Responses of Physiological Traits and Soil Properties in Pinus thunbergia and Euonymus japonicus Saplings under Drought and Cadmium (Cd) Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
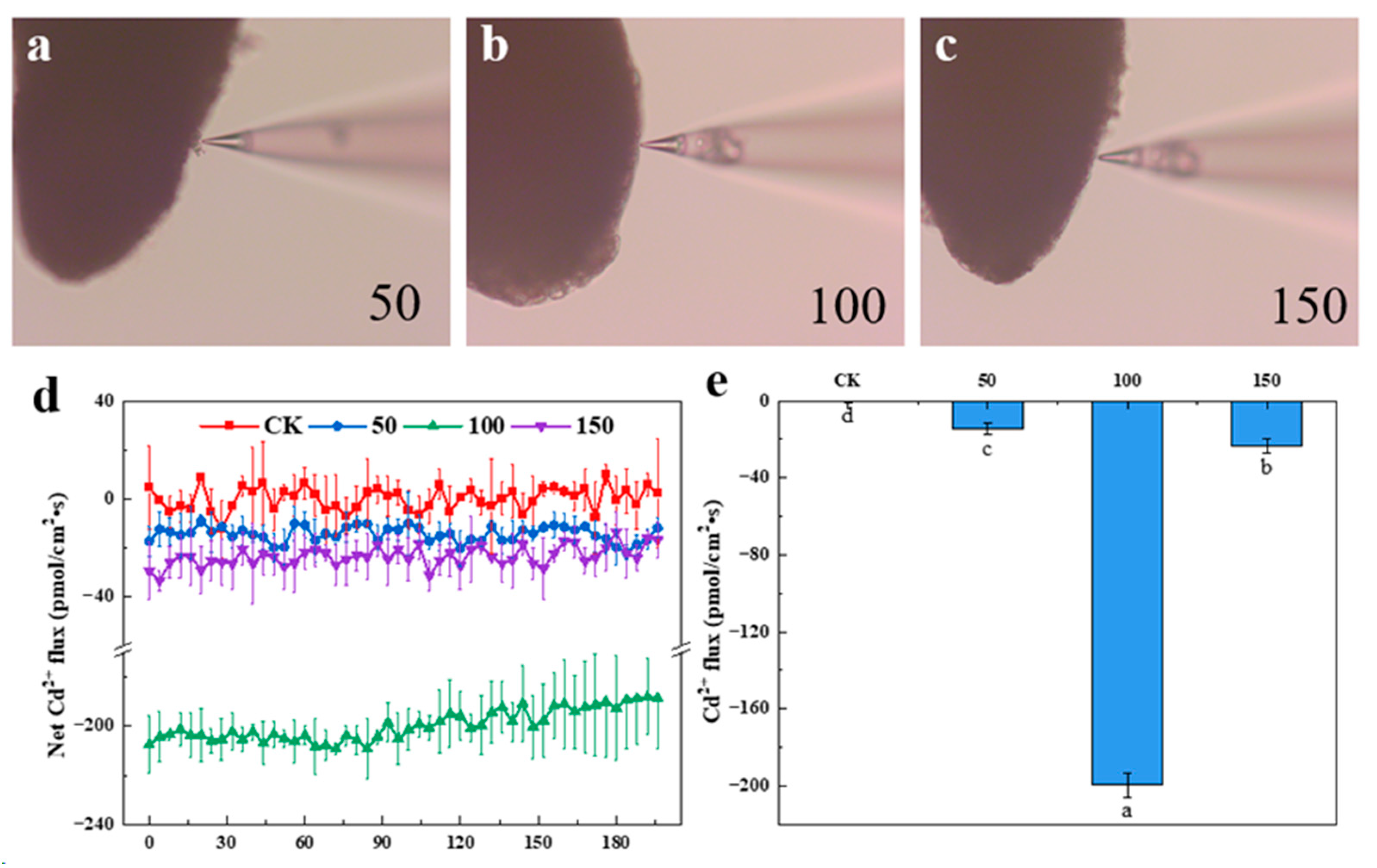
2.2. Measurement of the Root Cd2+ Flux
2.3. Determination of Cd Content of Different Organs
2.4. In Situ Observation of Cd Distribution in Stem Xylem and Phloem
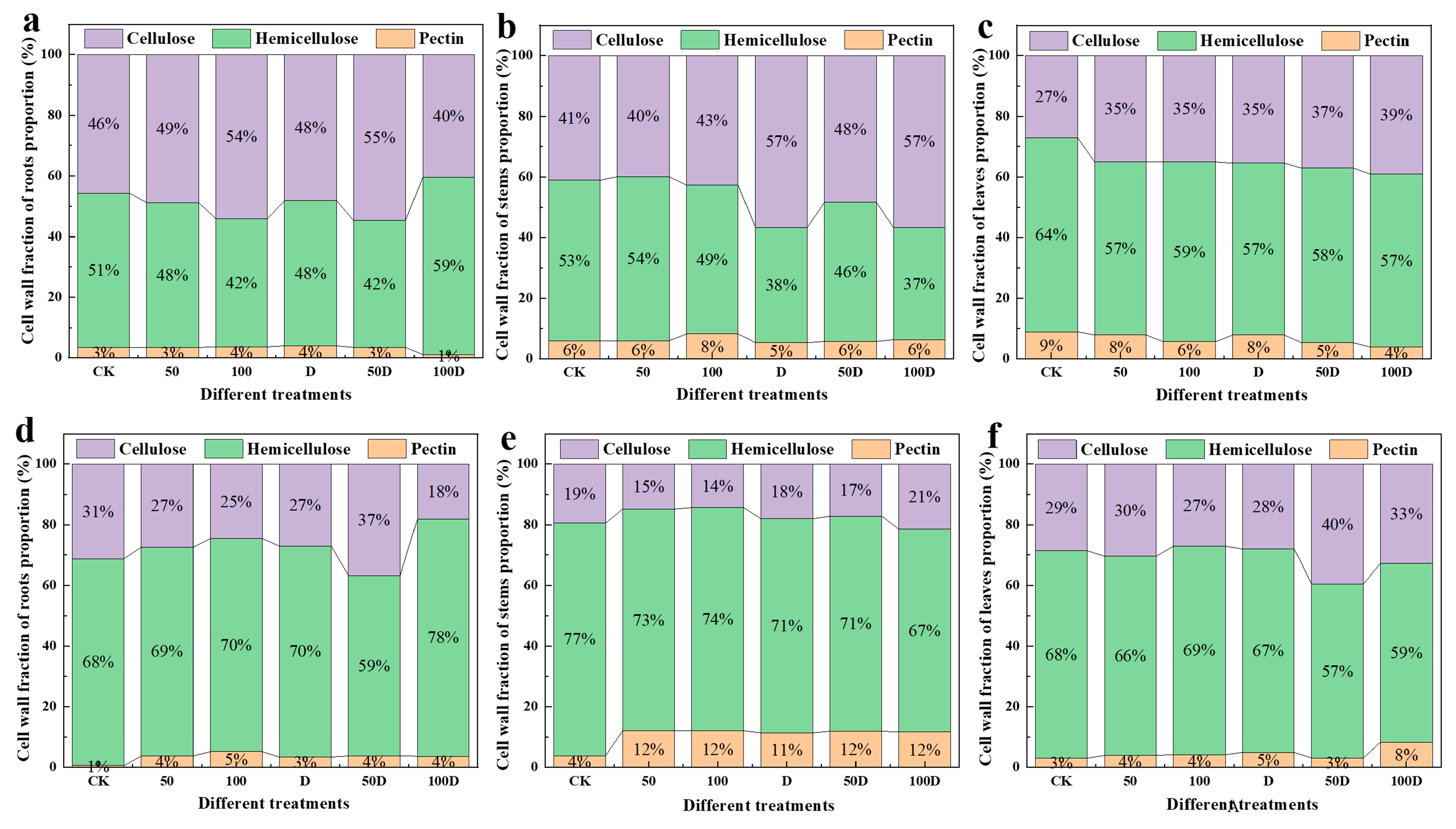
2.5. Determination of Cell Wall Component Fraction
2.6. Determination of Leaf Chlorophyll Content
2.7. Determination of Phytohormone Content (ABA, GA3, IAA, JA)
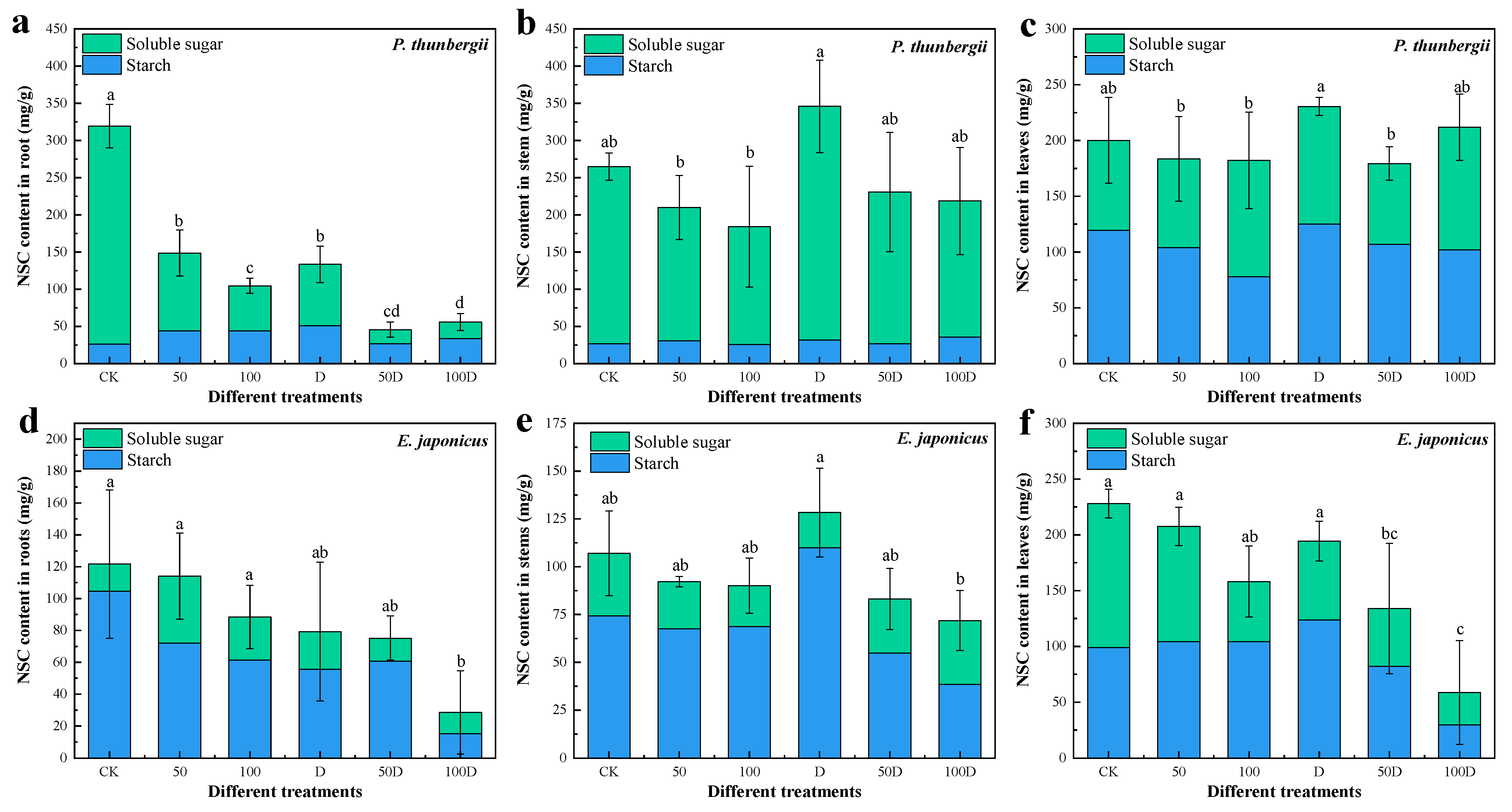
2.8. Determination of NSC Content
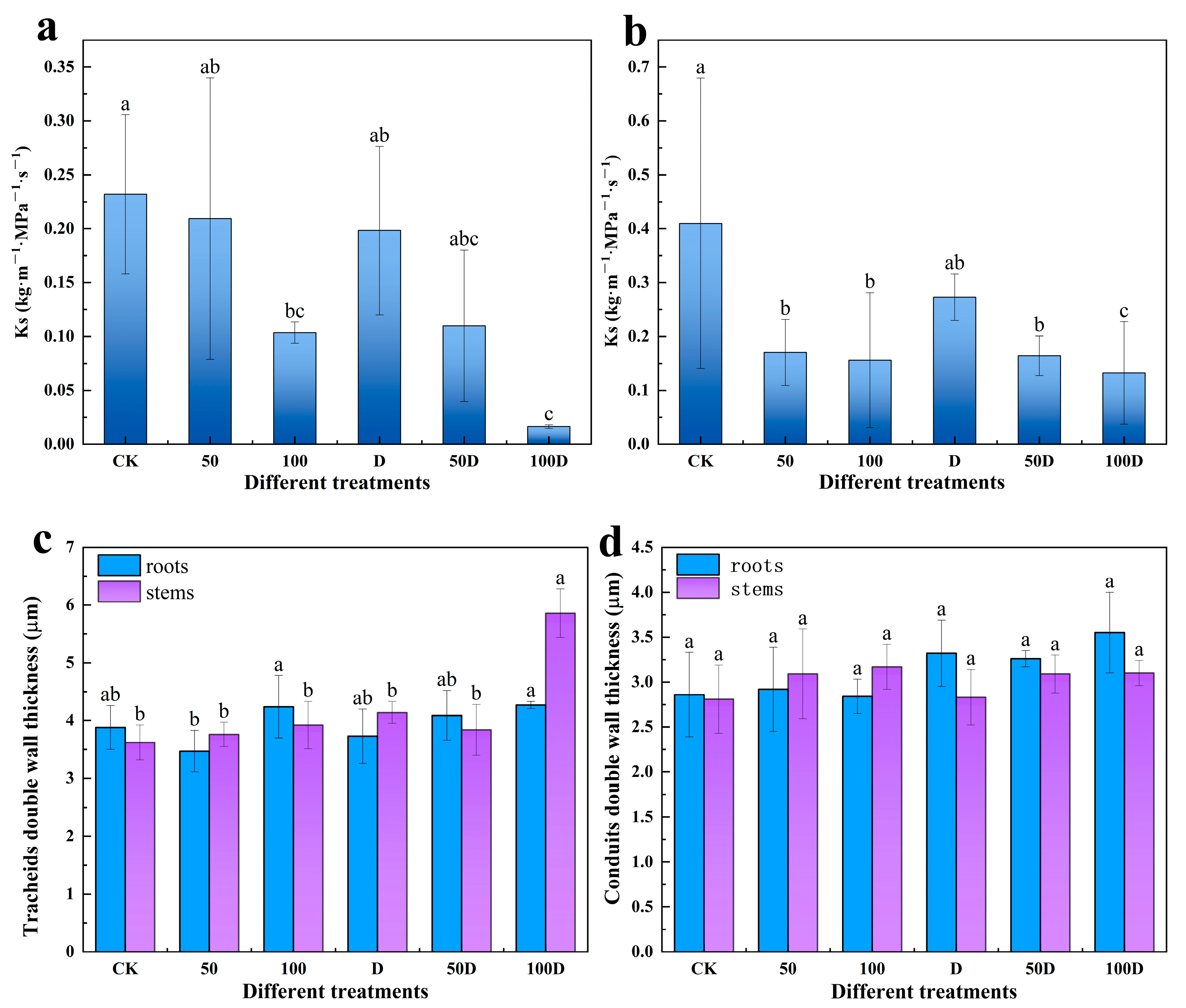
2.9. Determination of Xylem Hydraulic Traits
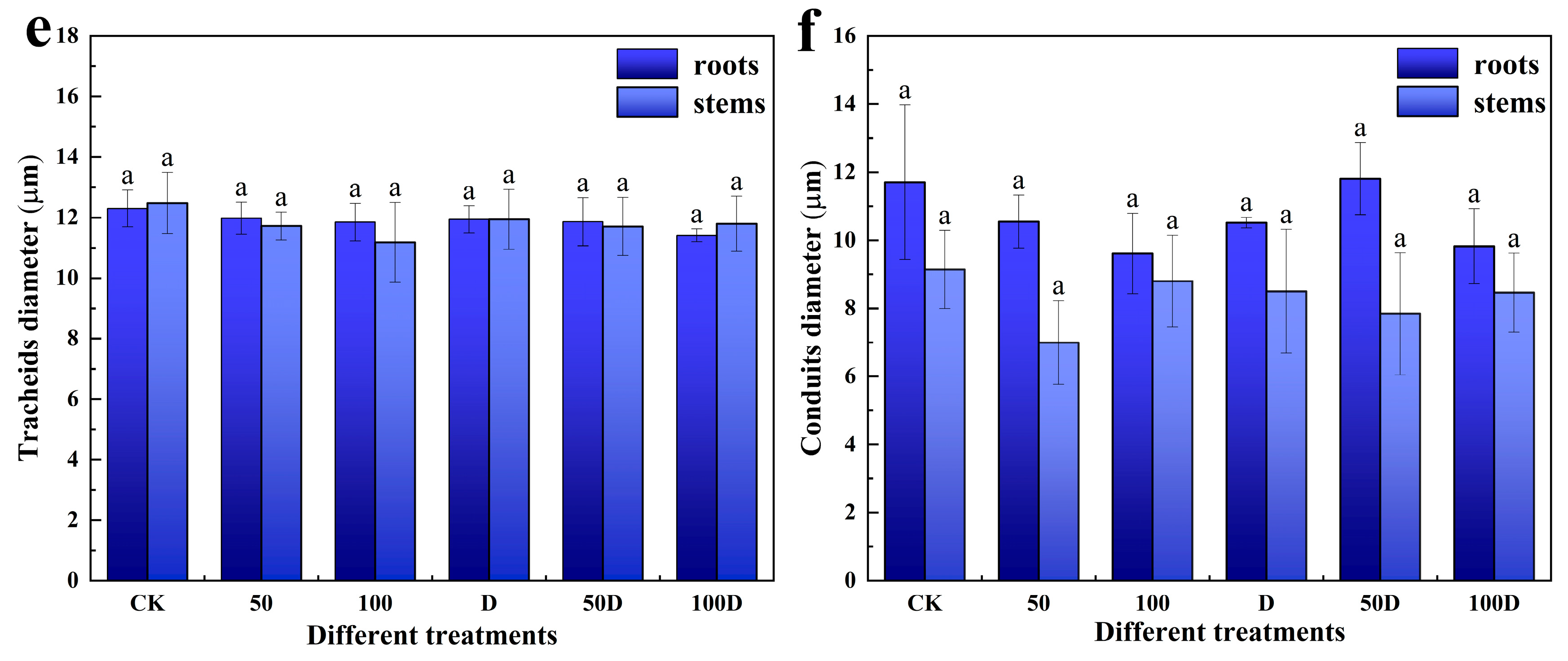
2.10. Determination of Xylem Anatomical Traits
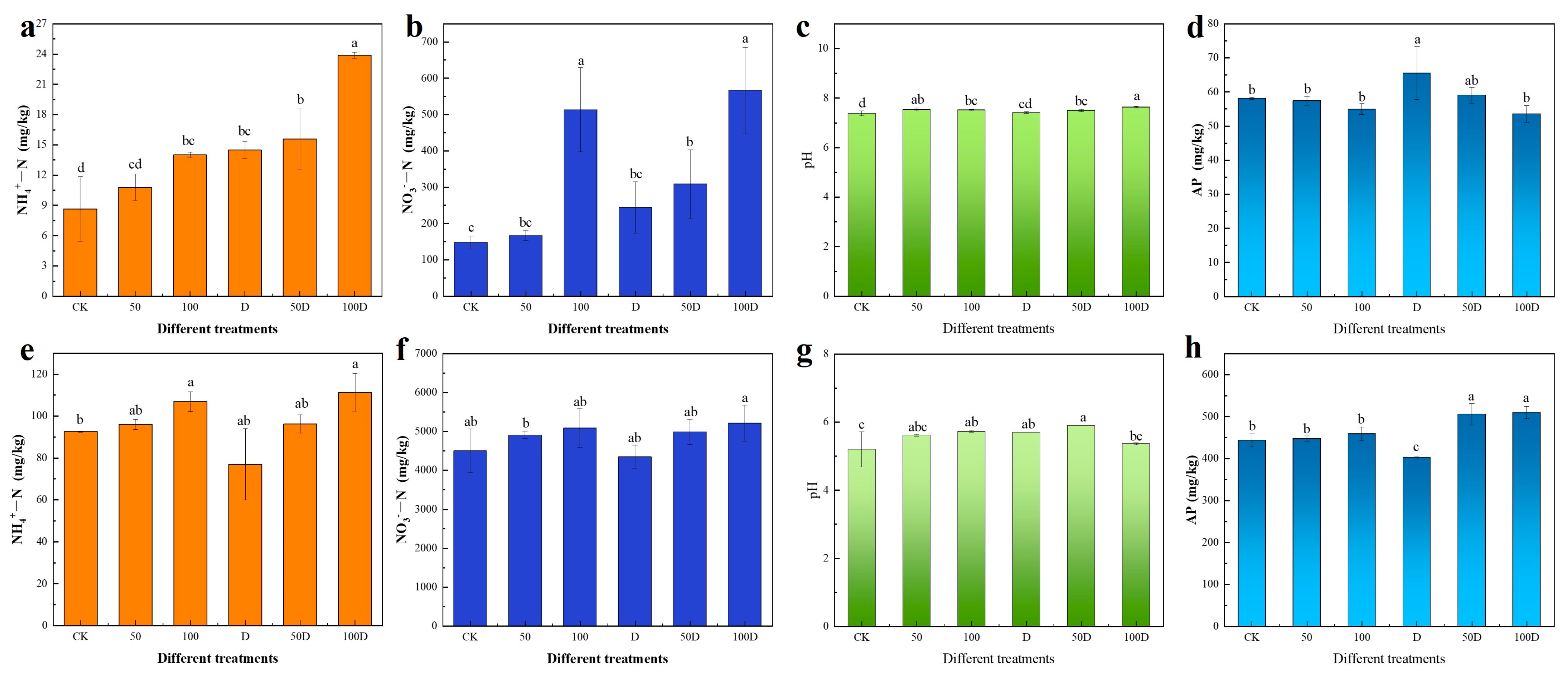
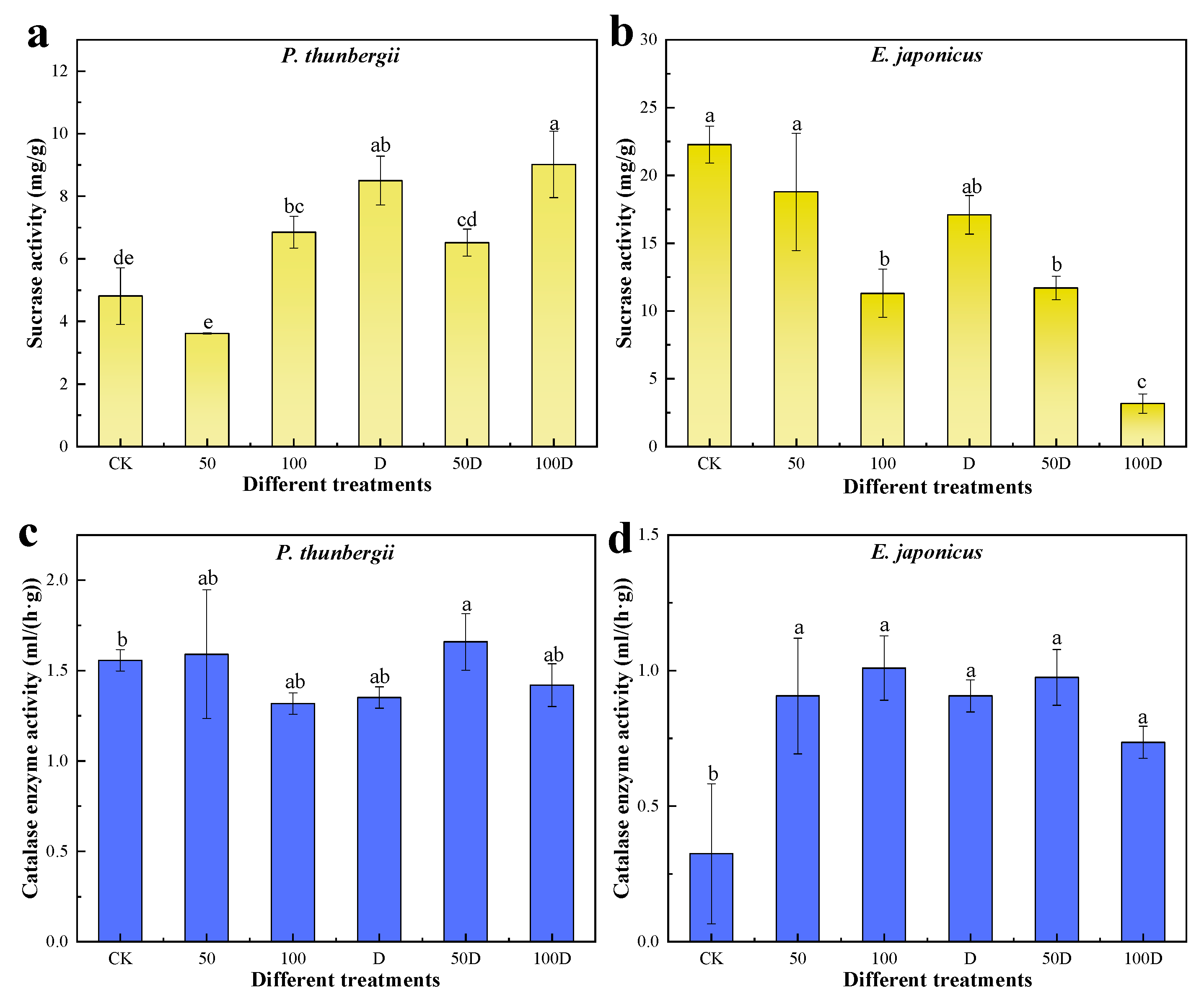
2.11. Determination of Soil Nutrient (N, P) Content and Enzyme Activity
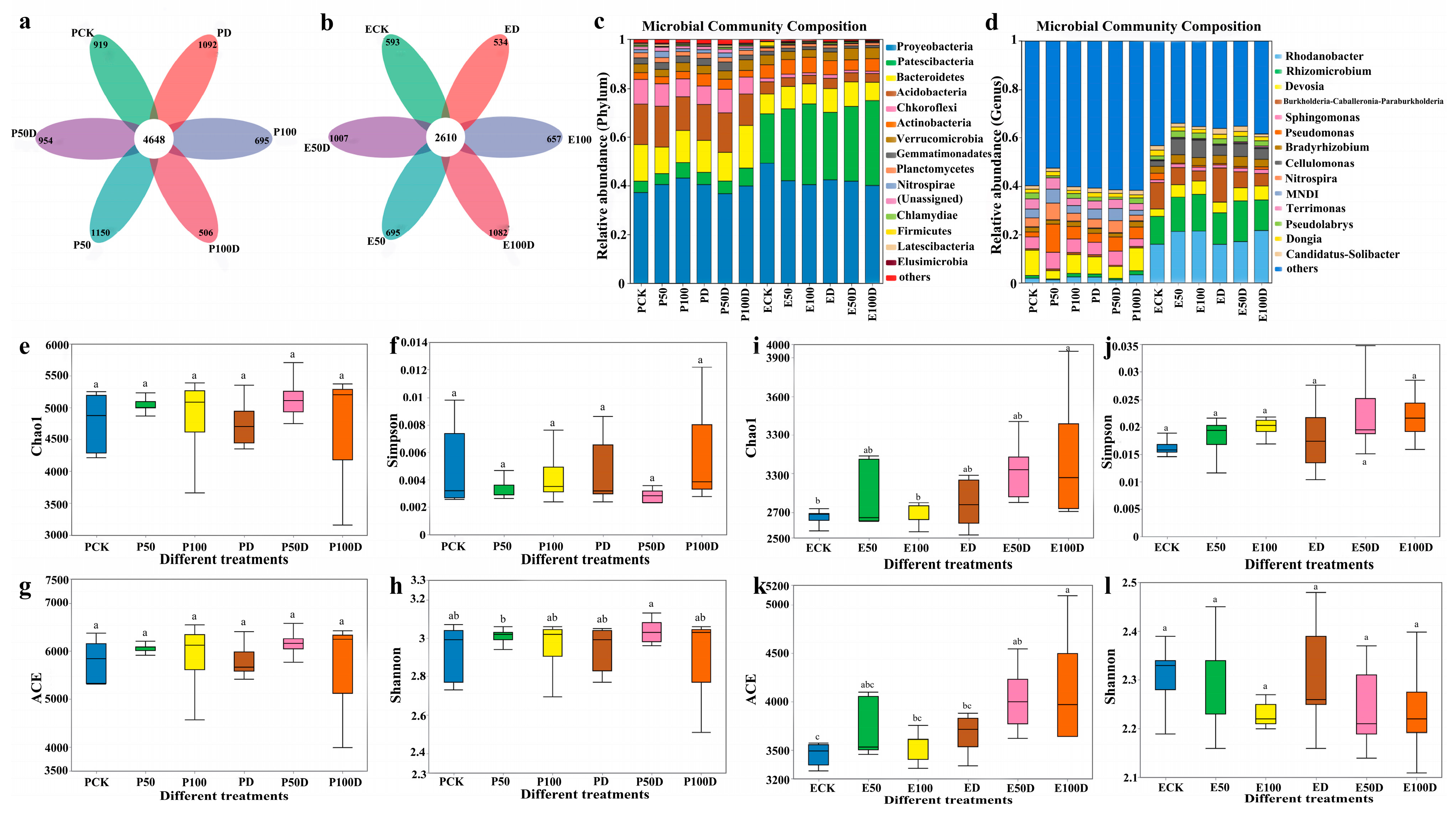
2.12. Structure of Rhizobacterial Community
2.13. Statistical Analysis
3. Results
3.1. Cd Uptake and Accumulation in P. thunbergii and E. japonicus Saplings
3.2. Physiological Responses of P. thunbergii and E. japonicus Saplings under Cd and Drought Stress
3.3. Response of P. thunbergii and E. japonicus Soil Properties to Drought and Cd Stress
4. Discussion
4.1. Cd Uptake and Accumulation in P. thunbergii and E. japonicus Saplings
4.2. Physiological Responses of P. thunbergii and E. japonicus under Cd and Drought Stress
4.3. Response of Soil Properties around Roots of P. thunbergii and E. japonicus to Drought and Cd Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Tao, Q.; Jupa, R.; Liu, Y.; Wu, K.; Song, Y.; Li, J.; Huang, Y.; Zou, L.; Liang, Y. Role of vertical transmission of shoot endophytes in root-associated microbiome assembly and heavy metal hyperaccumulation in Sedum alfredii. Environ. Sci. Technol. 2019, 53, 6954–6963. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, J.-C.; Yu, Y.; Cheng, X.; Du, Y.-L.; Zhao, Q.; Du, J.-D. Interactive effects of drought and cadmium stress on adzuki bean seedling growth, DNA damage repair, and Cd accumulation. Sci. Hortic. 2024, 324, 112624. [Google Scholar] [CrossRef]
- Yang, J.; Li, K.; Zheng, W.; Zhang, H.; Cao, X.; Lan, Y.; Yang, C.; Li, C. Characterization of early transcriptional responses to cadmium in the root and leaf of Cd-resistant Salix matsudana Koidz. BMC Genom. 2015, 16, 705. [Google Scholar] [CrossRef] [PubMed]
- De Silva, N.D.G.; Cholewa, E.; Ryser, P. Effects of combined drought and heavy metal stresses on xylem structure and hydraulic conductivity in red maple (Acer rubrum L.). J. Exp. Bot. 2012, 63, 5957–5966. [Google Scholar] [CrossRef]
- Disante, K.B.; Fuentes, D.; Cortina, J. Response to drought of Zn-stressed Quercus suber L. seedlings. Environ. Exp. Bot. 2011, 70, 96–103. [Google Scholar] [CrossRef]
