Functional Traits Affect the Contribution of Individual Species to Beta Diversity in the Tropical Karst Seasonal Rainforest of South China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Methods
2.2.1. Functional Traits
2.2.2. Environmental Data
2.3. Explanatory Variables
2.3.1. Species Niche Properties
2.3.2. Functional Distinctiveness
2.4. Statistical Analyses
2.4.1. Constructing Sites × Species Matrix
2.4.2. Calculating the SCBD Values
2.4.3. Statistical Modeling
2.4.4. Beta Regression to Analyze
3. Results
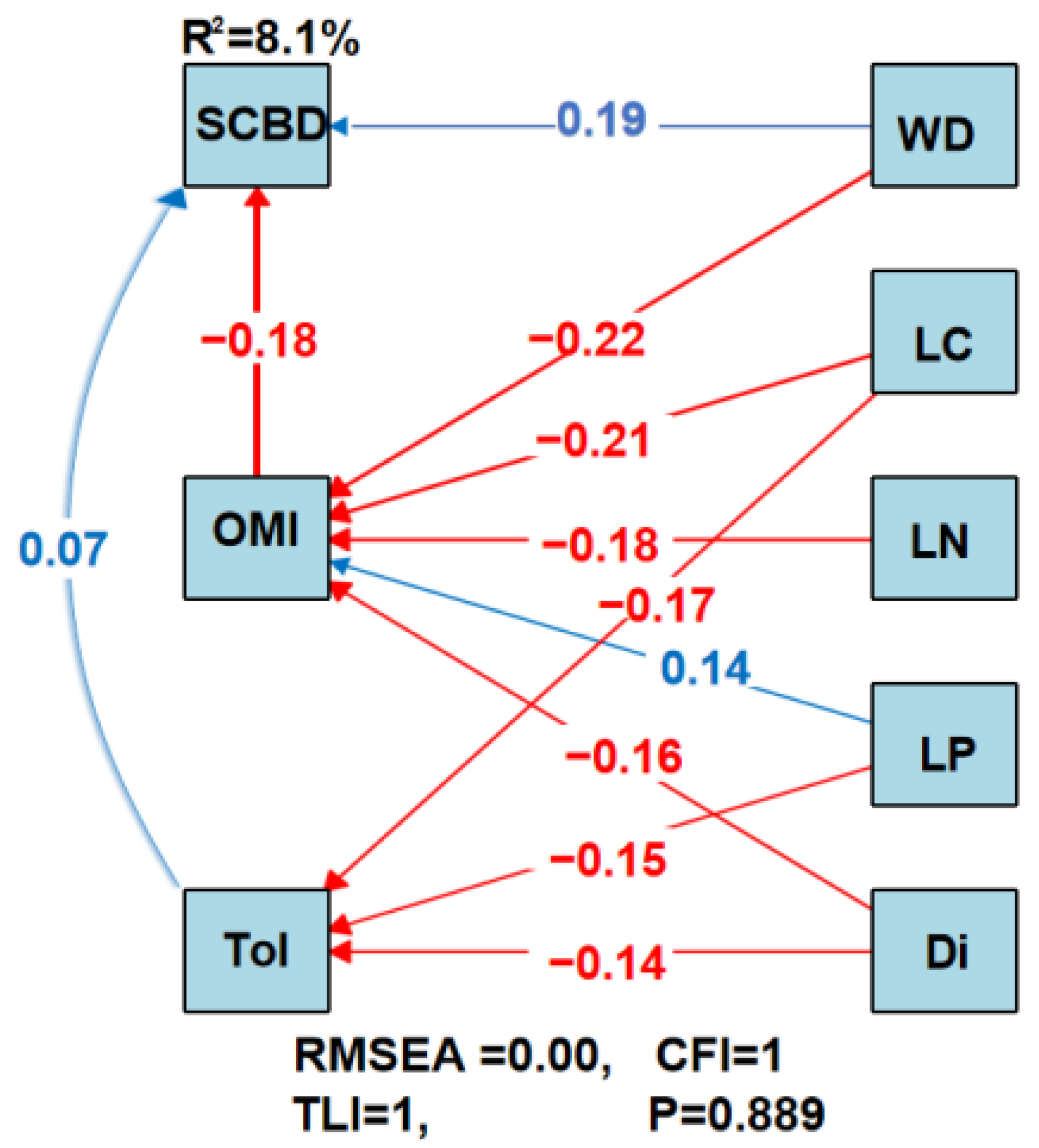
3.1. Distribution of SCBD
3.2. Impact of Species Traits on SCBD
4. Discussion
4.1. Distribution of SCBD
4.2. Impact of Species Ecological Niche Characteristics on SCBD
4.3. The Impact of Plant Functional Traits on SCBD
4.4. The Impact of Functional Distinctiveness on SCBD
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- White, H.J.; McKeon, C.M.; Pakeman, R.J.; Buckley, Y.M. The contribution of geographically common and rare species to the spatial distribution of biodiversity. Glob. Ecol. Biogeogr. 2023, 32, 1730–1747. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Wang, J.; Huang, Y.; Freedman, Z.; Fu, S.; Liu, K.; Wang, H.; Li, X.; Yao, M. Local community assembly mechanisms shape soil bacterial β diversity patterns along a latitudinal gradient. Nat. Commun. 2020, 11, 5428. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Lima, M.G.M.; Espinosa, S.; Ahumada, J.A.; Jansen, P.A.; Spironello, W.R.; Hurtado, J.; Juen, L.; Peres, C.A. Site and species contribution to β-diversity in terrestrial mammal communities: Evidence from multiple Neotropical forest sites. Sci. Total Env. Environ. 2021, 789, 147946. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Borcard, D.; Peres-Neto, P.R. Analyzing beta diversity: Partitioning the spatial variation of community composition data. Ecol. Monogr. 2005, 75, 435–450. [Google Scholar] [CrossRef]
- Myers, J.A.; LaManna, J.A. The promise and pitfalls of β-diversity in ecology and conservation. J. Veg. Sci. 2016, 27, 1081–1083. [Google Scholar] [CrossRef]
- Heino, J.; Grönroos, M. Exploring species and site contributions to beta diversity in stream insect assemblages. Oecologia 2017, 183, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, Y.; Li, B.; Luo, W.; Chu, C.; Wang, Y. How does functional distinctiveness affect single species contribu-tion to β diversity? Evidence from a subtropical forest plot in southern China. Ecol. Indic. 2023, 146, 109736. [Google Scholar] [CrossRef]
- Da Silva, P.G.; Hernández, M.I.M.; Heino, J. Disentangling the correlates of species and site contributions to beta diversity in dung beetle assemblages. Divers. Distrib. 2018, 24, 1674–1686. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Shenbrot, G.I.; Warburton, E.M.; Van Der Mescht, L.; Surkova, E.N.; Medvedev, S.G.; Pechnikova, N.; Er-molova, N.; Kotti, B.K.; Khokhlova, I.S. Species and site contributions to β-diversity in fleas parasitic on the Palearctic small mammals: Ecology, geography and host species composition matter the most. Parasitology 2019, 146, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Heino, J.; Yu, F.; He, Y.; Liu, F.; Wang, J. Spatial patterns of site and species contributions to β diversity in river-ine fish assemblages. Ecol. Indic. 2022, 145, 109728. [Google Scholar] [CrossRef]
