Spatial–Temporal Dynamics of a Germinable Seed Bank of a Semi-Arid Vegetation in the Brazilian Northeast
Abstract
1. Introduction
2. Materials and Methods
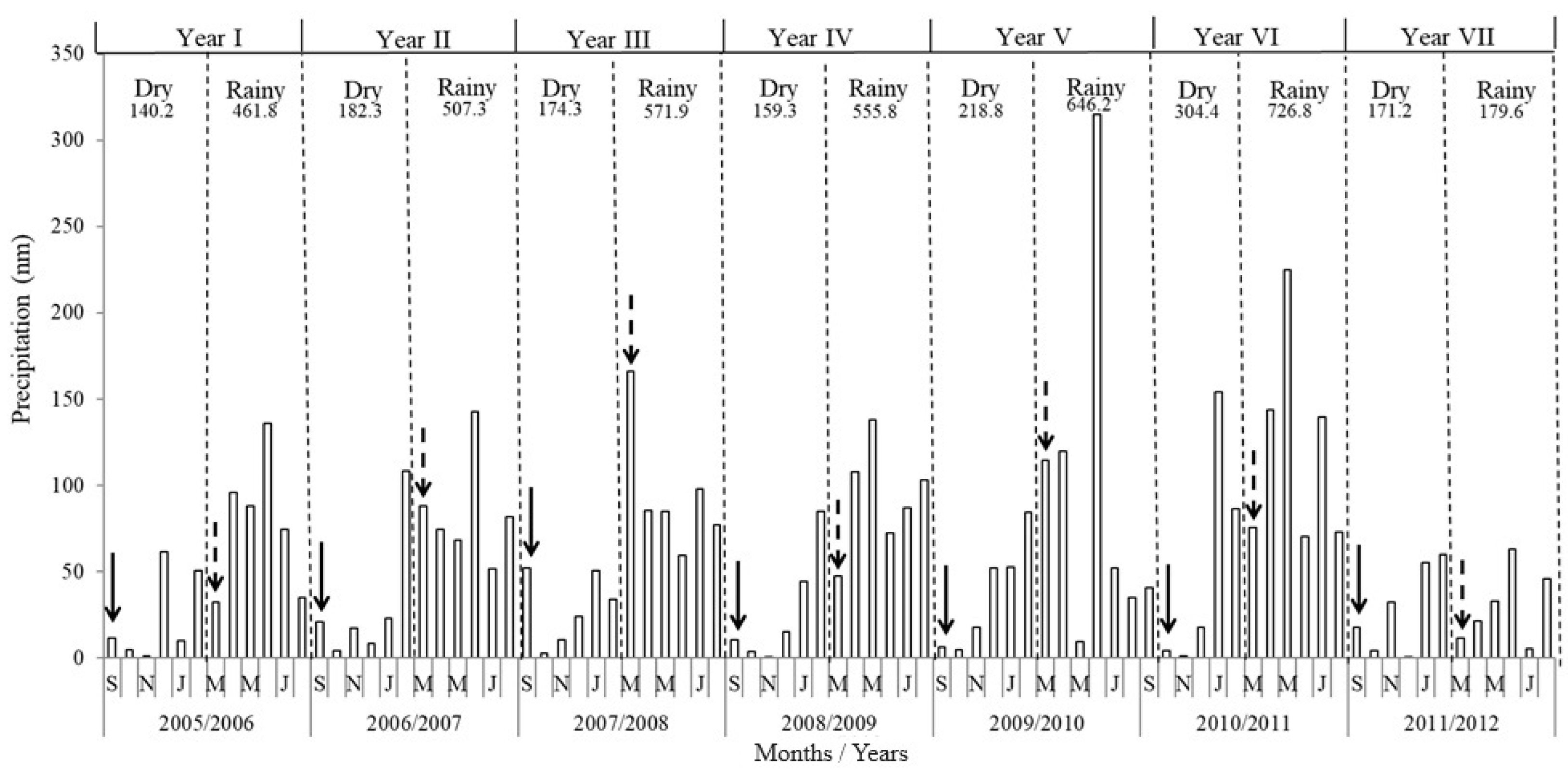
2.1. Study Area
2.2. Seed Bank Samplings
2.3. Seed Bank Analyses
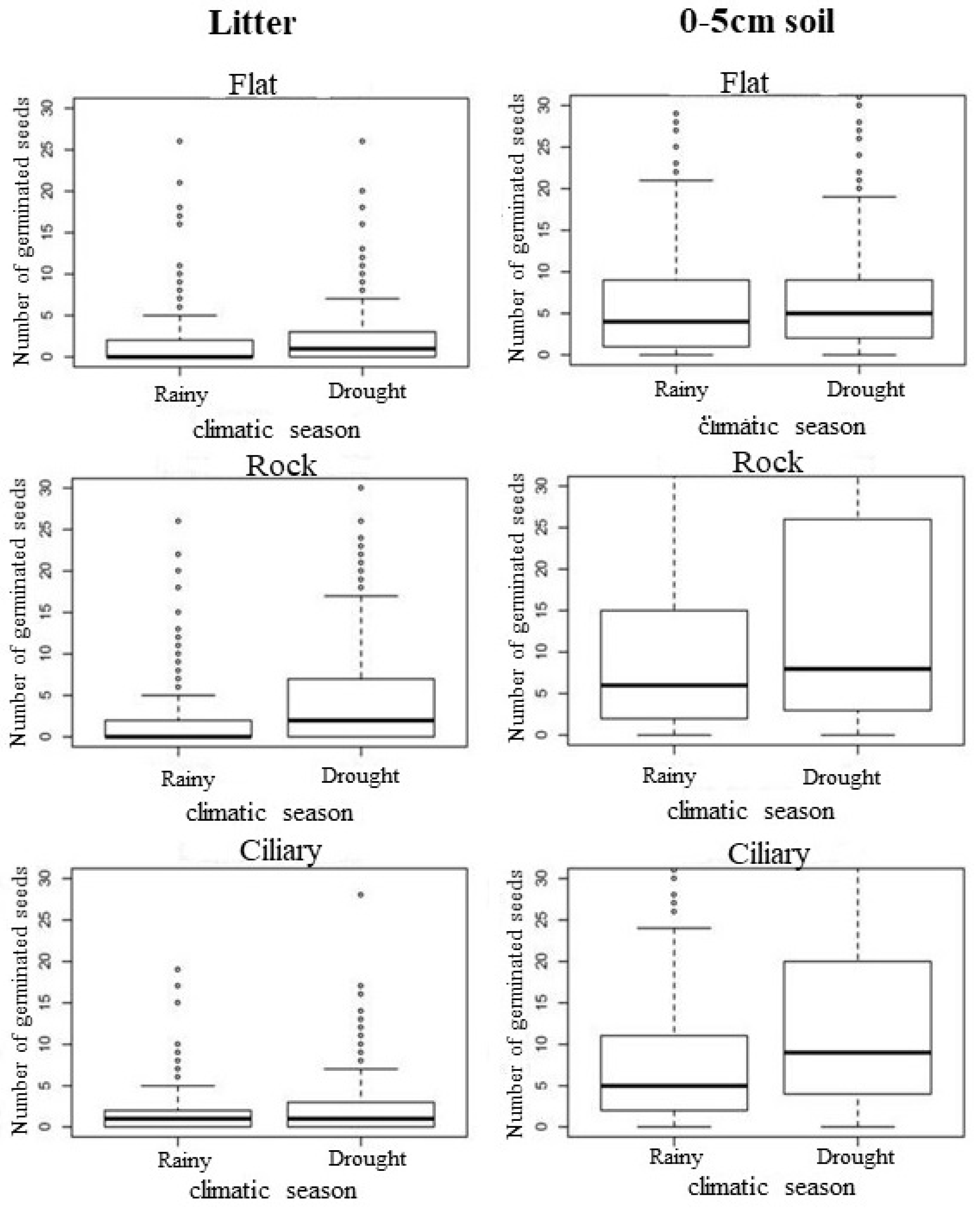
3. Results
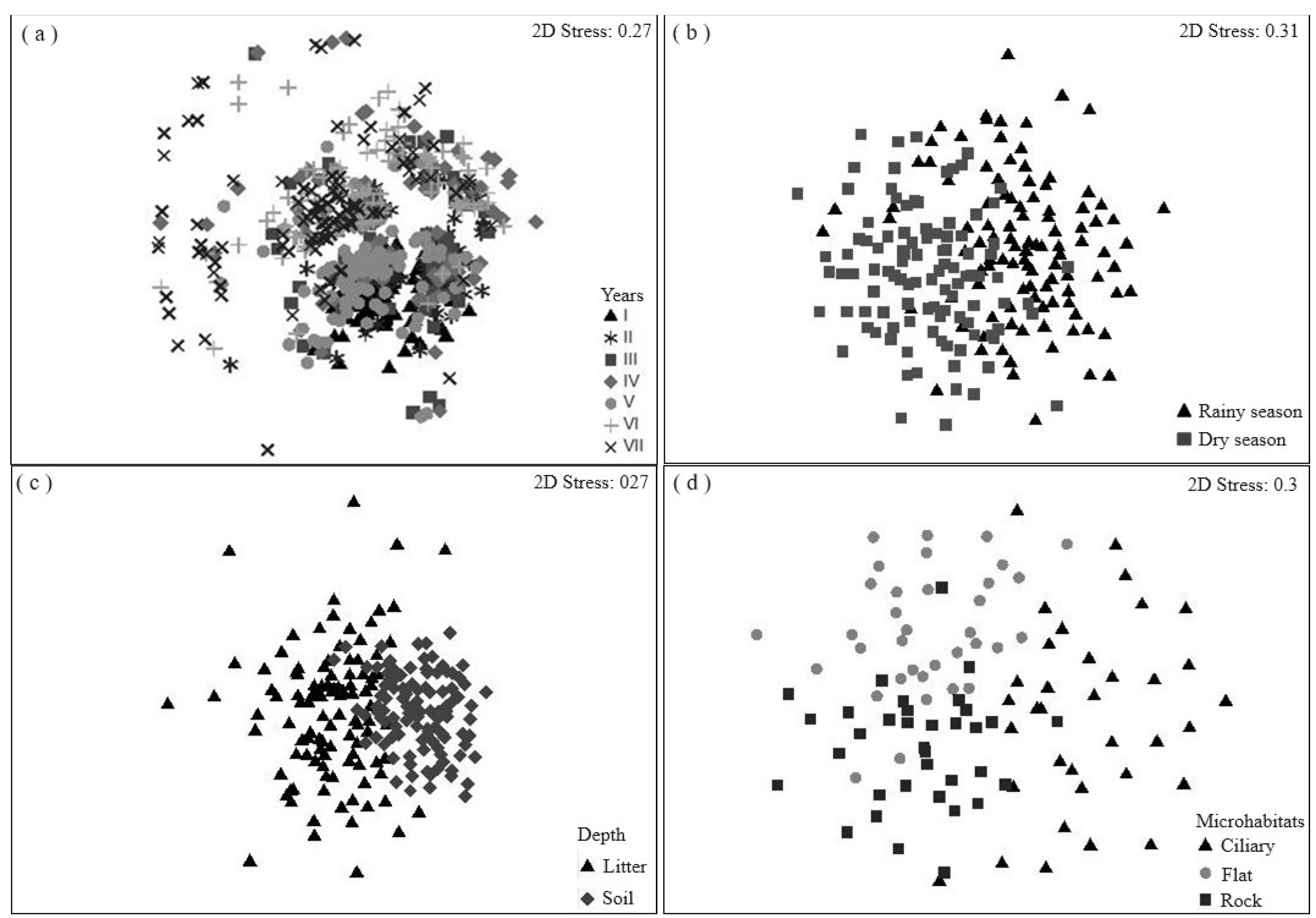
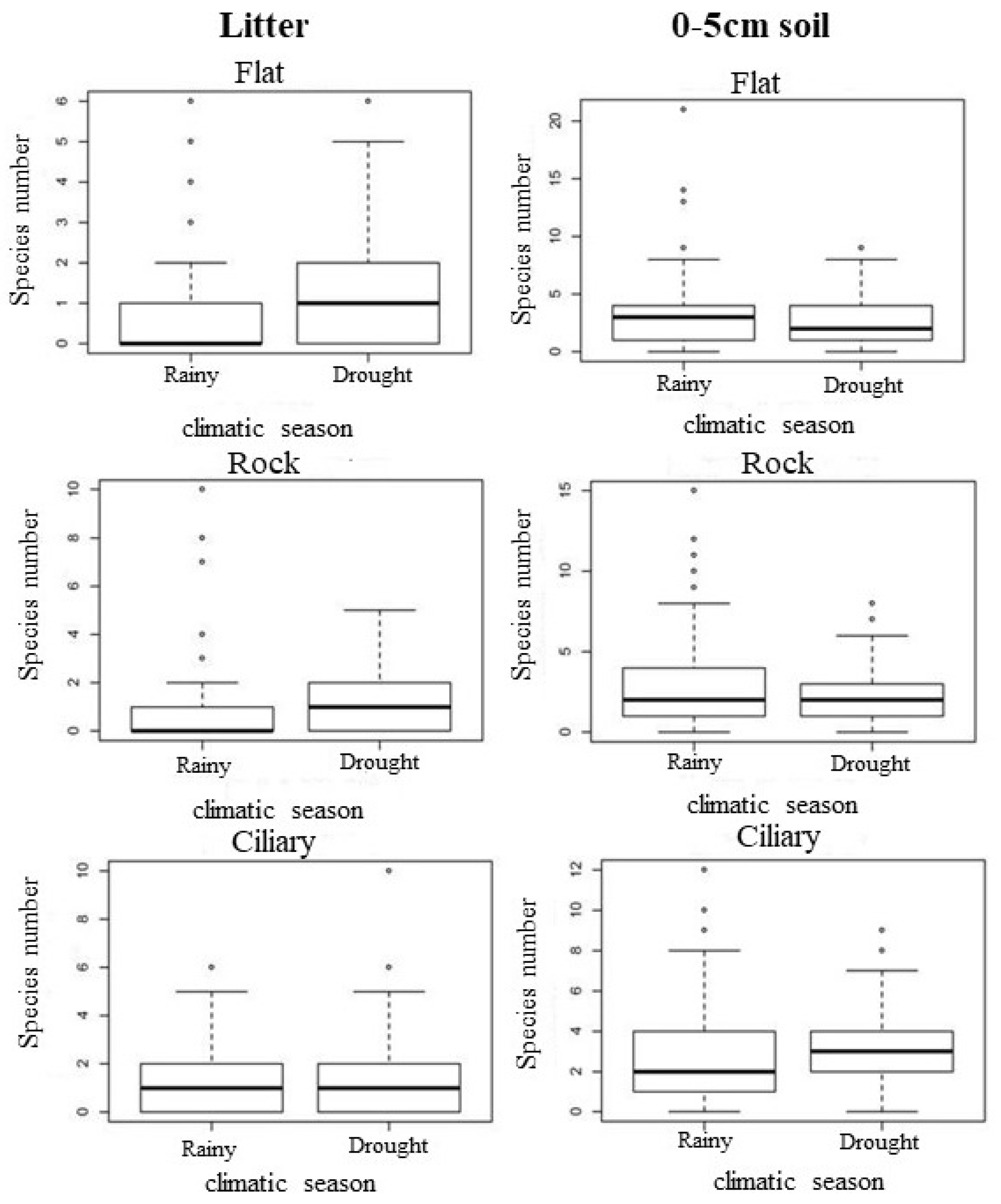
3.1. Species Richness and Floristic Composition
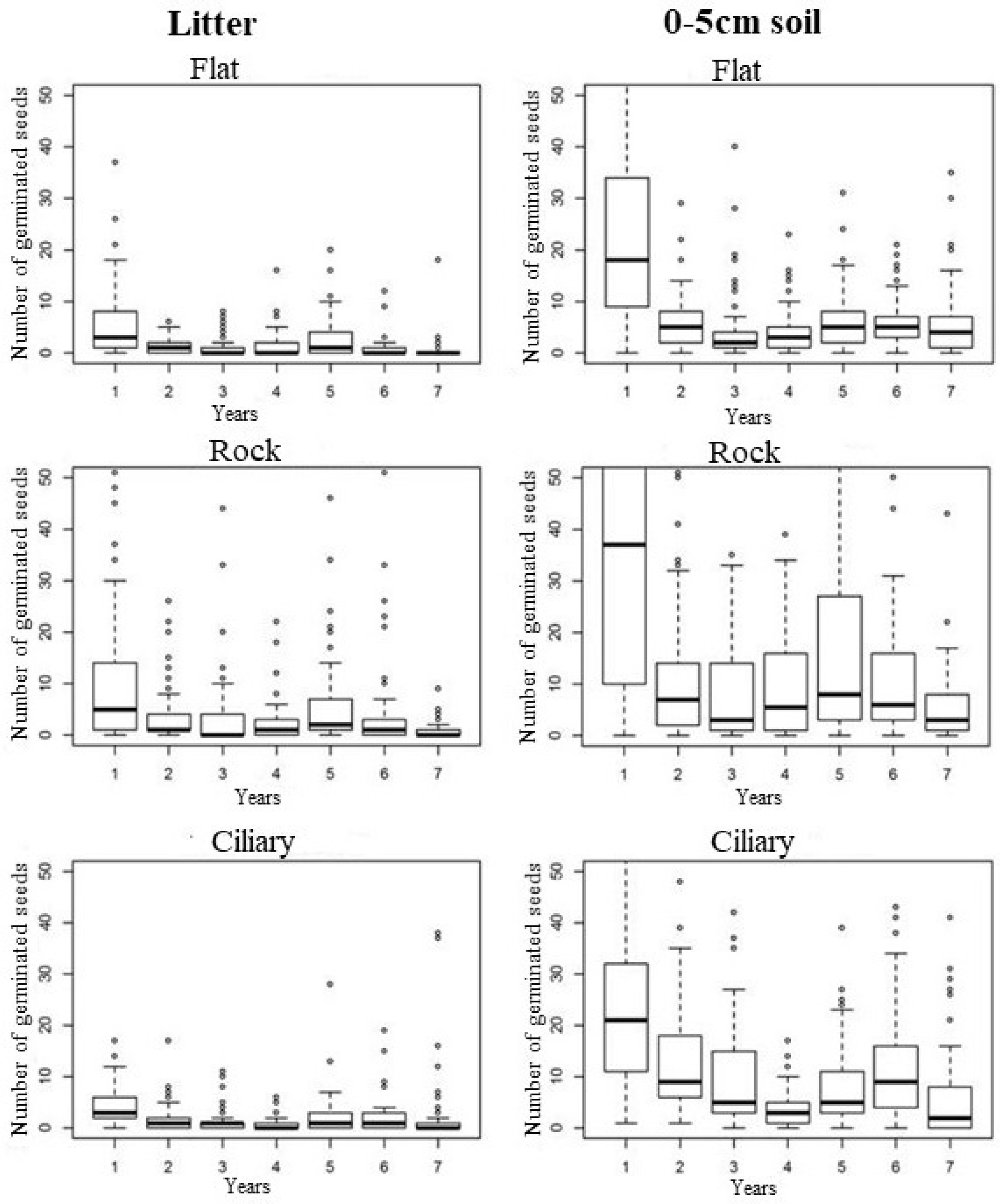
3.2. Seedling Emergence
4. Discussion
4.1. Temporal Variation Effect on the Seed Bank
4.2. Effect of Spatial Variation on the Seed Bank
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamin, N.; Quaye, M.; Westberg, E.; Barazoni, O. Soil seed bank among-years genetic diversity in arid populations of Eruca sativa Miller (Brassicaceae). J. Arid Environ. 2013, 91, 151–154. [Google Scholar] [CrossRef]
- Vandvik, V.; Klanderud, K.; Meineri, E.; Maren, I.E.; Topper, J. Seed banks are biodiversity reservoirs: Species-area relationships above versus below ground. Oikos 2016, 125, 218–228. [Google Scholar] [CrossRef]
- Hopfensperger, K.N. A review of similarity between seed bank and standing vegetation across ecosystems. Oikos 2007, 116, 1438–1448. [Google Scholar] [CrossRef]
- Quevedo-Robledo, L.; Pucheta, E.; Ribas-Fernandéz, Y. Influences of inter-year rainfall variability and microhabitat on the germinable seed bank of annual plants in a Sandy Monte Desert. J. Arid Environ. 2009, 74, 167–172. [Google Scholar] [CrossRef]
