Minor Effects of Canopy and Understory Nitrogen Addition on Soil Organic Carbon Turnover Time in Moso Bamboo Forests
Abstract
1. Introduction
2. Materials and Methods
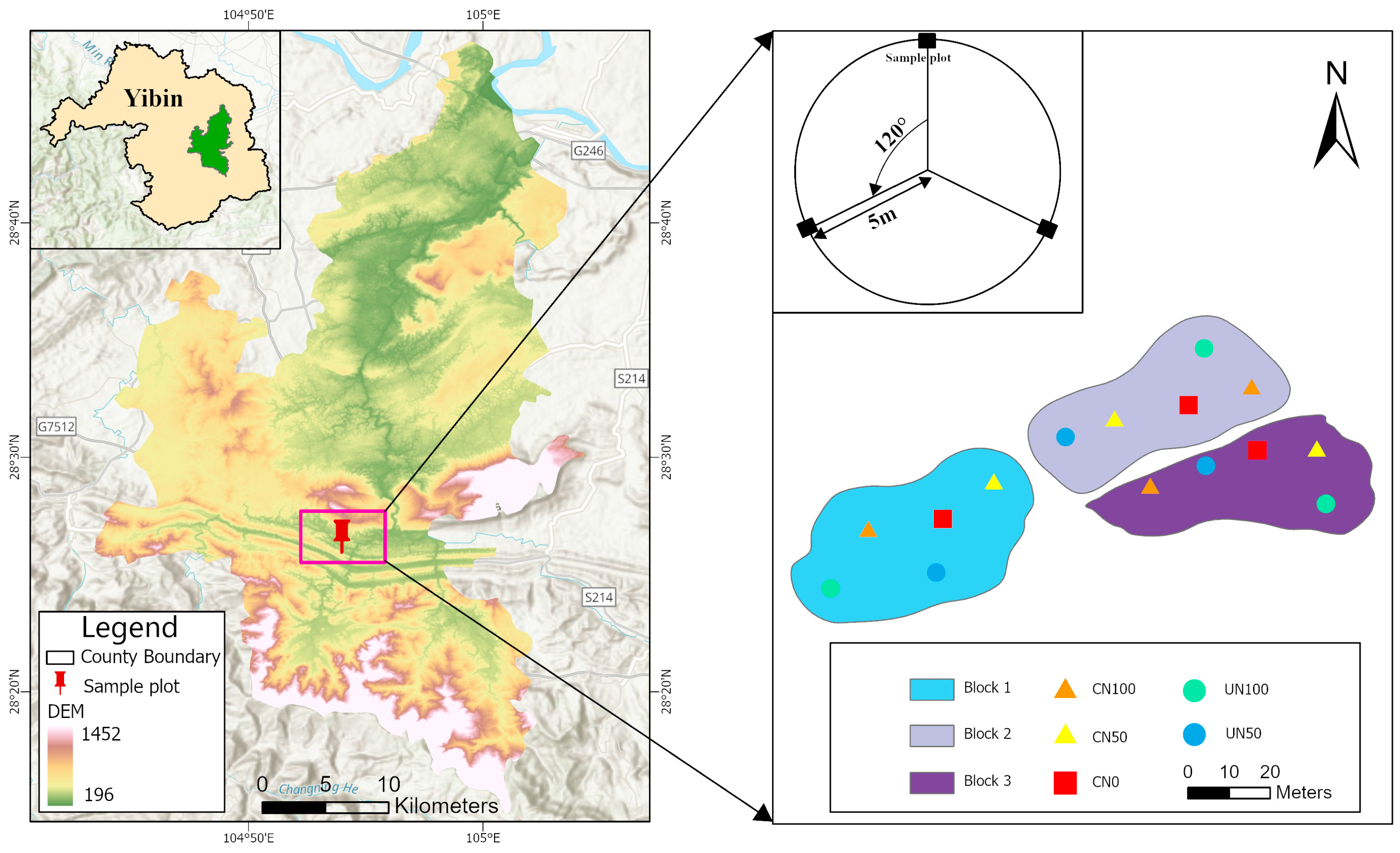
2.1. Study Site
2.2. Study Design
2.3. Soil Sampling and Analysis
2.4. Data Analysis
3. Results
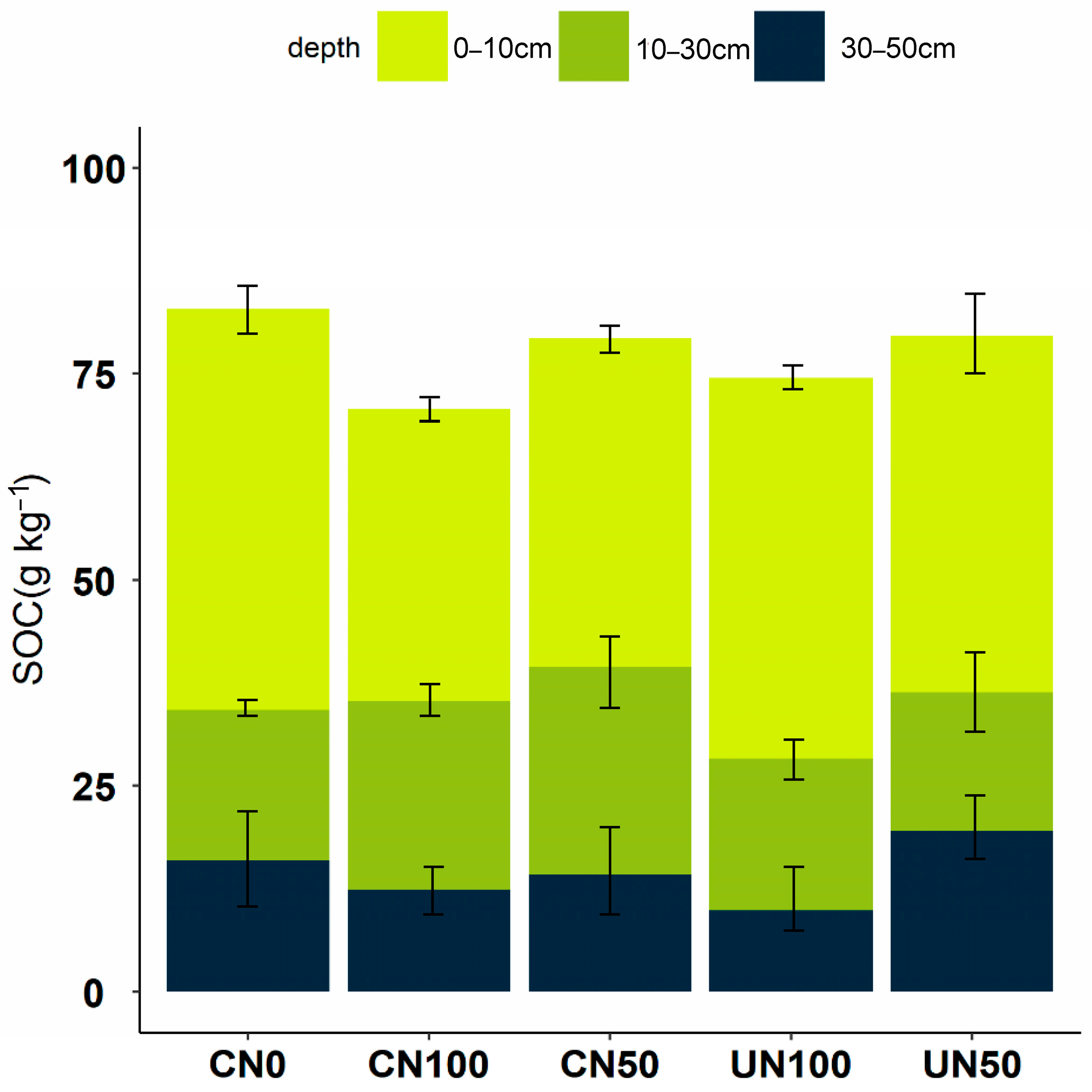
3.1. Effects of N Addition on SOC Contents and SOC Fractions
3.2. Effects of N Addition on SOC Stocks and τsoil
3.3. Influence of Environmental Factors on τsoil
4. Discussion
4.1. Effects of N Addition on SOC Contents and SOC Fractions
4.2. Effects of N Addition on τsoil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulte-Uebbing, L.; de Vries, W. Global-scale impacts of nitrogen deposition on tree carbon sequestration in tropical, temperate, and boreal forests: A meta-analysis. Glob. Chang. Biol. 2018, 24, e416–e431. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.M.; Leach, A.M.; de Vries, W. Consequences of human modification of the global nitrogen cycle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20130116. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Vitousek, P.M.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Turner, B.L.; Zhou, G.; Mo, J. Nitrogen deposition accelerates soil carbon sequestration in tropical forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2020790118. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, X.; Ju, C.; Chen, H.Y.H.; Wilsey, B.J.; Luo, Y.; Fan, W. Long-term, amplified responses of soil organic carbon to nitrogen addition worldwide. Glob. Chang. Biol. 2021, 27, 1170–1180. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, H.; Liu, T.; Zhang, W.; Shao, Y.; Ha, D.; Li, Y.; Zhang, C.; Cai, X.A.; Rao, X.; et al. Consistent effects of canopy vs. Understory nitrogen addition on the soil exchangeable cations and microbial community in two contrasting forests. Sci. Total Environ. 2016, 553, 349–357. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, G.; Wang, E. Global subsoil organic carbon turnover times dominantly controlled by soil properties rather than climate. Nat. Commun. 2019, 10, 3688. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, F.; Dong, L.; Wang, X.; Han, L.; Olesen, J.E. Effects of exogenous organic/inorganic nitrogen addition on carbon pool distribution and transformation in grassland soil. Sci. Total Environ. 2023, 858, 159919. [Google Scholar] [CrossRef]
