Comparative Transcriptome Analysis of Different Mulberry Varieties to Reveal Candidate Genes and Small Secreted Peptides Involved in the Sclerotiniose Response
Abstract
1. Introduction
2. Materials and Methods
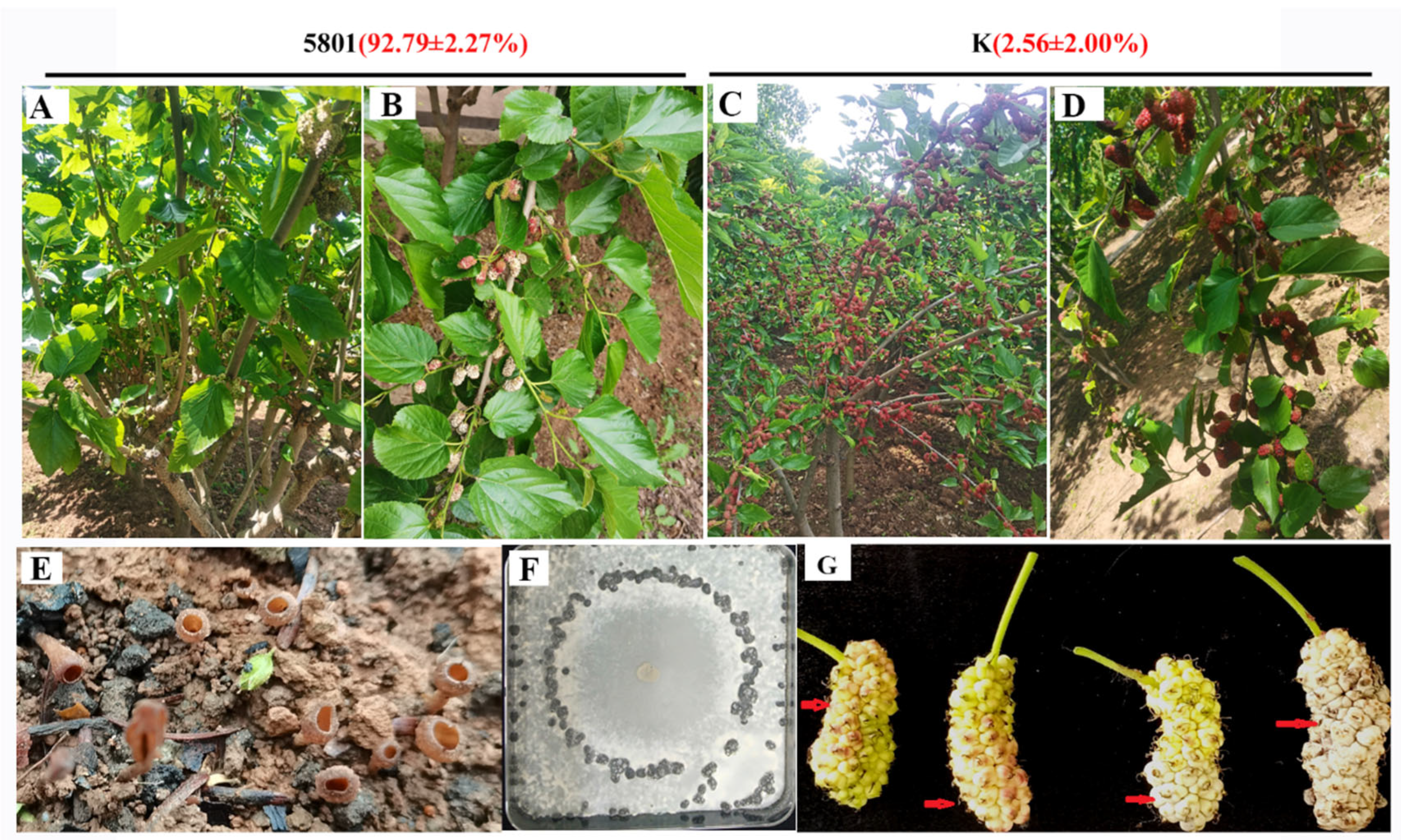
2.1. Plant Materials
2.2. Data Collection and Genome-Wide Annotation of SSPs
2.3. RNA-seq Analysis
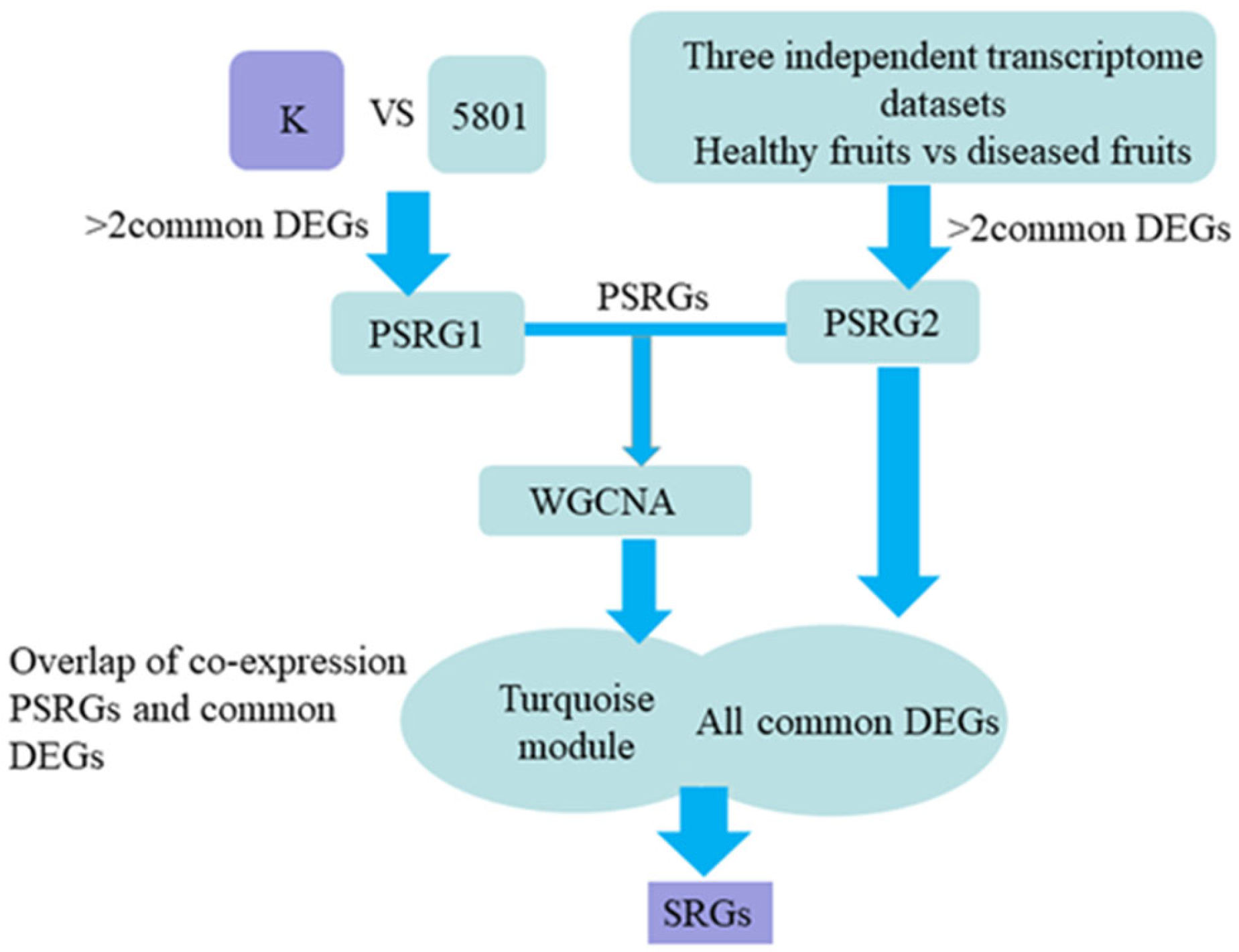
2.4. Workflow for Screening Sclerotiniose-Response Genes (SRGs) in Mulberry
2.5. Isolation of RNA and cDNA Synthesis
2.6. RT-qPCR Analysis
2.7. Obtaining Transient Transgenic Nicotiana Benthamiana
2.8. Obtaining VIGS Transgenic Mulberry
2.9. Estimation of Plant Resistance to C. shiraiana Infection
3. Results
3.1. Genome-Wide Annotation of SSPs in Mulberry
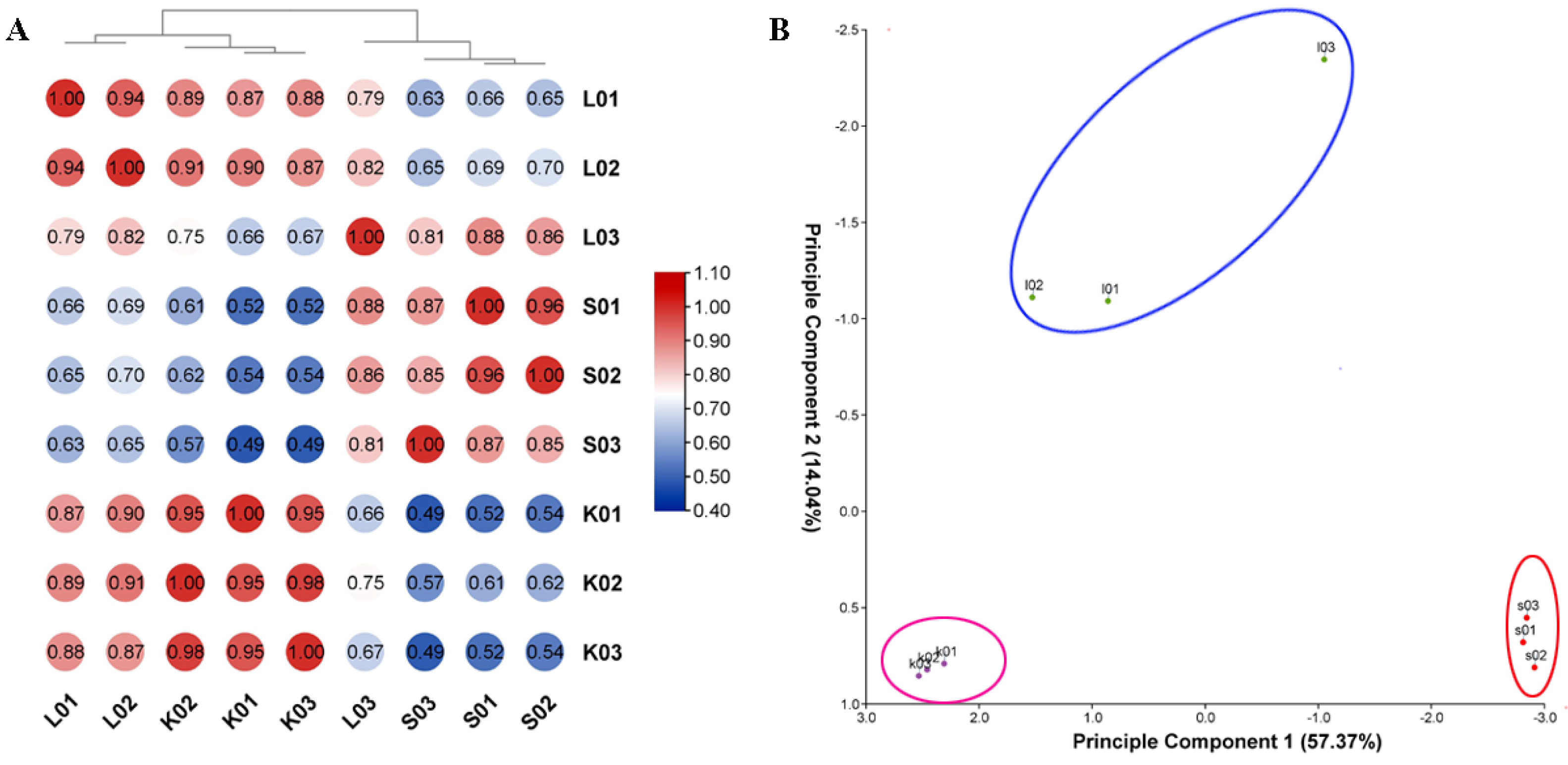
3.2. RNA-seq of Mulberry Varieties Susceptible and Resistant to Mulberry Sclerotiniose
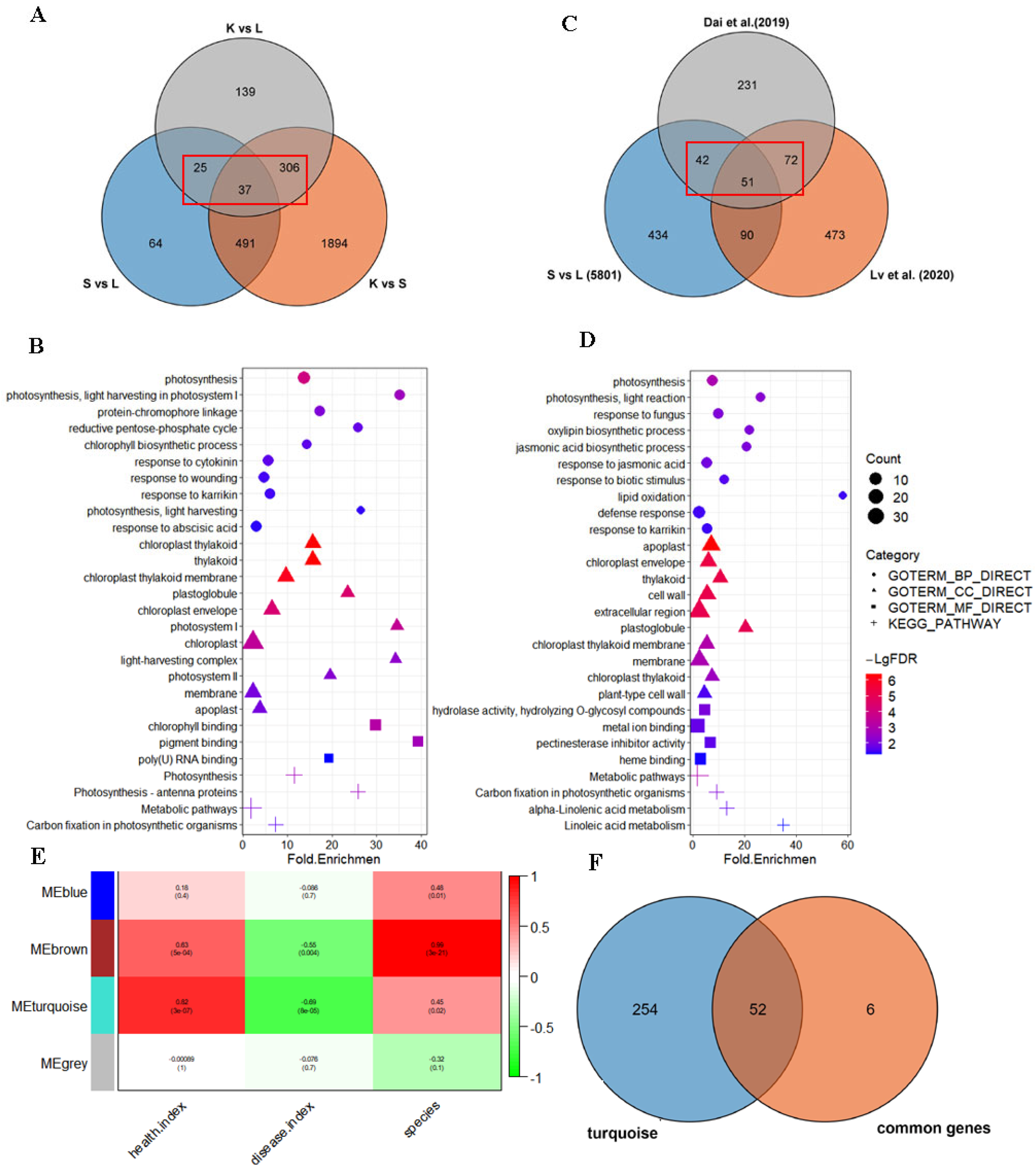
3.3. Comparative Transcriptome Analysis Revealed the Candidate Genes Involved in the Response to Mulberry Sclerotiniose
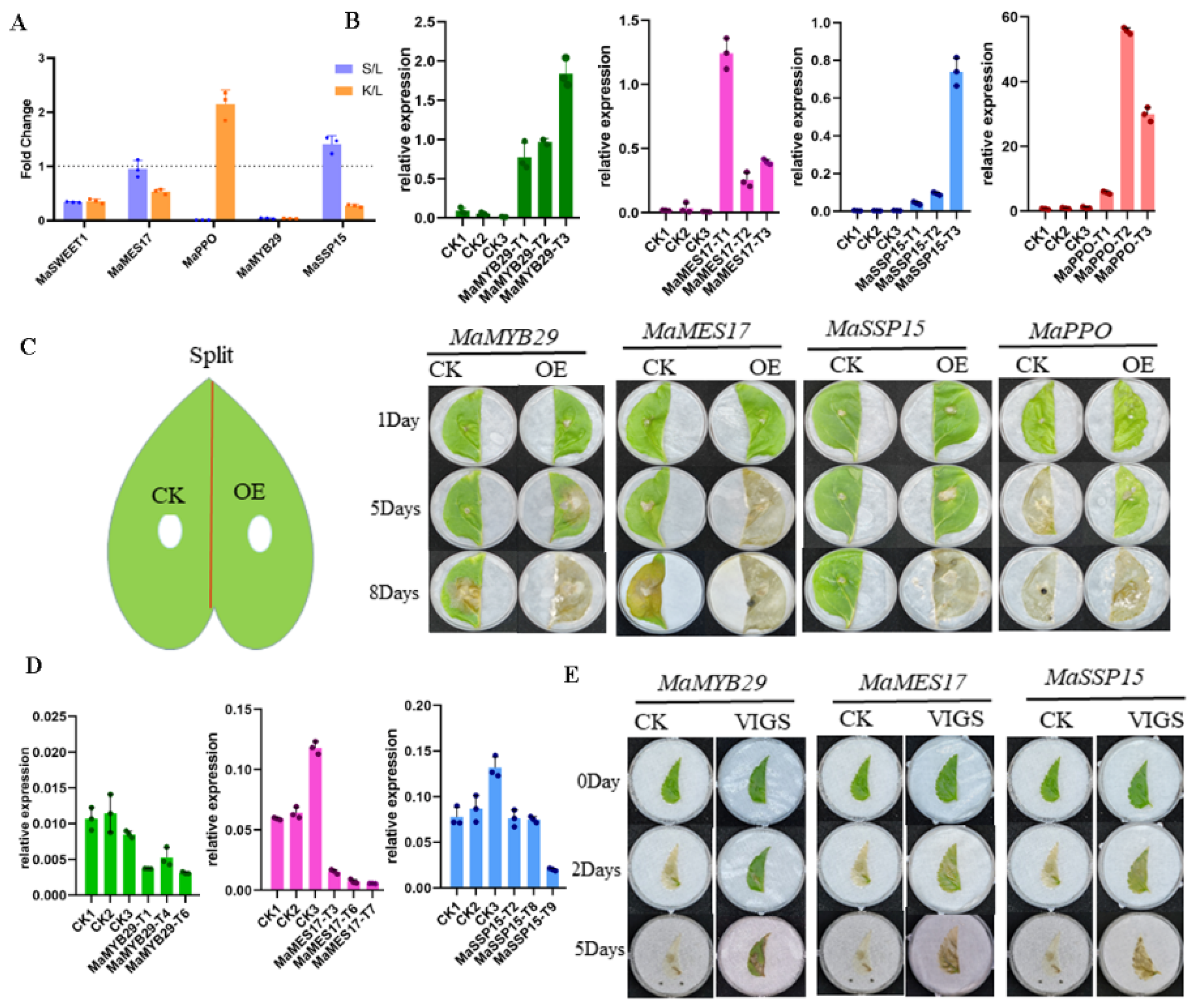
3.4. Functional Characterization of SRGs in Mulberry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vijayan, K.; Tikader, A.; Weiguo, Z.; Nair, C.V.; Ercisli, S.; Tsou, C.-H. Morus. In Wild Crop Relatives: Genomic and Breeding Resources; Springer: Berlin/Heidelberg, Germany, 2011; pp. 75–95. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.; Linhardt, R.J.; Liao, S.; Wu, H.; Zou, Y. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Wang, D.; Dong, Z.; Zhang, Y.; Guo, K.; Guo, P.; Zhao, P.; Xia, Q. Proteomics Provides Insight into the Interaction between Mulberry and Silkworm. J. Proteome Res. 2017, 16, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Luo, R.; Dai, X.; Liu, H.; Yu, G.; Han, S.; Lu, X.; Su, C.; Chen, Q.; Song, Q.; et al. Chromosome-level reference genome and population genomic analysis provide insight into the evolution and improvement of domesticated mulberry (Morus alba L.). Mol. Plant 2020, 13, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Zhang, C.; Qi, X.; Zhao, S.; Tao, Y.; Yang, G.; Lee, T.; Wang, X.; Cai, Q.; Li, D. Draft genome sequence of the mulberry tree Morus notabilis. Nat. Commun. 2013, 4, 2445. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Hao, L.; Ma, B.; He, Z.; Luo, Y.; Xin, Y.; He, N. Ciboria carunculoides Suppresses Mulberry Immune Responses Through Regulation of Salicylic Acid Signaling. Front. Plant Sci. 2021, 12, 658590. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Gao, H.; Zheng, Z.; Zhao, X.; Zhang, M.; Jiao, F.; Su, C.; Qian, Y. Integrated Transcriptomic and Un-Targeted Metabolomics Analysis Reveals Mulberry Fruit (Morus atropurpurea) in Response to Sclerotiniose Pathogen Ciboria shiraiana Infection. Int. J. Mol. Sci. 2020, 21, 1789. [Google Scholar] [CrossRef] [PubMed]
- Lü, R.; Zhao, A.; Yu, J.; Wang, C.; Liu, C.; Cai, Y.; Yu, M. Biological and epidemiological characteristics of the pathogen of hypertrophy sorosis scleroteniosis, Ciboria shiraiana. Wei Sheng Wu Xue Bao= Acta Microbiol. Sin. 2017, 57, 388–398. [Google Scholar]