- Santos, D.M.; Silva, K.A.; Albuquerque, U.P.; Santos, J.M.F.F.; Lopes, C.G.R.; Araújo, E.L. Can spatial variation and inter-annual variation in precipitation explain the seed density and species richness of the germinable soil seed bank in a tropical dry forest in north-eastern Brazil? Flora 2013, 208, 445–452. [Google Scholar] [CrossRef]
- Silva, K.A.; Santos, D.M.; Santos, J.M.F.F.; Albuquerque, U.P.; Ferraz, E.M.N.; Araújo, E.L. Spatio-temporal variation in a seed bank of a semi-arid region in northeastern Brazil. Acta Oecol. 2013, 46, 25–32. [Google Scholar] [CrossRef]
- Sotomayor, D.A.; Gutiérrez, J.R. Seed bank of desert annual plants along an aridity gradient in the southern Atacama coastal desert. J. Veg. Sci. 2015, 26, 1148–1158. [Google Scholar] [CrossRef]
- Peters, D.P.C. Plant species dominance at a grassland-Shrubland ecotone: And individual-based gap dynamics model of herbaceous and species woody. Ecol. Model. 2002, 152, 5–32. [Google Scholar] [CrossRef]
- Facelli, J.M.; Chesson, P.; Barnes, N. Differences in seed biology of annual plants in arid lands: A key ingredient of the storage effect. Ecology 2005, 86, 2998–3006. [Google Scholar] [CrossRef]
- Traba, J.; Azcárate, F.M.; Peco, B. The fate of seeds in Mediterranean soil seed bank in relation to their traits. J. Veg. Sci. 2006, 17, 5–10. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Hammouda, O.; Lovett-Doust, J.; Gomaa, N.H. Variations of the germinable soil seed bank along the altitudinal gradient in the northwestern Red Sea region. Acta Ecol. Sin. 2009, 29, 20–29. [Google Scholar] [CrossRef]