- De Bello, F.; Lavorel, S.; Díaz, S.; Harrington, R.; Cornelissen, J.H.; Bardgett, R.D.; Berg, M.P.; Cipriotti, P.; Feld, C.K.; He-ring, D. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Violle, C.; Thuiller, W.; Mouquet, N.; Munoz, F.; Kraft, N.J.B.; Cadotte, M.W.; Livingstone, S.W.; Mouillot, D. Functional Rarity: The Ecology of Outliers. Trends Ecol. Evol. 2017, 32, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Kondratyeva, A.; Knapp, S.; Durka, W.; Kühn, I.; Vallet, J.; Machon, N.; Martin, G.; Motard, E.; Grandcolas, P.; Pavoine, S. Urbanization Effects on Biodiversity Revealed by a Two-Scale Analysis of Species Functional Uniqueness vs. Redundancy. Front. Ecol. Evol. 2020, 8, 73. [Google Scholar] [CrossRef]
- Gaüzère, P.; Blonder, B.; Denelle, P.; Fournier, B.; Grenié, M.; Delalandre, L.; Münkemüller, T.; Munoz, F.; Violle, C.; Thuiller, W. The functional trait distinctiveness of plant species is scale dependent. Ecography 2023, 2023, e6504. [Google Scholar] [CrossRef]
- Grenié, M.; Denelle, P.; Tucker, C.M.; Munoz, F.; Violle, C. funrar: An R package to characterize functional rarity. Divers. Distrib. 2017, 23, 1365–1371. [Google Scholar] [CrossRef]
- Marino, N.A.; Cereghino, R.; Gilbert, B.; Petermann, J.S.; Srivastava, D.S.; de Omena, P.M.; Bautista, F.O.; Guzman, L.M.; Romero, G.Q.; Trzcinski, M.K. Species niches, not traits, determine abundance and occupancy patterns: A multi-site syn-thesis. Global Ecol. Biogeogr. 2020, 29, 295–308. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, B.; Mallik, A.U.; Huang, F.; Xiang, W.; Ding, T.; Wen, S.; Lu, S.; Li, D.; He, Y.; et al. Topo-graphic species–habitat associations of tree species in a heterogeneous tropical karst seasonal rain forest, China. J. Plant Ecol. 2016, 10, 450–460. [Google Scholar]
- Guo, Y.; Chen, H.Y.H.; Mallik, A.U.; Wang, B.; Li, D.; Xiang, W.; Li, X. Predominance of abiotic drivers in the relationship between species diversity and litterfall production in a tropical karst seasonal rainforest. For. Ecol. Manag. 2019, 449, 117452. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar]
- Xingming, Z.; Bin, W.; Ting, C.; Yili, G.; Xiankun, L. Revealing the relative importance among plant species, slope posi-tions, and soil types on rhizosphere microbial communities in northern tropical karst and non-karst seasonal rainforests of China. Front. Microbiol. 2023, 14, 1103550. [Google Scholar]
- Ruixia, M.; Jianxing, L.; Yili, G.; Bin, W.; Wusheng, X.; Dongxing, L.; Fuzhao, H.; Fang, L.; Shujun, W.; Shuhua, L.; et al. Re-cruitment dynamics in a tropical karst seasonal rain forest: Revealing complex processes from spatial patterns. For. Ecol. Manag. 2024, 553, 121610. [Google Scholar]
- Guo, Y.; Wang, B.; Li, D.; Mallik, A.U.; Xiang, W.; Ding, T.; Wen, S.; Lu, S.; Huang, F.; He, Y.; et al. Effects of topography and spatial processes on structuring tree species composition in a diverse heterogeneous tropical karst seasonal rainforest. Flora 2017, 231, 21–28. [Google Scholar] [CrossRef]
- Hu, G.; Pang, Q.; Hu, C.; Xu, C.; Zhang, Z.; Zhong, C. Beta Diversity Patterns and Determinants among Vertical Layers of Tropical Seasonal Rainforest in Karst Peak-Cluster Depressions. Forests 2024, 15, 365. [Google Scholar] [CrossRef]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Bai, K.; Lv, S.; Ning, S.; Zeng, D.; Guo, Y.; Wang, B. Leaf nutrient concentrations associated with phylogeny, leaf habit and soil chemistry in tropical karst seasonal rainforest tree species. Plant Soil. 2019, 434, 305–326. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167. [Google Scholar] [CrossRef]
