Abstract
Fire can significantly affect the structure and function of forest soil microorganisms. Therefore, it is important to study the effects of different fire intensities on soil microbial carbon source utilization capacity in cold-temperate larch forests to protect and utilize forest ecosystems. In this study, we investigated the effects of different burning intensities on the carbon utilization capacity of soil microorganisms in fire sites from 2010 and 2000 using Biolog-Eco technology. Our findings revealed that (1) fire significantly increased soil pH, AN (available nitrogen), and AK (available potassium) (p < 0.05); (2) fire significantly increased the average color change rate (AWCD) of soil microorganisms (p < 0.05); (3) the Shannon index of soil microorganisms increased significantly, whereas the Simpson index and the McIntosh index decreased significantly after the fire—however, the McIntosh index in the 10M site was not altered; (4) the metabolic functions of soil microbial communities differed significantly among different fire intensities—MC (moisture content), TN (total nitrogen), and AK were the most influential soil environmental factors in the soil microbial community; and (5) mid-term fire restoration significantly increased microbial responses to carbohydrates, amino acids, esters, alcohols, amines, and acids, while late-fire burn sites significantly increased the microbial utilization intensity of amino acids, esters, and acids. In conclusion, fire significantly altered the functional diversity of soil microorganisms and microbial activities related to carbon source substrate utilization. Additionally, the ability of microorganisms to utilize a single carbon source substrate was also altered.
1. Introduction
Fire is an important disturbance factor in forest ecosystems, capable of destroying large areas of forest resources and affecting the development and succession of the entire ecosystem [1]. With global warming, the frequency, area, and intensity of forest fires are expected to increase. Soil microorganisms play a crucial role in forest ecosystems by participating in material cycling, soil structure formation, organic matter decomposition, and nutrient transformation [2]. The effects of forest fires on soil microorganisms are complex and significant, influenced by the type and load of combustible materials, fire intensity, fire duration, stand conditions, and the state of vegetation restoration after the fire [3]. Fires can lead to a decrease in the number of microorganisms, changes in community structure, and reduced activity [4]. However, fires can also release nutrients and alter soil properties, potentially creating better conditions for microbial growth and reproduction [5].
Biolog-Eco microplate technology analyzes the microbial utilization of various carbon source substrates to gain broad insights into the microbial metabolic profiles within a sample [6]. This rapid and straightforward method has been increasingly used in recent years to study the functional diversity of microorganisms [7]. Previous studies have demonstrated that the composition of soil microbial communities changes after fire burning [8,9,10], and the ability of soil microbes to utilize carbon sources significantly decreases as the intensity of fire burning increases [11]. Velasco et al. [12] investigated the effect of fire burning on the functional diversity of soil microbial communities in Mediterranean rangelands using the Biolog-Eco plates method. The study revealed that soil moisture content (MC) had a greater impact on soil microbial functional diversity than other environmental factors. Li et al. [13] demonstrated that low-to-medium fire intensity could maintain high community functional diversity. Xu [14] reported that the ability of soil microorganisms to use carbon sources decreased significantly immediately after a fire. However, with increased recovery time, this ability in fire-disturbed sites eventually returned to levels similar to those in undisturbed soil. Although numerous studies have shown that fire disturbance significantly alters soil microbial carbon utilization capacity and functional diversity, researchers have reached different conclusions due to variations in fire intensity, fire type (wildfire and controlled fire), recovery time, soil properties, and plant cover type. These discrepancies highlight the importance of studying the effects of different fire intensities and recovery times on soil microbial communities in specific areas.
The Daxing’anling region is characterized by rapid snowmelt, dry air, low rainfall, and high wind speeds, combined with dead herbaceous plants, all of which contribute to the frequent occurrence of forest fires [15]. Larix gmelinii, known for its cold-resistant characteristics, is one of the most widely distributed and planted tree species in this region [16]. Previous studies on fire-burned sites in the Daxing’anling forest area have primarily focused on nitrogen estimation in birch leaves [17], dead combustible load [18], methane flux during vegetation restoration [19], renewal of soil phosphorus-solubilizing microorganisms and active seedlings [20], and spatial distribution patterns [21]. However, the restoration and protection of forest ecosystems after a fire should not only consider plant diversity but also soil microbial diversity, which has recently garnered increasing attention in soil ecology research. Moreover, there is a lack of systematic research on the characteristics of loam microorganisms in the Daxing’anling region and the effects of various environmental factors on this typical forest ecosystem.
In this study, Biolog-Eco analysis was conducted to investigate changes in soil microbial carbon utilization capacity under different fire disturbance intensities in the 2010 (mid-stage fire) and 2000 (late-stage fire) fire trails in Huzhong National Nature Reserve, Heilongjiang Province. Here, we hypothesized that (1) fire significantly reduces the ability of soil microbes to utilize carbon sources, and (2) soil MC is an important environmental factor influencing soil microbial carbon source utilization. Our study sought to provide insights into the mechanisms through which fire affects soil microbial dynamics, particularly in the context of mid-to-late-season fire recovery. This study is crucial for ecological research and provides a theoretical basis to gain a more in-depth understanding of the soil carbon and nitrogen cycle in the future.
2. Materials and Methods
2.1. Study Area
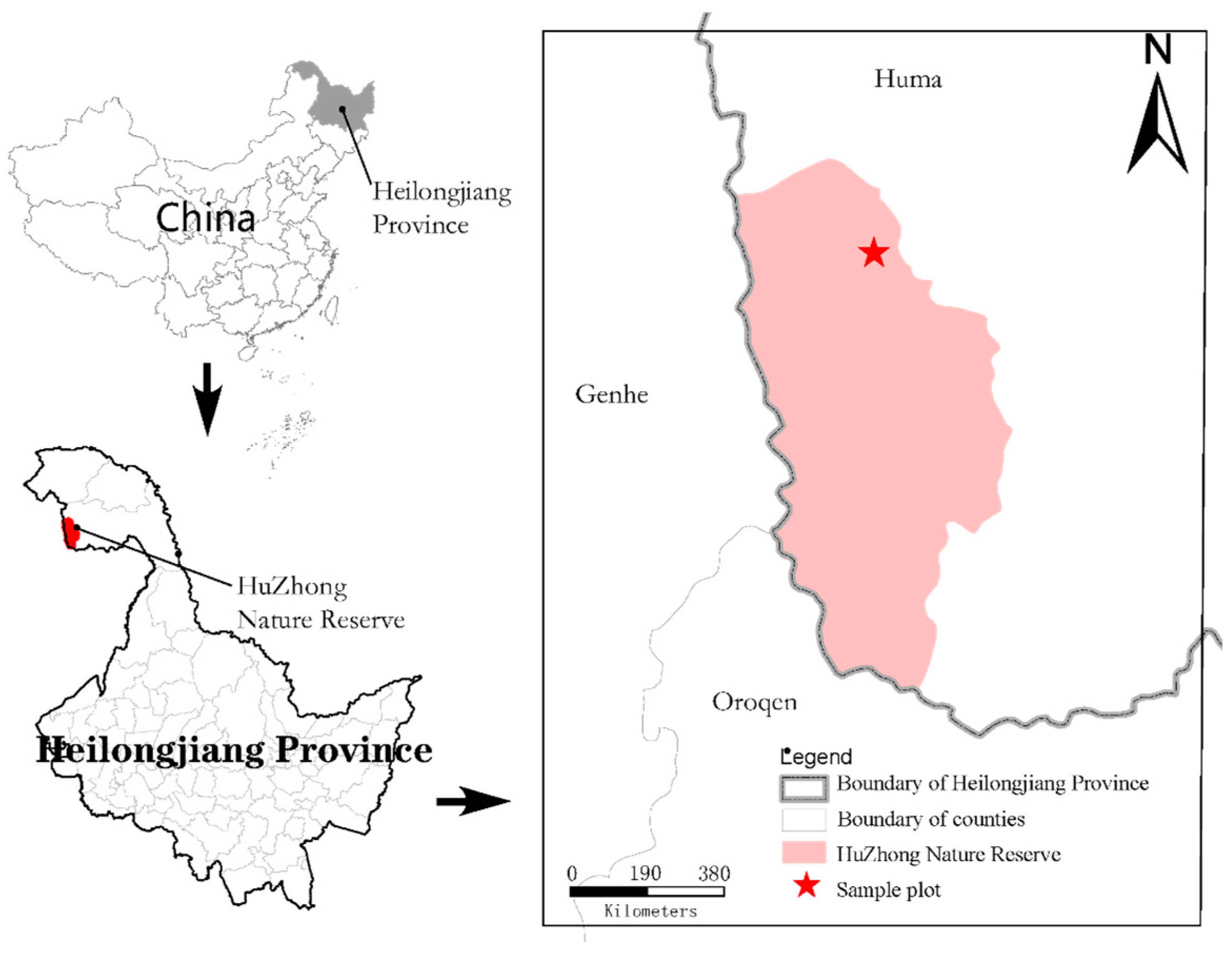
The Heilongjiang Huzhong National Nature Reserve is located in the Daxing’anling area of Heilongjiang Province, nestled between the main vein of the Daxing’anling Mountains and the Yilhuli Mountains. The western boundary is marked by the main vein of the Daxing’anling Mountains, adjoining the Inner Mongolia Daxing’anling Khan Horse National Nature Reserve [22]. The northwestern part connects with the Inner Mongolia Along Mountain Forestry Bureau, whereas the southern part neighbors the Inner Mongolia Ganhe Forestry Bureau. The eastern, northeastern, and northern sections are situated within the Hulzhong District of the Daxing’anling Mountains in Heilongjiang Province, under the administrative jurisdiction of the Hulzhong District. Its geographical coordinates are 122°42′14″~123°18′05″ E, 51°17′42″~51°56’31″ N (Figure 1). The reserve experiences a cold-temperate continental monsoon climate with significant seasonal temperature variations. Spring brings a sudden temperature rise, followed by a warm and short summer with abundant rainfall. Autumn exhibits an abrupt decrease in temperature, resulting in a long, cold, and snowy winter. The average annual temperature is −4 °C. The average annual precipitation is 458.3 mm, with an average annual relative humidity of 71% and an average annual evaporation of 911 mm. The frost-free period typically lasts approximately 80–100 days. The total area of the reserve is 167,213 hectares, with 54,087 hectares designated as the core area, 45,493 hectares as the buffer area, and 67,633 hectares as the experimental area. The reserve stretches 63 km from north to south and 32 km from east to west. Woodland covers 166,725 hectares, accounting for 99.71% of the total area, with a forest coverage rate of 91.05%. The understory soil is predominantly brown coniferous forest soil, with other boggy soils and meadow soils also present. The zonal vegetation of Huzhong Nature Reserve is dominated by typical cold-temperate bright coniferous forests. Phytogeographically, it is part of the pan-northern flora within the Eurasian forest flora sub-region and the Daxing’anling region. The vegetation system is a typical component of the East Siberian vegetation system. The sample site mainly includes trees such as larch, birch, and aspen, as well as shrubs such as Rhododendron dauricum, Ledum palustre, and Spiraea salicifolia. Herbaceous plants include Vicia ramuliflora, Sanguisorba tenuifolia, Geranium platyanthum, and Calamagrostis angustifolia. The primary focus of the Huzhong Nature Reserve is the preservation of cold-temperate bright coniferous forests and rare and endangered wild animals and plants. This region is among the most pristine and complete cold-temperate bright coniferous forests in China [23].