- Cabin, R.J.; Marshall, D.C. The demographic role of soil seed banks. I. Spatial and temporal comparisons of below- and above-ground populations of the desert mustard Lesquerella fendleri. J. Ecol. 2000, 88, 233–292. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Chacon, P.; Cavieres, L.A. Relationship between seed bank expression, adult longevity and aridity in species of Chaetanthera (asteraceae) in Central Chile. Ann. Bot. 2006, 98, 591–600. [Google Scholar] [CrossRef]
- Valdez-Hernández, M.; Andrade, J.L.; Jackson, P.C.; Rebolledo-Vieyra, M. Phenology of five tree species of a tropical dry forest in Yucatan, Mexico: Effects of environmental and physiological factors. Plant Soil 2010, 329, 155–171. [Google Scholar] [CrossRef]
- Yu, S.; Bell, D.; Sternberg, M.; Kutiel, P. The effect of microhabitats on vegetation and its relationships with seedlings and soil seed bank in a Mediterranean coastal sand dune community. J. Arid Environ. 2008, 72, 2040–2053. [Google Scholar] [CrossRef]
- Erfanzadeh, R.; Shahbazian, R.; Zali, H. Role of plant patches in preserving flora from the soil seed bank in an overgrazed high-mountain habitat in Northern Iran. J. Agric. Sci. Technol. 2014, 19, 229–238. [Google Scholar]
