Climate as a Driver of Aboveground Biomass Density Variation: A Study of Ten Pine Species in Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Study Area and Species Selection
2.2. Database Acquisition
2.3. Aboveground Biomass Estimation and Data Cleaning
| Species | Equation | R2 | n | Author |
|---|---|---|---|---|
| P. arizónica | 0.97 | 66 | [47] | |
| P. ayacahuite | 0.97 | 58 | [47] | |
| P. cembroides | 0.98 | 30 | [48] | |
| P. devoniana | 0.98 | 20 | [49] | |
| P. leiophylla | 0.93 | 27 | [47] | |
| P. montezumae | 0.99 | 16 | [50] | |
| P. oocarpa | 0.96 | 33 | [51] | |
| P. patula | 0.99 | 25 | [52] | |
| P. pseudostrobus | 0.99 | 20 | [49] | |
| P. teocote | 0.99 | 56 | [47] |
2.4. Statistical Analysis
3. Results
3.1. Basic Comparative Analysis
3.2. Distribution Patterns and Climatic Tolerances
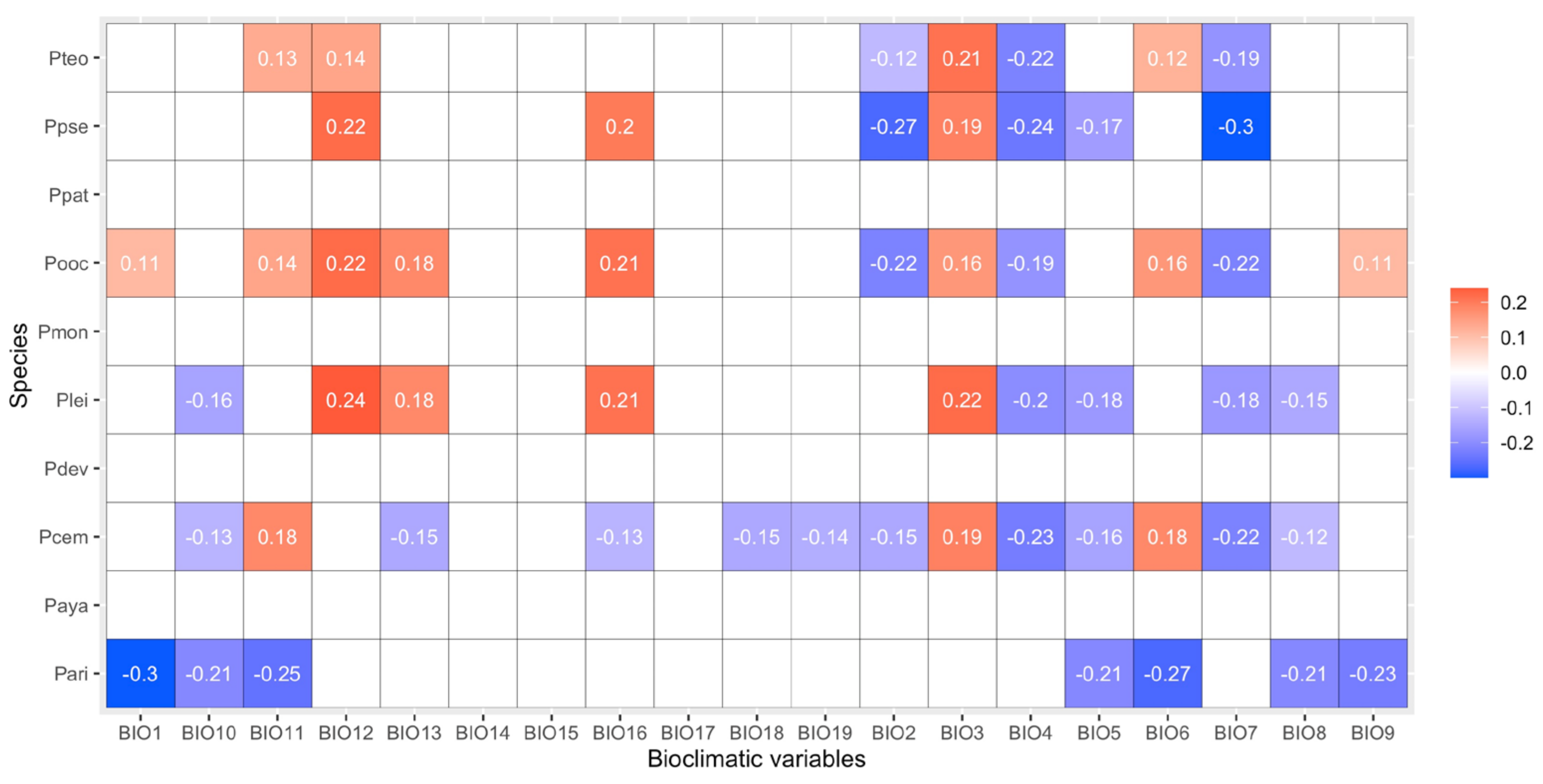
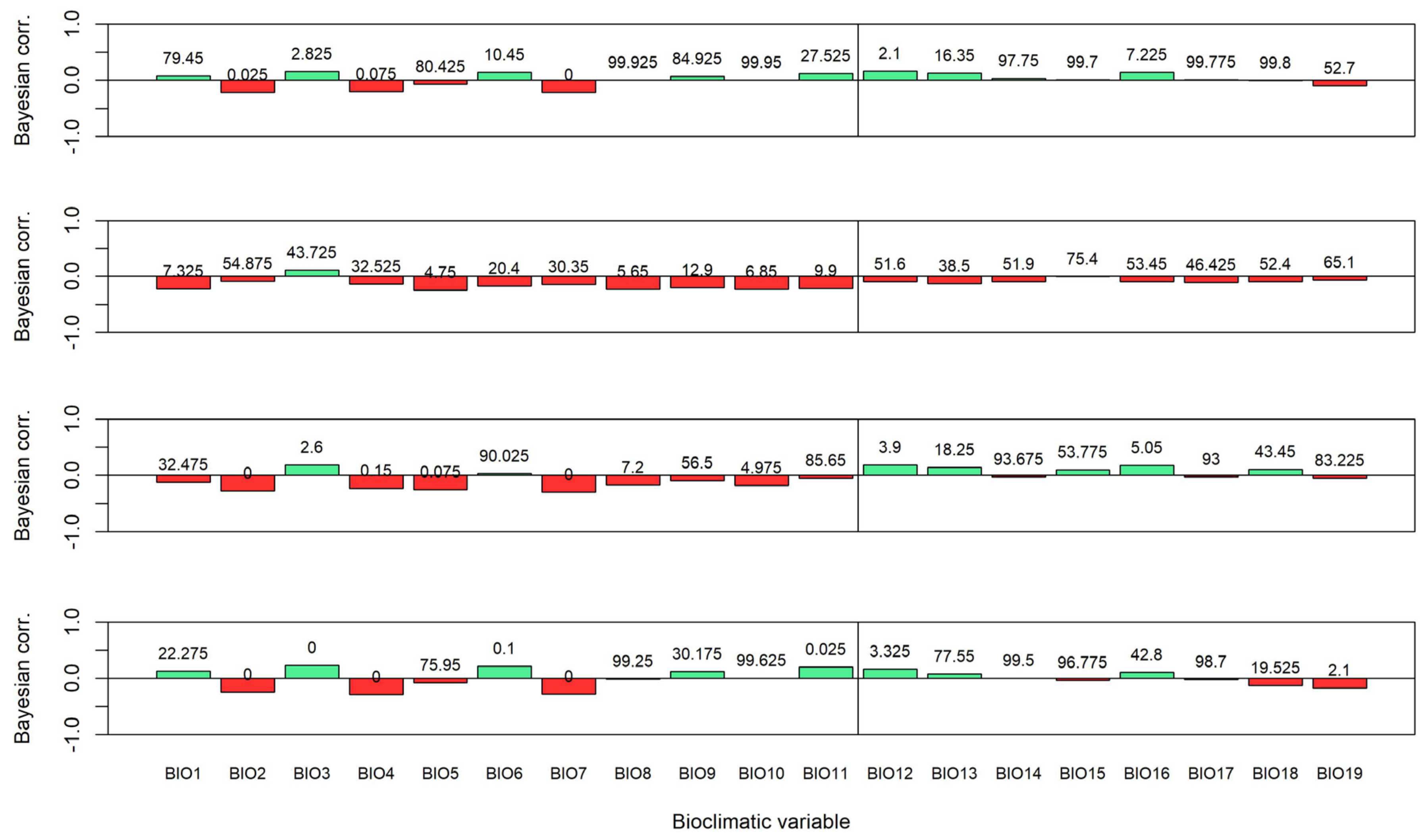
3.3. Influence of Bioclimatic Variables on Aboveground Biomass Density
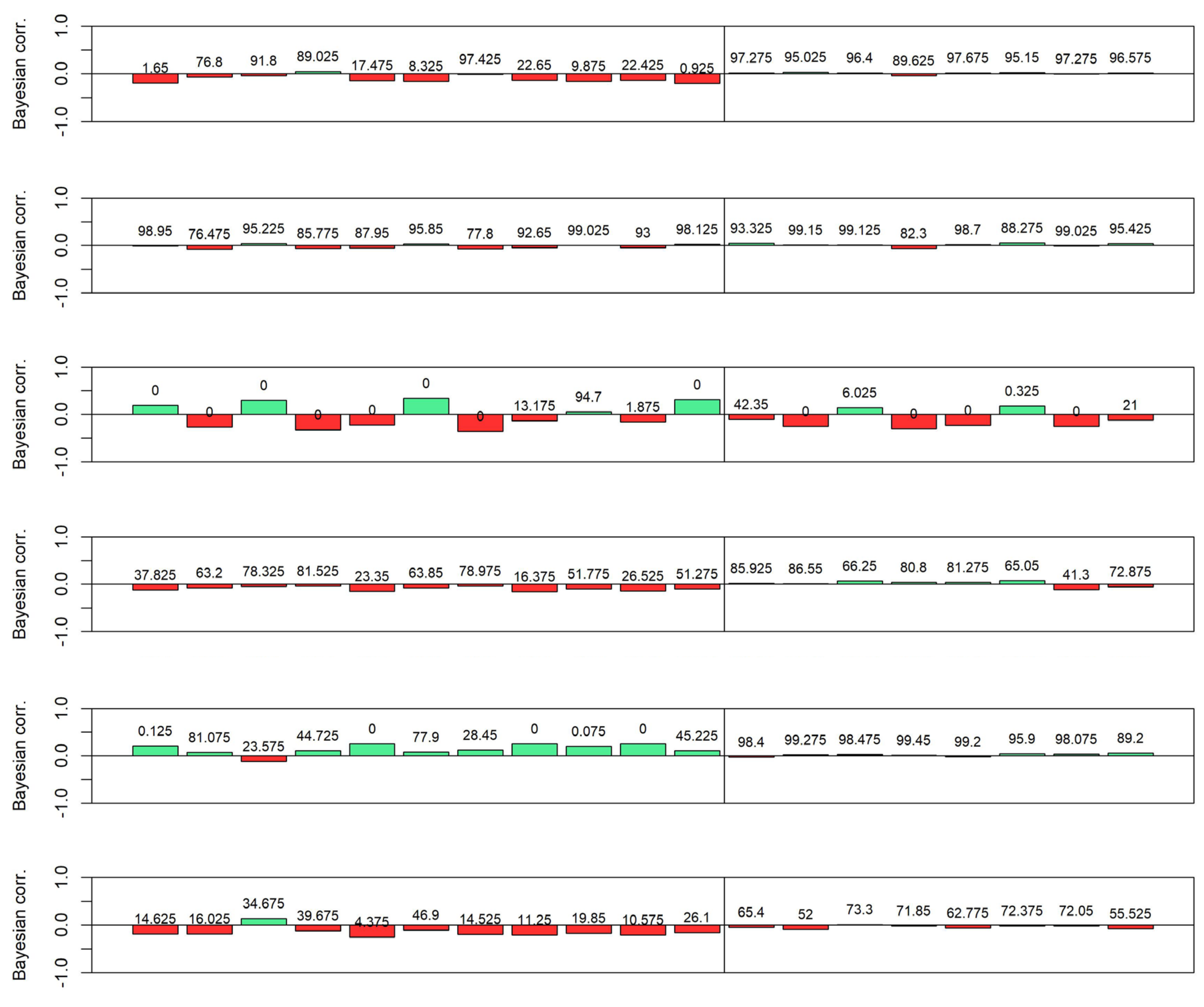
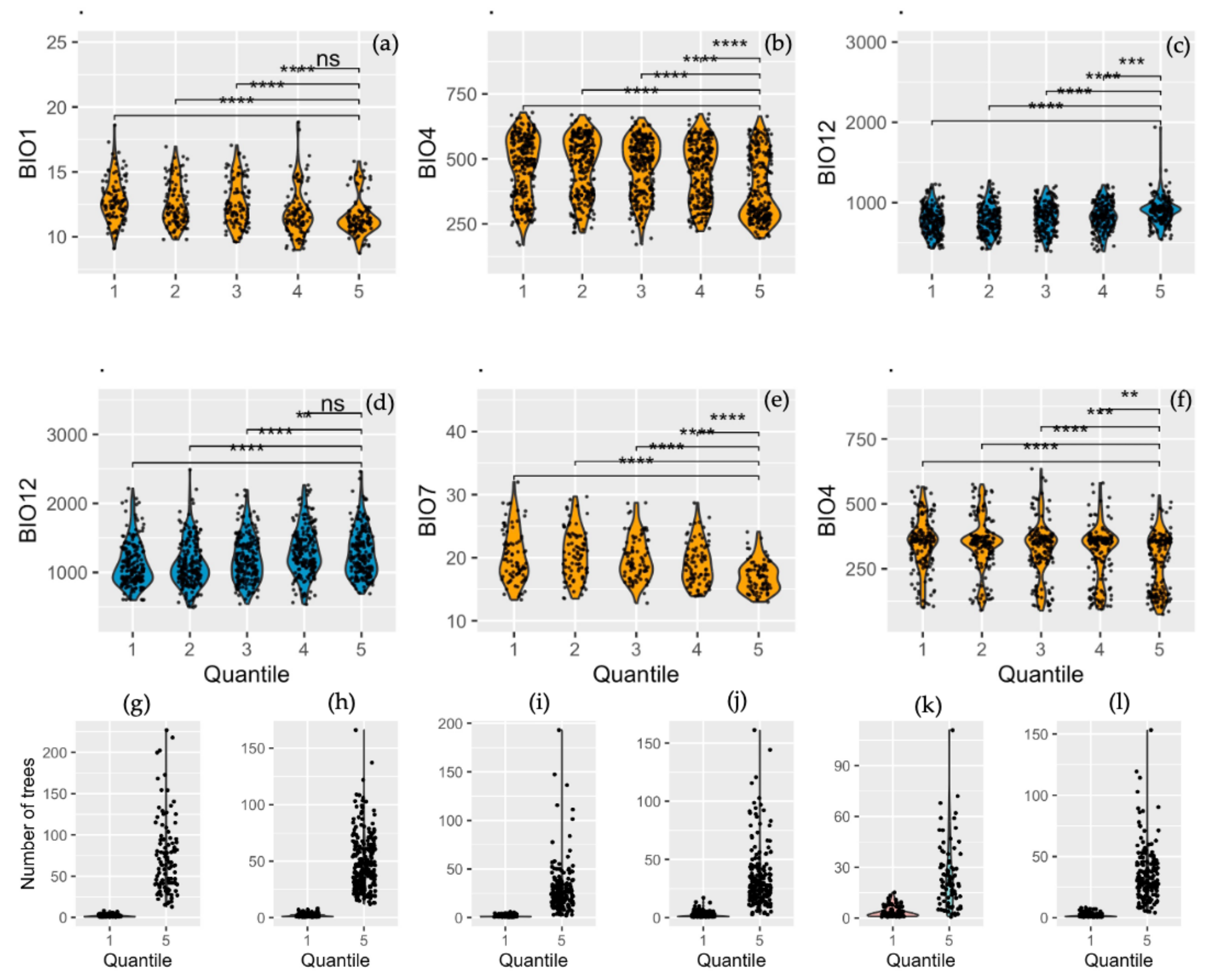
3.4. Quantile-Based Analysis of AGBd Response to Climate Variables
4. Discussion
Biomass Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable Code | Description |
| BIO1 | Annual Mean Temperature (°C) |
| BIO2 | Mean Diurnal Range (Mean of Monthly (°C) |
| BIO3 | Isothermality (BIO2/BIO7) (×100) |
| BIO4 | Temperature Seasonality (SD ×100) |
| BIO5 | Max Temperature of Warmest Month (°C) |
| BIO6 | Min Temperature of Coldest Month (°C) |
| BIO7 | Temperature Annual Range (BIO5-BIO6) |
| BIO8 | Mean Temperature of Wettest Quarter (°C) |
| BIO9 | Mean Temperature of Driest Quarter (°C) |
| BIO10 | Mean Temperature of Warmest Quarter (°C) |
| BIO11 | Mean Temperature of Coldest Quarter (°C) |
| BIO12 | Annual Precipitation (mm) |
| BIO13 | Precipitation of Wettest Month (mm) |
| BIO14 | Precipitation of Driest Month (mm) |
| BIO15 | Precipitation Seasonality (Coefficient of Variation) |
| BIO16 | Precipitation of Wettest Quarter (mm) |
| BIO17 | Precipitation of Driest Quarter (mm) |
| BIO18 | Precipitation of Warmest Quarter (mm) |
| BIO19 | Precipitation of Coldest Quarter (mm) |
Appendix B
| Specie | Bioclimatic Variable | Rho | Specie | Bioclimatic Variable | Rho |
| P. arizonica | P. oocarpa | BIO1 | 0.11 * | ||
| BIO1 | −0.3 *** | BIO2 | −0.22 *** | ||
| BIO5 | −0.21 *** | BIO3 | 0.16 *** | ||
| BIO6 | −0.27 *** | BIO4 | −0.19 *** | ||
| BIO8 | −0.21 *** | BIO6 | 0.16 *** | ||
| BIO9 | −0.23 *** | BIO7 | −0.22 *** | ||
| BIO10 | −0.21 *** | BIO9 | 0.11 * | ||
| BIO11 | −0.25 *** | BIO11 | 0.14 *** | ||
| BIO12 | 0.22 *** | ||||
| BIO13 | 0.18 *** | ||||
| BIO16 | 0.21 *** | ||||
| P. cembroides | BIO2 | −0.15 *** | P. pseudostrobus | ||
| BIO3 | 0.19 *** | BIO2 | −0.27 *** | ||
| BIO4 | −0.23 *** | BIO3 | 0.19 ** | ||
| BIO5 | −0.16 *** | BIO4 | −0.24 *** | ||
| BIO6 | 0.18 *** | BIO5 | −0.17 * | ||
| BIO7 | −0.22 *** | BIO7 | −0.3 *** | ||
| BIO8 | −0.12 *** | BIO12 | 0.22 *** | ||
| BIO10 | −0.13 *** | BIO16 | 0.2 *** | ||
| BIO11 | 0.18 *** | ||||
| BIO13 | −0.15 *** | ||||