- Lv, Z.; He, Z.; Hao, L.; Kang, X.; Ma, B.; Li, H.; Luo, Y.; Yuan, J.; He, N. Genome Sequencing Analysis of Scleromitrula shiraiana, a Causal Agent of Mulberry Sclerotial Disease with Narrow Host Range. Front. Microbiol. 2020, 11, 603927. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Jin, X.; Shi, X.; Xue, Y.; Jiang, J.; Yuan, C.; Du, Y.; Liu, X.; Xie, R.; Liu, X.; et al. Transcriptomic Analysis Reveals Candidate Genes Responsive to Sclerotinia scleroterum and Cloning of the Ss-Inducible Chitinase Genes in Morus laevigata. Int. J. Mol. Sci. 2020, 21, 8358. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W. The Cup Fungus, Ciboria carunculoides, Pathogenic on Mulberry Fruits. Mycologia 1945, 37, 476–491. [Google Scholar]
- Hong, S.K.; Wan, G.K.; Sung, G.B.; Nam, S.H. Identification and Distribution of Two Fungal Species Causing Sclerotial Disease on Mulberry Fruits in Korea. Mycobiology 2007, 35, 87–90. [Google Scholar] [CrossRef][Green Version]
- Zhu, P.; Kou, M.; Liu, C.; Zhang, S.; Lu, R.; Xia, Z.; Yu, M.; Zhao, A. Genome Sequencing of Ciboria shiraiana Provides Insights into the Pathogenic Mechanisms of Hypertrophy Sorosis scleroteniosis. Mol. Plant Microbe Interact. 2021, 34, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Bolton, M.D.; Thomma, B.P.H.J.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2005, 7, 1–16. [Google Scholar] [CrossRef]
- Lü, R.; Zhao, A.; Jin, X.; Du, Y.; Wu, W.; Wang, X.; Yu, M. A primary experiment on the control of mulberry fruit sclerotiniosis using herbicide glyphosate. Sci. Seric. 2011, 37, 907–913. [Google Scholar]
- Lu, Z.; Kang, X.; Xiang, Z.; He, N. Laccase Gene Sh-lac Is Involved in the Growth and Melanin Biosynthesis of Scleromitrula shiraiana. Phytopathology 2017, 107, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Feng, B.; He, P.; Shan, L. From Chaos to Harmony: Responses and Signaling Upon Microbial Pattern Recognition. Annu. Rev. Phytopathol. 2017, 55, 109. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Wang, Z.; Li, Z.; Luo, G.; Wang, Y.; Tang, C. Transcriptomic and proteomic analyses of mulberry (Morus atropurpurea) fruit response to Ciboria carunculoides. J. Proteom. 2019, 193, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Jourquin, J.; Fukaki, H.; Beeckman, T. Peptide-Receptor Signaling Controls Lateral Root Development. Plant Physiol. 2020, 182, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Hardtke, C.S. Peptide Signaling Pathways in Vascular Differentiation. Plant Physiol. 2020, 182, 1636–1644. [Google Scholar] [CrossRef]
- Takahashi, F.; Hanada, K.; Kondo, T.; Shinozaki, K. Hormone-like peptides and small coding genes in plant stress signaling and development. Curr. Opin. Plant Biol. 2019, 51, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.L.; de Souza, C.M.; de Oliveira, K.B.S.; Dias, S.C.; Franco, O.L. The role of antimicrobial peptides in plant immunity. J. Exp. Bot. 2018, 69, 4997–5011. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lee, C.Y.; Cheng, K.T.; Chang, W.H.; Huang, R.N.; Nam, H.G.; Chen, Y.R. Quantitative peptidomics study reveals that a wound-induced peptide from PR-1 regulates immune signaling in tomato. Plant Cell 2014, 26, 4135–4148. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; van der Hoorn, R.A.L. Plant Biology: Distinct New Players in Processing Peptide Hormones during Abscission. Curr. Biol. 2020, 30, R715–R717. [Google Scholar] [CrossRef] [PubMed]