- Roberts, D.W.; Cooper, S.V. Concepts and techniques of vegetation mapping. In Land Classification Based on Vegetation; Applications for Resource Management: Moscow, Russia, 1989; pp. 90–96. [Google Scholar]
- Scrimgeour, C. Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-8593-3586-0. [Google Scholar]
- Dolédec, S.; Chessel, D.; Carpentier, C.G. Niche Separation in Community Analysis: A New Method. Ecology 2000, 81, 2914–2927. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Dray, S.; Dufour, A. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Y. RMSEA, CFI, and TLI in structural equation modeling with ordered categorical data: The story they tell depends on the estimation methods. Behav. Res. Methods 2019, 51, 409–428. [Google Scholar] [CrossRef]
- Cribari-Neto, F.; Zeileis, A. Beta Regression in R. J. Stat. Softw. 2010, 34, 1–24. [Google Scholar] [CrossRef]
- Szabó, B.; Lengyel, E.; Padisák, J.; Stenger-Kovács, C. Benthic diatom metacommunity across small freshwater lakes: Driving mechanisms, β-diversity and ecological uniqueness. Hydrobiologia 2019, 828, 183–198. [Google Scholar] [CrossRef]
- Castaño Quintero, S.; Velasco, J.; González Voyer, A.; Martínez Meyer, E.; Yáñez Arenas, C. Niche position and niche breadth effects on population abundances: A case study of New World Warblers (Parulidae). Ecol. Evol. 2024, 14, e11108. [Google Scholar] [CrossRef] [PubMed]
- Vela Díaz, D.M.; Blundo, C.; Cayola, L.; Fuentes, A.F.; Malizia, L.R.; Myers, J.A.; Pither, J.; Pither, J. Untangling the im-portance of niche breadth and niche position as drivers of tree species abundance and occupancy across biogeographic re-gions. Glob. Ecol. Biogeogr. 2020, 29, 1542–1553. [Google Scholar] [CrossRef]
- François, M.; Klausmeier, C.A.; Pierre, G.; Gaurav, K.; Elena, L.; Nicolas, M.; Annette, O.; Wilfried, T.; Algar, A.C.; Arnaud, A.; et al. The ecological causes of functional distinctiveness in communities. Ecol. Lett. 2023, 26, 1452–1465. [Google Scholar]
- Zhu, Y.J.; Shan, D.; Zhang, X.; Liu, Y.S.; Shi, Z.J.; Yang, X.H. Advances in joint species distribution models to re-veal community structure and its environmental response. Chin. J. Appl. Ecol. 2018, 29, 4217–4225. [Google Scholar]
- Mori, A.S.; Isbell, F.; Seidl, R. β-diversity, community assembly, and ecosystem functioning. Trends Ecol. Evol. 2018, 33, 549–564. [Google Scholar] [CrossRef] [PubMed]
- van Der Plas, F.; Manning, P.; Soliveres, S.; Allan, E.; Scherer-Lorenzen, M.; Verheyen, K.; Wirth, C.; Zavala, M.A.; Am-poorter, E.; Baeten, L. Biotic homogenization can decrease landscape-scale forest multifunctionality. Proc. Natl. Acad. Sci. USA 2016, 113, 3557–3562. [Google Scholar] [CrossRef]



| Family | Genus | Species | OMI | Tol | SCBD | Abundance | Occupancy |
|---|---|---|---|---|---|---|---|
| Magnoliaceae | Lirianthe | fistulosa | 2.7737 | 5.7028 | 0.0034 | 154 | 46 |
| Annonaceae | Alphonsea | mollis | 2.0966 | 2.5104 | 0.0016 | 68 | 49 |
| Annonaceae | Alphonsea | monogyna | 0.4583 | 3.5079 | 0.0077 | 504 | 169 |
| Annonaceae | Desmos | chinensis | 1.5410 | 1.3923 | 0.0025 | 126 | 48 |