- Shi, G.; Xia, S.; Ye, J.; Huang, Y.; Liu, C.; Zhang, Z. PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology. Environ. Exp. Bot. 2015, 111, 127–134. [Google Scholar] [CrossRef]
- Bashir, W.; Anwar, S.; Zhao, Q.; Hussain, I.; Xie, F. Interactive effect of drought and cadmium stress on soybean root morphology and gene expression. Ecotoxicol. Environ. Saf. 2019, 175, 90–101. [Google Scholar] [CrossRef]
- Li, S.; Lu, S.; Wang, J.; Chen, Z.; Zhang, Y.; Duan, J.; Liu, P.; Wang, X.; Guo, J. Responses of physiological, morphological and anatomical traits to abiotic stress in woody plants. Forests 2023, 14, 1784. [Google Scholar] [CrossRef]
- Kharazian, P.; Bacchetta, G.; Cappai, G.; Piredda, M.; De Giudici, G. An integrated geochemical and mineralogical investigation on soil-plant system of Pinus halepensis pioneer tree growing on heavy metal polluted mine tailing. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2023, 157, 272–285. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, G.; Liu, C.; Lin, Y.; Chen, X.; Li, H.; Fu, Q.; Guo, B. Intercropping of Euonymus japonicus with Photinia× fraseri Improves Phytoremediation Efficiency in Cd/Cu/Zn Contaminated Field. Biology 2022, 11, 1133. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, H.; Qin, S.; He, J.; Lyu, D.; Li, H. Net cadmium flux and gene expression in relation to differences in cadmium accumulation and translocation in four apple rootstocks. Environ. Exp. Bot. 2016, 130, 95–105. [Google Scholar] [CrossRef]
- Guo, Z.; Zeng, P.; Xiao, X.; Peng, C. Physiological, anatomical, and transcriptional responses of mulberry (Morus alba L.) to Cd stress in contaminated soil. Environ. Pollut. 2021, 284, 117387. [Google Scholar] [CrossRef] [PubMed]
- Gall, H.L.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Han, G.H.; Huang, R.N.; Hong, L.H.; Xu, J.X.; Hong, Y.G.; Wu, Y.H.; Chen, W.W. The transcription factor NAC102 confers cadmium tolerance by regulating WAKL11 expression and cell wall pectin metabolism in Arabidopsis. J. Integr. Plant Biol. 2023, 65, 2262–2278. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-L.; Wang, H.-F.; Wang, P.; Yu, C.-G.; Luo, S.-Q.; Zhang, Y.-F.; Xie, Y.-F. Effects of cadmium stress on the antioxidant system and chlorophyll fluorescence characteristics of two Taxodium clones. Plant Cell Rep. 2018, 37, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Furze, M.E.; Wainwright, D.K.; Huggett, B.A.; Knipfer, T.; McElrone, A.J.; Brodersen, C.R. Ecologically driven selection of nonstructural carbohydrate storage in oak trees. New Phytol. 2021, 232, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees–from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Tomasella, M.; Casolo, V.; Aichner, N.; Petruzzellis, F.; Savi, T.; Trifilò, P.; Nardini, A. Non-structural carbohydrate and hydraulic dynamics during drought and recovery in Fraxinus ornus and Ostrya carpinifolia saplings. Plant Physiol. Biochem. 