- Valenta, K.; Steffens, T.S.; Rafaliarison, R.R.; Chapman, C.A.; Lehman, S.M. Seed banks in Savanna, forest fragments and a continuous forest edges differ in a tropical dry forest in Madagascar. Biotropica 2015, 47, 435–440. [Google Scholar] [CrossRef]
- Donnell, J.O.; Fryirs, K.; Leishman, M.R. Can the regeneration of vegetation from riparian seed banks support biogeomorphic succession and the geomorphic recovery of degraded river channels? River Res. Appl. 2015, 31, 834–846. [Google Scholar] [CrossRef]
- Donnell, J.O.; Fryirs, K.; Leishman, M.R. Seed banks as a source of vegetation regeneration to support the recovery of degraded rivers: A comparison of river reaches of varying condition. Sci. Total Environ. 2016, 542, 591–602. [Google Scholar] [CrossRef]
- Caballero, I.; Olano, J.M.; Escudero, A.; Loidi, J. Seed bank spatial structure in semi-arid environments: Beyond the patch-bare area dichotomy. Plant Ecol. 2008, 195, 215–223. [Google Scholar] [CrossRef]
- Dreber, N.; Oldeland, J.; Gretil, M.V.; Rooyen, V. Species, functional groups and community structure in seed banks of the arid Nama Karoo: Grazing impacts and implications for rangelands restoration. Agric. Ecosyst. Environ. 2011, 141, 399–409. [Google Scholar] [CrossRef]
- Mayor, M.D.; Bóo, R.M.; Pelaéz, D.V.; Elía, O.R.; Tomáz, M.A. Influence of shrub cover on germination, dormancy and viability of buried and unburied seeds of Piptochaetium napostaense (Speg.) Hackel. J. Arid Environ. 2007, 68, 509–521. [Google Scholar] [CrossRef]
