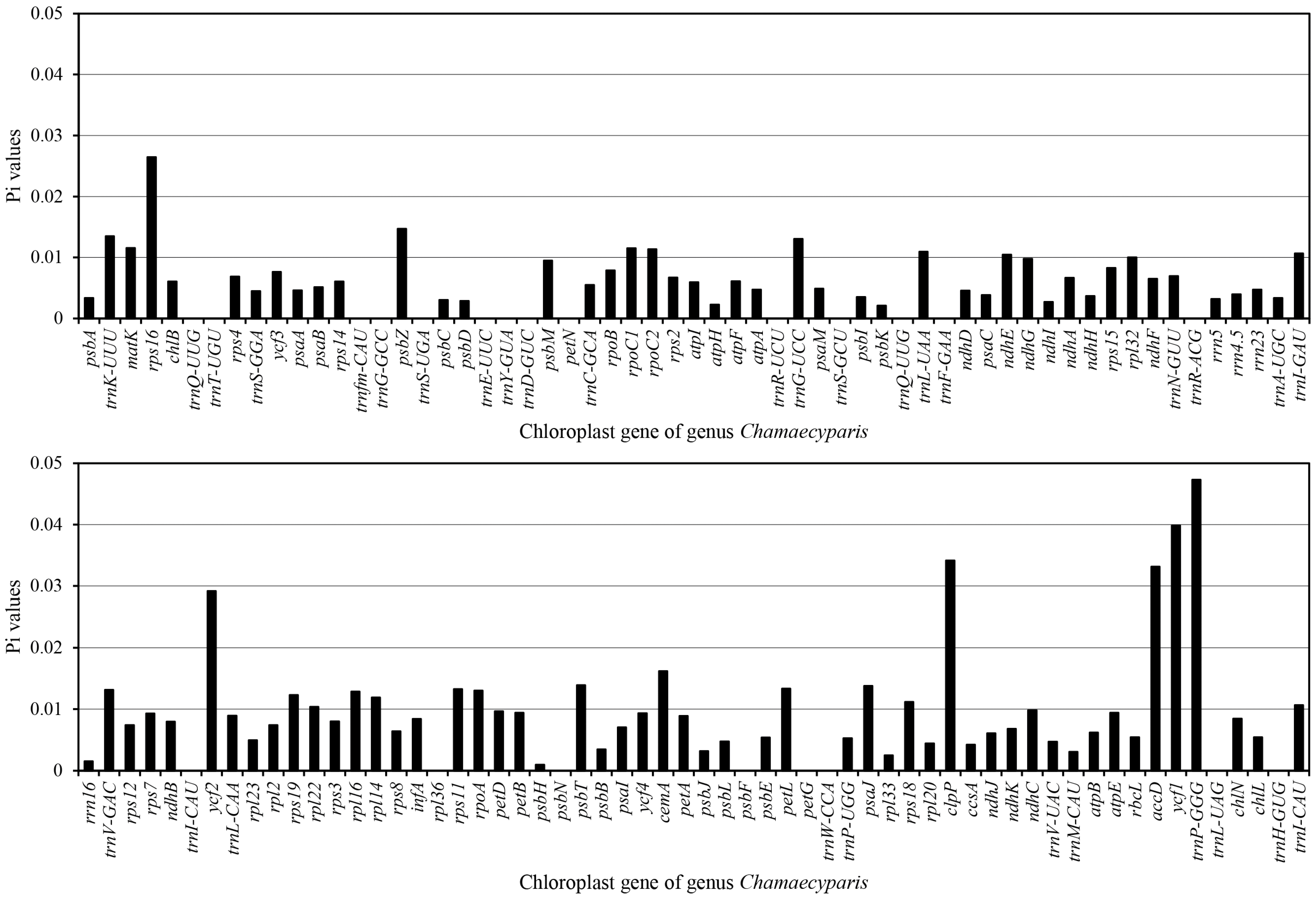
3.1. Evaluation of Nucleotide Diversity of Chloroplast Genomes within Chamaecyparis
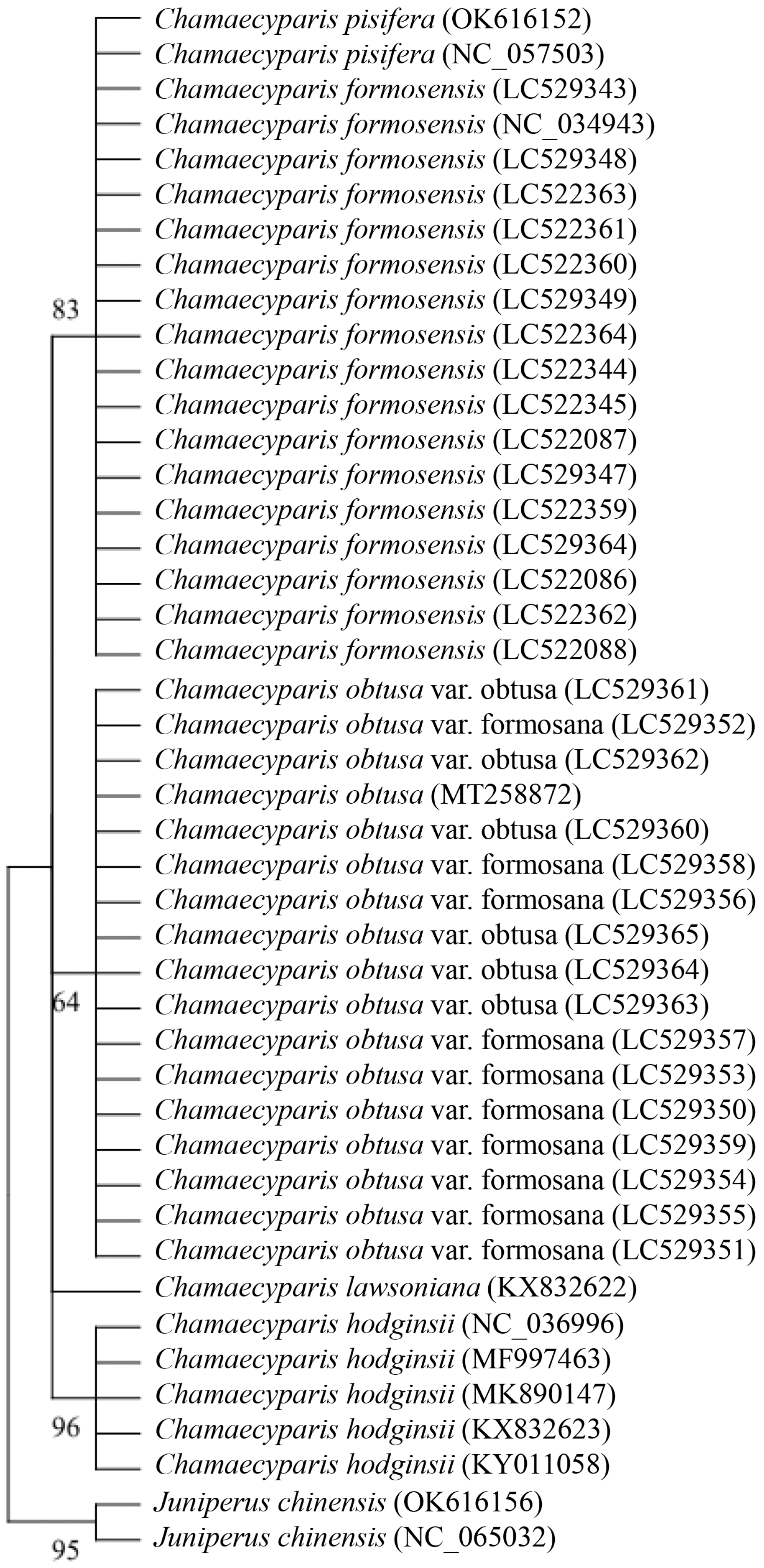
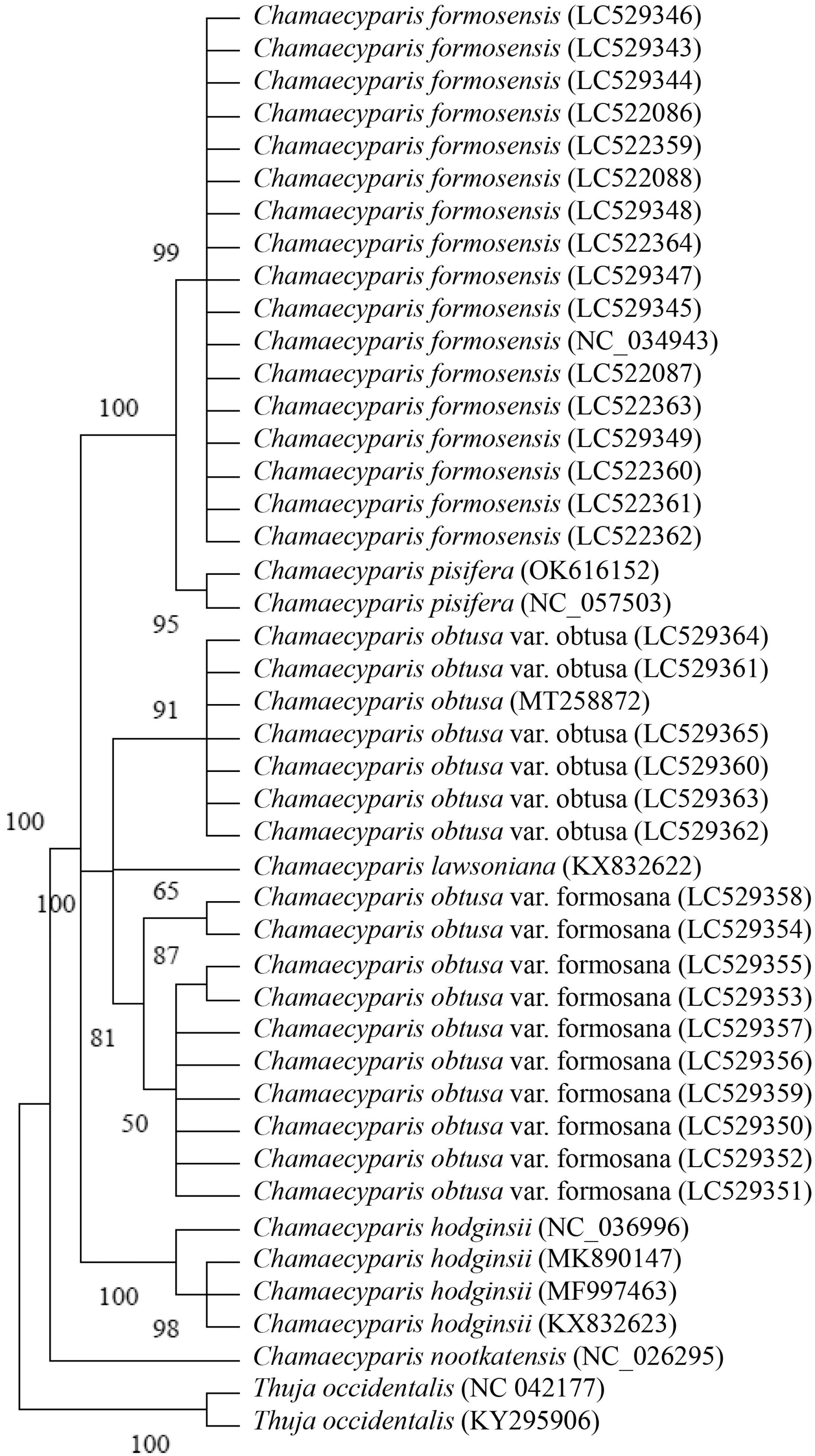
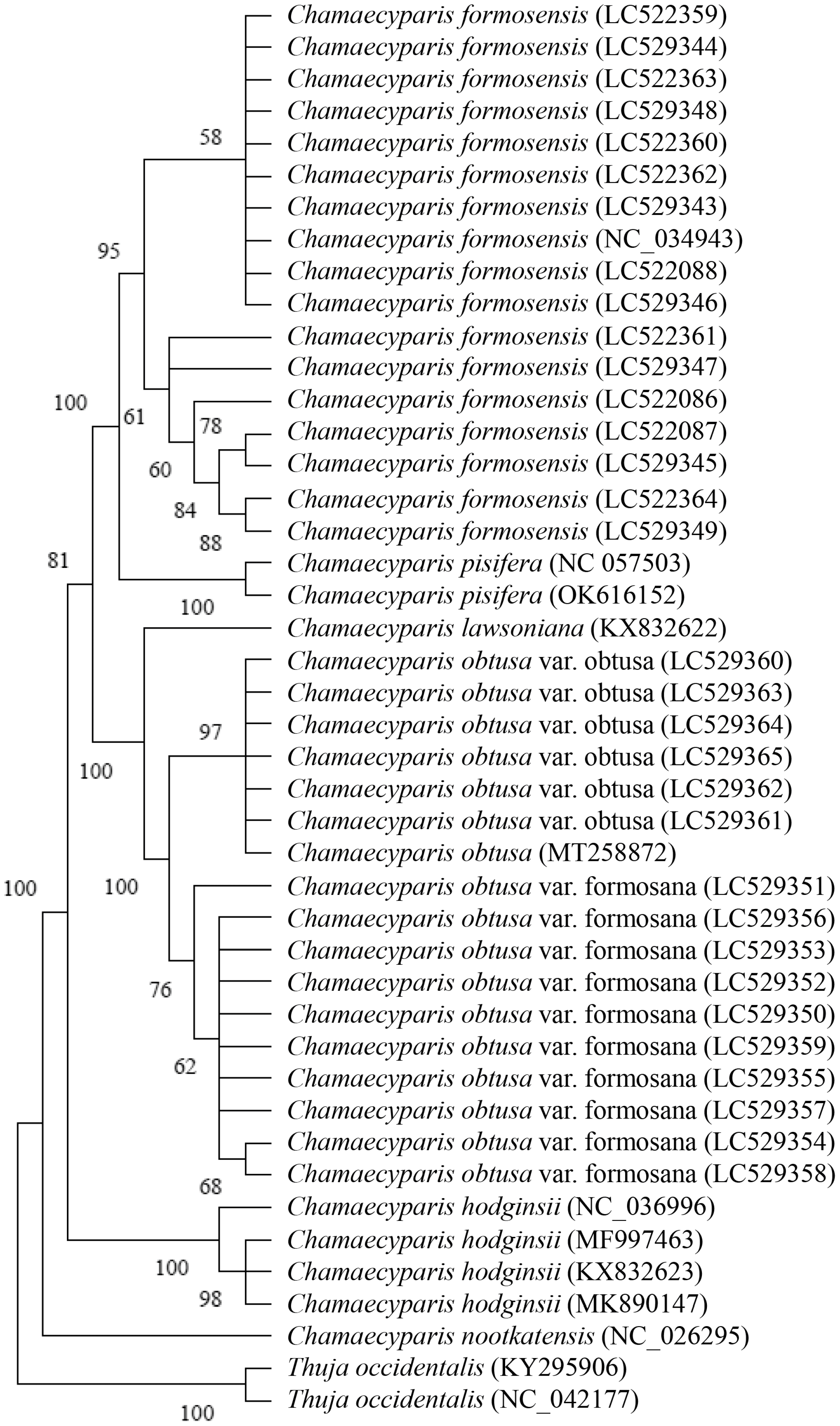
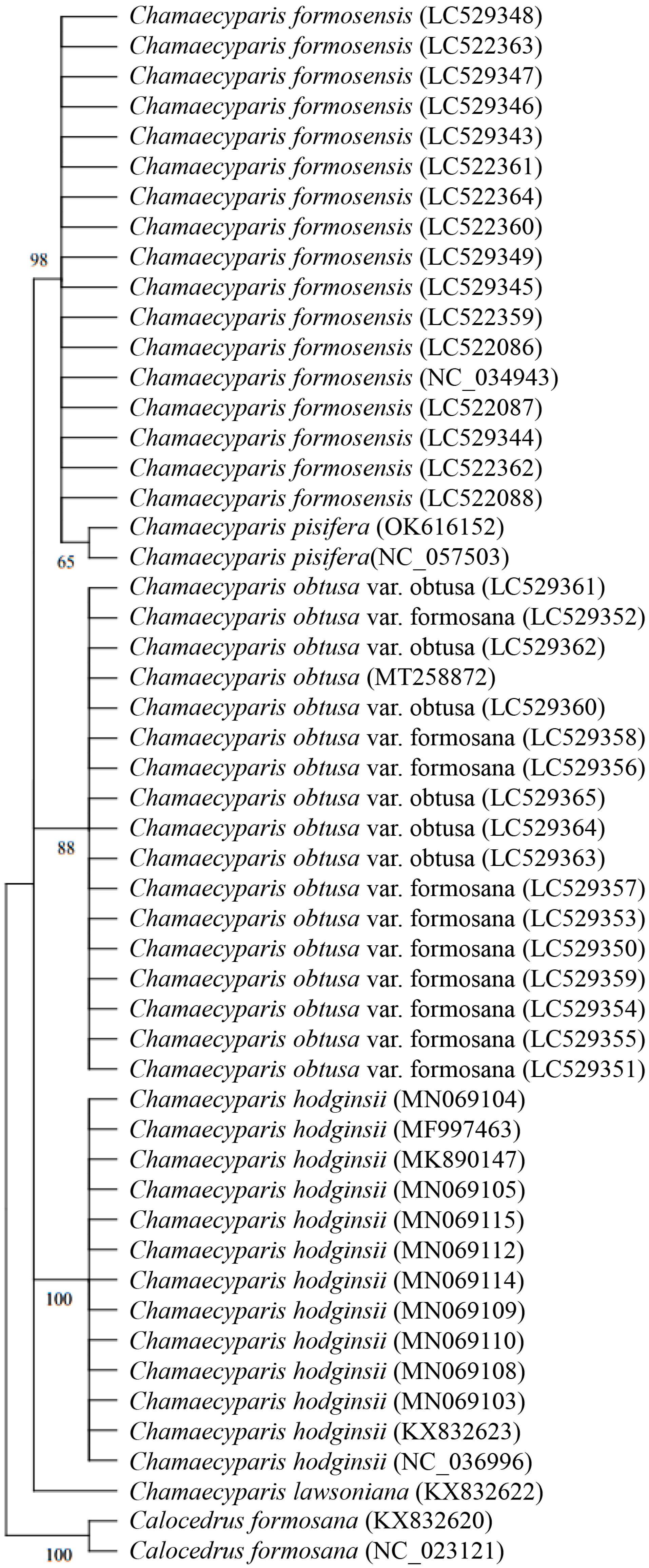
3.2. Phylogenetic Analysis of Five Species of Chamaecyparis Using trnP-GGG
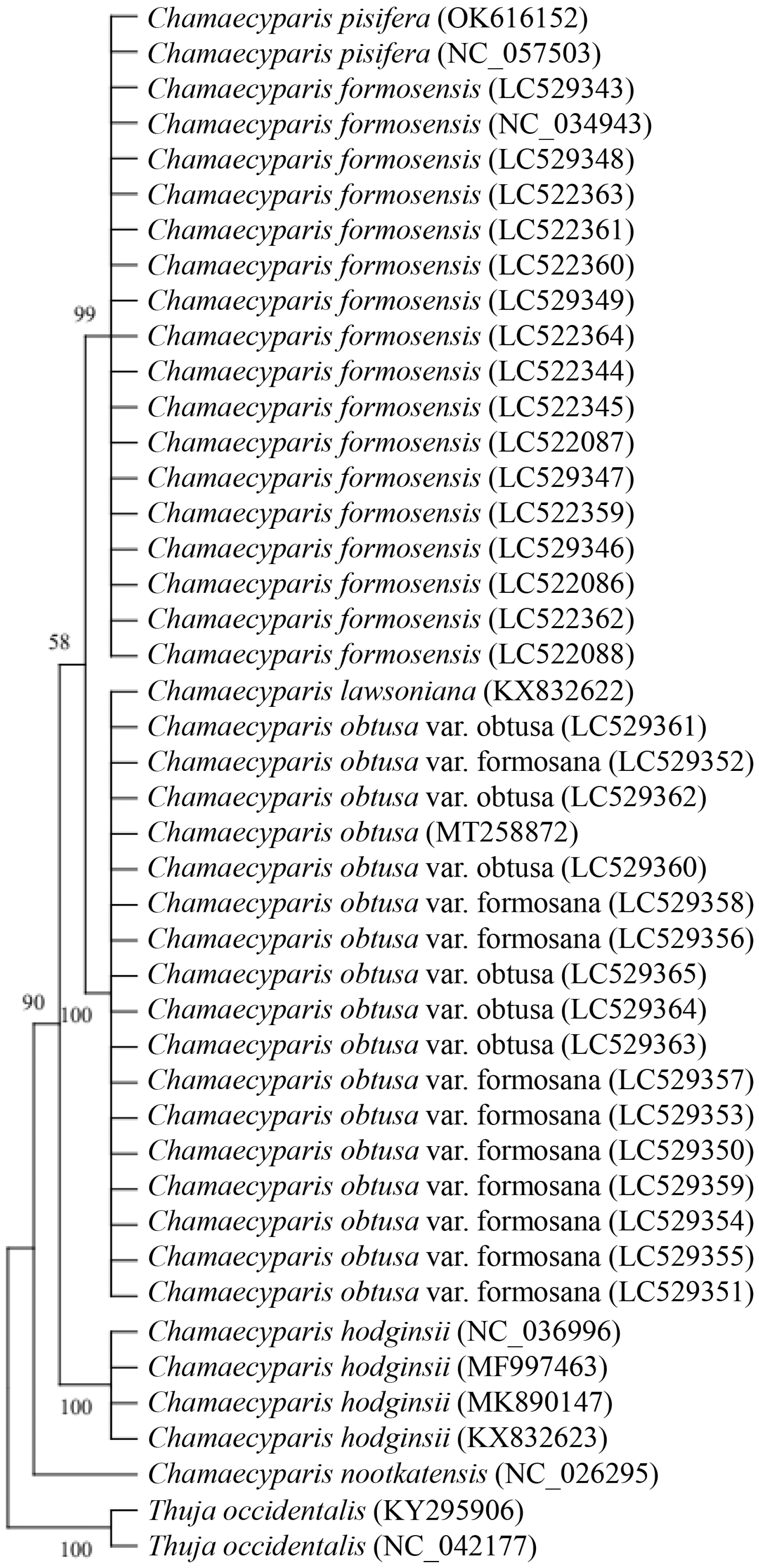
3.3. Phylogenetic Analysis of Five Species of Chamaecyparis Using ycf1b
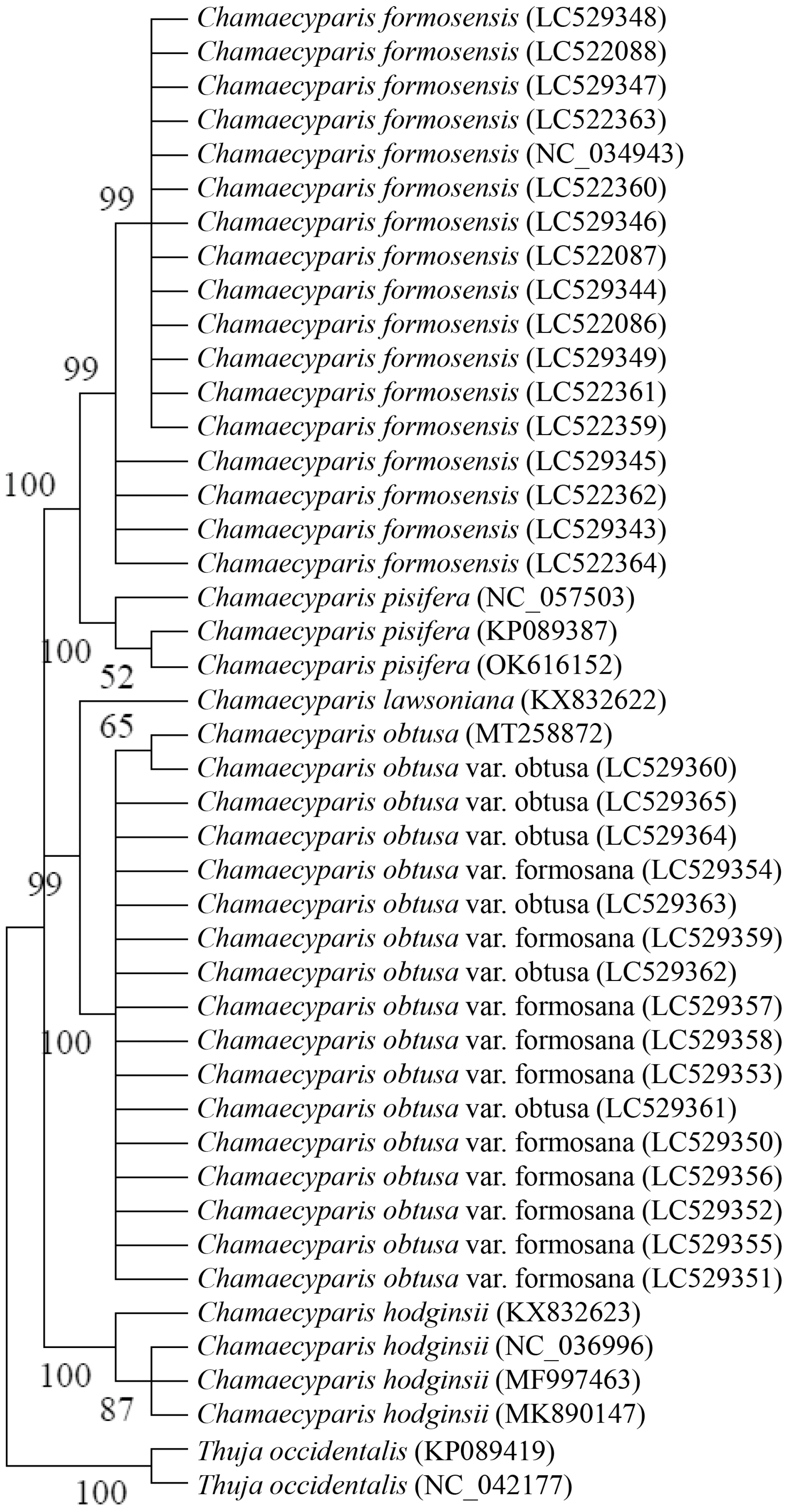
3.4. Phylogenetic Analysis of Six Species of Chamaecyparis Using clpP
3.5. Phylogenetic Analysis of Six Species of Chamaecyparis Using accD
3.6. Phylogenetic Analysis of Six Species of Chamaecyparis Using ycf2
3.7. Phylogenetic Analysis of Five Species of Chamaecyparis Using rps16