- Scharlemann, J.; Tanner, E.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Schwarz, M.T.; Bischoff, S.; Blaser, S.; Boch, S.; Schmitt, B.; Thieme, L.; Fischer, M.; Michalzik, B.; Schulze, E.-D.; Siemens, J.; et al. More efficient aboveground nitrogen use in more diverse central european forest canopies. For. Ecol. Manag. 2014, 313, 274–282. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, G.; Liu, X.; Hao, T.; Tan, W. Responses of soil organic carbon turnover to nitrogen deposition are associated with nitrogen input rates: Derived from soil 14C evidences. Environ. Poll. 2018, 238, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, D.; Lan, Y.; Meng, J.; Jiang, L.; Sun, Q.; Cao, D.; Sun, Y.; Chen, W. Labile organic carbon fractions and carbon pool management index in a 3-year field study with biochar amendment. J. Soils Sediments 2018, 18, 1569–1578. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Jiang, Z.; Ding, J.; Sun, X.; Xu, J. Effects of biochar application on soil organic carbon composition and enzyme activity in paddy soil under water-saving irrigation. Int. J. Environ. Res. Public Health 2020, 17, 333. [Google Scholar] [CrossRef] [PubMed]
- Leifeld, J.; Kögel-Knabner, I. Soil organic matter fractions as early indicators for carbon stock changes under different land-use? Geoderma 2005, 124, 143–155. [Google Scholar] [CrossRef]
- Garten, C.T.; Hanson, P.J. Measured forest soil c stocks and estimated turnover times along an elevation gradient. Geoderma 2006, 136, 342–352. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Xia, J.; He, N.; Li, M.; Niu, S. Soil and vegetation carbon turnover times from tropical to boreal forests. Funct. Ecol. 2018, 32, 71–82. [Google Scholar] [CrossRef]
- Trugman, A.T.; Medvigy, D.; Mankin, J.S.; Anderegg, W.R.L. Soil moisture stress as a major driver of carbon cycle uncertainty. Geophys. Res. Lett. 2018, 45, 6495–6503. [Google Scholar] [CrossRef]
- Wagner, S.; Cattle, S.R.; Scholten, T. Soil-aggregate formation as influenced by clay content and organic-matter amendment. J. Soil Sci. Plant Nutr. 2007, 170, 173–180. [Google Scholar] [CrossRef]
- Walker, T.W.N.; Kaiser, C.; Strasser, F.; Herbold, C.W.; Leblans, N.I.W.; Woebken, D.; Janssens, I.A.; Sigurdsson, B.D.; Richter, A. Microbial temperature sensitivity and biomass change explain soil carbon loss with warming. Nat. Clim. Chang. 2018, 8, 885–889. [Google Scholar] [CrossRef]
- Giardina, C.P.; Ryan, M.G. Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 2000, 404, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Posada, J.M.; Schuur, E.A. Relationships among precipitation regime, nutrient availability, and carbon turnover in tropical rain forests. Oecologia 2011, 165, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wen, Z.; Yin, R.; Deng, P.; Gao, Y.; Xu, H.; Xu, X. Soil organic carbon turnover response to nitrogen and phosphorus additions in eastern china: Evidence from stable carbon isotopes. Forests 2023, 14, 1314. [Google Scholar] [CrossRef]