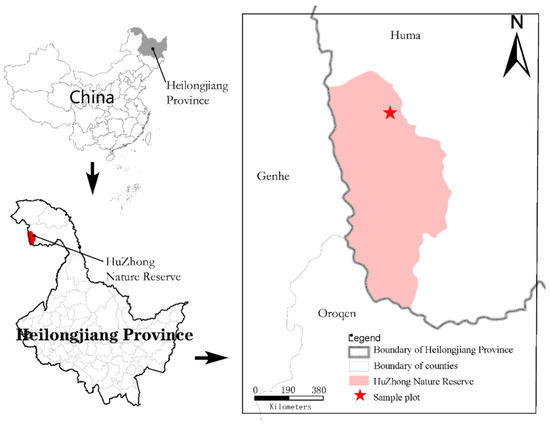
Figure 1.
Heilongjiang Huzhong National Nature Reserve.
2.2. Sample Plot and Sample Collection
According to records from the Fire Prevention Office of Heilongjiang Huzhong National Nature Reserve, the 2010 (indicated as “10” in this study) and 2000 (indicated as “00” in this study) fire burn sites were selected as test areas, with fire-affected areas of 3633.8 and 9216.6 hectares, respectively. The fires were caused by lightning strikes. During the investigation and classification of fire intensity at these sites, three standard sample plots representing low (L), moderate (M), and high (H) fire intensities were established based on fire intensity classification characteristics (Table 1) [15]. Unburned sample plots with vegetation, slope direction, slope gradient, and soil properties as similar as possible to those of the fire-burned sites were selected as control sample plots (CKs). These control plots were located 200 m away on the opposite side of the road from the fire-burned sites. Each sample plot measured 20 m × 20 m, and three replicate plots were set up for each fire intensity and the control, resulting in a total of 21 experimental plots. Samples were collected in July 2023. The sample plots are all recovered Larix gmelinii forests with natural growth. Before soil sampling, dead leaves were removed with a shovel to avoid external contamination. Soil samples were collected from the 0–20 cm soil layer using a bottom-to-top approach. Stones, gravel, and plant residues were removed before passing the samples through a 2 mm nylon sieve. The soils were mixed and placed in sterile self-sealing bags. A total of 21 soil samples were transported back to the laboratory in an insulated box with ice packs for Biolog-Eco analysis and measurement of soil physical and chemical properties [24].

Table 1.
Criteria for classifying different fire intensities.
2.3. Determination of Soil Physical and Chemical Properties
Soil MC was determined via the drying method; soil pH was detected using a soil–water ratio of 1:2.5 [25]; soil total nitrogen (TN) and organic carbon (SOC) were measured using a carbon and nitrogen analyzer (Multi N/C 2100S, Analytik Jena AG, Jena, Germany) [26]; soil available nitrogen (AN) was measured via the alkaline dissolution diffusion method [27]; and soil available potassium (AK) was measured via the flame photometric method [28].
2.4. Determination of Functional Diversity of Soil Microbial Communities
The carbon metabolism capacity of soil microbial communities at the fire burn site was examined using the Biolog-Eco microtiter plate culture method. This method provides insights into the physiological characteristics of microorganisms by determining variations in their utilization of different carbon sources and has been effectively applied to study microbial community changes on both spatial and temporal scales. The principle of this method is to culture microbial communities directly in microplates and monitor the changes in light absorption induced by the microbes in real time [29,30].
A portion of the soil samples was activated at 25 °C for one day. After activation, 10 g of fresh soil was placed in a 200 mL triangular flask, after which 90 mL of 0.85% sterile NaCl solution was added. Next, the flask was sealed and mixed, then placed in a shaker to oscillate for 0.5 h at 200 rpm. The soil suspension was then diluted to a concentration of 10−3 and inoculated into the microtiter plates using a pipette. Each group of experiments was repeated three times. The microtiter plates were incubated continuously at 25 °C for 216 h. During the incubation process, the absorbance value at a wavelength of 590 nm was recorded at 24 h intervals [31].
2.5. Data Analysis
The overall activity of soil microorganisms is reflected by the average well color development (AWCD) value. The AWCD value indicates the strength of soil microorganisms’ ability to utilize a single carbon source on the Biolog-Eco microplates [32]. The Shannon index characterizes the abundance of microbial communities in the soil, the Simpson index assesses the dominance of microbial communities, and the McIntosh index reflects the homogeneity of microbial communities. These three indices collectively characterize the functional diversity of microbial communities [33]. After incubating the microorganisms in the Biolog-ECO microplates for 192 h, almost all microorganisms in the soil have participated in carbon source metabolism processes, providing a comprehensive snapshot of the carbon metabolism characteristics of soil microorganisms.
AWCD value and the data derived from 192 h of incubation were used to calculate the functional diversity of the soil microbial community using the following formulas:
where Ci is the absorbance value of carbon source pores at 590 nm; and R is the absorbance value of control pores. In the calculation, all carbon source wells with Ci − R ≤ 0 were recorded as 0. Pi is the ratio of the difference between the absorbance value of the ith carbon source well and the control well (ni) to the sum of the relative absorbance values of all the wells [12,34].
The Biolog-Eco plate contains 31 carbon sources, which can be categorized into six major groups: seven carbohydrates, six amino acids, four esters, three alcohols, three amines, and eight acids [34]. Soil microbial functional diversity index, different utilization capacities of 6 types of carbon sources and different utilization capacities of 31 single carbon sources were calculated based on the absorbance values of 192 h was conducted using Microsoft Excel 2010. One-way analysis of variance (ANOVA) was conducted using the SPSS software (version 25.0), with the significance level set at 0.05. Scatter plots and bar charts with trend lines were generated using Microsoft Excel 2010. Soil microbial alpha diversity, principal coordinate analysis (PCoA), and heatmaps were all created in R using the ‘vegan’ package [35]. Correlation analysis of six different types of carbon sources, and soil physicochemical properties was based on Pearson’s correlation coefficient. Redundancy analysis (RDA) of basic soil physicochemical properties and mean absorbance of 31 carbon sources was performed using CANOCO 4.5 [36].
3. Results
3.1. Differences in Physicochemical Properties of Soils in Fire Burn Sites
As shown in Table 2, soil pH, AN, and AK were significantly increased at the 2010 fire burn site compared to the control. Additionally, MC, SOC, and TN were significantly increased under moderate and high fire intensities, while MC and SOC were significantly decreased and TN was not significantly different under low fire intensity.

Table 2.
Physicochemical properties of soils. Different letters represent significant differences (p < 0.05).
At the 2000 fire burn site, soil pH, SOC, TN, AN, and AK were significantly increased compared to the control. Soil MC was significantly lower under low and high fire intensities but was not significantly different under moderate fire intensity.