2019, 145, 1–9. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernandez-Zapata, J.C.; Nicolás, J.J.M.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Zhao, C.; Jiang, X.; Gao, D. Responses of non-structural carbohydrates and biomass in plant to heavy metal treatment. Sci. Total Environ. 2024, 909, 168559. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, C.; Dannoura, M.; Desalme, D.; Angeli, N.; Takanashi, S.; Kominami, Y.; Epron, D. Drought affects the fate of non-structural carbohydrates in hinoki cypress. Tree Physiol. 2022, 42, 784–796. [Google Scholar] [CrossRef]
- Tomasella, M.; Petrussa, E.; Petruzzellis, F.; Nardini, A.; Casolo, V. The possible role of non-structural carbohydrates in the regulation of tree hydraulics. Int. J. Mol. Sci. 2019, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Paula, S. The role of nonstructural carbohydrates storage in forest resilience under climate change. Curr. For. Rep. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Z. Cell wall polysaccharide-mediated cadmium tolerance between two Arabidopsis thaliana ecotypes. Front. Plant Sci. 2020, 11, 522497. [Google Scholar] [CrossRef]
- Guo, J.; Qin, S.; Rengel, Z.; Gao, W.; Nie, Z.; Liu, H.; Li, C.; Zhao, P. Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol. Environ. Saf. 2019, 172, 380–387. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Xue, J.; Clearwater, M.J.; Meason, D.F.; Clinton, P.W.; Domec, J.C. Aquaporin regulation in roots controls plant hydraulic conductance, stomatal conductance, and leaf water potential in Pinus radiata under water stress. Plant Cell Environ. 2019, 42, 717–729. [Google Scholar] [CrossRef]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant. 2016, 38, 257. [Google Scholar] [CrossRef]
- Parsamanesh, S.; Sadeghi, H. Modeling the interactions between inter-correlated variables of plant and soil micro-ecology responses under simultaneous cadmium stress and drought. J. Clean. Prod. 2023, 418, 138163. [Google Scholar] [CrossRef]
- Jin, K.; White, P.J.; Whalley, W.R.; Shen, J.; Shi, L. Shaping an optimal soil by root–soil interaction. Trends Plant Sci. 2017, 22, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xu, D.; Kong, T.; Wang, D. Characteristics of Enzyme Activities during Phytoremediation of Cd-Contaminated Soil. Sustainability 2022, 14, 9350. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Li, S.-W.; Leng, Y.; Kang, X.-H. Structural and functional responses of bacterial and fungal communities to multiple heavy metal exposure in arid loess. Sci. Total Environ. 2020, 723, 138081. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.L.; Sanchez-Marín, A.; Hernández, T.; García, C. Effect of cadmium on microbial activity and a ryegrass crop in two semiarid soils. Environ. Manag. 2006, 37, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhao, X.; Yang, F.; Chen, J.; Mo, Q.; Cui, S.; Chen, C.; He, S.; Li, Z. Impact of fertilization and grazing on soil N and enzyme activities in a karst pasture ecosystem. Geoderma 2023, 437, 116578. [Google Scholar] [CrossRef]