- Araújo, E.L.; Castro, C.C.; Albuquerque, U.P. Dynamics of Brazilian caatinga—A review concerning the plants, environment and people. Funct. Ecosist. Commun. 2007, 1, 15–28. [Google Scholar]
- Lucena, R.F.P.; Nascimento, V.T.; Araújo, E.L.; Albuquerque, U.P. Local uses of native plants in an area of caatinga vegetation (Pernambuco, NE-Brazil). Ethnobot. Res. Appl. 2008, 6, 3–13. [Google Scholar] [CrossRef]
- Köppen, W. Grundriss der Klimakunde; Walter de Gruyter: Berlin, Germany, 1931; 390p. [Google Scholar]
- Reis, A.M.S.; Araújo, E.L.; Ferraz, E.M.N.; Moura, A.N. Variações interanuais na florística e fitossociologia do componente herbáceo de uma área de caatinga, Pernambuco, Brasil. Acta Bot. Bras. 2006, 29, 497–508. [Google Scholar]
- Alcoforado-Filho, F.G.; Sampaio, E.V.S.B.; Rodal, M.J.N. Florística fitossociologia de um remanescente de vegetação caducifólia espinhosa arbórea em Caruaru. Acta Bot. Bras. 2003, 17, 287–303. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecologia—De Individuos A Ecossistemas, 4th ed.; Artmed Editora: Porto Alegre, Brazil, 2007; pp. 1–752. [Google Scholar]
- Santos, D.M.; Santos, J.M.F.F.; Silva, K.A.; Araújo, V.K.R.; Araújo, E.L. Composition, species richness and density of the germinable seed bank over 4 years in young and mature forests in Brazilian semiarid regions. J. Arid Environ. 2016, 129, 93–101. [Google Scholar] [CrossRef]