| BIO16 | −0.13 *** | ||||
| P. leiophylla | BIO18 | −0.15 *** | P. teocote | ||
| BIO19 | −0.14 *** | ||||
| BIO3 | 0.22 *** | ||||
| BIO4 | −0.20 *** | BIO2 | −0.12 * | ||
| BIO5 | −0.18 *** | BIO3 | 0.21 *** | ||
| BIO7 | −0.18 *** | BIO4 | −0.22 *** | ||
| BIO8 | −0.15 *** | BIO7 | −0.19 *** | ||
| BIO10 | −0.16 *** | BIO11 | 0.13 ** | ||
| BIO12 | 0.24 *** | BIO12 | 0.14 ** | ||
| BIO13 | 0.18 *** | ||||
| BIO16 | 0.21 *** | ||||
| Note: Significance of Spearman’s correlation; * p < 0.05, ** p < 0.01, *** p < 0.001. | |||||
Appendix C
| QUANTILE 1 | ||||||
| P. arizonica | P. cembroides | P. leiophylla | P. oocarpa | P. pseudostrubus | P. teocote | |
| Bioclimatic variable | BIO1 | BIO4 | BIO12 | BIO12 | BIO7 | BIO4 |
| Min | 9.10 | 168.80 | 431.00 | 600.00 | 13.30 | 101.19 |
| Max | 18.60 | 678.78 | 1230.00 | 2216.00 | 32.00 | 564.99 |
| Mean | 12.86 | 458.46 | 778.01 | 1121.35 | 20.22 | 347.51 |
| Mediana | 12.67 | 490.94 | 774.00 | 1050.50 | 19.20 | 358.27 |
| SD | 1.67 | 120.52 | 171.03 | 317.93 | 4.10 | 99.50 |
| QUANTILE 2 | ||||||
| P. arizonica | P. cembroides | P. leiophylla | P. oocarpa | P. pseudostrubus | P. teocote | |
| Bioclimatic variable | BIO1 | BIO4 | BIO12 | BIO12 | BIO7 | BIO4 |
| Min | 9.80 | 216.53 | 422.00 | 494.00 | 13.50 | 90.44 |
| Max | 16.95 | 669.43 | 1269.00 | 2490.00 | 29.70 | 575.05 |
| Mean | 12.55 | 472.08 | 784.34 | 1130.05 | 20.55 | 345.69 |
| Mediana | 12.20 | 496.24 | 771.00 | 1062.00 | 20.40 | 354.40 |
| SD | 1.66 | 114.29 | 176.30 | 331.83 | 3.85 | 104.14 |
| QUANTILE 3 | ||||||
| P. arizonica | P. cembroides | P. leiophylla | P. oocarpa | P. pseudostrubus | P. teocote | |
| Bioclimatic variable | BIO1 | BIO4 | BIO12 | BIO12 | BIO7 | BIO4 |
| Min | 9.60 | 170.86 | 394.00 | 544.00 | 12.80 | 89.06 |
| Max | 17.05 | 658.56 | 1206.00 | 2195.00 | 28.70 | 634.82 |
| Mean | 12.50 | 468.18 | 814.92 | 1195.24 | 19.86 | 323.20 |
| Mediana | 12.17 | 494.95 | 829.00 | 1165.00 | 19.10 | 347.05 |
| SD | 1.72 | 108.17 | 178.56 | 329.96 | 3.37 | 106.57 |
| QUANTILE 4 | ||||||
| P. arizonica | P. cembroides | P. leiophylla | P. oocarpa | P. pseudostrubus | P. teocote | |
| Bioclimatic variable | BIO1 | BIO4 | BIO12 | BIO12 | BIO7 | BIO4 |
| Min | 8.99 | 222.35 | 394.00 | 538.00 | 13.90 | 94.44 |
| Max | 18.84 | 672.84 | 1219.00 | 2265.00 | 28.70 | 579.92 |
| Mean | 11.93 | 454.05 | 830.28 | 1300.00 | 19.06 | 311.09 |
| Mediana | 11.65 | 476.88 | 828.00 | 1273.00 | 18.70 | 349.60 |
| SD | 1.84 | 113.42 | 171.85 | 344.91 | 3.35 | 106.51 |
| QUANTILE 5 | ||||||
| P. arizonica | P. cembroides | P. leiophylla | P. oocarpa | P. pseudostrubus | P. teocote | |
| Bioclimatic variable | BIO1 | BIO4 | BIO12 | BIO12 | BIO7 | BIO4 |
| Min | 8.74 | 195.01 | 542.00 | 692.00 | 13.00 | 74.84 |
| Max | 15.82 | 664.21 | 1940.00 | 2462.00 | 24.10 | 532.58 |
| Mean | 11.57 | 387.17 | 898.22 | 1288.27 | 16.97 | 272.41 |
| Mediana | 11.23 | 350.06 | 904.50 | 1235.00 | 16.90 | 303.20 |
| SD | 1.47 | 124.36 | 162.16 | 338.90 | 2.36 | 110.99 |
References