- Yang, R.; Ma, G.; Liu, C.; Wang, C.; Kang, X.; Wu, M.; Zhang, B. Effects of different heavy metal pollution levels on microbial community structure and risk assessment in Zn-Pb mining soils. Environ. Sci. Pollut. Res. 2023, 30, 52749–52761. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.-T.; Li, G.-l.; Zhou, J.-J.; Ji, R.-Q.; Meng, L.-P. Fungi community structure associated with Korean pine forests and the varying tendency with four forest land age. Nova Hedwig. 2020, 111, 391–415. [Google Scholar] [CrossRef]
- Chang, W.-S.; Van De Mortel, M.; Nielsen, L.; Nino de Guzman, G.; Li, X.; Halverson, L.J. Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water-limiting conditions. J. Bacteriol. 2007, 189, 8290–8299. [Google Scholar] [CrossRef]
- Gu, Z.; Hu, C.; Gan, Y.; Zhou, J.; Tian, G.; Gao, L. Role of Microbes in Alleviating Crop Drought Stress: A Review. Plants 2024, 13, 384. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. Front. Plant Sci. 2018, 9, 336111. [Google Scholar] [CrossRef]
- Brown, R.L.; Hangs, R.; Schoenau, J.; Bedard-Haughn, A. Soil nitrogen and phosphorus dynamics and uptake by wheat grown in drained prairie soils under three moisture scenarios. Soil Sci. Soc. Am. J. 2017, 81, 1496–1504. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Chi, Y.; Chu, S.; Hayat, K.; Zhi, Y.; Hayat, S.; Terziev, D.; Zhang, D.; Zhou, P. Optimization of NPK fertilization combined with phytoremediation of cadmium contaminated soil by orthogonal experiment. Ecotoxicol. Environ. Saf. 2020, 189, 109997. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Wang, J.; Lu, S.; Liu, Z.; Jia, H.; Wei, T.; Guo, J. The response of physiological and xylem anatomical traits under cadmium stress in Pinus thunbergii seedlings. Tree Physiol. 2024, 44, tpae046. [Google Scholar] [CrossRef] [PubMed]
- You-Ming, Y.; Chu-Nian, X.; Bao-Min, W.; Jun-Zhen, J. Effects of plant growth regulators on secondary wall thickening of cotton fibres. Plant Growth Regul. 2001, 35, 233–237. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Z.; Liu, S.; Cao, K.; Niu, B.; Liu, X.; Gao, X. Multi-year throughfall reduction enhanced the growth and non-structural carbohydrate storage of roots at the expenses of above-ground growth in a warm-temperate natural oak forest. For. Ecosyst. 2023, 10, 100118. [Google Scholar] [CrossRef]