3.2. Characterization of Carbon Source Utilization by Soil Microbial Communities in Fire Burn Sites
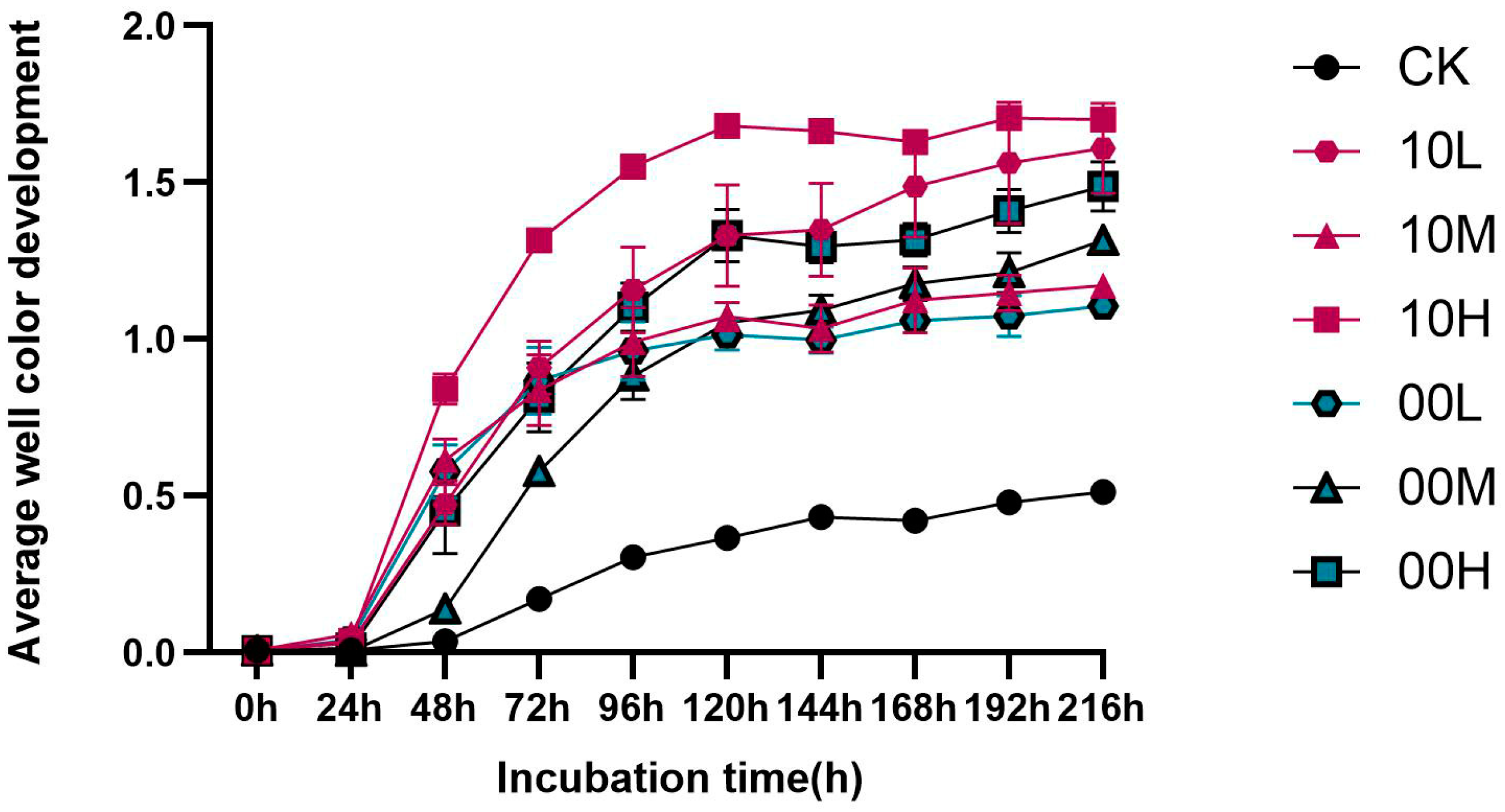
The AWCD value is used as an indicator of the utilization of carbon sources by microbial communities, reflecting soil microbial activity and functional diversity. A larger AWCD value indicates a higher density and activity of soil microorganisms [32]. As illustrated in Figure 2, the AWCD values of soil microbial communities under different fire intensity disturbances increased with the extension of incubation time. Specifically, the AWCD values increased rapidly from 0 to 120 h, indicating that the metabolic activity of microorganisms was most vigorous during this period. After 144 h, the increase in AWCD values slowed down, and it leveled off after 192 h. Generally, the AWCD values of soil microbial communities under different levels of fire disturbances tended to increase with incubation time.
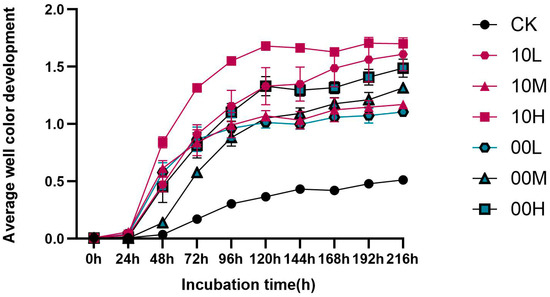
Figure 2.
Changes in the AWCD values of the soil microbial samples collected from different fire burn sites during the incubation time. CK: control (no fire history); 10L: low fire in 2010; 10M: moderate fire in 2010; 10H: high fire in 2010; 00L: low fire in 2000; 00M: moderate fire in 2000; 00H: high fire in 2000.
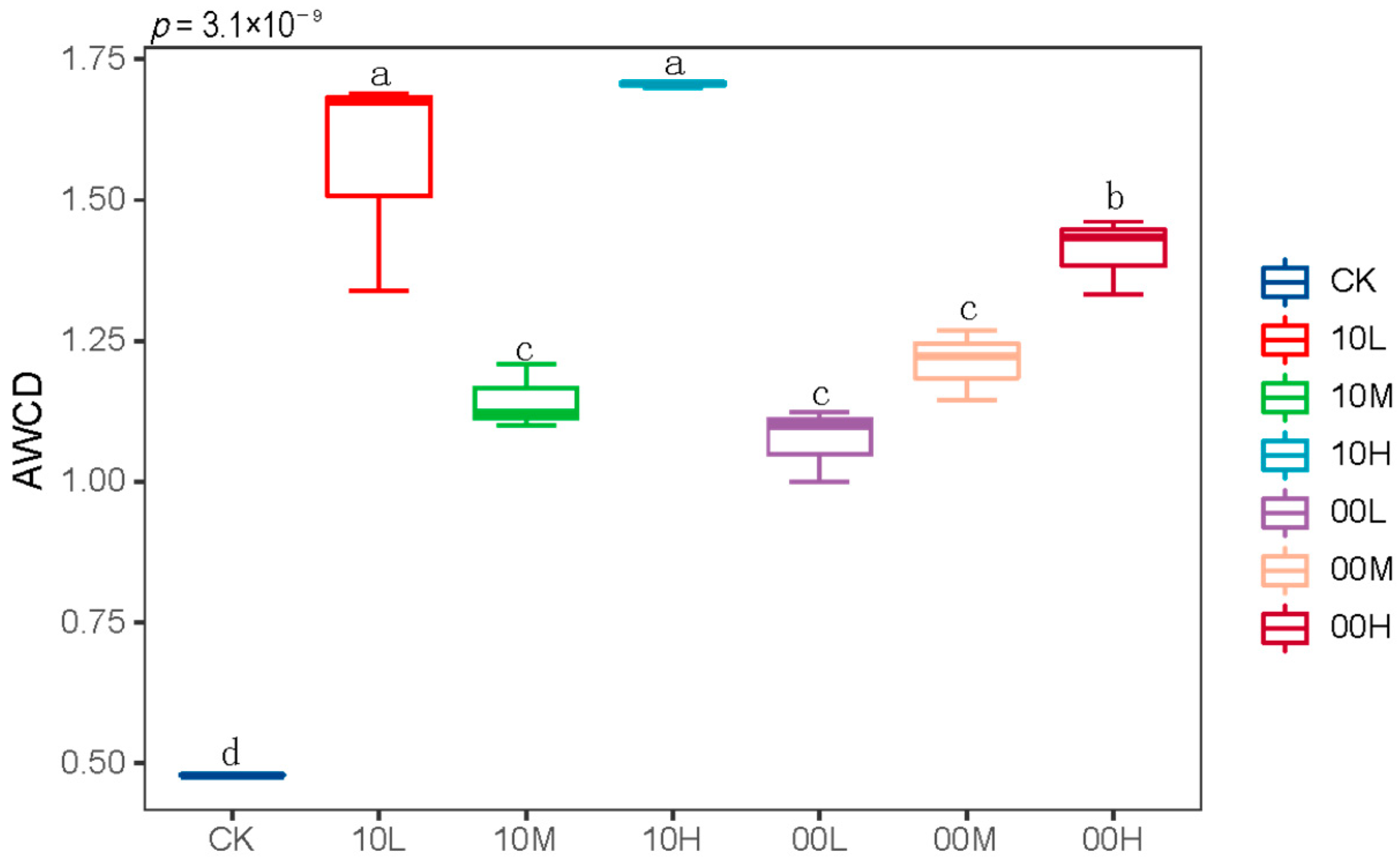
As shown in Figure 3, fire significantly increased the AWCD values of soil microorganisms. This suggests that fire enhances the carbon source metabolism capacity of soil microorganisms.
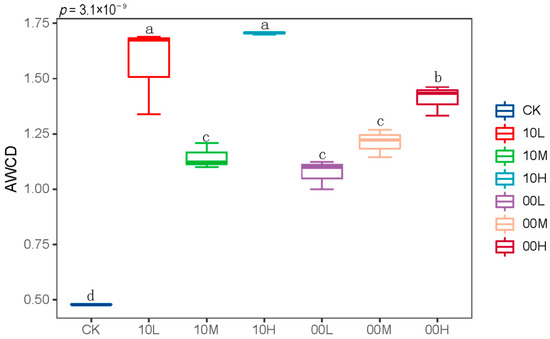
Figure 3.
AWCD values of soil microorganisms at 192 h. CK: control (no fire history); 10L: low fire in 2010; 10M: moderate fire in 2010; 10H: high fire in 2010; 00L: low fire in 2000; 00M: moderate fire in 2000; 00H: high fire in 2000. Different letters represent significant differences (p < 0.05).