- Motomitsu, A.; Sawa, S.; Ishida, T. Plant peptide hormone signalling. Essays Biochem. 2015, 58, 115–131. [Google Scholar] [PubMed]
- Boschiero, C.; Dai, X.; Lundquist, P.K.; Roy, S.; Christian de Bang, T.; Zhang, S.; Zhuang, Z.; Torres-Jerez, I.; Udvardi, M.K.; Scheible, W.R.; et al. MtSSPdb: The Medicago truncatula Small Secreted Peptide Database. Plant Physiol. 2020, 183, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Boschiero, C.; Lundquist, P.K.; Roy, S.; Dai, X.; Zhao, P.X.; Scheible, W.R. Identification and Functional Investigation of Genome-Encoded, Small, Secreted Peptides in Plants. Curr. Protoc. Plant Biol. 2019, 4, e20098. [Google Scholar] [CrossRef] [PubMed]
- Langdon, B.W. Performance of genetic programming optimised Bowtie2 on genome comparison and analytic testing (GCAT) benchmarks. BioData Min. 2015, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Nat. Preced. 2010, 5. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Reddy, R.A.; Ponnuvel, K.M.; Rohela, G.K.; Shabnam, A.A.; Ghosh, M.K.; Mishra, R.K. Selection of suitable reference genes for quantitative real-time PCR gene expression analysis in Mulberry (Morus alba L.) under different abiotic stresses. Mol. Biol. Rep. 2019, 46, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Zeng, Y.; Shen, W.; Tuo, D.; Li, X.; Zhou, P. Nimble Cloning: A Simple, Versatile, and Efficient System for Standardized Molecular Cloning. Front. Bioeng. Biotechnol. 2019, 7, 460. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Liang, Y.; Lee, M.Y.; Pidatala, V.R.; Mortimer, J.C.; Scheller, H.V. Agrobacterium-mediated transient transfor-mation of sorghum leaves for accelerating functional genomics and genome editing studies. BMC research notes 2020, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, L.; Dominic, K.; Wang, T.; Fan, T.; Hu, F.; Wang, Y.; Zhang, L.; Li, L.; Zhao, W. Mulberry (Morus alba) MmSK gene enhances tolerance to drought stress in transgenic mulberry. Plant Physiol. Biochem. 2018, 132, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nelson, R.S.; Sherwood, J.L. Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection. BioTechniques 1994, 16, 664–668, 670. [Google Scholar] [PubMed]
- Zhu, P.; Zhang, S.; Li, R.; Liu, C.; Fan, W.; Hu, T.; Zhao, A. Host-Induced Gene Silencing of a G Protein α Subunit Gene CsGpa1 Involved in Pathogen Appressoria Formation and Virulence Improves Tobacco Resistance to Ciboria shiraiana. J. Fungi 2021, 7, 1053. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Huang, S.; Feng, Y.; Fu, R.; Tang, F.; Zheng, L.; Li, P.; Chao, N.; Liu, L. SWEET transporters and their potential roles in response to abiotic and biotic stresses in mulberry. Beverage Plant Res. 2023, 3, 1–13. [Google Scholar] [CrossRef]