- Nirola, R.; Megharaj, M.; Beecham, S.; Aryal, R.; Thavamani, P.; Vankateswarlu, K.; Saint, C. Remediation of metalliferous mines, revegetation challenges and emerging prospects in semi-arid and arid conditions. Environ. Sci. Pollut. Res. 2016, 23, 20131–20150. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Liu, L.; Yan, D.; He, Y. Physiological stress responses, mineral element uptake and phytoremediation potential of Morus alba L. in cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 189, 109973. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Yang, L.; Jin, L.; Song, Y.; Jiang, S.; Qin, L. Transport pathways of cadmium (Cd) and its regulatory mechanisms in plant. Acta Ecol. Sin 2015, 35, 7921–7929. [Google Scholar]
- Lan, X.-Y.; He, Q.-S.; Yang, B.; Yan, Y.-Y.; Li, X.-Y.; Xu, F.-L. Influence of Cd exposure on H+ and Cd2+ fluxes in the leaf, stem and root of a novel aquatic hyperaccumulator-Microsorum pteropus. Chemosphere 2020, 249, 126552. [Google Scholar] [CrossRef]
- Colzi, I.; Doumett, S.; Del Bubba, M.; Fornaini, J.; Arnetoli, M.; Gabbrielli, R.; Gonnelli, C. On the role of the cell wall in the phenomenon of copper tolerance in Silene paradoxa L. Environ. Exp. Bot. 2011, 72, 77–83. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Wei, T.; Zhou, R.; Muhammad, H.; Hua, L.; Ren, X.; Guo, J.; Ding, Y. Accumulation and fixation of Cd by tomato cell wall pectin under Cd stress. Environ. Exp. Bot. 2019, 167, 103829. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Ali, Q.; Ali, S.; Arif, M.S.; Hussain, S.; Rizvi, H. Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol. Environ. Saf. 2014, 104, 285–293. [Google Scholar] [CrossRef]
- Sezgin, A.; Altuntaş, C.; Demiralay, M.; Cinemre, S.; Terzi, R. Exogenous alpha lipoic acid can stimulate photosystem II activity and the gene expressions of carbon fixation and chlorophyll metabolism enzymes in maize seedlings under drought. J. Plant Physiol. 2019, 232, 65–73. [Google Scholar] [CrossRef]
- Tuver, G.Y.; Ekinci, M.; Yildirim, E. Morphological, physiological and biochemical responses to combined cadmium and drought stress in radish (Raphanus sativus L.). Rend. Lincei. Sci. Fis. E Nat. 2022, 33, 419–429. [Google Scholar] [CrossRef]
- Nawaz, G.; Kang, H. Rice OsRH58, a chloroplast DEAD-box RNA helicase, improves salt or drought stress tolerance in Arabidopsis by affecting chloroplast translation. BMC Plant Biol. 2019, 19, 17. [Google Scholar] [CrossRef]
- Thomas, J.C.; Perron, M.; LaRosa, P.C.; Smigocki, A.C. Cytokinin and the regulation of a tobacco metallothionein-like gene during copper stress. Physiol. Plant. 2005, 123, 262–271. [Google Scholar] [CrossRef]
- Sharma, S.S.; Kumar, V. Responses of wild type and abscisic acid mutants of Arabidopsis thaliana to cadmium. J. Plant Physiol. 2002, 159, 1323–1327. [Google Scholar] [CrossRef]
- Kamínek, M.; Motyka, V.; Vaňková, R. Regulation of cytokinin content in plant cells. Physiol. Plant. 1997, 101, 689–700. [Google Scholar] [CrossRef]