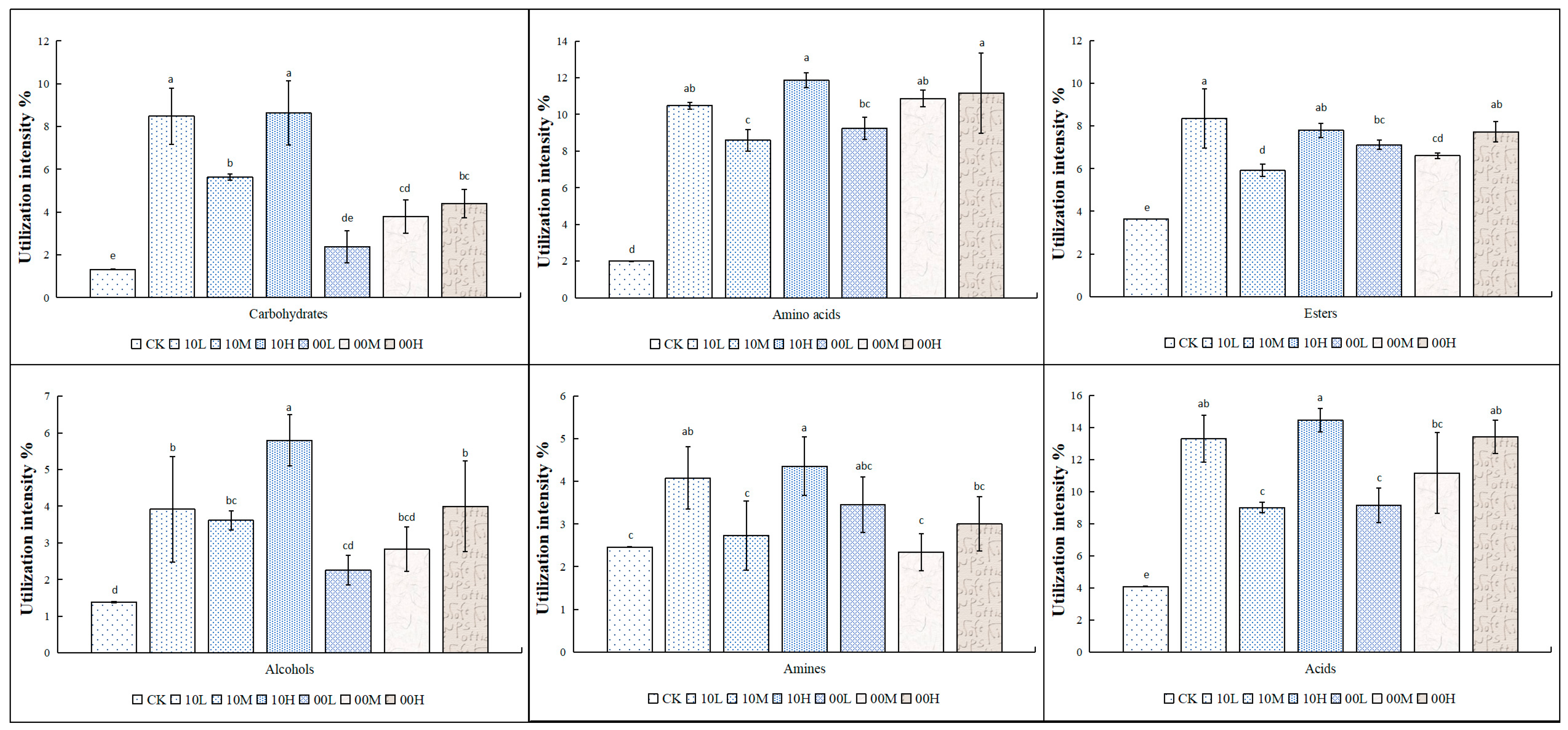
Figure 4 presents the experimental results, which reflect the degree of utilization of different carbon sources by soil microorganisms at different intensities in the mid- and late-fire burn sites. Our findings revealed that the intensity of microbial utilization of carbohydrates, amino acids, esters, alcohols, and acids in the 2010 fire burn sites increased significantly compared to the control. However, the intensity of microbial utilization of amines did not show any significant difference after moderate fire restoration compared to the control. Specifically, the intensity of amine utilization by microorganisms was significantly increased under low and high fire intensities compared to the control, but there was no significant difference in amine utilization under moderate fire conditions.
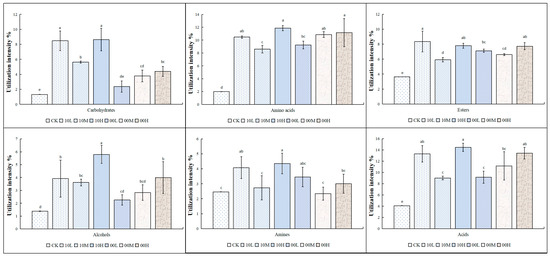
Figure 4.
Utilization of different carbon sources by the soil microbial communities of different fire-burned sites. CK: control (no fire history); 10L: low fire in 2010; 10M: moderate fire in 2010; 10H: high fire in 2010; 00L: low fire in 2000; 00M: moderate fire in 2000; 00H: high fire in 2000. Different letters represent significant differences (p < 0.05).
In the 2000 fire burn sites, the utilization intensities of amino acids, esters, and acids were significantly higher than in the unfired control. The utilization intensities of carbohydrates by microorganisms increased significantly after moderate and severe burns, with no significant difference observed after light burns compared to the control. The utilization intensity of alcohols by microorganisms increased significantly after severe burns, but no significant differences were observed after light and moderate burns compared to the control. The intensity of microbial utilization of amines showed no significant difference after the fire.
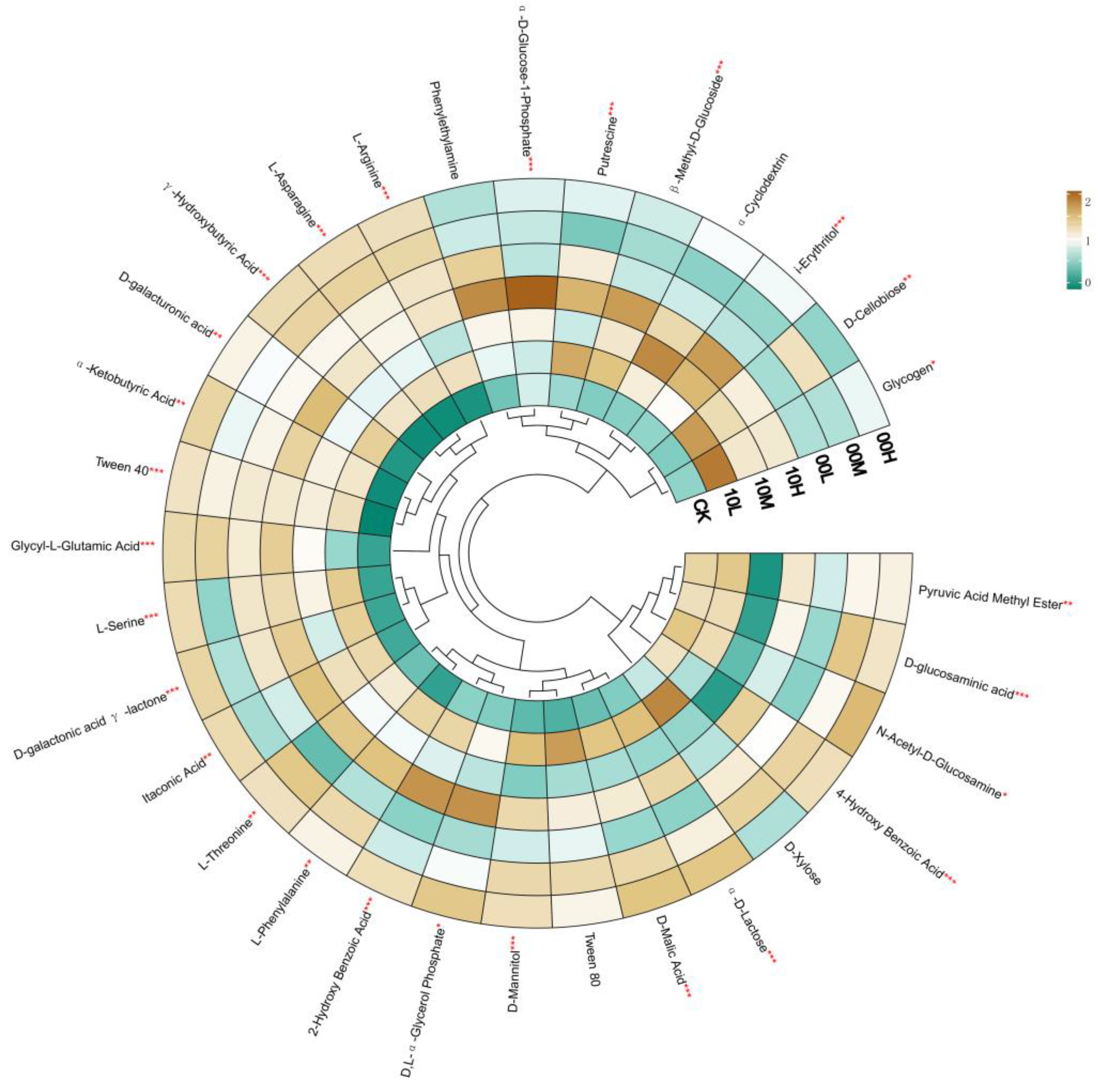
Differences in carbon source utilization by soil microorganisms at mid-to-late-stage fire traces under different fire intensities were observed, with color changes in the thermograms revealing variations in the microorganisms’ ability to utilize the different carbon sources in the microplates. As shown in Figure 5, the heatmap results of soil microbial community metabolic activity in this study can be categorized into five groups. Group I: the AWCD values of pyruvic acid metal ester, D-glucosamine acid, N-acetyl-D-glucosamine, and 4-hydroxy benzoic acid in 10M are lower than those in the other fire-burned areas; Group II: the AWCD values of D-xylose, α-D-lactose, D-malic acid, Tween 80, and D, L-α-glycerol phosphate and 2-hydroxy benzoic acid in 10M and 10H were significantly higher than those in other fire-burned areas; Group III: the AWCD values of D-galactic acid gamma lactone and itaconic acid in 10H were significantly higher than those in the other fire-burned areas; Group IV: the AWCD values of glycyl-L glutamic acid, alpha ketobutyric acid, and D-galacturonic acid in 10H; Tween 40 in 10L; and γ-hydroxybutyric acid, L-asparagine, and L-arginine in 00M and 00H were significantly higher than those in other fire-burned areas; Group V: the AWCD values of phenylethylamine, α-D-glucose-1-phosphate, β-methyl-D-glucoside, i-erythritol, and D-cellobiose in 10H; α-cyclodextrin in 10M; and putrescine and glycogen in 10L were significantly higher than those in other fire-burned areas. These results indicate significant differences in soil microbial activity and carbon source utilization preferences in fire-burned areas with different intensities and recovery times.