- Yang, X.; Duan, P.; Hicks, L.; Wang, K.; Li, D. Mechanisms underlying the responses of microbial carbon and nitrogen use efficiencies to nitrogen addition are mediated by topography in a subtropical forest. Sci. Total Environ. 2023, 880, 163236. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, Y.; Hu, J.; Tang, C.; Zhang, S.; Fu, S.; Jiang, P.; Ge, T.; Luo, Y.; Song, X.; et al. Rates of soil respiration components in response to inorganic and organic fertilizers in an intensively-managed moso bamboo forest. Geoderma 2021, 403, 115212. [Google Scholar] [CrossRef]
- Song, X.; Gu, H.; Wang, M.; Zhou, G.; Li, Q. Management practices regulate the response of moso bamboo foliar stoichiometry to nitrogen deposition. Sci. Rep. 2016, 6, 24107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Q.; Lv, J.; Peng, C.; Gu, Z.; Qi, L.; Song, X.; Song, X. Management scheme influence and nitrogen addition effects on soil CO2, CH4, and N2O fluxes in a moso bamboo plantation. For. Ecosys. 2021, 8, 6. [Google Scholar] [CrossRef]
- Lei, Z.; Li, Q.; Song, X.; Wang, W.; Zhang, Z.; Peng, C.; Tian, L. Biochar mitigates dissolved organic carbon loss but does not affect dissolved organic nitrogen leaching loss caused by nitrogen deposition in moso bamboo plantations. Glob. Ecol. Conserv. 2018, 16, e00494. [Google Scholar] [CrossRef]
- Song, X.; Peng, C.; Ciais, P.; Li, Q.; Xiang, W.; Xiao, W.; Zhou, G.; Deng, L. Nitrogen addition increased CO2 uptake more than non-CO2 greenhouse gases emissions in a moso bamboo forest. Sci. Adv. 2020, 6, eaaw5790. [Google Scholar] [CrossRef]
- Li, Y.; Feng, P. Bamboo resources in china based on the ninth national forest inventory data. World Bamboo Ratt. 2019, 17, 45–48, (In Chinese with English Abstract). [Google Scholar]
- Guerrieri, R.; Templer, P.; Magnani, F. Canopy exchange and modification of nitrogen fluxes in forest ecosystems. Curr. For. Rep. 2021, 7, 115–137. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, X.; Wang, Y.; Guo, Z.; He, D.; Fu, S.; Wan, S.; Ye, Q.; Zhang, W.; Liu, W.; et al. Responses of litter, organic and mineral soil enzyme kinetics to 6 years of canopy and understory nitrogen additions in a temperate forest. Sci. Total Environ. 2020, 712, 136383. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ren, W.; Hou, E.; Tang, S.; Zhang, L.; Liu, Z.; Lin, Y.; Fu, S.; Wen, D.; Kuang, Y. Different effects of canopy and understory nitrogen addition on soil organic carbon and its related processes in a subtropical forest. J. Soils Sediments 2020, 21, 235–244. [Google Scholar] [CrossRef]
- Lu, X.; Ren, W.; Hou, E.; Zhang, L.; Wen, D.; Liu, Z.; Lin, Y.; Wang, J.; Kuang, Y. Negative effects of canopy N addition on soil organic carbon in wet season are primarily detected in uppermost soils of a subtropical forest. Glob. Ecol. Conserv. 2019, 17, e00543. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.E.I.; Zhu, W.; Gundersen, P.E.R.; Fang, Y.; Li, D.; Wang, H.U.I. Nitrogen addition reduces soil respiration in a mature tropical forest in southern china. Glob. Chang. Biol. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Rocha, I.; Stevens, C.J.; Manrique, E.; Luciañez, M.J. Simulated nitrogen deposition affects soil fauna from a semiarid mediterranean ecosystem in central spain. Biol. Fertil. Soils 2014, 50, 191–196. [Google Scholar] [CrossRef]