- Iori, V.; Pietrini, F.; Bianconi, D.; Mughini, G.; Massacci, A.; Zacchini, M. Analysis of biometric, physiological, and biochemical traits to evaluate the cadmium phytoremediation ability of eucalypt plants under hydroponics. Iforest-Biogeosci. For. 2017, 10, 416. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Pang, J.; Turner, N.C.; Khan, T.; Du, Y.-L.; Xiong, J.-L.; Colmer, T.D.; Devilla, R.; Stefanova, K.; Siddique, K.H. Response of chickpea (Cicer arietinum L.) to terminal drought: Leaf stomatal conductance, pod abscisic acid concentration, and seed set. J. Exp. Bot. 2017, 68, 1973–1985. [Google Scholar]
- Per, T.S.; Masood, A.; Khan, N.A. Nitric oxide improves S-assimilation and GSH production to prevent inhibitory effects of cadmium stress on photosynthesis in mustard (Brassica juncea L.). Nitric Oxide 2017, 68, 111–124. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.-H. Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef]
- Stobrawa, K.; Lorenc-Plucińska, G. Changes in carbohydrate metabolism in fine roots of the native European black poplar (Populus nigra L.) in a heavy-metal-polluted environment. Sci. Total Environ. 2007, 373, 157–165. [Google Scholar] [CrossRef]
- Guo, X.; Peng, C.; Li, T.; Huang, J.; Song, H.; Zhu, Q.; Wang, M. The effects of drought and re-watering on non-structural carbohydrates of Pinus tabulaeformis seedlings. Biology 2021, 10, 281. [Google Scholar] [CrossRef]
- Li, L.; Yan, X.; Li, J.; Tian, Y. Physiological and FtCHS gene expression responses to PEG-simulated drought and cadmium stresses in tartary buckwheat seedlings. J. Plant Growth Regul. 2021, 41, 3518–3529. [Google Scholar] [CrossRef]
- Gauthey, A.; Peters, J.M.; López, R.; Carins-Murphy, M.R.; Rodriguez-Dominguez, C.M.; Tissue, D.T.; Medlyn, B.E.; Brodribb, T.J.; Choat, B. Mechanisms of xylem hydraulic recovery after drought in Eucalyptus saligna. Plant Cell Environ. 2022, 45, 1216–1228. [Google Scholar] [CrossRef]
- Torun, H.; Cetin, B.; Stojnic, S.; Petrík, P. Salicylic acid alleviates the effects of cadmium and drought stress by regulating water status, ions, and antioxidant defense in Pterocarya fraxinifolia. Front. Plant Sci. 2024, 14, 1339201. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Luo, J.; Lux, A.; Kováč, J.; Wen, Y.; Zhou, Y.; Jan, J.; Liang, Y.; Li, T. The apoplasmic pathway via the root apex and lateral roots contributes to Cd hyperaccumulation in the hyperaccumulator Sedum alfredii. J. Exp. Bot. 2017, 68, 739–751. [Google Scholar]
- Sperry, J.S.; Hacke, U.G.; Pittermann, J. Size and function in conifer tracheids and angiosperm vessels. Am. J. Bot. 2006, 93, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Monni, S.; Uhlig, C.; Hansen, E.; Magel, E. Ecophysiological responses of Empetrum nigrum to heavy metal pollution. Environ. Pollut. 2001, 112, 121–129. [Google Scholar] [CrossRef]
- Knipfer, T.; Barrios-Masias, F.H.; Cuneo, I.F.; Bouda, M.; Albuquerque, C.P.; Brodersen, C.R.; Kluepfel, D.A.; McElrone, A.J. Variations in xylem embolism susceptibility under drought between intact saplings of three walnut species. Tree Physiol. 2018, 38, 1180–1192. [Google Scholar] [CrossRef]