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boekhout van Solinge, T. Researching illegal logging and deforestation. Int. J. Crime Justice Soc. Democr. 2014, 3, 35–48. [Google Scholar] [CrossRef]
- Hasyim, S.; Abdullah, R.; Ibrahim, H. Forest damage and preservation through forest resources management in Indonesia. GeoJournal 2021, 86, 2183–2189. [Google Scholar] [CrossRef]
- Reboredo, F. Socio-economic, environmental, and governance impacts of illegal logging. Environ. Syst. Decis. 2013, 33, 295–304. [Google Scholar] [CrossRef]
- Khalid, F.; Babar Taj, M.; Jamil, A.; Kamal, H.; Afzal, T.; Jamshed Iqbal, M.; Khan, T.; Ashiq, M.; Raheel, A.; Sharif, M. Multiple impacts of illegal logging: A key to deforestation over the globe. Biomed. J. Sci. Tech. Res. 2019, 20, 15430–15435. [Google Scholar] [CrossRef]
- Koch, G.; Haag, V.; Heinz, I.; Richter, H.-G.; Schmitt, U. Control of internationally traded timber-the role of macroscopic and microscopic wood identification against illegal logging. J. Forensic Res. 2015, 6, 1000317. [Google Scholar] [CrossRef]
- Dormontt, E.E.; Boner, M.; Braun, B.; Breulmann, G.; Degen, B.; Espinoza, E.; Gardner, S.; Guillery, P.; Hermanson, J.C.; Koch, G.; et al. Forensic timber identification: It’s time to integrate disciplines to combat illegal logging. Biol. Conserv. 2015, 191, 790–798. [Google Scholar] [CrossRef]
- Gismondi, A.; Fanali, F.; Labarga, J.M.M.; Caiola, M.G.; Canini, A. Crocus sativus L. genomics and different DNA barcode applications. Plant Syst. Evol. 2013, 299, 1859–1863. [Google Scholar] [CrossRef]
- Gavrilut, I.; Halalisan, A.-F.; Giurca, A.; Sotirov, M. The interaction between FSC certification and the implementation of the EU timber regulation in Romania. Forests 2016, 7, 3. [Google Scholar] [CrossRef]