- Souza, V.C.; Lorenzi, H. Botânica Sistemática: Guia Ilustrado para Identificação das Famílias de Fanerógamas Nativas e Exóticas no Brasil, Baseado em APGIV, 3rd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2012; pp. 1–768. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Primer v6: User Manual/Tutorial, 6th ed.; Plymouth Marine Laboratory: Plymouth, UK, 2006; pp. 1–190. [Google Scholar]
- Dobson, A.J. An Introduction to Generalized Linear Models, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2002; pp. 50–62. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Lazaró, R. Seed bank and understorey species composition in a semi-arid environment: The effect of shrub age and rainfall. Ann. Bot. 2000, 86, 807–813. [Google Scholar] [CrossRef]
- López, R.P. Soil seed bank in the semi-arid Prepuna of Bolivia. Plant Ecol. 2003, 168, 85–92. [Google Scholar] [CrossRef]
- Ooi, M.K.J. Seed bank persistence and climate change. Seed Sci. Res. 2012, 22, 53–60. [Google Scholar] [CrossRef]


| Variable Response | Explanatory Variations and Their Interactions | DF | Deviance Explained by the Variable | Residual DF | Residual Deviance | p-Value | PRD | CPRD | CPRD% |
|---|---|---|---|---|---|---|---|---|---|
| Species richness | NULL | NA | NA | 2939 | 5,762,075,032 | ||||
| Depth | 1 | 113,201,889 | 2938 | 4,630,056,142 | 0.0000 | 0.196 | 0.196 | 19.6 | |
| Previous year’s precipitation | 1 | 2,430,666,244 | 2937 | 4,386,989,517 | 0.0000 | 0.042 | 0.239 | 4.2 | |
| Precipitation of the year | 1 | 239,359,418 | 2936 | 4,147,630,099 | 0.0000 | 0.042 | 0.280 | 4.2 | |
| Climatic station precipitation | 1 | 3,437,730,606 | 2935 | 4,144,192,369 | 0.0637 | 0.001 | 0.281 | 0.1 | |