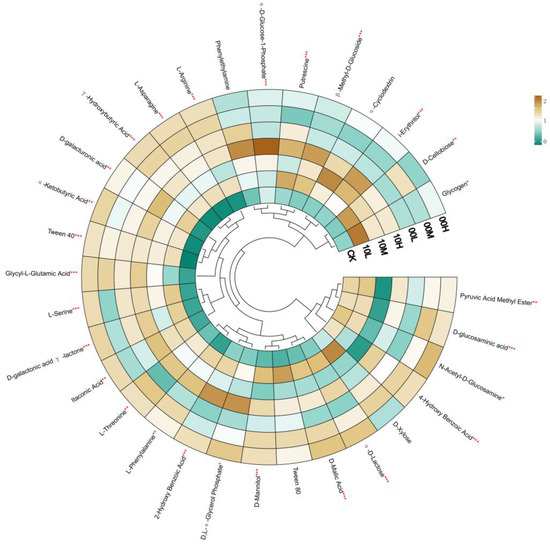
Figure 5.
Heatmap of the metabolic activity of the soil microbial community based on 192 h AWCD values. CK: control (no fire history); 10L: low fire in 2010; 10M: moderate fire in 2010; 10H: high fire in 2010; 00L: low fire in 2000; 00M: moderate fire in 2000; 00H: high fire in 2000. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.3. Diversity of Microbial Metabolism in Soils of Fire-Burned Sites
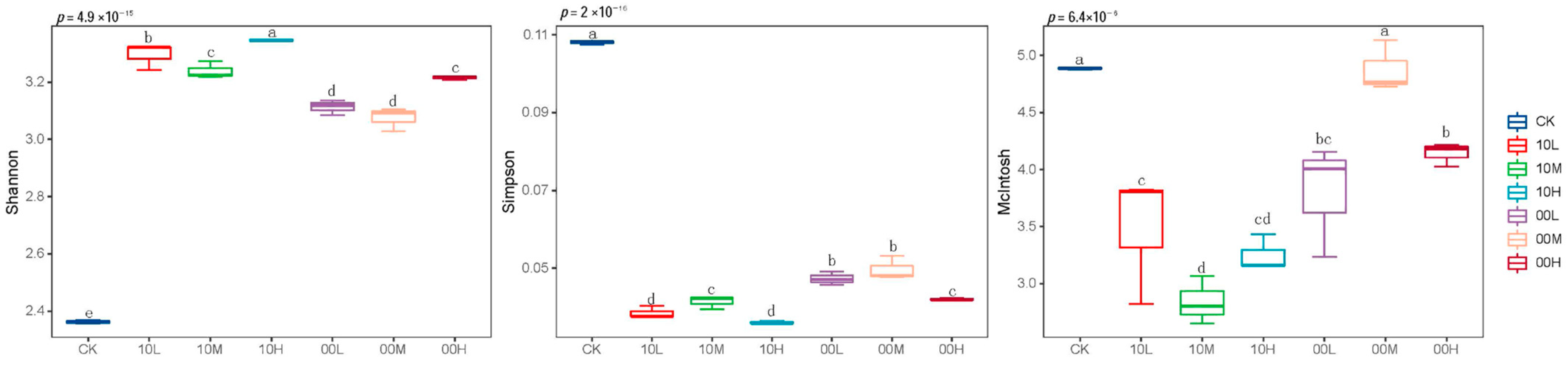
To further determine the impact of fire on soil microbial functional diversity, alpha diversity analysis was conducted based on 192 h AWCD values. As illustrated in Figure 6, fire significantly increased the Shannon index of soil microorganisms, while significantly reducing their Simpson index and McIntosh index. However, under moderate fire disturbance in 2000 compared with the control group, there was no significant difference in the McIntosh index of soil microorganisms.
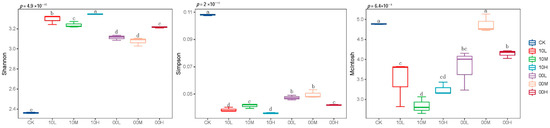
Figure 6.
Alpha diversity of the microbial population of different fire burn sites. CK: control (no fire history); 10L: low fire in 2010; 10M: moderate fire in 2010; 10H: high fire in 2010; 00L: low fire in 2000; 00M: moderate fire in 2000; 00H: high fire in 2000. Different letters represent significant differences (p < 0.05).
3.4. Principal Coordinate Analysis of Soil Microbial Communities
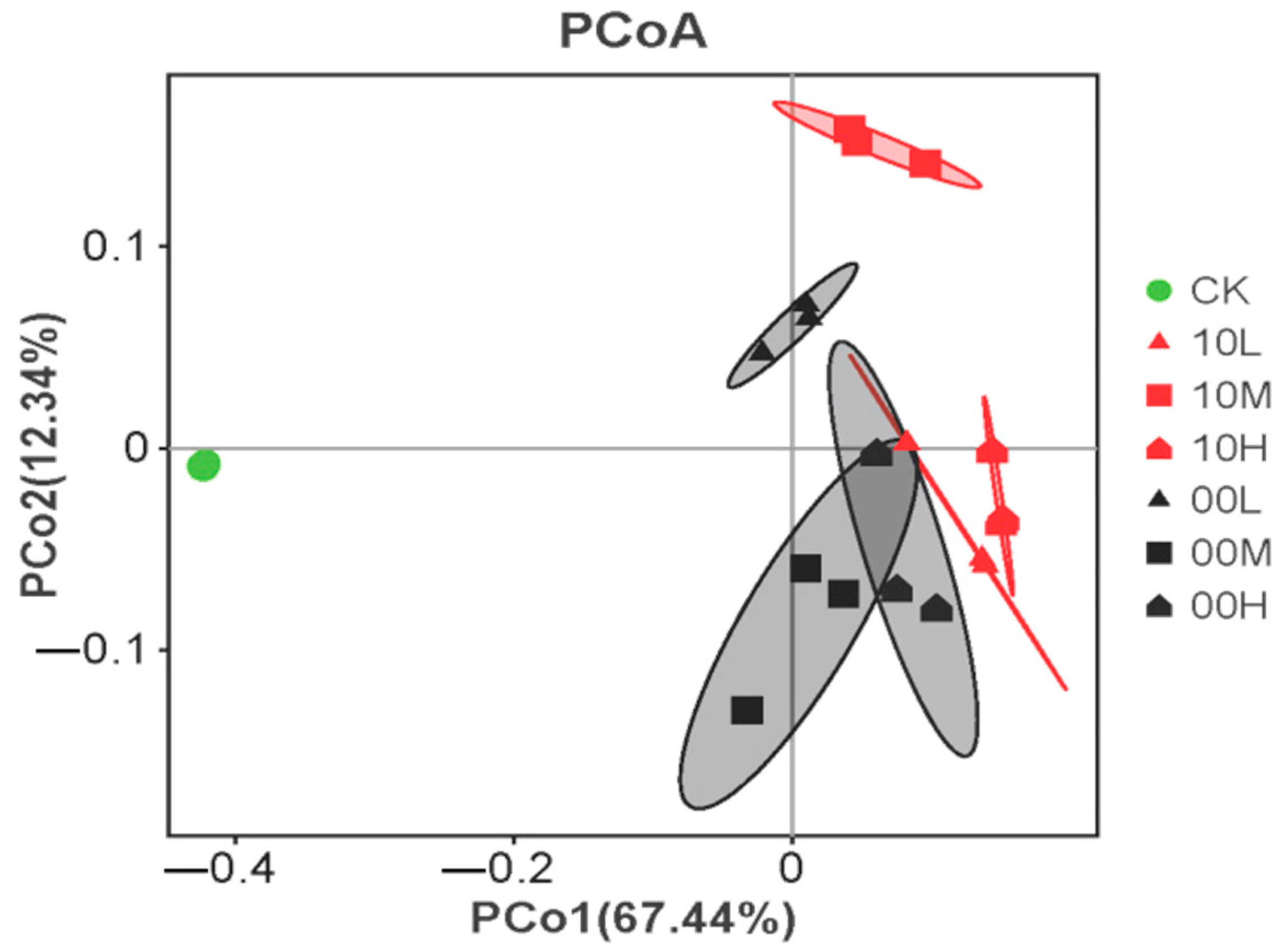
Principal coordinate analysis of the Biolog-Eco plate results revealed key insights into the mechanisms through which microbial communities respond to fire, effectively reflecting the structural characteristics of soil microbial communities [37]. Jones et al. [38] suggested that differences in the spatial location of samples are associated with the ability of microorganisms to utilize carbon substrates. Specifically, differences in the coordinates of various PC axes in PC space among samples were linked to the ability to utilize carbon sources that were aggregated along those PC axes [39]. Using the AWCD values measured after 192 h of incubation, which were normalized, principal coordinate analysis was performed using methods described in the relevant literature [40,41].