- Pezdevšek Malovrh, Š.; Bećirović, D.; Marić, B.; Nedeljković, J.; Posavec, S.; Petrović, N.; Avdibegović, M. Contribution of forest stewardship council certification to sustainable forest management of state forests in selected southeast European countries. Forests 2019, 10, 648. [Google Scholar] [CrossRef]
- Koch, G.; Richter, H.-G.; Schmitt, U. Design and application of CITESwoodID computer-aided identification and description of CITES-protected timbers. IAWA J. 2011, 32, 213–220. [Google Scholar] [CrossRef]
- Zobel, D.B.; Hawk, G.M. The environment of Chamaecyparis lawsoniana. Am. Midl. Nat. 1980, 103, 280–297. [Google Scholar] [CrossRef]
- Igarashi, T.; Kiyono, Y. The potential of hinoki (Chamaecyparis obtusa [Sieb. et Zucc.] Endlicher) plantation forests for the restoration of the original plant community in Japan. For. Ecol. Manag. 2008, 255, 183–192. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Cheng, S.-S.; Chang, S.-T. Chemotaxonomic identification of Chamaecyparis formosensis Matsumura and Chamaecyparis obtusa var. formosana (Hayata) Rehder using characteristic compounds of wood essential oils. Biochem. Syst. Ecol. 2022, 105, 104525. [Google Scholar] [CrossRef]
- Wang, W.P.; Hwang, C.Y.; Lin, T.P.; Hwang, S.Y. Historical biogeography and phylogenetic relationships of the genus Chamaecyparis (Cupressaceae) inferred from chloroplast DNA polymorphism. Plant Syst. Evol. 2003, 241, 13–28. [Google Scholar] [CrossRef]
- Zang, M.; Su, Q.; Weng, Y.; Lu, L.; Zheng, X.; Ye, D.; Zheng, R.; Cheng, T.; Shi, J.; Chen, J. Complete chloroplast genome of Fokienia hodginsii (Dunn) Henry et Thomas: Insights into repeat regions variation and phylogenetic relationships in Cupressophyta. Forests 2019, 10, 528. [Google Scholar] [CrossRef]
- Li, C.-F.; Zelený, D.; Chytrý, M.; Chen, M.-Y.; Chen, T.-Y.; Chiou, C.-R.; Hsia, Y.-J.; Liu, H.-Y.; Yang, S.-Z.; Yeh, C.-L.; et al. Chamaecyparis montane cloud forest in Taiwan: Ecology and vegetation classification. Ecol. Res. 2015, 30, 771–791. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Wu, C.-L.; Chu, F.-H.; Chien, S.-C.; Kuo, Y.-H.; Shyur, L.-F.; Chang, S.-T. Chemical composition and antifungal activity of essential oil isolated from Chamaecyparis formosensis Matsum. wood. Holzforschung 2005, 59, 295–299. [Google Scholar] [CrossRef]
- Zobel, D.B.; Roth, L.F.; Hawk, G.M. Ecology, Pathology, and Management of Port-Orford-Cedar; U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Washington, DC, USA, 1982.