- SEMARNAT. Anuario Estadístico de la Producción Forestal 2017–2018; SEMARNAT: Mexico City, Mexico, 2021. [Google Scholar]
- Galicia, L.; Chávez-Vergara, B.M.; Kolb, M.; Jasso-Flores, R.I.; Rodríguez-Bustos, L.A.; Solís, L.E.; de la Cruz, V.G.; Pérez-Campuzano, E.; Villanueva, Y.A. Perspectives of the socioecological approach in the preservation, utilization and the payment of environmental services of the temperate forests of Mexico. Madera Bosques 2018, 24, e2421443. [Google Scholar] [CrossRef]
- Perry, J. The Pines of Mexico and Central America; Timber Press: Portland, OR, USA, 1991. [Google Scholar]
- Sánchez-González, A. Una visión actual de la diversidad y distribución actual de los pinos de México. Madera Bosques 2008, 14, 107–120. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, Distribution, and Biomass of the World’s forests. Annu. Rev. Ecol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Martin, A.R.; Doraisami, M.; Thomas, S.C. Global patterns in wood carbon concentration across the world’s trees and forests. Nat. Geosci. 2018, 11, 915–920. [Google Scholar] [CrossRef]
- IPCC. Uso de la tierra, cambio de uso de la tierra y silvicultura. In Resumen para Responsables de Políticas; IPCC: Valencia, Spain, 2000; Volume 24. [Google Scholar]
- Ullah, F.; Gilani, H.; Sanaei, A.; Hussain, K.; Ali, A. Stand structure determines aboveground biomass across temperate forest types and species mixture along a local-scale elevational gradient. For. Ecol. Manag. 2021, 486, 118984. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Veiga, P.; Saatchi, S.; Tansey, K.; Balzter, H. Magnitude, spatial distribution and uncertainty of forest biomass stocks in Mexico. Remote Sens. Environ. 2016, 183, 265–281. [Google Scholar] [CrossRef]
- Coomes, D.; Simonson, W.; Burslem, D. Forests and Global Change; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781107041851. [Google Scholar]
- Ali, A.; Lin, S.L.; He, J.K.; Kong, F.M.; Yu, J.H.; Jiang, H.S. Climatic water availability is the main limiting factor of biotic attributes across large-scale elevational gradients in tropical forests. Sci. Total Environ. 2019, 647, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Z.; Jin, G. Impacts of stand density on tree crown structure and biomass: A global meta-analysis. Agric. For. Meteorol. 2022, 326, 109181. [Google Scholar] [CrossRef]
- Fotis, A.T.; Murphy, S.J.; Ricart, R.D.; Krishnadas, M.; Whitacre, J.; Wenzel, J.W.; Queenborough, S.A.; Comita, L.S. Above-ground biomass is driven by mass-ratio effects and stand structural attributes in a temperate deciduous forest. J. Ecol. 2018, 106, 561–570. [Google Scholar] [CrossRef]
- Balima, L.H.; Kouamé, F.N.G.; Bayen, P.; Ganamé, M.; Nacoulma, B.M.I.; Thiombiano, A.; Soro, D. Influence of climate and forest attributes on aboveground carbon storage in Burkina Faso, West Africa. Environ. Chall. 2021, 4, 100123. [Google Scholar] [CrossRef]
- Arasa-Gisbert, R.; Vayreda, J.; Román-Cuesta, R.M.; Villela, S.A.; Mayorga, R.; Retana, J. Forest diversity plays a key role in determining the stand carbon stocks of Mexican forests. For. Ecol. Manag. 2018, 415–416, 160–171. [Google Scholar] [CrossRef]