- Fletcher, J.C. Recent Advances in Arabidopsis CLE Peptide Signaling. Trends Plant Sci. 2020, 25, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, D.; Xia, Y.; Li, Z.; Niu, N.; Ma, S.; Wang, J.; Song, Y.; Zhang, G. Identification and Functional Analysis of the CLAVATA3/EMBRYO SURROUNDING REGION (CLE) Gene Family in Wheat. Int. J. Mol. Sci. 2019, 20, 4319. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Xu, H.; Ye, S.; Wang, S.; Li, L.; Zhang, S.; Wang, X. AtGASA6 Serves as an Integrator of Gibberellin-, Abscisic Acid- and Glucose-Signaling during Seed Germination in Arabidopsis. Plant Physiol. 2015, 169, 15–00858. [Google Scholar] [CrossRef] [PubMed]
- Moyano-Canete, E.; Bellido, M.L.; Garcia-Caparros, N.; Medina-Puche, L.; Amil-Ruiz, F.; Gonzalez-Reyes, J.A.; Caballero, J.L.; Munoz-Blanco, J.; Blanco-Portales, R. FaGAST2, a strawberry ripening-related gene, acts together with FaGAST1 to determine cell size of the fruit receptacle. Plant Cell Physiol. 2013, 54, 218–236. [Google Scholar] [CrossRef]
- de la Fuente, J.I.; Amaya, I.; Castillejo, C.; Sanchez-Sevilla, J.F.; Quesada, M.A.; Botella, M.A.; Valpuesta, V. The strawberry gene FaGAST affects plant growth through inhibition of cell elongation. J. Exp. Bot. 2006, 57, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 2021, 181, 112588. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]

| SSP Family Name | Description | Class | Mode of Action | Number of Genes in M. alba |
|---|---|---|---|---|
| 2SA | 2S Albumin | Cys-rich | Antimicrobial | 0 |
| 401p11 | 401p11 | sORF | Signal | 0 |
| 908p11 | 908p11 | sORF | Signal | 0 |
| ARACIN | ARACIN | Cys-rich | Antimicrobial | 10 |
| BBPI | Bowman–Birk Peptidase Inhibitor | Cys-rich | Peptidase inhibitor | 0 |
| BRAZZEIN | BRAZZEIN | Cys-rich | Antimicrobial | 0 |
| CAPE | CAP-derived Peptide | Functional Precursor | Signal | 42 |
| CEP | C-terminally Encoded Peptide | PTM | Signal | 31 |
| CIF | Casparian Strip Integrity Factor | PTM | Signal | 0 |
| CLE | Clavata/Embryo Surrounding Region | PTM | Signal | 137 |
| CTLA | Cytotoxic T-lymphocyte antigen-2 alpha | non-Cys, non-PTM | Peptidase inhibitor | 0 |
| CYC | CYCLOTIDE (CYC) | Cys-rich | Antimicrobial; oeptidase inhibitor | 1 |
| EAL | Egg Appartus-Like (EAL) | Cys-rich | Signal | 0 |
| EC | Egg Cell 1-Like | Cys-rich | Signal | 19 |
| ECA | Egg Cell Appartus | 50 | ||
| ENOD40 | Early Nodulin 40 | sORF | Signal | 0 |
| EPFL | Epidermal Patterning Factor-Like | Cys-rich | Signal | 39 |
| ES | Embryo Sac (ES) | Cys-rich | Signal; antimicrobial | 2 |
| ESF | Embryo Surrounding Factor (ESF) | Cys-rich | Signal | 5 |
| GASA | Gibberellic Acid Stimulated in Arabidopsis | Cys-rich | Signal | 68 |
| GLV | Golven/Root Growth Factor | PTM | Signal | 32 |
| GmPEP | Plant Elicitor Peptides | non-Cys, non-PTM | Signal | 2 |
| HAIRPININ | alfa-HAIRPININ (HAIRPININ) | Cys-rich | Antimicrobial; peptidase inhibitor | 3 |