- Morikawa, T.; Ashitani, T.; Sekine, N.; Kusumoto, N.; Takahashi, K. Bioactivities of extracts from Chamaecyparis obtusa branch heartwood. J. Wood Sci. 2012, 58, 544–549. [Google Scholar] [CrossRef]
- Chien, T.-C.; Lo, S.-F.; Ho, C.-L. Chemical composition and anti-inflammatory activity of Chamaecyparis obtusa f. formosana wood essential oil from Taiwan. Nat. Prod. Commun. 2014, 9, 1934578X1400900537. [Google Scholar] [CrossRef]
- Huang, C., Jr.; Chu, F.-H.; Huang, Y.-S.; Tu, Y.-C.; Hung, Y.-M.; Tseng, Y.-H.; Pu, C.-E.; Hsu, C.T.; Chao, C.-H.; Chou, Y.-S.; et al. SSR individual identification system construction and population genetics analysis for Chamaecyparis formosensis. Sci. Rep. 2022, 12, 4126. [Google Scholar] [CrossRef]
- Huang, C., Jr.; Chu, F.-H.; Huang, Y.-S.; Hung, Y.-M.; Tseng, Y.-H.; Pu, C.-E.; Chao, C.-H.; Chou, Y.-S.; Liu, S.-C.; You, Y.T.; et al. Development and technical application of SSR-based individual identification system for Chamaecyparis taiwanensis against illegal logging convictions. Sci. Rep. 2020, 10, 22095. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.-J.; Park, B.-D. Wood species identification of documentary woodblocks of Songok clan of the Milseong Park, Gyeongju, Korea. J. Korean Wood Sci. Technol. 2018, 46, 270–277. [Google Scholar] [CrossRef]
- Yeon, J.-A.; Park, W.-K. Species identification of wooden elements used for Daewungjeon hall in the Bukjijangsa temple, Daegu, Korea. J. Korean Wood Sci. Technol. 2013, 41, 201–210. [Google Scholar] [CrossRef][Green Version]
- Kim, S.C.; Oh, J.A. Identification of wood members in Seoul streetcar no.381. J. Korean Wood Sci. Technol. 2011, 39, 33–40. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, M.O.; Park, W.K. Species identification of wooden elements used for Daewungjeon hall in the Woonsoosa temple, Busan. J. Korean Wood Sci. Technol. 2014, 42, 244–250. [Google Scholar] [CrossRef]
- Noshiro, S. Identification of Japanese species of Cupressaceae from wood structure. Jpn. J. Hist. Bot. 2011, 19, 125–132. [Google Scholar] [CrossRef]
- Jiao, L.; Lu, Y.; He, T.; Guo, J.; Yin, Y. DNA barcoding for wood identification: Global review of the last decade and future perspective. IAWA J. 2020, 41, 620–643. [Google Scholar] [CrossRef]
- Group, C.P.W.; Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef]
- Hilu, K.W.; Liang, G. The matK gene: Sequence variation and application in plant systematics. Am. J. Bot. 1997, 84, 830–839. [Google Scholar] [CrossRef]
- Liao, P.-C.; Lin, T.-P.; Hwang, S.-Y. Reexamination of the pattern of geographical disjunction of Chamaecyparis (Cupressaceae) in North America and East Asia. Bot. Stud. 2010, 51, 511–520. [Google Scholar]
- Fajarningsih, N.D. Internal transcribed spacer (ITS) as DNA barcoding to identify fungal species: A Review. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2016, 11, 37–44. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2002, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Sánchez-DelBarrio, J.C.; Messeguer, X.; Rozas, R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 2003, 19, 2496–2497. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, T.-J. Genetic species identification using ycf1b, rbcL, and trnH-psbA in the genus Pinus as a complementary method for anatomical wood species identification. Forests 2023, 14, 1095. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Pang, X.; Liu, C.; Shi, L.; Liu, R.; Liang, D.; Li, H.; Cherny, S.S.; Chen, S. Utility of the trnH–psbA intergenic spacer region and its combinations as plant DNA Bbarcodes: A meta-analysis. PLoS ONE 2012, 7, e48833. [Google Scholar] [CrossRef] [PubMed]
- Collard, B.C.Y.; Mackill, D.J. Start codon targeted (SCoT) polymorphism: A simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Raman, G.; Choi, K.S.; Park, S. Phylogenetic relationships of the fern Cyrtomium falcatum (Dryopteridaceae) from Dokdo island based on chloroplast genome sequencing. Genes 2016, 7, 115. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Guo, Z.; Mo, J.; Cui, J.; Hu, H.; Xu, J. Comparative analyses and phylogenetic relationships between Cryptomeria fortunei and related species based on complete chloroplast genomes. Phyton-Int. J. Exp. Bot. 2020, 89, 957–986. [Google Scholar] [CrossRef]