| Annonaceae | Goniothalamus | donnaiensis | 8.5599 | 3.3864 | 0.0017 | 71 | 29 |
| Annonaceae | Miliusa | balansae | 4.9616 | 0.7396 | 0.0003 | 20 | 10 |
| Annonaceae | Mitrephora | macclurei | 7.9938 | 21.3198 | 0.0003 | 13 | 4 |
| Annonaceae | Orophea | polycarpa | 0.7544 | 1.7086 | 0.0323 | 2336 | 167 |
| Lauraceae | Cinnamomum | saxatile | 1.3120 | 3.5064 | 0.0013 | 63 | 35 |
| Lauraceae | Cryptocarya | lyoniifolia | 4.7864 | 3.2563 | 0.0047 | 231 | 94 |
| Lauraceae | Litsea | foveola | 1.2293 | 1.6186 | 0.0040 | 283 | 123 |
| Lauraceae | Litsea | glutinosa | 3.6175 | 0.2567 | 0.0001 | 4 | 4 |
| Lauraceae | Litsea | variabilis | 3.1188 | 2.0604 | 0.0013 | 50 | 20 |
| Lauraceae | Neolitsea | hainanensis | 3.3917 | 0.5653 | 0.0001 | 4 | 3 |
| Lauraceae | Phoebe | calcarea | 0.6926 | 0.8499 | 0.0006 | 25 | 16 |
| Capparaceae | Capparis | subsessilis | 19.4964 | 1.1943 | 0.0002 | 4 | 2 |
| Capparaceae | Capparis | urophylla | 0.6876 | 0.5002 | 0.0010 | 51 | 34 |
| Violaceae | Rinorea | bengalensis | 0.5558 | 0.6363 | 0.0411 | 2769 | 98 |
| Linaceae | Tirpitzia | ovoidea | 21.3642 | 1.2255 | 0.0078 | 514 | 33 |
| Lythraceae | Lagerstroemia | caudata | 2.3910 | 5.7955 | 0.0017 | 78 | 45 |
| Pittosporaceae | Pittosporum | pulchrum | 20.3276 | 1.7033 | 0.0041 | 269 | 23 |
| Salicaceae | Bennettiodendron | leprosipes | 9.3751 | 2.9852 | 0.0098 | 424 | 68 |
| Achariaceae | Hydnocarpus | hainanensis | 0.1843 | 1.2017 | 0.0216 | 2551 | 292 |
| Salicaceae | Xylosma | longifolia | 2.2536 | 3.3728 | 0.0007 | 31 | 21 |
| Salicaceae | Homalium | sabiifolium | 13.1345 | 3.6274 | 0.0009 | 50 | 16 |
| Theaceae | Camellia | flavida | 6.5399 | 2.6925 | 0.0050 | 235 | 21 |
| Actinidiaceae | Saurauia | tristyla | 18.3052 | 3.2746 | 0.0005 | 19 | 8 |
| Myrtaceae | Decaspermum | gracilentum | 6.4694 | 1.0052 | 0.0115 | 684 | 85 |
| Myrtaceae | Syzygium | chunianum | 1.8107 | 0.6665 | 0.0002 | 9 | 9 |
| Myrtaceae | Syzygium | hainanense | 2.8723 | 0.0000 | 0.0001 | 3 | 1 |
| Melastomataceae | Memecylon | scutellatum | 6.4861 | 4.4803 | 0.0179 | 1618 | 207 |
| Hypericaceae | Cratoxylum | formosum | 2.1060 | 1.8615 | 0.0010 | 48 | 39 |
| Clusiaceae | Garcinia | bracteata | 6.7035 | 0.6009 | 0.0000 | 2 | 2 |
| Clusiaceae | Garcinia | paucinervis | 0.2379 | 1.2100 | 0.0135 | 2021 | 319 |
| Malvaceae | Hainania | trichospermus | 1.4394 | 1.1525 | 0.0152 | 1139 | 185 |
| Malvaceae | Excentrodendron | tonkinense | 0.7990 | 0.9469 | 0.0197 | 1786 | 216 |
| Malvaceae | Grewia | henryi | 1.4429 | 1.1094 | 0.0006 | 32 | 21 |
| Malvaceae | Firmiana | simplex | 4.0572 | 7.2913 | 0.0001 | 5 | 5 |
| Malvaceae | Pterospermum | truncatolobatum | 0.7737 | 1.4338 | 0.0220 | 1977 | 204 |
| Malvaceae | Reevesia | glaucophylla | 4.5306 | 1.4507 | 0.0006 | 30 | 15 |
| Malvaceae | Sterculia | euosma | 14.1766 | 5.0915 | 0.0010 | 47 | 26 |
| Malvaceae | Sterculia | monosperma | 0.5953 | 1.4198 | 0.0414 | 7265 | 336 |
| Malvaceae | Bombax | ceiba | 4.0580 | 1.1816 | 0.0001 | 3 | 2 |
| Euphorbiaceae | Acalypha | kerrii | 0.2209 | 0.6969 | 0.0065 | 388 | 90 |
| Phyllanthaceae | Antidesma | bunius | 3.3454 | 0.9661 | 0.0002 | 9 | 7 |
| Phyllanthaceae | Antidesma | japonicum | 3.0975 | 2.3460 | 0.0312 | 2385 | 230 |
| Phyllanthaceae | Antidesma | montanum | 3.4436 | 2.4558 | 0.0016 | 71 | 36 |