- Álvarez-Dávila, E.; Cayuela, L.; González-Caro, S.; Aldana, A.M.; Stevenson, P.R.; Phillips, O.; Cogollo, Á.; Peñuela, M.C.; Von Hildebrand, P.; Jiménez, E.; et al. Forest biomass density across large climate gradients in northern South America is related to water availability but not with temperature. PLoS ONE 2017, 12, e0171072. [Google Scholar] [CrossRef]
- Jia, B.; Guo, W.; He, J.; Sun, M.; Chai, L.; Liu, J.; Wang, X. Topography, Diversity, and Forest Structure Attributes Drive Aboveground Carbon Storage in Different Forest Types in Northeast China. Forests 2022, 13, 455. [Google Scholar] [CrossRef]
- Usuga, J.C.L.; Toro, J.A.R.; Alzate, M.V.R.; de Jesús Lema Tapias, Á. Estimation of biomass and carbon stocks in plants, soil and forest floor in different tropical forests. For. Ecol. Manag. 2010, 260, 1906–1913. [Google Scholar] [CrossRef]
- Chen, X.; Luo, M.; Larjavaara, M. Effects of climate and plant functional types on forest above-ground biomass accumulation. Carbon Balance Manag. 2023, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Luo, Y.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl. Acad. Sci. USA 2014, 111, 13721–13726. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.C.; Penman, T.D.; Arndt, S.K.; Roxburgh, S.H.; Bennett, L.T. Climate more important than soils for predicting forest biomass at the continental scale. Ecography 2020, 43, 1692–1705. [Google Scholar] [CrossRef]
- Ma, Y.; Eziz, A.; Halik, Ü.; Abliz, A.; Kurban, A. Precipitation and Temperature Influence the Relationship between Stand Structural Characteristics and Aboveground Biomass of Forests—A Meta-Analysis. Forests 2023, 14, 896. [Google Scholar] [CrossRef]
- IPCC. Summary for Policy Markers:Climate Change 2023: Synthesis Report; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Harvey, J.E.; Smiljanić, M.; Scharnweber, T.; Buras, A.; Cedro, A.; Cruz-García, R.; Drobyshev, I.; Janecka, K.; Jansons, Ā.; Kaczka, R.; et al. Tree growth influenced by warming winter climate and summer moisture availability in northern temperate forests. Glob. Change Biol. 2020, 26, 2505–2518. [Google Scholar] [CrossRef]
- Alfaro-Ramírez, U.; Ramírez-Albores, E.; Vargas-Hernández, J.; Franco-Maass, S.; Pérez-Suárez, M. Potential reduction of Hartweg´s Pine (Pinus hartwegii Lindl.) geographic distribution. PLoS ONE 2020, 15, e0229178. [Google Scholar] [CrossRef] [PubMed]
- Villers-Ruiz, L.; Trejo-Vázquez, I. Assessment of the vulnerability of forest ecosystems to climate change in Mexico. Clim. Res. 1997, 9, 87–93. [Google Scholar] [CrossRef]
- Rodríguez-Zúñiga, J.; Flores-Ortiz, C.M.; González-Guillén, M.D.J.; Lira-Saade, R.; Rodríguez-Arévalo, N.I.; Dávila-Aranda, P.D.; Ulian, T. Cost Analysis of Seed Conservation of Commercial Pine Species Vulnerable to Climate Change in Mexico. Forests 2022, 13, 539. [Google Scholar] [CrossRef]
- INEGI. México en Cifras. Available online: https://www.inegi.org.mx/app/areasgeograficas/#collapse-Res (accessed on 13 March 2024).