- Valentovičová, K.; Halušková, Ľ.; Huttová, J.; Mistrík, I.; Tamás, L. Effect of heavy metals and temperature on the oxalate oxidase activity and lignification of metaxylem vessels in barley roots. Environ. Exp. Bot. 2009, 66, 457–462. [Google Scholar] [CrossRef]
- Badagliacca, G.; Benítez, E.; Amato, G.; Badalucco, L.; Giambalvo, D.; Laudicina, V.A.; Ruisi, P. Long-term effects of contrasting tillage on soil organic carbon, nitrous oxide and ammonia emissions in a Mediterranean Vertisol under different crop sequences. Sci. Total Environ. 2018, 619, 18–27. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Wang, Y.; Yeh, K.-C. Role of root exudates in metal acquisition and tolerance. Curr. Opin. Plant Biol. 2017, 39, 66–72. [Google Scholar] [CrossRef]
- Rosenstock, N.P.; Berner, C.; Smits, M.M.; Krám, P.; Wallander, H. The role of phosphorus, magnesium and potassium availability in soil fungal exploration of mineral nutrient sources in Norway spruce forests. New Phytol. 2016, 211, 542–553. [Google Scholar]
- Wang, C.; Wang, J.; Niu, X.; Yang, Y.; Malik, K.; Jin, J.; Zhao, C.; Tang, R.; Zheng, R.; Huang, R. Phosphorus addition modifies the bacterial community structure in rhizosphere of Achnatherum inebrians by influencing the soil properties and modulates the Epichloë gansuensis-mediated root exudate profiles. Plant Soil 2023, 491, 543–560. [Google Scholar]
- Zhu, D.; Zhang, Z.-H.; Wang, Z.-H. Effects of heavy metals on soil microbial community of different land use types. J. Mt. Sci. 2023, 20, 3582–3595. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Yang, T.; Lv, G.; Li, W.; Chen, Y.; Wu, D. Mechanisms underlying the succession of plant rhizosphere microbial community structure and function in an alpine open-pit coal mining disturbance zone. J. Environ. Manag. 2023, 325, 116571. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wei, Y.; Deng, Z.; Hao, X.; Li, F. Linear models for describing relations between sensitive bacterial taxa and ecological risk from heavy metals in soils of coal mines in semi-arid region. Appl. Soil Ecol. 2024, 193, 105122. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, B.; Guo, Y.; Tian, S.; Zhang, Z.; Hou, X. Assessment of the Pollution of Soil Heavy Metal (loid) s and Its Relation with Soil Microorganisms in Wetland Soils. Sustainability 2022, 14, 12164. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, J.; Li, H.; Sunahara, G.; Li, M.; Tang, C.; Duran, R.; Ma, B.; Liu, H.; Feng, L. Effects of three plant growth-promoting bacterial symbiosis with ryegrass for remediation of Cd, Pb, and Zn soil in a mining area. J. Environ. Manag. 2024, 353, 120167. [Google Scholar] [CrossRef]
- Łukowski, A.; Dec, D. Influence of Zn, Cd, and Cu fractions on enzymatic activity of arable soils. Environ. Monit. Assess. 2018, 190, 278. [Google Scholar] [CrossRef] [PubMed]