| Phyllanthaceae | Baccaurea | ramiflora | 17.8377 | 3.6234 | 0.0013 | 50 | 23 |
| Phyllanthaceae | Bischofia | javanica | 7.1519 | 2.4342 | 0.0029 | 120 | 59 |
| Phyllanthaceae | Breynia | fruticosa | 12.3668 | 0.0000 | 0.0000 | 1 | 1 |
| Phyllanthaceae | Bridelia | balansae | 3.5676 | 4.1806 | 0.0026 | 118 | 60 |
| Phyllanthaceae | Bridelia | glauca | 3.7060 | 0.7323 | 0.0002 | 7 | 7 |
| Phyllanthaceae | Bridelia | tomentosa | 2.0770 | 0.9531 | 0.0002 | 11 | 9 |
| Euphorbiaceae | Cephalomappa | sinensis | 2.3070 | 6.1148 | 0.0279 | 1625 | 100 |
| Euphorbiaceae | Claoxylon | indicum | 3.5511 | 0.0000 | 0.0000 | 2 | 1 |
| Phyllanthaceae | Cleistanthus | petelotii | 6.1533 | 17.2701 | 0.0148 | 623 | 39 |
| Phyllanthaceae | Cleistanthus | sumatranus | 1.4978 | 0.7679 | 0.1167 | 10,344 | 193 |
| Euphorbiaceae | Croton | euryphyllus | 13.4244 | 4.2763 | 0.0065 | 351 | 76 |
| Putranjivaceae | Drypetes | congestiflora | 1.0949 | 1.3855 | 0.0105 | 797 | 202 |
| Putranjivaceae | Drypetes | perreticulata | 0.3357 | 1.2542 | 0.0101 | 747 | 176 |
| Phyllanthaceae | Flueggea | virosa | 8.6238 | 0.6769 | 0.0007 | 37 | 12 |
| Phyllanthaceae | Glochidion | coccineum | 0.9633 | 0.9644 | 0.0003 | 13 | 13 |
| Phyllanthaceae | Glochidion | ellipticum | 2.1397 | 8.1872 | 0.0005 | 22 | 17 |
| Phyllanthaceae | Glochidion | eriocarpum | 6.7703 | 1.3602 | 0.0010 | 44 | 31 |
| Phyllanthaceae | Glochidion | lanceolarium | 4.5042 | 0.1109 | 0.0002 | 6 | 3 |
| Euphorbiaceae | Mallotus | barbatus | 18.1800 | 2.9402 | 0.0003 | 14 | 8 |
| Euphorbiaceae | Mallotus | conspurcatus | 11.9856 | 1.8932 | 0.0003 | 16 | 11 |
| Euphorbiaceae | Mallotus | philippensis | 4.7272 | 0.2811 | 0.0001 | 4 | 3 |
| Euphorbiaceae | Mallotus | repandus | 4.1010 | 0.8797 | 0.0002 | 11 | 8 |
| Euphorbiaceae | Mallotus | yunnanensis | 6.6121 | 1.8236 | 0.0116 | 723 | 73 |
| Phyllanthaceae | Phyllanthodendron | breynioides | 15.5937 | 0.9962 | 0.0001 | 7 | 3 |
| Phyllanthaceae | Phyllanthus | emblica | 8.1405 | 0.0000 | 0.0000 | 1 | 1 |
| Phyllanthaceae | Phyllanthus | reticulatus | 18.6518 | 3.1836 | 0.0004 | 18 | 5 |
| Euphorbiaceae | Sapium | cochinchinensis | 29.6479 | 0.2504 | 0.0000 | 2 | 2 |
| Euphorbiaceae | Sapium | rotundifolia | 9.3766 | 3.5024 | 0.0018 | 84 | 47 |
| Euphorbiaceae | Strophioblachia | fimbricalyx | 3.2134 | 2.8171 | 0.0006 | 21 | 8 |
| Euphorbiaceae | Trigonostemon | bonianus | 11.4924 | 14.1410 | 0.0002 | 8 | 7 |
| Euphorbiaceae | - | sp | 4.1441 | 0.6807 | 0.0002 | 14 | 8 |
| Rosaceae | Laurocerasus | zippeliana | 1.4110 | 1.1041 | 0.0035 | 211 | 95 |
| Rosaceae | Pygeum | topengii | 3.2315 | 1.9965 | 0.0005 | 23 | 17 |
| Fabaceae | Adenanthera | microsperma | 1.3727 | 2.7576 | 0.0016 | 82 | 55 |
| Fabaceae | Albizia | odoratissima | 1.2674 | 1.6587 | 0.0005 | 24 | 20 |
| Mimosaceae | Cylindrokelupha | robinsonii | 6.9191 | 2.8315 | 0.0089 | 439 | 105 |
| Fabaceae | Acrocarpus | fraxinifolius | 6.1384 | 0.0000 | 0.0000 | 1 | 1 |
| Fabaceae | Gleditsia | fera | 0.8312 | 1.2611 | 0.0012 | 58 | 36 |
| Fabaceae | Gleditsia | australis | 3.7466 | 0.6928 | 0.0003 | 9 | 6 |
| Fabaceae | Saraca | dives | 11.2354 | 3.7537 | 0.0041 | 168 | 41 |
| Fabaceae | Zenia | insignis | 3.1396 | 0.0000 | 0.0000 | 1 | 1 |
| Fabaceae | Campylotropis | bonii | 14.8007 | 0.0000 | 0.0000 | 2 | 1 |
| Fabaceae | Erythrina | stricta | 1.8081 | 1.5567 | 0.0045 | 254 | 132 |