- DOF. NORMA Oficial Mexicana de Emergencia NOM, EM 001-SARH3-1994; Diario Oficial de la Federación: Mexico City, Mexico, 1994; pp. 5–7. [Google Scholar]
- SEMARNAT; CONAFOR. La Producción de Resina de Pino en México; Comisión Nacional Forestal: Zapopan, Mexico, 2012. [Google Scholar]
- CONAFOR; CONABIO. Pinus montezumae Lamb. Available online: http://www.conafor.gob.mx:8080/documentos/docs/13/971Pinus%20montezumae.pdf (accessed on 21 June 2024).
- CONAFOR; CONABIO. Pinus oocarpa Shiede. Available online: http://www.conafor.gob.mx:8080/documentos/docs/13/974Pinus%20oocarpa.pdf (accessed on 21 June 2024).
- Sáenz-Romero, C.; Guzmán-Reyna, R.R.; Rehfeldt, G.E. Altitudinal genetic variation among Pinus oocarpa populations in Michoacán, Mexico. Implications for seed zoning, conservation, tree breeding and global warming. For. Ecol. Manag. 2006, 229, 340–350. [Google Scholar] [CrossRef]
- CONAFOR; CONABIO. Pinus devoniana Lindley. Available online: http://www.conafor.gob.mx:8080/documentos/docs/13/957Pinus%20devoniana.pdf (accessed on 21 June 2024).
- Gómez-Romero, M.; Soto–Correa, J.; Blanco-García, J.A.; Sáenz–Romero, C.; Villegas, J.; Linding-Cisneros, R. Estudio de especies de pino para restauración de sitios degradados. Agrociencia 2012, 46, 795–807. [Google Scholar]
- CONAFOR; CONABIO. Pinus cembroides Zucc. Available online: http://www.conafor.gob.mx:8080/documentos/docs/13/955Pinus%20cembroides.pdf (accessed on 21 June 2024).
- SEMARNAT; CONAFOR; CONACYT. Catálogo de Recursos Forestales Maderables y no Maderables Árido, Tropical y Templado; CONAFOR: Zapopan, Mexico, 2008. [Google Scholar]
- Constante García, V.; Villanueva Díaz, J.; Cerano Paredes, J.; Cornejo Oviedo, E.H.; Valencia Manzo, S. Dendrocronología de Pinus cembroides Zucc. y reconstrucción de precipitación estacional para el Sureste de Coahuila. Cienc. For. México 2009, 34, 17–39. [Google Scholar]
- CONAFOR; CONABIO. Pinus patula Schl. et Cham. Available online: http://www.conafor.gob.mx:8080/documentos/docs/13/975Pinus%20patula.pdf (accessed on 21 June 2024).
- CONAFOR; CONABIO. Pinus ayacahuite Ehren. Available online: http://www.conafor.gob.mx:8080/documentos/docs/13/953Pinus%20ayacahuite.pdf (accessed on 21 June 2024).
- CONAFOR; CONABIO. Pinus arizonica Engelm. Available online: http://www.conafor.gob.mx:8080/documentos/docs/13/952Pinus%20arizonica.pdf (accessed on 21 June 2024).
- CONAFOR. Procedimientos de Muestreo Version 19.0; SEMARNAT-CONAFOR: Guadalajara, Mexico, 2017. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Rzedowski, J. Vegetación de México; Edición Digital; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2006. [Google Scholar]
- Návar, J. Allometric equations for tree species and carbon stocks for forests of northwestern Mexico. For. Ecol. Manag. 2009, 257, 427–434. [Google Scholar] [CrossRef]
- Guerrero, L. Cuantificación de Biomasa, Carbono y Producción de Oxígeno de Pinus cembroides Zucc. Bachelor’s Thesis, Universidad Autónoma Agraria Antonio Narro, En Mazapil, Zacatecas, Mexico, 2013. [Google Scholar]
- Méndez-González, J.; Luckie-Navarrete, S.L.; Capó-Arteaga, M.A.; Nájera-Luna, J.A. Ecuaciones alométricas y estimación de incrementos en biomasa aérea y carbono en una plantación mixta de Pinus devoniana Lindl. y Pinus pseudostrobus Lindl., En Guanajuato, México. Agrociencia 2011, 45, 479–491. [Google Scholar]
- Carrillo-Anzúres, F.; Acosta-Mireles, M.; Flores-Ayala, E.; Juárez-Bravo, J.E.; Bonilla-Padilla, E. Estimación de biomasa y carbono en dos especies arboreas en La Sierra Nevada, México. Rev. Mex. Cienc. Agrícolas 2018, 5, 779–793. [Google Scholar] [CrossRef]
- Navarro-Martínez, J.; Godínez-Jaimes, F.; López-López, M.Á.; Rosas-Acevedo, J.L.; Juárez-López, A.L.; Reyes-Umaña, M. Ajuste de ecuaciones alométricas para estimar biomasa aérea en Pinus oocarpa y Quercus resinosa en Guerrero, México. Madera Bosques 2020, 26, e2611964. [Google Scholar] [CrossRef]
- Díaz-Franco, R.; Acosta-Mireles, M.; Carrillo-Anzures, F.; Buendía-Rodríguez, E.; Flores-Ayala, E.; Etchevers-Barra, J.D. Determinación de ecuaciones alométricas para estimar biomasa y carbono en Pinus patula Schl. et Cham. Madera Bosques 2007, 13, 25–34. [Google Scholar] [CrossRef]