| P. thunbergii | E. japonicus | |||||
|---|---|---|---|---|---|---|
| Groups | Roots | Stems | Leaves | Roots | Stems | Leaves |
| CK | 1.23 ± 1.02 c | 0.27 ± 0.12 b | 0.29 ± 0.02 b | 1.76 ± 1.15 c | 0.21 ± 0.14 b | 1.22 ± 0.82 a |
| 50 | 18.16 ± 4.08 b | 1.61 ± 0.36 b | 0.80 ± 0.58 b | 6.55 ± 3.68 ab | 0.66 ± 0.42 b | 1.73 ± 1.65 a |
| 100 | 42.75 ± 8.42 a | 12.93 ± 7.94 a | 9.65 ± 3.42 a | 8.96 ± 4.00 a | 3.03 ± 1.81 ab | 2.83 ± 1.92 a |
| D | 1.91 ± 1.24 c | 0.40 ± 0.19 b | 0.93 ± 0.86 b | 0.91 ± 0.79 c | 0.18 ± 0.02 b | 0.96 ± 0.38 a |
| 50D | 22.14 ± 5.00 b | 2.46 ± 1.64 b | 1.25 ± 0.11 b | 3.05 ± 1.73 bc | 4.20 ± 3.41 a | 3.07 ± 1.69 a |
| 100D | 10.75 ± 9.80 bc | 8.82 ± 2.13 a | 1.42 ± 0.75 b | 4.96 ± 2.68 abc | 3.44 ± 1.20 ab | 2.43 ± 1.55 a |
| Species | Parameter | 50 | 100 | 50D | 100D |
|---|---|---|---|---|---|
| P. thunbergii | TF from roots to stems | 0.08 ± 0.02 a | 0.40 ± 0.17 a | 0.16 ± 0.14 a | 1.26 ± 0.72 b |
| TF from stems to leaves | 0.57 ± 0.42 a | 0.62 ± 0.08 a | 0.74 ± 0.04 a | 0.15 ± 0.04 a | |
| BCF for roots | 0.27 ± 0.19 ab | 0.43 ± 0.08 a | 0.39 ± 0.16 a | 0.11 ± 0.09 b | |
| BCF for stems | 0.03 ± 0.01 b | 0.15 ± 0.05 a | 0.05 ± 0.03 b | 0.09 ± 0.02 b | |
| BCF for leaves | 0.02 ± 0.01 ab | 0.09 ± 0.03 a | 0.03 ± 0.02 ab | 0.01 ± 0.007 b | |
| E. japonicus | TF from roots to stems | 0.14 ± 0.11 a | 0.56 ± 0.39 a | 1.37 ± 1.16 a | 0.97 ± 0.69 a |
| TF from stems to leaves | 1.63 ± 0.01 a | 1.42 ± 1.10 a | 1.16 ± 0.76 a | 0.73 ± 0.24 a | |
| BCF for roots | 0.13 ± 0.07 a | 0.09 ± 0.04 a | 0.06 ± 0.03 a | 0.05 ± 0.03 a | |
| BCF for stems | 0.01 ± 0.002 a | 0.03 ± 0.01 a | 0.08 ± 0.06 a | 0.03 ± 0.02 a | |
| BCF for leaves | 0.04 ± 0.01 a | 0.03 ± 0.01 a | 0.06 ± 0.03 a | 0.02 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, J.; Lu, S.; Li, H.; Guo, J. Responses of Physiological Traits and Soil Properties in Pinus thunbergia and Euonymus japonicus Saplings under Drought and Cadmium (Cd) Stress. Forests 2024, 15, 1141. https://doi.org/10.3390/f15071141
Li S, Wang J, Lu S, Li H, Guo J. Responses of Physiological Traits and Soil Properties in Pinus thunbergia and Euonymus japonicus Saplings under Drought and Cadmium (Cd) Stress. Forests. 2024; 15(7):1141. https://doi.org/10.3390/f15071141
Chicago/Turabian StyleLi, Shan, Jing Wang, Sen Lu, Huan Li, and Junkang Guo. 2024. "Responses of Physiological Traits and Soil Properties in Pinus thunbergia and Euonymus japonicus Saplings under Drought and Cadmium (Cd) Stress" Forests 15, no. 7: 1141. https://doi.org/10.3390/f15071141
APA StyleLi, S., Wang, J., Lu, S., Li, H., & Guo, J. (2024). Responses of Physiological Traits and Soil Properties in Pinus thunbergia and Euonymus japonicus Saplings under Drought and Cadmium (Cd) Stress. Forests, 15(7), 1141. https://doi.org/10.3390/f15071141