| Fagaceae | Lithocarpus | areca | 27.8181 | 1.0906 | 0.0004 | 9 | 3 |
| Cannabaceae | Aphananthe | aspera | 2.8524 | 4.8486 | 0.0002 | 10 | 9 |
| Cannabaceae | Celtis | biondii | 5.6941 | 3.6628 | 0.0016 | 77 | 39 |
| Cannabaceae | Celtis | sinensis | 2.2313 | 0.1924 | 0.0002 | 9 | 7 |
| Cannabaceae | Celtis | timorensis | 1.0203 | 1.9088 | 0.0099 | 879 | 235 |
| Ulmaceae | Ulmus | lanceifolia | 4.5180 | 1.4114 | 0.0005 | 24 | 10 |
| Moraceae | Artocarpus | hypargyreus | 9.4386 | 0.0000 | 0.0000 | 1 | 1 |
| Moraceae | Artocarpus | tonkinensis | 0.5413 | 1.1298 | 0.0005 | 24 | 20 |
| Moraceae | Broussonetia | papyrifera | 3.4315 | 0.5463 | 0.0001 | 4 | 4 |
| Moraceae | Ficus | auriculata | 9.9516 | 1.8701 | 0.0006 | 25 | 15 |
| Moraceae | Ficus | trivia | 25.8864 | 1.5262 | 0.0002 | 16 | 10 |
| Moraceae | Ficus | cyrtophylla | 6.1959 | 0.4720 | 0.0005 | 16 | 10 |
| Moraceae | Ficus | erecta | 5.8253 | 0.0000 | 0.0000 | 1 | 1 |
| Moraceae | Ficus | glaberrima | 1.2356 | 4.0438 | 0.0024 | 108 | 52 |
| Moraceae | Ficus | hispida | 11.6521 | 2.1282 | 0.0581 | 2603 | 88 |
| Moraceae | Ficus | microcarpa | 2.7327 | 2.0681 | 0.0011 | 61 | 32 |
| Moraceae | Ficus | oligodon | 4.9979 | 2.4026 | 0.0063 | 277 | 56 |
| Moraceae | Ficus | orthoneura | 4.0602 | 1.7475 | 0.0003 | 16 | 12 |
| Moraceae | Ficus | tinctoria | 2.3093 | 1.6849 | 0.0029 | 132 | 57 |
| Moraceae | Ficus | virens | 1.6212 | 2.4634 | 0.0016 | 84 | 47 |
| Moraceae | Cudrania | tricuspidata | 2.0825 | 0.6992 | 0.0002 | 7 | 5 |
| Moraceae | Morus | macroura | 5.3547 | 0.0000 | 0.0000 | 1 | 1 |
| Moraceae | Streblus | tonkinensis | 1.2871 | 0.9345 | 0.0229 | 1016 | 68 |
| Urticaceae | Boehmeria | nivea | 1.0366 | 2.0099 | 0.0062 | 351 | 91 |
| Urticaceae | Oreocnide | kwangsiensis | 3.2924 | 1.0319 | 0.0004 | 18 | 7 |
| Aquifoliaceae | Ilex | memecylifolia | 2.9661 | 0.0000 | 0.0000 | 1 | 1 |
| Celastraceae | Euonymus | dielsianus | 6.4955 | 4.3918 | 0.0057 | 286 | 70 |
| Celastraceae | Glyptopetalum | rhytidophyllum | 3.4693 | 0.0000 | 0.0000 | 1 | 1 |
| Celastraceae | Maytenus | confertiflora | 1.9793 | 1.7703 | 0.0002 | 11 | 5 |
| Icacinaceae | Apodytes | dimidiata | 4.2729 | 2.0617 | 0.0064 | 375 | 97 |
| Stemonuraceae | Gomphandra | tetrandra | 11.7720 | 10.1052 | 0.0016 | 66 | 16 |
| Opiliaceae | Champereia | manillana | 0.2476 | 1.0775 | 0.0102 | 1315 | 285 |
| Rhamnaceae | Rhamnus | coriophylla | 21.7867 | 1.2658 | 0.0003 | 24 | 10 |
| Rhamnaceae | Ziziphus | incurva | 2.2219 | 1.1406 | 0.0019 | 88 | 53 |
| Vitaceae | Leea | indica | 5.3925 | 1.1149 | 0.0023 | 97 | 38 |
| Rutaceae | Citrus | maxima | 7.5878 | 0.0000 | 0.0001 | 2 | 1 |
| Rutaceae | Clausena | anisum-olens | 2.5844 | 8.1492 | 0.0030 | 175 | 76 |
| Rutaceae | Clausena | dunniana | 13.7348 | 3.2465 | 0.0079 | 485 | 68 |
| Rutaceae | Glycosmis | esquirolii | 5.4778 | 0.5624 | 0.0002 | 8 | 3 |
| Rutaceae | Micromelum | integerrimum | 5.1666 | 1.1699 | 0.0001 | 3 | 3 |
| Rutaceae | Evodia | glabrifolium | 6.4009 | 0.6022 | 0.0002 | 8 | 7 |
| Simaroubaceae | Picrasma | quassioides | 10.8477 | 0.0000 | 0.0000 | 1 | 1 |
| Burseraceae | Garuga | forrestii | 1.6781 | 1.4372 | 0.0002 | 11 | 10 |
| Burseraceae | Garuga | pinnata | 0.7895 | 1.2887 | 0.0009 | 45 | 35 |
| Meliaceae | Amoora | lawii | 0.2009 | 0.5850 | 0.0010 | 50 | 39 |
| Meliaceae | Aglaia | odorata | 0.9734 | 1.8666 | 0.0085 | 740 | 193 |