As shown in Figure 7, Principal Axis 1 (PCo1) explains 67.44% of the variance of all variables, whereas Principal Axis 2 (PCo2) explains 12.34% of the variance, with a cumulative explanation reaching 79.78%. Samples from 10M and 00L are mainly distributed on the positive half of the main coordinate axis PCo1, suggesting that the soil microbial communities in 10M and 00L exhibit similar carbon source utilization abilities. Conversely, samples from 10L, 10H, 00M, and 00H are predominantly distributed on the negative half of the main coordinate axis PCo1, indicating that the soil microbial communities in these areas share similar abilities to utilize carbon sources. Interestingly, only the CK sample is distributed on the negative half of the coordinate axis PCo2, suggesting that fire significantly alters the soil microbial utilization ability of carbon sources.
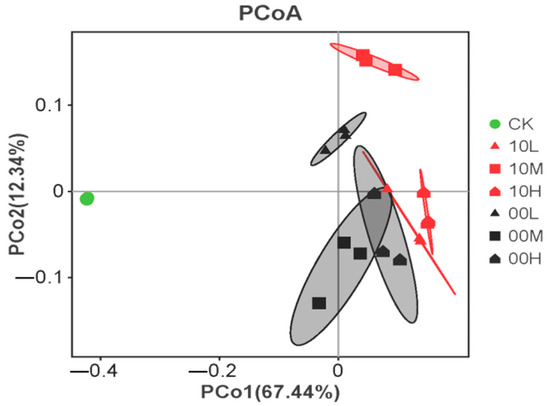
Figure 7.
Principal coordinate analysis of carbon utilization. CK: control (no fire history); 10L: low fire in 2010; 10M: moderate fire in 2010; 10H: high fire in 2010; 00L: low fire in 2000; 00M: moderate fire in 2000; 00H: high fire in 2000.
3.5. Relationship between Soil Microbial Carbon Source Utilization Activity and Soil Physicochemical Properties
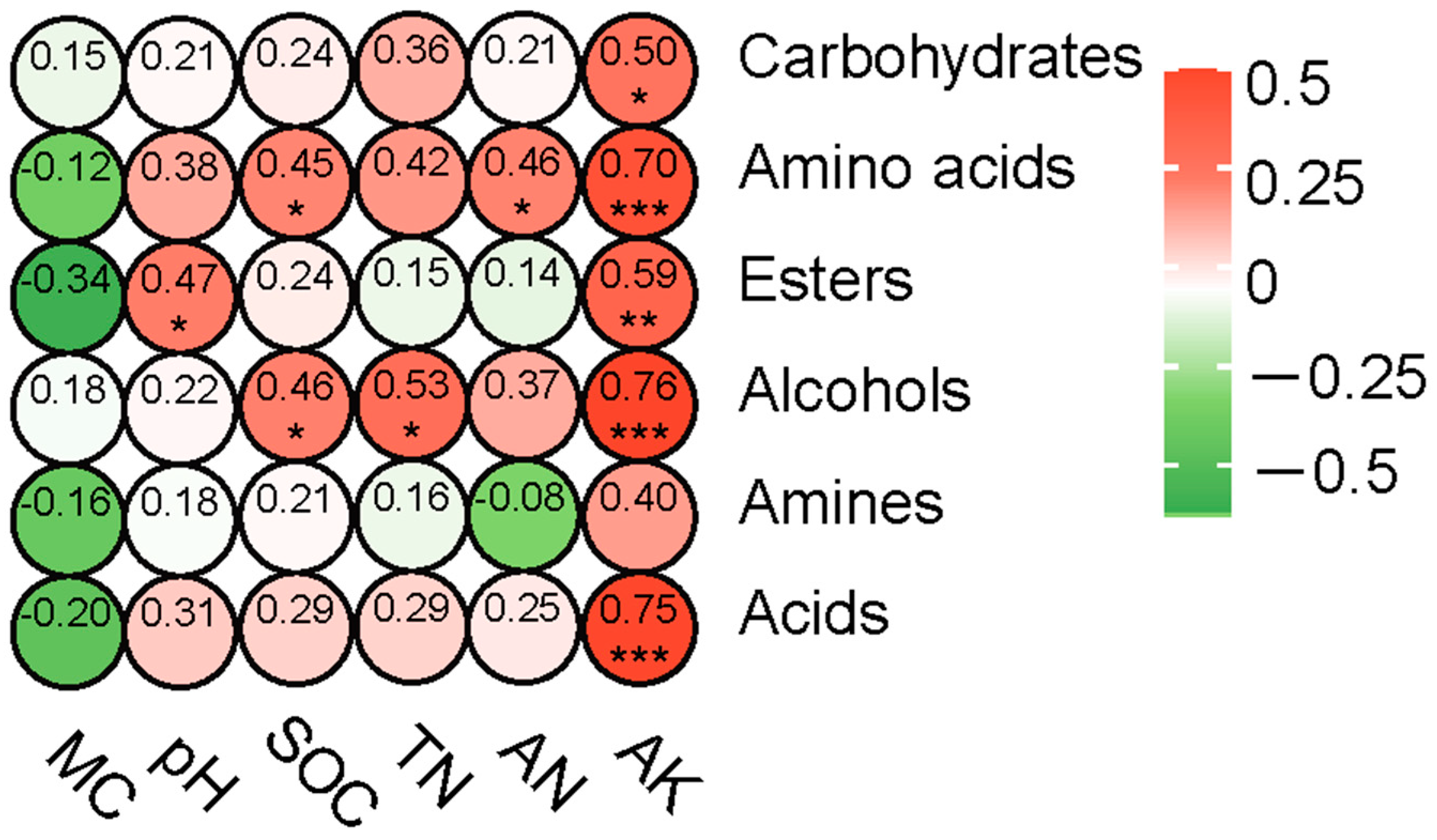
To further clarify the relationship between different soil physicochemical properties and the ability of soil microorganisms to utilize carbon sources, a correlation heatmap analysis was conducted using Pearson’s correlation coefficient. This analysis correlated the ability of soil microorganisms to utilize six major types of carbon sources with different soil physicochemical properties. As shown in Figure 8, the microbial utilization of carbohydrates and acids was significantly and positively correlated with AK. Microbial utilization of amino acids was significantly and positively correlated with SOC, AN, and AK. Microbial utilization of esters was significantly and positively correlated with pH and AK. Additionally, microbial utilization of alcohols was significantly and positively correlated with SOC, TN, and AK. Taken together, these results indicate that the ability of soil microorganisms to utilize carbon sources in the fire burn site is influenced by the physicochemical properties of the soil.
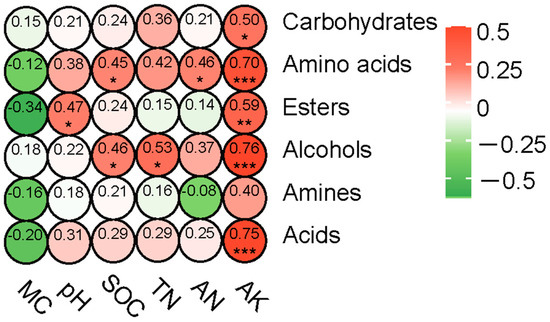
Figure 8.
Heatmap illustrating the correlation between the ability of soil microorganisms to utilize six different carbon sources and soil physicochemical factors. * p < 0.05, ** p < 0.01, *** p < 0.001.