| HEVEIN | HEVEIN | Cys-rich | Antimicrobial | 7 |
| HypSys | Hydroxyproline-Rich Systemin (HypSys) | PTM | Antimicrobial; Signal | 2 |
| Ib-AMP | Ib-AMP | Cys-rich | Antimicrobial | 2 |
| IDA | Inflorescence Deficient in Abscission | PTM | Signal | 43 |
| IMA | Iron Man (IMA) | 0 | ||
| Kaz | Kazal family inhibitors | Cys-rich | Peptidase inhibitor | 0 |
| KNOTTIN/LURE/PDF | KNOTTIN/LURE/Plant Defensin | Cys-rich | Antimicrobial; peptidase inhibitor | 31 |
| KOD | Kiss of Death (KOD) | sORF | Signal | 0 |
| Kunitz | Kunitz-P trypsin inhibitor | Cys-rich | Peptidase inhibitor | 0 |
| LAT52-POE | LAT52/Pollen Ole e 1 Allergen | Cys-rich | Signal | 14 |
| LCR | Low-molecular weight Cys-rich | Cys-rich | Unknown | 40 |
| Legin | Leginsulin | Cys-rich | Signal | 0 |
| LP | LEED..PEED | non-Cys, non-PTM | Unknown | 0 |
| MBP-1 | Antimicrobial Peptide MBP-1 (MBP-1) | Cys-rich | Antimicrobial | 0 |
| MEG | Maternally Expressed Gene | Cys-rich | Signal | 0 |
| MTI | Mustard Trypsin Inhibitor (MTI) | Cys-rich | Peptidase inhibitor | 0 |
| MtPEP | Plant Elicitor Peptides | non-Cys, non-PTM | Signal | 0 |
| MtSUBPEP | Subtilisin-embedded Plant Elicitor Peptide | Functional Precursor | Signal | 2 |
| N26 | Nodulin26 | Cys-rich | Signal | 0 |
| NCR-A | Nodule-specific Cysteine Rich Group A | Cys-rich | Unknown | 0 |
| NCR-B | Nodule-specific Cysteine Rich Group B | Cys-rich | Unknown | 0 |
| NodGRP | Nodule-specific Glycine-rich Protein | non-Cys, non-PTM | Unknown | 0 |
| nsLTP | Non-specific Lipid Transfer Protein | Cys-rich | Signal | 47 |
| OSIP108 | Oxidative Stress-Induced Peptide 108 (OSIP108) | sORF | Antimicrobial | 0 |
| PCP-A | Pollen Coat Protein Group A (PCP-A) | Cys-rich | Signal | 0 |
| PCP-B | Pollen Coat Protein Group B (PCP-B) | Cys-rich | Signal | 0 |
| PCY | Plantcyanin/Chemocyanin | Cys-rich | Signal | 0 |
| PDL | Plant Defensin-like | Cys-rich | Antimicrobial | 0 |
| PDP | PawS-derived Peptide (PDP) | Functional Precursor; cyclotide | Peptidase inhibitor | 17 |
| PhyCys | Phytocystatin | non-Cys, non-PTM | Peptidase inhibitor | 0 |
| PIP | PAMP-induced Secreted Peptide | PTM | Signal | 27 |
| PLS | Polaris (PLS) | sORF | Signal | 0 |
| PNP | Plant Natriuretic Peptide | non-Cys, non-PTM | Signal | 14 |
| PRP485 | Pro-rich Protein Group 485 (PRP485) | non-Cys, non-PTM | - | 0 |
| PRP669 | Pro-rich Protein Group 669 | non-Cys, non-PTM | Unknown | 0 |
| PSK | Phytosulfokine | PTM | Signal | 19 |
| PSY | Plant Peptide Containing Sulfated Tyrosine | PTM | Signal | 47 |
| RALF | Rapid Alkalinization Factor | Cys-rich | Signal | 106 |
| RC | Root Cap | Cys-rich | Signal | 0 |
| RTFL/DVL | Rotundifolia/Devil | sORF | Signal | 16 |
| SCR | S-locus Cysteine Rich (SCR) | Cys-rich | Signal | 0 |
| SCRL | S-Locus Cysteine Rich-Like | Cys-rich | Signal | 21 |
| STIG-GRI | Stigma1/GRI | Cys-rich | Signal | 46 |
| SubIn | Subtilisin inhibitor | non-Cys, non-PTM | Peptidase inhibitor | 0 |
| SYS | Systemin (SYS) | non-Cys, non-PTM | Signal | 2 |
| T2SPI | Potato type II proteinase inhibitor | Cys-rich | Peptidase inhibitor | 0 |