| Meliaceae | Aphanamixis | polystachya | 2.0877 | 0.9764 | 0.0003 | 12 | 11 |
| Meliaceae | Chukrasia | tabularis | 0.5828 | 1.6676 | 0.0008 | 37 | 36 |
| Meliaceae | Cipadessa | cinerascens | 1.8393 | 1.7090 | 0.0060 | 292 | 90 |
| Meliaceae | Dysoxylum | mollissimum | 4.0752 | 1.3528 | 0.0001 | 2 | 2 |
| Meliaceae | Toona | sinensis | 11.7777 | 0.0000 | 0.0000 | 1 | 1 |
| Meliaceae | Walsura | robusta | 0.6196 | 1.0994 | 0.0191 | 1478 | 195 |
| Meliaceae | - | sp | 6.7189 | 1.2770 | 0.0002 | 10 | 4 |
| Sapindaceae | Allophylus | caudatus | 1.2007 | 1.3291 | 0.0002 | 13 | 11 |
| Sapindaceae | Amesiodendron | chinense | 1.8686 | 0.7868 | 0.0018 | 73 | 24 |
| Sapindaceae | Boniodendron | minus | 9.1871 | 3.9048 | 0.0138 | 898 | 121 |
| Sapindaceae | Delavaya | toxocarpa | 2.4446 | 0.2571 | 0.0027 | 152 | 13 |
| Sapindaceae | Lepisanthes | cauliflora | 10.2858 | 3.3060 | 0.0024 | 98 | 36 |
| Sapindaceae | Lepisanthes | senegalensis | 0.5753 | 0.3596 | 0.0015 | 70 | 22 |
| Sapindaceae | Acer | tonkinense | 7.7082 | 0.6685 | 0.0017 | 71 | 17 |
| Sabiaceae | Meliosma | thorelii | 5.4164 | 0.2198 | 0.0001 | 2 | 2 |
| Anacardiaceae | Choerospondias | axillaris | 0.8667 | 1.6339 | 0.0007 | 29 | 27 |
| Anacardiaceae | Pistacia | chinensis | 4.0888 | 0.7604 | 0.0008 | 32 | 23 |
| Anacardiaceae | Pistacia | weinmanniifolia | 20.6699 | 4.1688 | 0.0068 | 419 | 42 |
| Anacardiaceae | Pistacia | cucphuongensis | 13.3562 | 2.1598 | 0.0001 | 2 | 2 |
| Anacardiaceae | Spondias | lakonensis | 0.7925 | 0.5068 | 0.0003 | 13 | 12 |
| Anacardiaceae | Toxicodendron | succedaneum | 3.4033 | 3.6991 | 0.0017 | 83 | 52 |
| Cornaceae | Alangium | chinense | 21.6297 | 16.4759 | 0.0004 | 18 | 10 |
| Cornaceae | Alangium | kurzii | 8.9845 | 1.6805 | 0.0009 | 45 | 24 |
| Araliaceae | Schefflera | leucanthum | 15.3874 | 0.9278 | 0.0008 | 42 | 8 |
| Araliaceae | Schefflera | locianum | 35.9328 | 0.0000 | 0.0000 | 1 | 1 |
| Araliaceae | Trevesia | palmata | 9.8484 | 1.3604 | 0.0000 | 2 | 2 |
| Ebenaceae | Diospyros | xiangguiensis | 1.5354 | 8.7952 | 0.0005 | 22 | 12 |
| Ebenaceae | Diospyros | eriantha | 2.0623 | 2.0652 | 0.0086 | 641 | 164 |
| Ebenaceae | Diospyros | saxatilis | 11.4827 | 3.1469 | 0.0011 | 64 | 33 |
| Ebenaceae | Diospyros | siderophylla | 4.5385 | 1.2474 | 0.0250 | 1780 | 153 |
| Sapotaceae | Chrysophyllum | lanceolata | 11.3745 | 0.0000 | 0.0001 | 2 | 1 |
| Sapotaceae | Madhuca | pasquieri | 1.0481 | 1.2840 | 0.0007 | 35 | 21 |
| Sapotaceae | Sinosideroxylon | pedunculatum | 11.8062 | 0.8704 | 0.0004 | 15 | 5 |
| Sapotaceae | Sinosideroxylon | pedunculatum | 16.8656 | 2.1904 | 0.0092 | 607 | 55 |
| Primulaceae | Ardisia | pseudocrispa | 27.9405 | 0.0000 | 0.0000 | 1 | 1 |
| Primulaceae | Ardisia | thyrsiflora | 7.2239 | 1.7877 | 0.0176 | 860 | 73 |
| Primulaceae | Maesa | balansae | 1.4668 | 1.4208 | 0.0056 | 325 | 85 |
| Primulaceae | Maesa | perlarius | 3.9635 | 1.4303 | 0.0032 | 130 | 50 |
| Primulaceae | Myrsine | seguinii | 5.9847 | 0.0032 | 0.0000 | 2 | 2 |
| Oleaceae | Chionanthus | guangxiensis | 23.2232 | 0.6822 | 0.0033 | 225 | 20 |
| Oleaceae | Chionanthus | ramiflorus | 1.9910 | 2.2328 | 0.0025 | 135 | 60 |
| Apocynaceae | Alstonia | scholaris | 3.4033 | 0.1865 | 0.0000 | 2 | 2 |
| Apocynaceae | Wrightia | coccinea | 0.6892 | 1.0941 | 0.0023 | 139 | 78 |