| Microhabitat | 2 | 1,090,314,181 | 2933 | 4,133,289,227 | 0.0043 | 0.002 | 0.283 | 0.2 | |
| Precipitation of the previous climatic season | 1 | 3,257,551,862 | 2932 | 4,130,031,675 | 0.0711 | 0.001 | 0.283 | 0.1 | |
| Emergence of seedlings | NULL | NA | NA | 2939 | 62,454 | NA | |||
| Depth | 1 | 10,043 | 2938 | 52,411 | 0.0000 | 0.161 | 0.161 | 16.1 | |
| Previous year’s precipitation | 1 | 4391 | 2937 | 48,020 | 0.0000 | 0.070 | 0.231 | 7.0 | |
| Microhabitat | 2 | 3840 | 2935 | 44,179 | 0.0000 | 0.061 | 0.293 | 6.1 | |
| Precipitation of the previous climatic season | 1 | 918 | 2934 | 43,261 | 0.0000 | 0.015 | 0.307 | 1.5 | |
| Precipitation of the year | 1 | 1966 | 2933 | 41,296 | 0.0000 | 0.031 | 0.339 | 3.1 | |
| Climatic station precipitation | 1 | 181 | 2932 | 41,115 | 0.0000 | 0.003 | 0.342 | 0.3 |
| Emergence of Seedlings | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flat | Rocky | Ciliary | ||||||||||
| Rainy | Dry | Rainy | Dry | Rainy | Dry | |||||||
| Year | Litter | Soil | Litter | Soil | Litter | Soil | Litter | Soil | Litter | Soil | Litter | Soil |
| I | 6 | 29 | 4 | 14 | 8 | 43 | 14 | 66 | 3 | 15 | 4 | 29 |
| II | 1 | 7 | 1 | 3 | 2 | 9 | 4 | 8 | 2 | 15 | 1 | 8 |
| III | 1 | 2 | 1 | 6 | 1 | 3 | 9 | 21 | 1 | 3 | 1 | 13 |
| IV | 1 | 3 | 1 | 4 | 1 | 11 | 2 | 7 | 1 | 3 | 1 | 3 |
| V | 1 | 4 | 3 | 7 | 2 | 12 | 12 | 25 | 1 | 5 | 3 | 9 |
| VI | 1 | 5 | 1 | 5 | 1 | 6 | 13 | 21 | 6 | 16 | 1 | 7 |