| TAX | Taximin | Cys-rich | Signal | 16 |
| THIONIN | THIONIN | Cys-rich | Antimicrobial | 13 |
| THL | Thionin-like | Cys-rich | Antimicrobial | 0 |
| TPD | Tapetum Determinant 1 | Cys-rich | Signal | 16 |
| TPDL | Tapetum Determinant 1-like | Cys-rich | Signal | 8 |
| Unkown | 91 | |||
| Total | 1088 |
| Dai et al. (2019) [20] | Lv et al. (2020) [10] | This Study | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Functional Annotation | Gene IDs | Modules | Homolog in Arabidopsis | Log2FC (S1/CK) | Log2FC (S2/CK) | Log2FC (S1/CK) | Log2FC (S2/CK) | Log2FC (S3/CK) | Log2FC (S/CK) | Log2FC (K/CK) |
| ABA | M.alba_G0017378 | Turquoise | AT4G38970 | −3.306 | −5.495 | 0.324 | −1.662 | −1.489 | −3.079 | 1.398 |
| ABA | M.alba_G0004617 | Turquoise | AT5G59320 | −1.814 | −8.467 | 0.561 | −2.507 | −1.365 | −4.499 | −0.515 |
| ABA | M.alba_G0006018 | Turquoise | AT4G37050 | −2.575 | −12.385 | 0.347 | −2.121 | −1.131 | −4.498 | −0.088 |
| JA | M.alba_G0015820 | Turquoise | AT3G45140 | −3.296 | −8.982 | 0.933 | −2.025 | −2.141 | −5.336 | 0.032 |
| JA | M.alba_G0014496 | Turquoise | AT1G55020 | −2.847 | −5.838 | 0.375 | −2.636 | −2.125 | −6.463 | 0.686 |
| JA | M.alba_G0017173 | Turquoise | AT5G42650 | −2.820 | −5.659 | 0.508 | −1.886 | −2.935 | −5.399 | 0.472 |
| JA-FUNGUS | M.alba_G0010603 | Turquoise | None | −2.469 | −9.319 | 0.220 | −1.745 | −2.432 | −5.001 | 1.042 |
| Photosynthesis | M.alba_G0003694 | Turquoise | AT2G39730 | −3.271 | −2.943 | 0.029 | −1.224 | −1.873 | −2.450 | 0.617 |
| Photosynthesis | M.alba_G0007619 | Turquoise | AT5G38410 | −2.911 | −3.327 | 0.179 | −1.514 | −1.668 | −2.233 | 0.846 |
| Photosynthesis | M.alba_G0004494 | Turquoise | AT3G08940 | −2.900 | −2.819 | 0.419 | −2.267 | −2.684 | −1.807 | 1.625 |
| Photosynthesis | M.alba_G0001233 | Turquoise | AT3G26650 | −2.883 | −3.690 | 0.071 | −1.642 | −1.433 | −2.797 | 1.290 |
| Photosynthesis | M.alba_G0008333 | Turquoise | AT5G35630 | −2.804 | −3.783 | 0.106 | −1.898 | −1.601 | −2.278 | 1.550 |
| Photosynthesis | M.alba_G0010904 | Turquoise | AT2G34430 | −2.318 | −2.964 | 0.087 | −2.371 | −1.955 | −2.243 | 2.160 |
| Photosynthesis | M.alba_G0002406 | Turquoise | AT1G06680 | −2.291 | −1.928 | 0.026 | −1.359 | −1.240 | −1.628 | 1.423 |
| Photosynthesis | M.alba_G0006044 | Turquoise | AT5G66570 | −2.035 | −1.980 | 0.172 | −1.126 | −1.330 | −1.507 | 1.007 |
| Fruit development | M.alba_G0020219 | Turquoise | AT1G02065 | −1.832 | −5.135 | 0.333 | −1.289 | −1.124 | −3.121 | 0.141 |
| Fruit development | M.alba_G0012049 | Grey | AT1G21460 | −1.268 | −2.016 | 0.984 | −0.653 | −0.268 | −1.028 | −0.696 |
| Cell wall | M.alba_G0005265 | Turquoise | AT1G63910 | −4.453 | −2.846 | 2.769 | 0.157 | −1.335 | −3.923 | −0.683 |
| Cell wall | M.alba_G0001161 | Turquoise | AT2G44480 | −2.172 | −8.627 | 1.059 | −2.144 | −2.460 | −3.636 | 0.387 |
| alpha/beta-Hydrolases | M.alba_G0013391 | Turquoise | AT1G56630 | −1.101 | −6.693 | 0.333 | −2.408 | −1.406 | −4.668 | 0.187 |
| Ethylene | M.alba_G0011799 | Turquoise | AT1G05010 | 2.349 | 2.901 | −1.468 | −0.271 | −0.056 | 2.122 | −0.715 |