- Moyroud, N.; Portet, F. Introduction to QGIS. In QGIS and Generic Tools; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; Chapter 1; pp. 1–17. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- De Mendiburo, F. Una Herramienta de Análisis Estadístico para la Investigación Agrícola; Universidad Nacional de Ingenieria: Rímac, Peru, 2009. [Google Scholar]
- Kassambara, A. rstatix:Pipe-Friendly Framework for Basic Statical Tests; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Makowski, D.; Ben-Shachar, M.S.; Lüdecke, D. bayestestR: Describing Effects and their Uncertainty, Existence and Significance within the Bayesian Framework. J. Open Source Softw. 2019, 4, 1541. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr: “ggplot2” Based Publication Ready Plots; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Wang, L.Q.; Ali, A. Climate regulates the functional traits—Aboveground biomass relationships at a community-level in forests: A global meta-analysis. Sci. Total Environ. 2021, 761, 143238. [Google Scholar] [CrossRef]
- Wang, L.-Q.; Ali, A.; Wang, L.-Q.; Ali, A. Functional identity regulates aboveground biomass better than trait diversity along abiotic conditions in global forest metacommunities. Ecography 2021, 2022, e05854. [Google Scholar] [CrossRef]
- Cañas, R.A.; Canales, J.; Muñoz-Hernández, C.; Granados, J.M.; Ávila, C.; García-Martín, M.L.; Cánovas, F.M. Understanding developmental and adaptive cues in pine through metabolite profiling and co-expression network analysis. J. Exp. Bot. 2015, 66, 3113. [Google Scholar] [CrossRef]
- Gómez-Mendoza, L.G.; Arriaga, L. Modeling the Effect of Climate Change on the Distribution of Oak and Pine Species of Mexico. Conserv. Biol. 2007, 21, 1545–1555. [Google Scholar] [CrossRef]
- Aceves-Rangel, L.; Méndez-González, J.; García-Aranda, M.A.; Nájera-Luna, J.A. Distribución potencial de 20 especies de pinos en México. Agrociencia 2018, 52, 1043–1057. [Google Scholar]
- Alfaro-Reyna, T.; Retana, J.; Arasa-Gisbert, R.; Vayreda, J.; Martínez-Vilalta, J. Recent dynamics of pine and oak forests in Mexico. Eur. J. For. Res. 2020, 139, 179–187. [Google Scholar] [CrossRef]
- Slik, J.W.F.; Aiba, S.I.; Brearley, F.Q.; Cannon, C.H.; Forshed, O.; Kitayama, K.; Nagamasu, H.; Nilus, R.; Payne, J.; Paoli, G.; et al. Environmental correlates of tree biomass, basal area, wood specific gravity and stem density gradients in Borneo’s tropical forests. Glob. Ecol. Biogeogr. 2010, 19, 50–60. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, G.; Wang, Q.; Zhang, Y. How temperature, precipitation and stand age control the biomass carbon density of global mature forests. Glob. Ecol. Biogeogr. 2014, 23, 323–333. [Google Scholar] [CrossRef]
- Escobar-Alonso, S.; Rodríguez-Trejo, D.A.; Escobar-Alonso, S.; Rodríguez Trejo, D.A. Estado del arte en la investigación sobre calidad de planta del género Pinus en México. Rev. Mex. Cienc. For. 2019, 10, 4–38. [Google Scholar] [CrossRef]
- De Frenne, P.; Lenoir, J.; Luoto, M.; Scheffers, B.R.; Zellweger, F.; Aalto, J.; Ashcroft, M.B.; Christiansen, D.M.; Decocq, G.; De Pauw, K.; et al. Forest microclimates and climate change: Importance, drivers and future research agenda. Glob. Change Biol. 2021, 27, 2279–2297. [Google Scholar] [CrossRef]
- Ali, A.; Sanaei, A.; Li, M.; Nalivan, O.A.; Ahmadaali, K.; Pour, M.J.; Valipour, A.; Karami, J.; Aminpour, M.; Kaboli, H.; et al. Impacts of climatic and edaphic factors on the diversity, structure and biomass of species-poor and structurally-complex forests. Sci. Total Environ. 2020, 706, 135719. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef]
- Ni, Y.; Jian, Z.; Zeng, L.; Liu, J.; Lei, L.; Zhu, J.; Xu, J.; Xiao, W. Climate, soil nutrients, and stand characteristics jointly determine large-scale patterns of biomass growth rates and allocation in Pinus massoniana plantations. For. Ecol. Manag. 2022, 504, 119839. [Google Scholar] [CrossRef]
- González-Cásares, M.; Acosta-Hernández, A.C.; Zúñiga-Vazquez, J.M.; Herrera-Soto, G.; Camarero, J.J. Responses of Growth to Climate and Drought in Two Sympatric Mexican Pine Species Marcos. In Latin American Dendroecology; Pompa-García, M., Camarero, J.J., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 61–77. ISBN 9783030369293. [Google Scholar]