- Frey, S.D.; Ollinger, S.; Nadelhoffer, K.; Bowden, R.; Brzostek, E.; Burton, A.; Caldwell, B.A.; Crow, S.; Goodale, C.L.; Grandy, A.S.; et al. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 2014, 121, 305–316. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, J.; Ma, S.; Feng, S.; Zhang, X.; Hu, D. Seasonal spatial pattern of soil respiration in a temperate urban forest in beijing. Urban For. Urban Green. 2015, 14, 1122–1130. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Mo, J.; Otieno, D.; Tenhunen, J.; Yan, J.; Liu, J.; Zhang, D. Effects of nitrogen deposition on soil organic carbon fractions in the subtropical forest ecosystems of s china. J. Plant Nutr. Soil Sci. 2012, 175, 947–953. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, P.; Liu, S.; Sun, T. Inhibition effects of n deposition on soil organic carbon decomposition was mediated by n types and soil nematode in a temperate forest. Appl. Soil Ecol. 2017, 120, 105–110. [Google Scholar] [CrossRef]
- Cai, C.; Yang, Z.; Liu, L.; Lai, Y.; Lei, J.; Fan, S.; Tang, X. Consistent effects of canopy vs. Understory nitrogen addition on soil respiration and net ecosystem production in moso bamboo forests. Forests 2021, 12, 1427. [Google Scholar] [CrossRef]
- Tian, H.; Yang, J.; Lu, C.; Xu, R.; Canadell, J.G.; Jackson, R.B.; Arneth, A.; Chang, J.; Chen, G.; Ciais, P.; et al. The global N2O model intercomparison project. Bull. Am. Meteorol. Soc. 2018, 99, 1231–1251. [Google Scholar] [CrossRef]
- Zhang, L.H.; Xie, Z.K.; Zhao, R.F.; Wang, Y.J. The impact of land use change on soil organic carbon and labile organic carbon stocks in the longzhong region of loess plateau. J. Arid Land 2012, 4, 241–250. [Google Scholar] [CrossRef]
- Luo, R.; Fan, J.; Wang, W.; Luo, J.; Kuzyakov, Y.; He, J.S.; Chu, H.; Ding, W. Nitrogen and phosphorus enrichment accelerates soil organic carbon loss in alpine grassland on the qinghai-tibetan plateau. Sci. Total Environ. 2019, 650, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Ma, H.; Luo, C.; Hu, T. Forest soil organic carbon fraction and its measure methods. J. Soil Sci. 2010, 41, 1018–1024. [Google Scholar]
- Chen, Z.; Xu, C.; An, Z.; Wang, Y.; Sun, D.; Wang, S. Research progress on fraction and analysis methods of soil carbon. Rock Miner. Anal. 2019, 38, 233–244. [Google Scholar]
- Haynes, R.J. Labile organic matter as an indicator of organic matter quality in arable and pastoral soils in new zealand. Soil Biol. Biochem. 2000, 32, 211–219. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.Org/ (accessed on 11 June 2019).