| Ethylene | M.alba_G0013301 | Turquoise | AT5G25190 | 4.295 | 3.806 | 0.030 | 2.312 | 2.359 | 3.409 | 0.816 |
| IAA | M.alba_G0003453 | Turquoise | AT3G10870 | 1.885 | 1.908 | 0.242 | 2.036 | 2.667 | 1.553 | 1.236 |
| Nutritional immunity | M.alba_G0002433 | Turquoise | AT1G05300 | 1.485 | 0.452 | −1.052 | 1.743 | 1.814 | 1.272 | −0.278 |
| Fruit development | M.alba_G0009820 | Turquoise | AT3G52600 | 3.641 | 6.036 | 1.287 | 5.102 | 4.232 | 4.238 | −0.898 |
| Antifungal | M.alba_G0011399 | Turquoise | AT2G19760 | 2.221 | 2.457 | −0.039 | 0.606 | 1.558 | 3.506 | −0.265 |
| Cell wall | M.alba_G0016996 | Turquoise | AT4G02330 | 3.416 | 2.584 | −1.027 | 2.312 | 3.036 | 2.587 | 0.245 |
| Cell wall | M.alba_G0013029 | Turquoise | AT1G20190 | 3.594 | 3.431 | 0.397 | 2.146 | 2.459 | 3.448 | −1.805 |
| alpha/beta-Hydrolases | M.alba_G0008183 | Turquoise | AT4G15960 | 2.211 | 2.428 | −0.113 | 3.410 | 3.431 | 2.978 | −2.447 |
| M.alba_G0013851 | Turquoise | None | 0.854 | 1.038 | −0.165 | 1.487 | 2.113 | 1.960 | −0.546 | |
| M.alba_G0005835 | Turquoise | None | 1.392 | 2.630 | −0.312 | 1.281 | 1.689 | 2.995 | −0.696 | |
| M.alba_G0012361 | Turquoise | AT1G72160 | 1.396 | 1.530 | −0.068 | 1.214 | 1.454 | 1.561 | −0.863 | |
| M.alba_G0013424 | Turquoise | AT3G16150 | 1.817 | 2.490 | −1.246 | 1.558 | 1.651 | 2.096 | 0.451 | |
| M.alba_G0011456 | Turquoise | AT1G73010 | 1.853 | 1.995 | −0.499 | 0.982 | 1.590 | 4.046 | 0.827 | |
| M.alba_G0008070 | Turquoise | None | 1.875 | 2.973 | 0.377 | 1.356 | 0.833 | 1.956 | −0.378 | |
| ABA | M.alba_G0012076 | Turquoise | AT3G11945 | 2.601 | 2.780 | −0.689 | 2.462 | 1.559 | 2.157 | −0.567 |
| M.alba_G0009086 | Grey | AT3G09925 | 2.810 | 0.387 | −0.956 | −1.125 | −1.529 | −3.017 | 0.072 | |
| M.alba_G0012741 | Turquoise | AT1G09910 | 3.666 | 4.493 | 0.302 | 2.382 | 2.871 | 2.530 | −0.888 | |
| M.alba_G0000303 | Turquoise | AT5G54760 | 4.545 | 4.793 | −1.040 | 1.943 | 3.118 | 2.965 | −1.757 | |
| Dai et al. (2019) [20] | Lv et al. (2020) [10] | This Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Symbols | Gene IDs | Modules | Class | Log2FC (S1/CK) | Log2FC (S2/CK) | Log2FC (S1/CK) | Log2FC (S2/CK) | Log2FC (S3/CK) | Log2FC (S/CK) | Log2FC (K/CK) | Log2FC (K/S) |
| SSP1 | maker-Chr12-exonerate_protein2genome-gene-102.32 | Turquoise | CLE | −0.727 | −11.867 | −0.027 | −3.690 | −4.089 | −6.623 | 0.610 | 7.234 |
| SSP2 | maker-Chr5-est_gff_StringTie-gene-35.3 | Turquoise | GASA | −0.545 | −11.311 | −1.898 | −4.865 | −4.746 | −6.502 | 0.952 | 7.454 |
| SSP3 | maker-Chr12-est_gff_StringTie-gene-47.1 | Turquoise | ns-LTP | −2.032 | −13.715 | 0.594 | −2.335 | −1.185 | −5.147 | −0.868 | 4.278 |
| SSP4 | maker-Chr12-est_gff_StringTie-gene-142.0 | Turquoise | NA | −4.798 | −3.015 | 2.929 | 0.473 | −1.072 | −4.295 | −1.143 | 3.152 |
| SSP5 | maker-Chr14-exonerate_protein2genome-gene-46.38 | Turquoise | CLE | −3.745 | −8.271 | 0.076 | −1.877 | −2.462 | −4.070 | 1.136 | 5.206 |
| SSP6 | maker-Chr12-est_gff_StringTie-gene-122.1 | Turquoise | NA | −0.319 | −10.777 | −0.257 | −1.504 | −1.659 | −3.587 | −0.381 | 3.206 |