| VII | 1 | 2 | 1 | 8 | 1 | 4 | 1 | 5 | 1 | 1 | 4 | 22 |
| Total | 11 | 52 | 12 | 46 | 15 | 88 | 54 | 154 | 13 | 57 | 15 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, D.M.d.; Silva, K.A.d.; Cunha, S.R.d.; Aguiar, B.A.d.S.; Santos, P.S.d.; Araujo, V.K.R.d.; Santos, J.M.F.F.d.; Araújo, E.d.L. Spatial–Temporal Dynamics of a Germinable Seed Bank of a Semi-Arid Vegetation in the Brazilian Northeast. Forests 2024, 15, 1108. https://doi.org/10.3390/f15071108
Santos DMd, Silva KAd, Cunha SRd, Aguiar BAdS, Santos PSd, Araujo VKRd, Santos JMFFd, Araújo EdL. Spatial–Temporal Dynamics of a Germinable Seed Bank of a Semi-Arid Vegetation in the Brazilian Northeast. Forests. 2024; 15(7):1108. https://doi.org/10.3390/f15071108
Chicago/Turabian StyleSantos, Danielle Melo dos, Kleber Andrade da Silva, Simone Rabelo da Cunha, Bruno Ayron de Souza Aguiar, Priscila Silva dos Santos, Vanessa Kelly Rodrigues de Araujo, Josiene Maria Falcão Fraga dos Santos, and Elcida de Lima Araújo. 2024. "Spatial–Temporal Dynamics of a Germinable Seed Bank of a Semi-Arid Vegetation in the Brazilian Northeast" Forests 15, no. 7: 1108. https://doi.org/10.3390/f15071108
APA StyleSantos, D. M. d., Silva, K. A. d., Cunha, S. R. d., Aguiar, B. A. d. S., Santos, P. S. d., Araujo, V. K. R. d., Santos, J. M. F. F. d., & Araújo, E. d. L. (2024). Spatial–Temporal Dynamics of a Germinable Seed Bank of a Semi-Arid Vegetation in the Brazilian Northeast. Forests, 15(7), 1108. https://doi.org/10.3390/f15071108