- Tian, H.; Lu, C.; Yang, J.; Banger, K.; Huntzinger, D.N.; Schwalm, C.R.; Michalak, A.M.; Cook, R.; Ciais, P.; Hayes, D.; et al. Global patterns and controls of soil organic carbon dynamics as simulated by multiple terrestrial biosphere models: Current status and future directions. Glob. Biogeochem. Cycles 2015, 29, 775–792. [Google Scholar] [CrossRef] [PubMed]
- Jouquet, P.; Harit, A.; Bottinelli, N.; Eldridge, D.J. Termite bioturbation: Fungal versus non-fungal building strategies lead to different soil sheeting stability. Soil Biol. Biochem. 2023, 176, 108868. [Google Scholar] [CrossRef]
- Wang, C.; Ma, L.; Zhang, Y.; Chen, N.; Wang, W. Spatiotemporal dynamics of wetlands and their driving factors based on pls-sem: A case study in wuhan. Sci. Total Environ. 2022, 806, 151310. [Google Scholar] [CrossRef]
- Lu, X.; Kuang, Y.; Mou, L.; Hou, E.; Fu, S.; Li, J. Canopy mitigates the effects of nitrogen deposition on soil carbon-related processes in a subtropical forest. Sci. Total Environ. 2021, 757, 143847. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Hu, T.; Zhang, J.; Li, R.; Dai, H.; Luo, S. Response of soil organic carbon and nutrients to simulated nitrogen deposition in pleioblastus amarus plantation, rainy area of west china. Acta Phytoecol. Sin. 2011, 35, 125–136. [Google Scholar] [CrossRef]
- Kuang, L.; Mou, Z.; Li, Y.; Lu, X.; Kuang, Y.; Wang, J.; Wang, F.; Cai, X.; Zhang, W.; Fu, S.; et al. Depth-driven responses of microbial residual carbon to nitrogen addition approaches in a tropical forest: Canopy addition versus understory addition. J. Environ. Manag. 2023, 340, 118009. [Google Scholar] [CrossRef] [PubMed]
- Churkina, G.; Trusilova, K.; Vetter, M.; Dentener, F. Contributions of nitrogen deposition and forest regrowth to terrestrial carbon uptake. Carbon Balance Manag. 2007, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.H.; Chen, G.; Peng, Y.; Hu, H.L.; Hu, T.X.; Zhang, J.; Li, X.W.; Liu, L.; Tang, Y. Soil biochemical responses to nitrogen addition in a bamboo forest. PLoS ONE 2014, 9, e102315. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, S.; Staelens, J.; Wuyts, K.; Van Wittenberghe, S.; Wuytack, T.; Verheyen, K.; Boeckx, P.; Samson, R. Canopy uptake of 15NH3 by four temperate tree species and the interaction with leaf properties. Water Air Soil Pollut. 2012, 223, 5643–5657. [Google Scholar] [CrossRef]
- Xiao, H. Atmospheric nitrogen deposition and nitrogen dynamics of forest ecosystems. Acta Ecol. Sin. 1999, 16, 90–99. [Google Scholar]