- Olsson, S.; Grivet, D.; Cid-Vian, J. Species-diagnostic markers in the genus Pinus: Evaluation of the chloroplast regions matK and ycf1. For. Syst. 2018, 27, e016. [Google Scholar] [CrossRef]
- Li, H.; Xiao, W.; Tong, T.; Li, Y.; Zhang, M.; Lin, X.; Zou, X.; Wu, Q.; Guo, X. The specific DNA barcodes based on chloroplast genes for species identification of Orchidaceae plants. Sci. Rep. 2021, 11, 1424. [Google Scholar] [CrossRef]
- Dong, W.; Liu, J.; Yu, J.; Wang, L.; Zhou, S. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLoS ONE 2012, 7, e35071. [Google Scholar] [CrossRef]
- Maurizi, M.R.; Clark, W.P.; Kim, S.H.; Gottesman, S. ClpP represents a unique family of serine proteases. J. Biol. Chem. 1990, 265, 12546–12552. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.; Yang, Y.; Zhuang, Q.; Chen, S.; Liu, Y.; Huang, S. The comparative studies of complete chloroplast genomes in Actinidia (Actinidiaceae): Novel insights into heterogenous variation, clpP gene annotation and phylogenetic relationships. Mol. Genet. Genom. 2022, 297, 535–551. [Google Scholar] [CrossRef]
- Harris, M.E.; Meyer, G.; Vandergon, T.; Vandergon, V.O. Loss of the acetyl-CoA carboxylase (accD) gene in Poales. Plant Mol. Biol. Report. 2013, 31, 21–31. [Google Scholar] [CrossRef]
- da Cruz, F.; Turchetto-Zolet, A.C.; Veto, N.; Mondin, C.A.; Sobral, M.; Almerão, M.; Margis, R. Phylogenetic analysis of the genus Hexachlamys (Myrtaceae) based on plastid and nuclear DNA sequences and their taxonomic implications. Bot. J. Linn. Soc. 2013, 172, 532–543. [Google Scholar] [CrossRef]
- Kikuchi, S.; Asakura, Y.; Imai, M.; Nakahira, Y.; Kotani, Y.; Hashiguchi, Y.; Nakai, Y.; Takafuji, K.; Bédard, J.; Hirabayashi-Ishioka, Y.; et al. A Ycf2-FtsHi heteromeric AAA-ATPase complex is required for chloroplast protein import. Plant Cell 2018, 30, 2677–2703. [Google Scholar] [CrossRef]
- Celiński, K.; Kijak, H.; Wojnicka-Półtorak, A.; Buczkowska-Chmielewska, K.; Sokołowska, J.; Chudzińska, E. Effectiveness of the DNA barcoding approach for closely related conifers discrimination: A case study of the Pinus mugo complex. Comptes Rendus Biol. 2017, 340, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Shradha, R.; Minoru, U.; Koh-ichi, K.; Nobuhiro, T. Different status of the gene for ribosomal protein S16 in the chloroplast genome during evolution of the genus Arabidopsis and closely related species. Genes Genet. Syst. 2010, 85, 319–326. [Google Scholar] [CrossRef]
- Wang, Q.-Z.; Zhou, S.-D.; Liu, T.-Y.; Pang, Y.-L.; Wu, Y.-K.; He, X.-J.J.A.B.C. Phylogeny and classification of Chinese Bupleurum based on nuclear ribosomal DNA internal transcribed spacer and rps16. Acta Biol. Cracoviensia 2008, 50, 105–116. [Google Scholar]
- Oliver, M.J.; Murdock, A.G.; Mishler, B.D.; Kuehl, J.V.; Boore, J.L.; Mandoli, D.F.; Everett, K.D.E.; Wolf, P.G.; Duffy, A.M.; Karol, K.G. Chloroplast genome sequence of the moss Tortula ruralis: Gene content, polymorphism, and structural arrangement relative to other green plant chloroplast genomes. BMC Genom. 2010, 11, 143. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Li, C.; Sun, J.; Zuo, Y.; Shi, S.; Cheng, T.; Guo, J.; Zhou, S. ycf1, the most promising plastid DNA barcode of land plants. Sci. Rep. 2015, 5, 8348. [Google Scholar] [CrossRef]
- Li, J.; Su, Y.; Wang, T. The repeat sequences and elevated substitution rates of the chloroplast accD gene in Cupressophytes. Front. Plant Sci. 2018, 9, 533. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Im, S.; Kim, T.-J. DNA Barcodes for Wood Identification of Anatomically Similar Species of Genus Chamaecyparis. Forests 2024, 15, 1106. https://doi.org/10.3390/f15071106
Kim M, Im S, Kim T-J. DNA Barcodes for Wood Identification of Anatomically Similar Species of Genus Chamaecyparis. Forests. 2024; 15(7):1106. https://doi.org/10.3390/f15071106
Chicago/Turabian StyleKim, Minjun, Seokhyun Im, and Tae-Jong Kim. 2024. "DNA Barcodes for Wood Identification of Anatomically Similar Species of Genus Chamaecyparis" Forests 15, no. 7: 1106. https://doi.org/10.3390/f15071106
APA StyleKim, M., Im, S., & Kim, T.-J. (2024). DNA Barcodes for Wood Identification of Anatomically Similar Species of Genus Chamaecyparis. Forests, 15(7), 1106. https://doi.org/10.3390/f15071106