- Pompa-García, M.; Hadad, M.A. Sensitivity of pines in Mexico to temperature varies with age. Atmósfera 2016, 29, 209–219. [Google Scholar] [CrossRef]
- Vivar-Vivar, E.D.; Pompa-García, M.; Camarero, J.J. Tree Rings Elucidate Differential Drought Responses in Stands of Three Mexican Pines. Forests 2024, 15, 994. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Change Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Vieilledent, G.; Gardi, O.; Grinand, C.; Burren, C.; Andriamanjato, M.; Camara, C.; Gardner, C.J.; Glass, L.; Rasolohery, A.; Rakoto Ratsimba, H.; et al. Bioclimatic envelope models predict a decrease in tropical forest carbon stocks with climate change in Madagascar. J. Ecol. 2016, 104, 703–715. [Google Scholar] [CrossRef]
- Rosas-Chavoya, M.; López-Serrano, P.M.; Vega-Nieva, D.J.; Hernández-Díaz, J.C.; Wehenkel, C.; Corral-Rivas, J.J. Estimating Above-Ground Biomass from Land Surface Temperature and Evapotranspiration Data at the Temperate Forests of Durango, Mexico. Forests 2023, 14, 299. [Google Scholar] [CrossRef]
- Toledo, M.; Peña-Claros, M.; Bongers, F.; Alarcón, A.; Balcázar, J.; Chuviña, J.; Leaño, C.; Licona, J.C.; Poorter, L. Distribution patterns of tropical woody species in response to climatic and edaphic gradients. J. Ecol. 2012, 100, 253–263. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Ji, Y.; Yu, M.; Li, X.; Duan, J.; Wang, Y.; Gao, J.; Guo, X. Climate Factors Affect Above–Belowground Biomass Allocation in Broad-Leaved and Coniferous Forests by Regulating Soil Nutrients. Plants 2023, 12, 3926. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Swenson, N.G.; Enquist, B.J.; White, E.P.; Phillips, O.L.; Jørgensen, P.M.; Weiser, M.D.; Monteagudo Mendoza, A.; Núñez Vargas, P. Variation in above-ground forest biomass across broad climatic gradients. Glob. Ecol. Biogeogr. 2011, 20, 744–754. [Google Scholar] [CrossRef]
- Lie, Z.; Xue, L.; Jacobs, D.F. Allocation of forest biomass across broad precipitation gradients in China’s forests. Sci. Rep. 2018, 8, 10536. [Google Scholar] [CrossRef] [PubMed]
- Sandel, B.; Goldstein, L.J.; Kraft, N.J.B.; Okie, J.G.; Shuldman, M.I.; Ackerly, D.D.; Cleland, E.E.; Suding, K.N. Contrasting trait responses in plant communities to experimental and geographic variation in precipitation. New Phytol. 2010, 188, 565–575. [Google Scholar] [CrossRef]
- Lin, D.; Xia, J.; Wan, S. Climate warming and biomass accumulation of terrestrial plants: A meta-analysis. New Phytol. 2010, 188, 187–198. [Google Scholar] [CrossRef]
- Saatchi, S.; Houghton, R.A.; Dos Santos Alvalá, R.C.; Soares, J.V.; Yu, Y. Distribution of aboveground live biomass in the Amazon basin. Glob. Change Biol. 2007, 13, 816–837. [Google Scholar] [CrossRef]
- IPCC. Synthesis Report, Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2023; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar]
- McKenney, D.W.; Pedlar, J.H.; Lawrence, K.; Campbell, K.; Hutchinson, M.F. Potential Impacts of Climate Change on the Distribution of North American Trees. Bioscience 2007, 57, 939–948. [Google Scholar] [CrossRef]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; López, R.; Sáenz-Romero, C.; Hartmann, H.; Breshears, D.D.; Allen, C.D. Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests. Nat. Commun. 2022, 13, 1761. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Robayo, A.P.; Ureta, C.; Gómez-Albores, M.A.; Meneses-Mosquera, A.K.; Téllez-Valdés, O.; Martínez-Meyer, E. One hundred years of climate change in Mexico. PLoS ONE 2020, 15, e0209808. [Google Scholar] [CrossRef] [PubMed]
- Larjavaara, M.; Lu, X.; Chen, X.; Vastaranta, M. Impact of rising temperatures on the biomass of humid old-growth forests of the world. Carbon Balance Manag. 2021, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, E.; Trejo, I. Efecto del cambio climático en la distribución potencial de cinco especies arbóreas de bosque templado en México. Rev. Mex. Biodivers. 2014, 85, 179–188. [Google Scholar] [CrossRef]
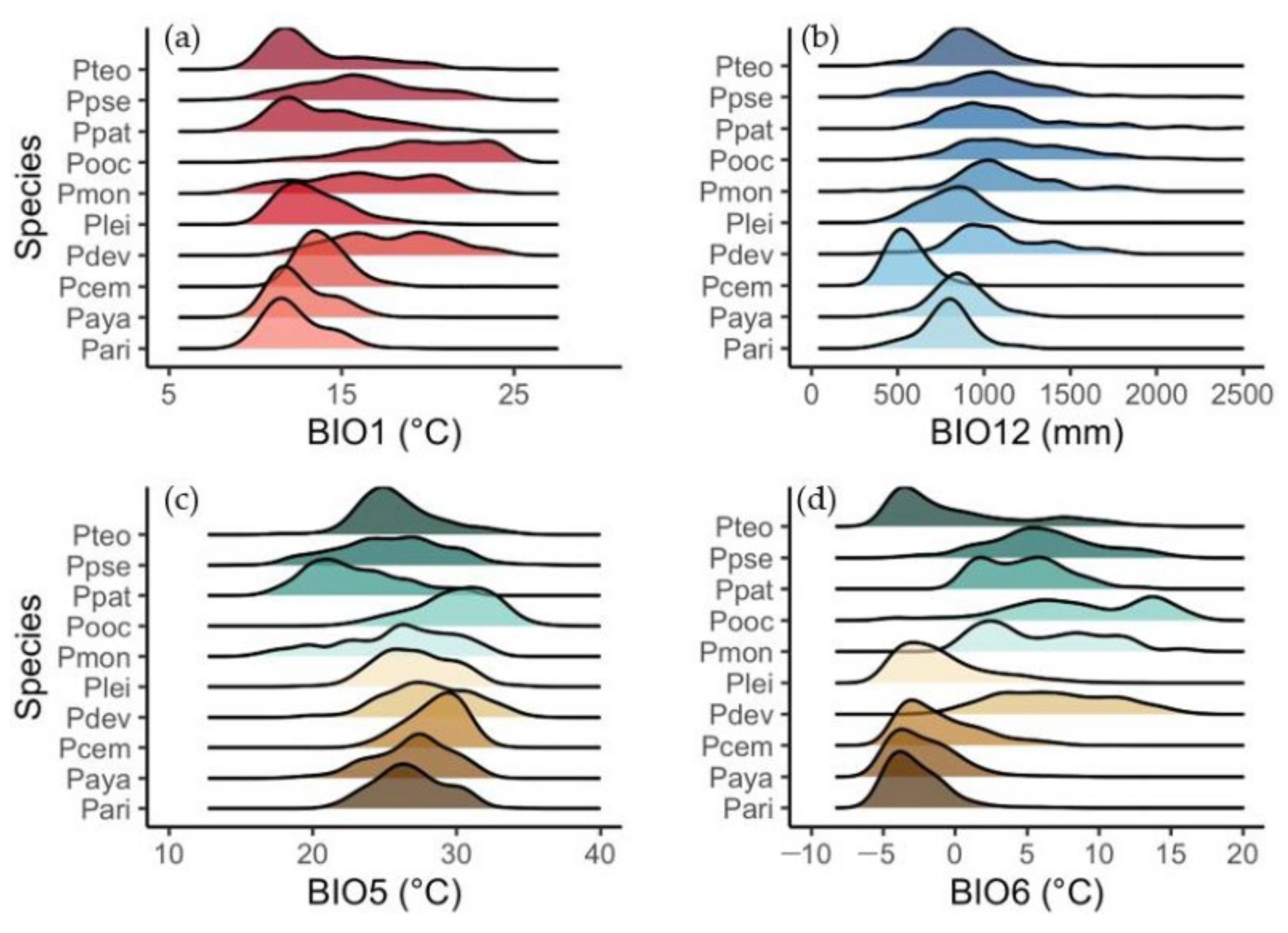
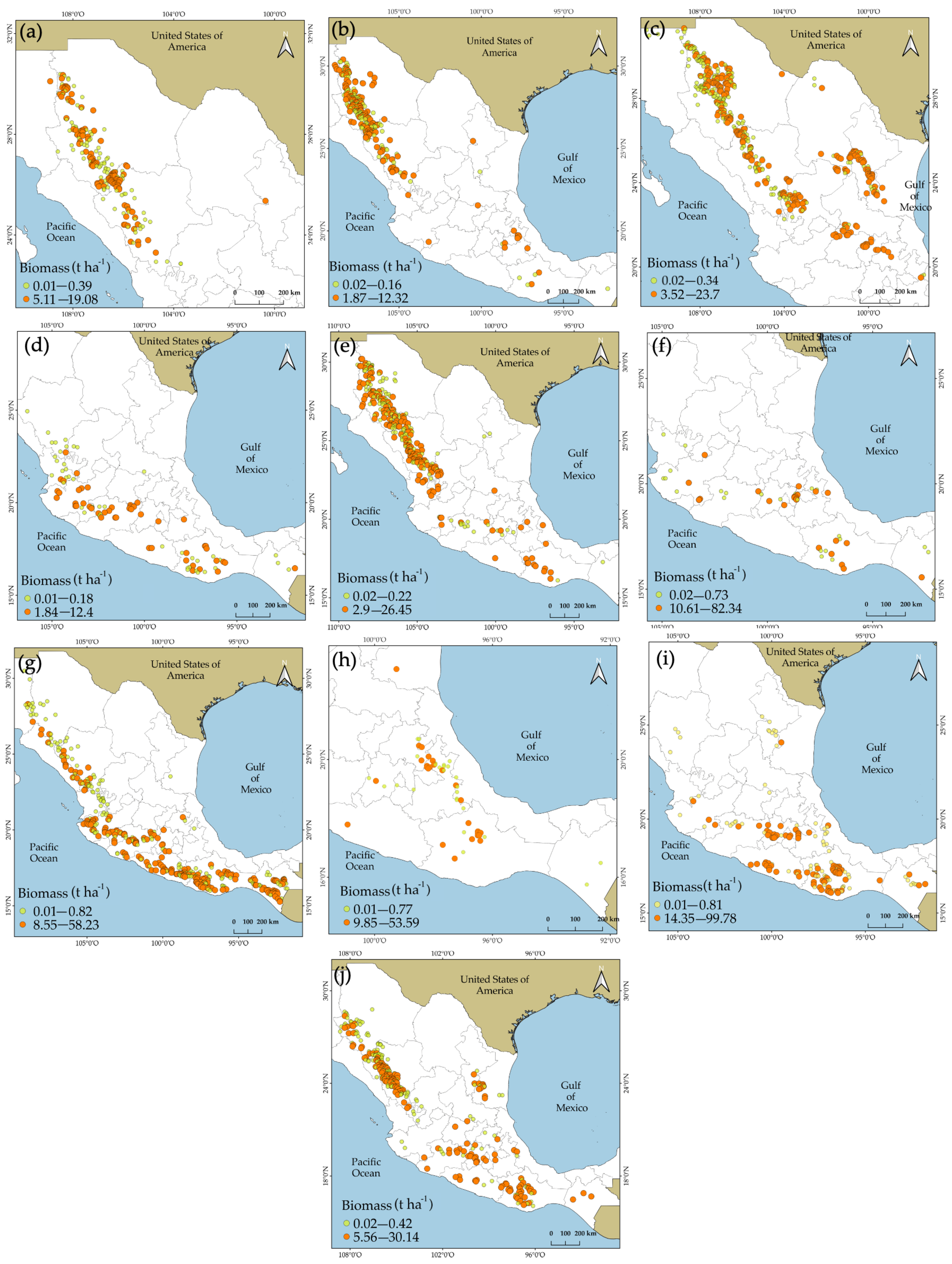
| Species | H (m) | DBH (cm) | AGBd (t ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | |
| P. arizonica | 0.20 | 29.50 | 8.57 e | 7.50 | 61.50 | 16.62 f | 0.013 | 19.07 | 3.11 c |
| P. ayacahuite | 0.20 | 30.00 | 8.89 d | 7.50 | 62.40 | 15.77 f | 0.022 | 12.32 | 1.14 f |
| P. cembroides | 0.20 | 16.20 | 5.52 f | 7.50 | 60.50 | 14.82 g | 0.002 | 14.30 | 2.33 d |
| P. devoniana | 0.20 | 41.50 | 12.31 ab | 7.50 | 85.10 | 24.55 a | 0.010 | 12.40 | 1.16 f |
| P. leiophylla | 0.20 | 35.00 | 9.60 d | 7.50 | 67.80 | 18.39 e | 0.020 | 26.45 | 1.92 e |
| P. montezumae | 0.20 | 35.70 | 12.32 ab | 7.50 | 97.00 | 24.76 ab | 0.020 | 82.33 | 8.08 a |
| P. oocarpa | 0.20 | 36.70 | 11.67 b | 7.50 | 89.00 | 22.61 b | 0.014 | 58.23 | 5.07 a |
| P. patula | 0.20 | 36.70 | 13.37 a | 7.50 | 84.90 | 20.88 c | 0.010 | 53.59 | 6.66 a |
| P. pseudostrobus | 0.20 | 44.40 | 13.27 a | 7.50 | 101.0 | 25.54 a | 0.010 | 99.78 | 9.01 a |
| P. teocote | 0.20 | 33.00 | 10.28 c | 7.50 | 71.10 | 19.21 d | 0.022 | 30.14 | 3.48 b |
| Parameter | Pari | Paya | Pcem | Pdev | Plei | Pmon | Pooc | Ppat | Ppse | Pteo |
|---|---|---|---|---|---|---|---|---|---|---|
| BIO1 | 2.11 e × 103 *** | 0.094 | 1.46 e × 109 *** | 0.857 | 2.17 e × 107 *** | 1.89 | 1.15 | 6.75 * | 2.84 | 50.51 *** |
| BIO2 | 0.353 | 0.608 | 2.04 e × 1018 *** | 0.313 | 0.696 | 2.05 | 1.46 e × 109 *** | 0.338 | 1.09 e × 106 *** | 2.97 e × 109 *** |
| BIO3 | 0.153 | 0.147 | 9.59 e × 1022 *** | 0.198 | 72.00 *** | 0.637 | 2.69 e × 104 *** | 0.456 | 239.75 *** | 3.32 e × 108 *** |
| BIO4 | 0.183 | 0.307 | 5.84 e × 1028 *** | 0.178 | 11.35 ** | 0.536 | 7.69 e × 107 *** | 0.742 | 1.13 e × 104 *** | 8.87 e × 1013 *** |
| BIO5 | 19.88 ** | 0.278 | 8.23 e × 1012 *** | 2.03 | 4.46 e × 1011 *** | 11.61 ** | 1.12 | 10.94 ** | 1.12 e × 105 *** | 0.91 |
| BIO6 | 115.62 *** | 0.142 | 8.54 e × 1031 *** | 0.31 | 0.922 | 0.424 | 1.47 e × 103 *** | 1.39 | 0.15 | 2.27 e × 107 *** |
| BIO7 | 0.106 | 0.542 | 1.17 e × 1035 *** | 0.19 | 42.36 *** | 2.38 | 9.90 e × 108 *** | 0.812 | 2.19 e × 107 *** | 2.72 e × 1013 *** |
| BIO8 | 10.05 ** | 0.186 | 4.15 e × 103 *** | 3.34 * | 4.11 e × 1011 *** | 2.94 | 0.074 | 7.46 * | 62.22 *** | 0.089 |
| BIO9 | 66.21 *** | 0.089 | 0.405 | 0.44 | 1.20 e × 107 *** | 1.33 | 0.737 | 3.12 * | 0.648 | 22.90 ** |
| BIO10 | 12.52 ** | 0.18 | 3.50 e × 105 *** | 1.64 | 4.39 e × 1011 *** | 3.29 * | 0.075 | 7.65 * | 141.23 *** | 0.082 |
| BIO11 | 4.99 e × 103 *** | 0.105 | 3.74 e × 1025 *** | 0.465 | 10.14 ** | 0.948 | 99.08 *** | 4.50 * | 0.194 | 1.46 e × 106 *** |
| BIO12 | 0.117 | 0.185 | 76.19 *** | 0.158 | 0.108 | 0.241 | 7.30 e × 104 *** | 0.36 | 156.21 *** | 5.38 e × 103 *** |
| BIO13 | 0.128 | 0.09 | 7.41 e × 1016 *** | 0.154 | 0.09 | 0.359 | 393.53 *** | 0.565 | 8.92* | 0.749 |
| BIO14 | 0.113 | 0.093 | 2.82 e × 104 *** | 0.276 | 0.104 | 0.212 | 0.138 | 0.362 | 0.13 | 0.083 |
| BIO15 | 0.173 | 0.381 | 2.29 e × 1025 *** | 0.19 | 0.084 | 0.215 | 0.077 | 0.205 | 0.749 | 0.138 |
| BIO16 | 0.11 | 0.099 | 3.14 e × 1013 *** | 0.179 | 0.092 | 0.272 | 2.87 e × 103 *** | 0.352 | 102.24 *** | 10.02 ** |
| BIO17 | 0.125 | 0.262 | 3.77 e × 107 *** | 0.287 | 0.182 | 0.215 | 0.079 | 0.422 | 0.136 | 0.094 |
| BIO18 | 0.103 | 0.091 | 3.75 e × 1016 *** | 0.719 | 0.125 | 0.216 | 0.074 | 0.361 | 1.26 | 90.19 *** |
| BIO19 | 0.112 | 0.138 | 977.44 *** | 0.228 | 0.37 | 0.313 | 9.29 * | 0.262 | 0.202 | 1.15 e × 104 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girón-Gutiérrez, D.; Méndez-González, J.; Osorno-Sánchez, T.G.; Cerano-Paredes, J.; Soto-Correa, J.C.; Cambrón-Sandoval, V.H. Climate as a Driver of Aboveground Biomass Density Variation: A Study of Ten Pine Species in Mexico. Forests 2024, 15, 1160. https://doi.org/10.3390/f15071160
Girón-Gutiérrez D, Méndez-González J, Osorno-Sánchez TG, Cerano-Paredes J, Soto-Correa JC, Cambrón-Sandoval VH. Climate as a Driver of Aboveground Biomass Density Variation: A Study of Ten Pine Species in Mexico. Forests. 2024; 15(7):1160. https://doi.org/10.3390/f15071160
Chicago/Turabian StyleGirón-Gutiérrez, Dioseline, Jorge Méndez-González, Tamara G. Osorno-Sánchez, Julián Cerano-Paredes, José C. Soto-Correa, and Víctor H. Cambrón-Sandoval. 2024. "Climate as a Driver of Aboveground Biomass Density Variation: A Study of Ten Pine Species in Mexico" Forests 15, no. 7: 1160. https://doi.org/10.3390/f15071160