- Tian, Y.; Tao, L.; Wang, J.; Lu, H.; Zhou, L.; Campbell, D.E.; Ren, H. Canopy and understory nitrogen additions did not significantly change the community structure of soil fauna under a mature subtropical forest. Sci. Total Environ. 2020, 718, 137438. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Yin, G.; Mo, X.; Mao, Q.; Mo, J.; Wang, J.; Lu, X. Do long-term high nitrogen inputs change the composition of soil dissolved organic matter in a primary tropical forest? Environ. Res. Lett. 2022, 17, 095015. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Chen, C.R.; Liu, Y.Q.; Xu, Z.H. Soil soluble organic carbon and nitrogen pools under mono- and mixed species forest ecosystems in subtropical china. J. Soils Sediments 2010, 10, 1071–1081. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Dou, S.; Shen, H.; Mei, L.; Zhang, Y.; Zeng, X.; Zhang, Y.; Pei, Y.; Ren, H.; et al. Divergent responses of soil carbon and nitrogen pools to short-term nitrogen addition between two plantations in northeast china. Int. J. Agric. Biol. Eng. 2019, 12, 82–90. [Google Scholar] [CrossRef]
- Jian, J.-N.; Liu, W.-C.; Zhu, Y.-F.; Li, J.-X.; Wen, Y.-H.; Liu, F.-H.; Ren, C.-J.; Han, X.-H. Effects of short-term nitrogen addition on soil organic carbon components in robinia pseudoacacia l. Plantation. Envrion. Sci. 2023, 44, 2767–2774, (In Chinese with English Abstract). [Google Scholar]
- Chen, X.; Liu, J.; Deng, Q.; Yan, J.; Zhang, D. Effects of elevated CO2 and nitrogen addition on soil organic carbon fractions in a subtropical forest. Plant Soil 2012, 357, 25–34. [Google Scholar] [CrossRef]
- Rovira, P.; Vallejo, V.R. Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: An acid hydrolysis approach. Geoderma 2002, 107, 109–141. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Q.; Li, Q.; Liao, C.; He, M.; Liu, F. Lignin characteristics in soil profiles in different plant communities in a subtropical mixed forest. J. Plant Ecol. 2018, 11, 560–568. [Google Scholar] [CrossRef]
- Tu, L.-H.; Hu, T.-X.; Zhang, J.; Li, R.-H.; Dai, H.-Z.; Luo, S.-H. Short-term simulated nitrogen deposition increases carbon sequestration in a pleioblastus amarus plantation. Plant Soil 2010, 340, 383–396. [Google Scholar] [CrossRef]
- Verstraeten, A.; De Vos, B.; Neirynck, J.; Roskams, P.; Hens, M. Impact of air-borne or canopy-derived dissolved organic carbon (doc) on forest soil solution doc in flanders, belgium. Atmos. Environ. 2014, 83, 155–165. [Google Scholar] [CrossRef]
- Tate, K.R.; Parshotam, A.; Ross, D.J. Soil carbon storage and turnover in temperate forests and grasslands—A new zealand perspective. J. Biogeo. 1995, 22, 695–700. [Google Scholar] [CrossRef]
- Cusack, D.F.; Torn, M.S.; McDowell, W.H.; Silver, W.L. The response of heterotrophic activity and carbon cycling to nitrogen additions and warming in two tropical soils. Glob. Chang. Biol. 2010, 16, 2555–2572. [Google Scholar] [CrossRef]
- Carvalhais, N.; Forkel, M.; Khomik, M.; Bellarby, J.; Jung, M.; Migliavacca, M.; Mu, M.; Saatchi, S.; Santoro, M.; Thurner, M.; et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 2014, 514, 213–217. [Google Scholar] [CrossRef]