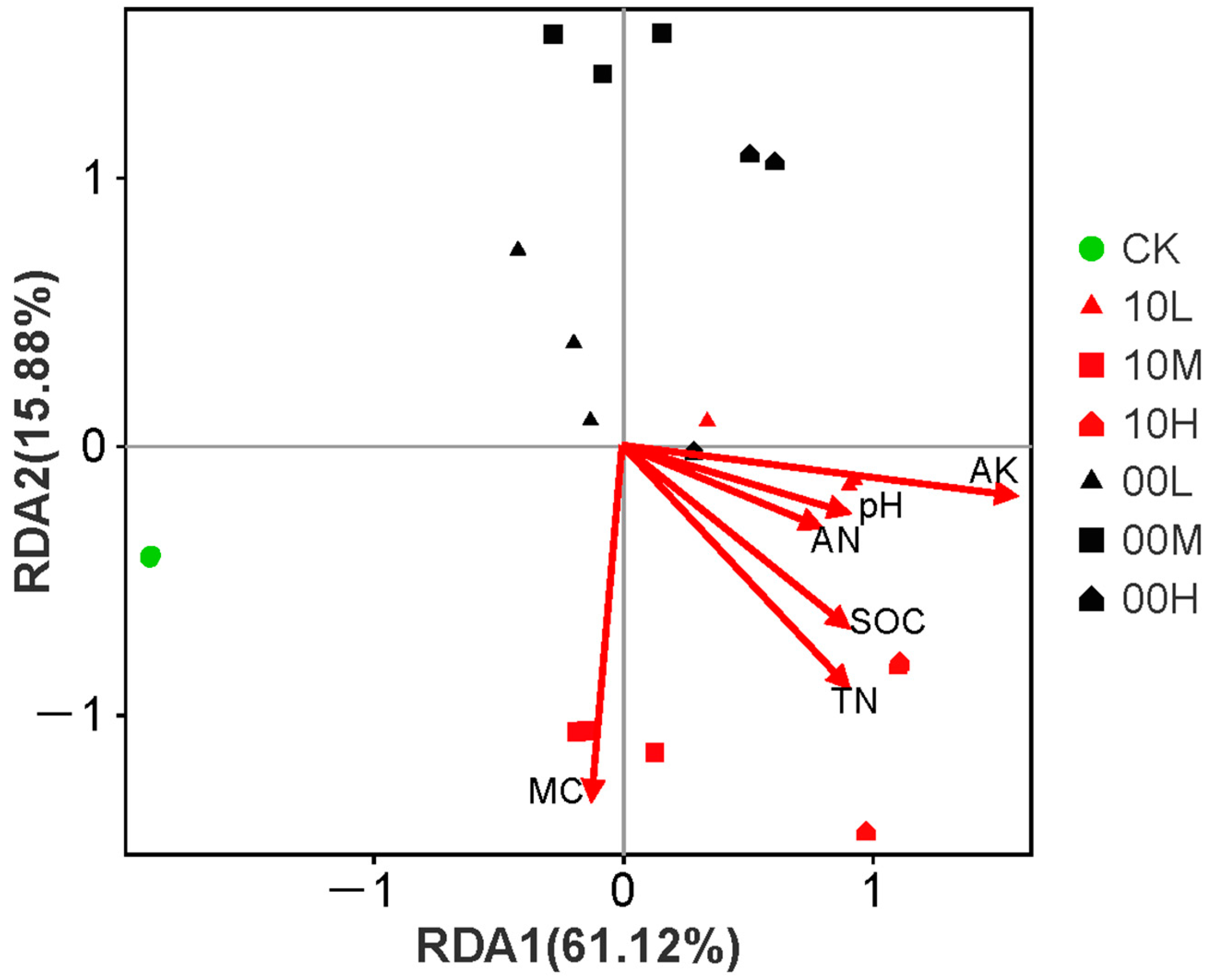
Redundancy analysis (RDA) was performed on the AWCD values of the soil microbial community in the fire burn site after 192 h of incubation. The first two RDA axes explained 60.76% and 15.74% of the variance, respectively (Figure 9). This analysis showed that the soil microbial community exhibited significant spatial differentiation in carbon source utilization after the fire, indicating that the fire affected both the types and capacities of soil microbial carbon source utilization. Additionally, RDA revealed that MC, TN, and AK were the most influential soil environmental factors for the soil microbial community (Table 3).
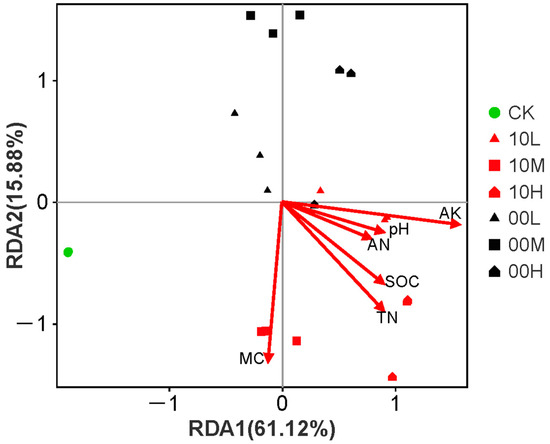
Figure 9.
Redundancy analysis of soil microbial community functions and environmental factors. CK: control (no fire history); 10L: low fire in 2010; 10M: moderate fire in 2010; 10H: high fire in 2010; 00L: low fire in 2000; 00M: moderate fire in 2000; 00H: high fire in 2000.

Table 3.
Correlation between soil microbial community function and soil physicochemical properties. * p < 0.05, ** p < 0.01.
4. Discussion
4.1. Effect of Fire on the Carbon Source Utilization Capacity of Soil Microorganisms
Fire can induce changes in the ability of microorganisms to utilize carbon sources, thereby altering the structure and function of soil microbial communities [42]. Differences in the AWCD of soil microorganisms reflect changes in the carbon metabolism activity of these microorganisms [43]. In this study, the average rate of color change in soil microbial communities increased with incubation time, and fire significantly enhanced the ability of microbes to utilize carbon sources. This finding is contrary to our hypothesis, likely because, on the one hand, the Daxing’anling region is located in a cold-temperate zone, where the growing season of larch forests is relatively short, leading to a limited supply of carbon and nutrients for soil microorganisms. However, high temperatures from fires accelerate microbial processes and increase the availability of readily decomposable organic matter, which promotes microbial growth and metabolism, and thus enhances the carbon utilization capacity of soil microorganisms [44]. On the other hand, in cold-temperate larch forests, which are mostly dominated by difficult-to-decompose coniferous litter, fire rapidly decomposes the litter on the ground surface, returning nutrients to the soil, increasing the accumulation of carbon sources, and making soil nutrients more readily available for microbial uptake and utilization. This, in turn, promotes microbial carbon metabolism activity [45,46].
Additionally, this study identified significant differences in the utilization of six carbon sources and variations in single carbon source utilization by soil microorganisms in the fire burn site. These differences can be attributed to variations in forest environment, soil nutrients, and the time of recovery from the fire, which influenced the soil microorganisms’ adaptations to different types of substrates [12]. This is reflected in the observed differences in the utilization capacity for different carbon source types. Castrillo et al. [47] concluded that differences in soil environment are key factors influencing the carbon source utilization capacity of soil microorganisms, which is consistent with the results of this study. Our findings (Figure 8) demonstrated that the intensity of the utilization of six carbon sources by soil microorganisms was significantly and positively correlated with pH, SOC, TN, and AK. Fire burns decompose organic matter in the soil, reducing acidic substances such as organic acids and leading to an increase in pH [48,49]. The growth and secretion of Larix gmelinii increase soil TN and return nitrogen to the soil in the form of apomictic material and root exudates [50]. Microorganisms require both carbon and nitrogen sources for growth and metabolism, and the increase in carbon and nitrogen inputs after the fire provided sufficient nutrient substrates for microbial growth, reproduction, and metabolic activities, thus increasing the intensity of carbon source utilization [51]. Additionally, the grass ash produced by the fire is rich in mineral elements such as potassium. These ashes settle into the soil and directly increase the content of AK, which affects the nutrient sources available to soil microorganisms and thus increases the intensity of carbon source utilization [52]. Collectively, these findings indicate that the soil environmental factors at the fire burn site are important determinants of the carbon source utilization capacity of the soil microbial community.
4.2. Effect of Fire on the Functional Diversity of Soil Microorganisms
The functional diversity index of soil microorganisms reflects the adaptive capacity and changing characteristics of microbial communities in different habitats [53]. An analysis of the Shannon diversity index, Simpson index, and McIntosh index revealed significant differences in the impact of different fire intensities on soil microbial community diversity. This implies variations in microbial species richness, dominance, and abundance under different fire intensities. Fire was found to significantly increase the Shannon index of soil microorganisms while decreasing the Simpson and McIntosh indices. These findings align with the research results of Zheng et al. [11] on the metabolic diversity of carbon sources in Daxing’anling forest soil microorganisms. The observed changes may be attributed to fire-induced alterations in the microenvironment [54], species adaptability [55], and niche differentiation of soil microorganisms [56], thereby affecting the utilization mode of carbon sources by microorganisms and subsequently influencing microbial diversity indices.
Our RDA and PCoA results revealed significant differences in the utilization of different carbon sources after a fire. Moreover, fire altered the metabolic capacity of forest soil microbial communities to metabolize carbon sources (Figure 7). This phenomenon can be attributed to changes in soil water content, soil carbon and nitrogen levels, and other physicochemical properties caused by the fire. In this study, soil MC, TN, and AK were found to significantly affect the functional diversity of soil microorganisms. Ding and Song et al. [57,58] found that total nitrogen was the main factor influencing soil microbial community structure in subtropical forests, which is consistent with our findings. Additionally, soil TN and AK are essential for maintaining various functions and material conversions in soil microorganisms, and significant changes in their contents play a key role in the functional diversity of the microbial community [59]. Furthermore, Li et al. [60] found that soil microbial community diversity in Southwest subtropical forests primarily depends on soil water content, which aligns with our hypothesis. Variations in soil water content can lead to changes in plant root development, microbial activity, and humus decomposition rates, all of which affect the efficiency of soil microbes in utilizing carbon sources [61]. Therefore, soil water content significantly influenced the functional diversity of soil microorganisms in the fire burn site in this study.
In summary, fire induces significant variations in the metabolic characteristics of soil microbial communities in cold temperate larch forests. It alters the utilization mode of carbon sources by microbial communities, thereby affecting the metabolic activity of soil microorganisms. However, Biolog-Eco analysis can only identify changes in microbial functional diversity from the perspective of metabolic characteristics. Therefore, future research should combine next-generation sequencing, metagenomic technology, and genome-wide association studies to explore the variations in the functional diversity of microbial communities.