| SSP7 | h26356.03 | Turquoise | RALF | −3.282 | −10.664 | 0.594 | −1.390 | −1.518 | −2.184 | 0.878 | 3.062 |
| SSP8 | h60346.02 | Turquoise | STIG | −2.817 | −3.409 | −0.221 | −1.770 | −1.476 | −2.177 | 0.867 | 3.044 |
| SSP9 | maker-Chr6-est_gff_StringTie-gene-51.4 | Turquoise | CAPE | −1.383 | −2.625 | 0.461 | −0.240 | −0.220 | −1.651 | 1.238 | 2.889 |
| SSP10 | maker-Chr5-est_gff_StringTie-gene-4.6 | Grey | PSK | −1.576 | −1.522 | 0.729 | −0.494 | −0.244 | −1.132 | −1.106 | 0.025 |
| SSP11 | maker-Chr14-est_gff_StringTie-gene-10.7 | Turquoise | RALF | 1.035 | 0.644 | 0.044 | 0.827 | 1.301 | 1.270 | −1.153 | −2.423 |
| SSP12 | maker-Chr13-exonerate_protein2genome-gene-49.4 | Grey | GASA | 0.875 | −9.421 | 0.837 | 4.227 | 3.390 | 1.432 | −1.092 | −2.523 |
| SSP13 | maker-Chr8-exonerate_protein2genome-gene-129.4 | Turquoise | CLE | 2.996 | 4.732 | −1.349 | 0.509 | 1.139 | 1.864 | −1.739 | −3.602 |
| SSP14 | maker-Chr8-exonerate_protein2genome-gene-3.26 | Turquoise | PSK | 1.981 | 3.301 | −1.966 | −1.432 | −1.020 | 1.980 | −1.355 | −3.335 |
| SSP15 | maker-Chr4-est_gff_StringTie-gene-90.0 | Turquoise | GASA | 4.097 | 4.695 | −0.393 | 2.575 | 3.170 | 2.222 | −0.878 | −3.099 |
| SSP16 | h55705.01 | Blue | CAPE | 2.123 | 2.509 | −2.226 | 0.399 | −0.675 | 2.408 | 2.205 | −0.202 |
| SSP17 | h7280.01 | Turquoise | DVL/RTFL | 1.990 | 0.769 | 0.045 | 0.202 | 1.416 | 2.535 | 0.727 | −1.808 |
| SSP18 | maker-Chr4-exonerate_protein2genome-gene-224.17 | Turquoise | PIP | −8.764 | 5.371 | 0.296 | 4.235 | 3.702 | 5.577 | 0.754 | −4.822 |
| SSP19 | maker-Chr5-est_gff_StringTie-gene-118.1 | Grey | GASA | 2.210 | 3.849 | 1.092 | 0.536 | −0.018 | 5.695 | 3.828 | −1.867 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Guo, Z.; Kang, X.; Li, S.; Huang, S.; Zheng, L.; Fu, R.; Yidilisi, K.; Chao, N. Comparative Transcriptome Analysis of Different Mulberry Varieties to Reveal Candidate Genes and Small Secreted Peptides Involved in the Sclerotiniose Response. Forests 2024, 15, 1126. https://doi.org/10.3390/f15071126
Liu L, Guo Z, Kang X, Li S, Huang S, Zheng L, Fu R, Yidilisi K, Chao N. Comparative Transcriptome Analysis of Different Mulberry Varieties to Reveal Candidate Genes and Small Secreted Peptides Involved in the Sclerotiniose Response. Forests. 2024; 15(7):1126. https://doi.org/10.3390/f15071126
Chicago/Turabian StyleLiu, Li, Zixuan Guo, Xiaoru Kang, Shan Li, Shuai Huang, Longyan Zheng, Rumeng Fu, Keermula Yidilisi, and Nan Chao. 2024. "Comparative Transcriptome Analysis of Different Mulberry Varieties to Reveal Candidate Genes and Small Secreted Peptides Involved in the Sclerotiniose Response" Forests 15, no. 7: 1126. https://doi.org/10.3390/f15071126
APA StyleLiu, L., Guo, Z., Kang, X., Li, S., Huang, S., Zheng, L., Fu, R., Yidilisi, K., & Chao, N. (2024). Comparative Transcriptome Analysis of Different Mulberry Varieties to Reveal Candidate Genes and Small Secreted Peptides Involved in the Sclerotiniose Response. Forests, 15(7), 1126. https://doi.org/10.3390/f15071126