- Ge, R.; He, H.; Zhang, L.; Ren, X.; Williams, M.; Yu, G.; Luke Smallman, T.; Zhou, T.; Li, P.; Xie, Z.; et al. Climate sensitivities of carbon turnover times in soil and vegetation: Understanding their effects on forest carbon sequestration. J. Geophys. Res. Biogeosci. 2022, 127, e2020JG005880. [Google Scholar] [CrossRef]
- Allison, S.D.; LeBauer, D.S.; Ofrecio, M.R.; Reyes, R.; Ta, A.M.; Tran, T.M. Low levels of nitrogen addition stimulate decomposition by boreal forest fungi. Soil Biol. Biochem. 2009, 41, 293–302. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.; Kim, D.Y.; Kim, J.G. Degradation characteristics of waste lubricants under different nutrient conditions. J. Hazard. Mater. 2007, 143, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Aspray, T.J.; Gluszek, A.; Carvalho, D. Effect of nitrogen amendment on respiration and respiratory quotient (rq) in three hydrocarbon contaminated soils of different type. Chemosphere 2008, 72, 947–951. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Gilliam, F.S.; Gundersen, P.; Zhang, W.; Chen, H.; Mo, J. Interactive effects of nitrogen and phosphorus on soil microbial communities in a tropical forest. PLoS ONE 2013, 8, e61188. [Google Scholar] [CrossRef]



| Factor | SOC | SOC Stock | τsoil |
|---|---|---|---|
| CN or UN | 0.939 | 0.963 | 0.777 |
| N addition rate | 0.528 | 0.212 | 0.139 |
| Soil depth | <0.001 | 0.640 | 0.514 |
| Approach × Rate | 0.830 | 0.932 | 0.697 |
| Approach × Soil depth | 0.450 | 0.459 | 0.516 |
| Rate × Soil depth | 0.316 | 0.177 | 0.199 |
| Approach × Rate × Soil depth | 0.502 | 0.516 | 0.596 |
| Factor | ROC | EOC | DOC | MBC |
|---|---|---|---|---|
| CN or UN | 0.505 | 0.094 | 0.379 | 0.268 |
| N addition rate | 0.568 | 0.009 | 0.894 | 0.229 |
| Approach × rate | 0.093 | 0.741 | 0.473 | 0.456 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, C.; He, S.; Long, B.; Zhou, Z.; Hong, J.; Cao, H.; Yang, Z.; Tang, X. Minor Effects of Canopy and Understory Nitrogen Addition on Soil Organic Carbon Turnover Time in Moso Bamboo Forests. Forests 2024, 15, 1144. https://doi.org/10.3390/f15071144
Zeng C, He S, Long B, Zhou Z, Hong J, Cao H, Yang Z, Tang X. Minor Effects of Canopy and Understory Nitrogen Addition on Soil Organic Carbon Turnover Time in Moso Bamboo Forests. Forests. 2024; 15(7):1144. https://doi.org/10.3390/f15071144
Chicago/Turabian StyleZeng, Changli, Shurui He, Boyin Long, Zhihang Zhou, Jie Hong, Huan Cao, Zhihan Yang, and Xiaolu Tang. 2024. "Minor Effects of Canopy and Understory Nitrogen Addition on Soil Organic Carbon Turnover Time in Moso Bamboo Forests" Forests 15, no. 7: 1144. https://doi.org/10.3390/f15071144
APA StyleZeng, C., He, S., Long, B., Zhou, Z., Hong, J., Cao, H., Yang, Z., & Tang, X. (2024). Minor Effects of Canopy and Understory Nitrogen Addition on Soil Organic Carbon Turnover Time in Moso Bamboo Forests. Forests, 15(7), 1144. https://doi.org/10.3390/f15071144