| Rubiaceae | Adina | pilulifera | 6.3763 | 1.3811 | 0.0001 | 4 | 4 |
| Rubiaceae | Aidia | cochinchinensis | 3.1568 | 1.3604 | 0.0004 | 22 | 6 |
| Rubiaceae | Canthium | dicocca | 6.8278 | 1.9429 | 0.0139 | 855 | 106 |
| Rubiaceae | Canthium | simile | 12.4970 | 4.2530 | 0.0031 | 186 | 49 |
| Rubiaceae | Catunaregam | spinosa | 1.7888 | 1.4582 | 0.0039 | 214 | 74 |
| Boraginaceae | Ehretia | tsangii | 1.5110 | 1.6638 | 0.0031 | 156 | 77 |
| Boraginaceae | Ehretia | acuminata | 9.4977 | 0.1606 | 0.0001 | 4 | 2 |
| Rubiaceae | Hymenodictyon | flaccidum | 5.4563 | 2.8533 | 0.0012 | 51 | 31 |
| Rubiaceae | Ixora | henryi | 14.2587 | 3.2446 | 0.0052 | 201 | 38 |
| Rubiaceae | Sinoadina | racemosa | 2.4946 | 1.5594 | 0.0010 | 50 | 33 |
| Rubiaceae | Tarenna | attenuata | 13.4637 | 4.5385 | 0.0021 | 128 | 37 |
| Rubiaceae | Wendlandia | oligantha | 4.1150 | 0.0800 | 0.0002 | 9 | 6 |
| Rubiaceae | Wendlandia | uvariifolia | 2.6551 | 1.0872 | 0.0013 | 56 | 31 |
| Adoxaceae | Viburnum | triplinerve | 17.5436 | 2.3779 | 0.0027 | 180 | 23 |
| Boraginaceae | Cordia | furcans | 3.5511 | 0.0000 | 0.0000 | 1 | 1 |
| Bignoniaceae | Dolichandrone | stipulate | 3.4735 | 1.1844 | 0.0039 | 212 | 26 |
| Bignoniaceae | Oroxylum | indicum | 2.0721 | 0.8372 | 0.0015 | 67 | 44 |
| Bignoniaceae | Radermachera | frondosa | 0.7636 | 1.8518 | 0.0007 | 33 | 25 |
| Bignoniaceae | Radermachera | sinica | 1.4756 | 3.1189 | 0.0013 | 59 | 38 |
| Bignoniaceae | Stereospermum | colais | 0.8353 | 1.6160 | 0.0025 | 127 | 74 |
| Lamiaceae | Callicarpa | longifolia | 1.6441 | 5.2615 | 0.0002 | 11 | 5 |
| Lamiaceae | Clerodendrum | wallichii | 1.7434 | 1.1507 | 0.0160 | 1041 | 184 |
| Lamiaceae | Premna | confinis | 19.3949 | 1.3329 | 0.0003 | 19 | 10 |
| Lamiaceae | Premna | fulva | 4.3661 | 0.0670 | 0.0002 | 4 | 2 |
| Lamiaceae | Vitex | kwangsiensis | 0.6866 | 1.6586 | 0.0305 | 4392 | 313 |
| Lamiaceae | Vitex | quinata | 6.6420 | 2.4395 | 0.0022 | 123 | 59 |
| Asparagaceae | Dracaena | cochinchinensis | 5.3429 | 4.0552 | 0.0119 | 646 | 111 |
| Arecaceae | Caryota | obtusa | 4.1325 | 2.5319 | 0.0013 | 51 | 42 |
| Data Situation | Item | Coefficient | p Value |
|---|---|---|---|
| Abundance data | Abundance | −0.0002 | p < 0.001 |
| Occupancy | 0.0090 | p < 0.001 | |
| Intercept | −5.8537 | p < 0.001 | |
| Presence–absence data | Abundance | 0.0002 | p < 0.001 |
| Occupancy | 0.0068 | p < 0.001 | |
| Intercept | −6.1157 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, B.; Li, J.; Lu, F.; Tao, W.; Li, D.; Guo, Y.; Tang, N.; Li, X.; Xiang, W. Functional Traits Affect the Contribution of Individual Species to Beta Diversity in the Tropical Karst Seasonal Rainforest of South China. Forests 2024, 15, 1125. https://doi.org/10.3390/f15071125
Wang Y, Wang B, Li J, Lu F, Tao W, Li D, Guo Y, Tang N, Li X, Xiang W. Functional Traits Affect the Contribution of Individual Species to Beta Diversity in the Tropical Karst Seasonal Rainforest of South China. Forests. 2024; 15(7):1125. https://doi.org/10.3390/f15071125
Chicago/Turabian StyleWang, Yanping, Bing Wang, Jianxing Li, Fang Lu, Wanglan Tao, Dongxing Li, Yili Guo, Nianwu Tang, Xiankun Li, and Wusheng Xiang. 2024. "Functional Traits Affect the Contribution of Individual Species to Beta Diversity in the Tropical Karst Seasonal Rainforest of South China" Forests 15, no. 7: 1125. https://doi.org/10.3390/f15071125