5. Conclusions
- (1)
- Significant differences in soil physicochemical properties were identified in fire burn sites of cold-temperate Larix gmelinii forests during the middle and late stages of restoration.
- (2)
- Fire significantly increased the carbon source utilization capacity of soil microorganisms.
- (3)
- Fire increased the Shannon index and decreased the Simpson and McIntosh diversity indices of soil microorganisms.
- (4)
- Soil MC, TN, and AK were the main factors influencing the functional diversity of soil microbial communities in middle- and late-stage cold-temperate Larix gmelinii forest fire burn sites.
Author Contributions
Data curation, Z.C., H.P., X.F., D.W. and X.L.; writing—original draft preparation, Z.C.; writing—review and editing, L.Y. and S.W.; funding acquisition, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
Forestry and grassland ecological protection and restoration funds project (GZCG2023-024); the Foundation of Heilongjiang Academy of Sciences (KY2023ZR03); and the Financial Special Project of Heilongjiang Province (CZKYF2024-1-A008).
Data Availability Statement
All data are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ma, W.; Feng, Z.; Cheng, Z.; Chen, S.; Wang, F. Identifying Forest Fire Driving Factors and Related Impacts in China Using Random Forest Algorithm. Forests 2020, 11, 507. [Google Scholar] [CrossRef]
- Wasserman, T.N.; Mueller, S.E. Climate influences on future fire severity: A synthesis of climate-fire interactions and impacts on fire regimes, high-severity fire, and forests in the western United States. Fire Ecol. 2023, 19, 43. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Martínez-Hidalgo, P.; Cobo-Díaz, J.F.; Villadas, P.J.; Martínez-Molina, E.; Toro, N.; Tringe, S.G.; Fernández-López, M. The rhizosphere microbiome of burned holm-oak: Potential role of the genus Arthrobacter in the recovery of burned soils. Sci. Rep. 2017, 7, 6008. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, S.; Wei, D.; Pan, H.; Fu, X.; Lu, X.; Yang, L. Current Status of Research on Wildland Fire Impacts on Soil Environment and Soil Organisms and Hotspots Visualization Analysis. Fire 2024, 7, 163. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, S.; Du, J.; Pan, H.; Lu, X.; Liu, Y.; Yang, L. Variations in the Diversity and Biomass of Soil Bacteria and Fungi under Different Fire Disturbances in the Taiga Forests of Northeastern China. Forests 2023, 14, 2063. [Google Scholar] [CrossRef]
- Mao, H.R.; Li, G.L.; Leng, K.; Sun, L.Y.; Liu, K.L.; Lin, Y.X.; Liu, J.; Xiang, X.J. Effects of core soil microbial taxa on soil carbon source utilization under different long-term fertilization treatments in Ultisol. Soil Ecol. Lett. 2024, 6, 240241. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Liu, Z.D.; Song, Y.Y.; Wang, X.W.; Yuan, J.B.; Li, M.T.; Lou, Y.J.; Gao, Z.L.; Song, C.C. Soil microbial functional diversity is primarily affected by soil nitrogen, salinity and alkalinity in wetland ecosystem. Appl. Soil Ecol. 2024, 199, 105407. [Google Scholar] [CrossRef]
- Lopez, A.M.; Avila, C.C.E.; Vanderroest, J.P.; Roth, H.K.; Fendorf, S.; Borch, T. Molecular insights and impacts of wildfire-induced soil chemical changes. Nat. Rev. Earth Environ. 2024, 5, 431–446. [Google Scholar] [CrossRef]
- Fox, S.; Taylor, M.K.; Callaham, M., Jr.; Jumpponen, A. Fire-excluded and frequently burned longleaf pine forests have contrasting soil microbial communities. For. Ecol. Manag. 2023, 551, 121519. [Google Scholar] [CrossRef]
- Greenwood, L.; Nimmo, D.G.; Egidi, E.; Price, J.N.; McIntosh, R.; Frew, A. Fire shapes fungal guild diversity and composition through direct and indirect pathways. Mol. Ecol. 2023, 32, 4921–4939. [Google Scholar] [CrossRef]
- Zheng, Q.; Cui, X.Y.; Di, X.Y.; Jin, S. Effects of Different Forest Fire Intensities on Microbial Community Functional Diversity in Forest Soil in Daxing’anling. Sci. Silvae Sin. 2012, 5, 95–100. [Google Scholar] [CrossRef]
- Velasco, A.G.V.; Probanza, A.; Mañero, F.J.G.; Treviño, A.C.; Moreno, J.M.; Garcia, J.A.L. Effect of fire and retardant on soil microbial activity and functional diversity in a Mediterranean pasture. Geoderma 2009, 153, 186–193. [Google Scholar] [CrossRef]
- Li, M.L.; Song, Z.P.; Liu, Y.H.; Wang, H.L. Effects of fire intensity on leaf functional traits and functional diversity of Larix gmelinii community. Chin. J. Appl. Ecol. 2019, 30, 4021–4030. [Google Scholar] [CrossRef]
- Xu, Y.H. Influences of Wildfire on Soil Microbial Community in a Shrubby Ecosystem. Master’s Thesis, Shihezi University, Shihezi, China, 2010. [Google Scholar]
- Zhong, C.; Guo, M.; Zhou, F.F.; Li, J.N.; Yu, F.B.; Guo, F.T.; Li, W.S. Forest succession trajectories after fires in valleys and on slopes in the Greater Khingan Mountains, China. J. For. Res. 2023, 34, 623–640. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhao, Y.L.; Xu, Y.; Ma, J.J.; Babalola, B.J.; Fan, Y.J. Ectomycorrhizal fungal communities associated with Larix gemelinii Rupr. in the Great Khingan Mountains, China. PeerJ 2021, 9, e11230. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Wang, B.; Zhang, P.J. Estimation of Leaf Nitrogen of Betula platyphylla in Burned Area of Daxing’anling, Inner Mongolia. For. Grassl. Resour. Res. 2021, 6, 90–96. [Google Scholar] [CrossRef]
- Ning, J.B.; Weng, Y.T.; Di, X.Y.; Yang, G. Rapid Determination Method for Swamp Meadow Surface Fuel Loads of Daxing’an Mountains. J. Northeast. For. Univ. 2018, 46, 44–48. [Google Scholar] [CrossRef]
- Cao, J.; Liang, D.Z.; Zhao, Y.S.; Xin, Y. Methane Flux and Affecting Factors in the Process of Vegetation Restoration in Burned Area of Great Xing’an Mountains. J. Northwest For. Univ. 2021, 36, 173–178. [Google Scholar] [CrossRef]
- Han, Z.M.; Xin, Y.; Zhao, Y.S. Soil Phosphate-dissolving Microorganisms in the Process of Vegetation Restoration in Burned Area of Daxing’an Mountains. J. Northeast. For. Univ. 2020, 48, 55–60+71. [Google Scholar] [CrossRef]
- Wang, Z.C.; Li, Y.X.; Meng, Y.B.; Wang, C. Responses of spatial distribution patterns and associations of Larix gmelinii and Populus davidiana mixed forests in Daxing’an mountains to different tending thinning intensities. J. Cent. South Univ. For. Technol. 2022, 42, 75–83+107. [Google Scholar] [CrossRef]
- Yang, L.B.; Jiang, Y.B.; Zhou, T.; Cui, F.X.; Zhu, D.G.; Xu, F. Efects of litter fall on soil fungal diversity under snow cover in the Greater Xing’an Mountains. Res. Environ. Sci. 2022, 35, 1037–1044. [Google Scholar] [CrossRef]
- Jiang, Y.B.; Yang, L.B.; Wu, S.; Zhou, T. Warming changes the composition and diversity of fungal communities in permafrost. Ann. Microbiol. 2023, 73, 7. [Google Scholar] [CrossRef]
- Yue, X.; Dun, X.J.; Cui, D.J.; Guo, X.D.; Song, X.Y.; Liu, L.; Wang, L.L.; Jiang, C.; Xu, J.W.; Li, S.M. Effects of Different Fire Intensities on Forest Soil Nutrients. Shandong Agric. Sci. 2018, 50, 72–76. [Google Scholar] [CrossRef]
- Kim, H.M.; Jung, J.Y.; Yergeau, E.; Hwang, C.Y.; Hinzman, L.; Nam, S.; Hong, S.G.; Kim, O.S.; Chun, J.; Lee, Y.K. Bacterial community structure and soil properties of a subarctic tundra soil in Council, Alaska. FEMS Microbiol. Ecol. 2014, 89, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Wear, E.K.; Wilbanks, E.G.; Nelson, C.E.; Carlson, C.A. Primer selection impacts specific population abundances but not community dynamics in a monthly time-series 16S rRNA gene amplicon analysis of coastal marine bacterioplankton. Environ. Microbiol. 2018, 20, 2709–2726. [Google Scholar] [CrossRef]
- Ade, L.J.; Hu, L.; Zi, H.B.; Wang, C.T.; Lerdau, M.; Dong, S.K. Efect of snowpack on the soil bacteria of alpine meadows in the Qinghai-Tibetan Plateau of China. Catena 2018, 164, 13–22. [Google Scholar] [CrossRef]
- Hu, W.G.; Zhang, Q.; Li, D.Y.; Cheng, G.; Mu, J.; Wu, Q.B.; Niu, F.; An, L.Z.; Feng, H.Y. Diversity and community structure of fungi through a permafrost core profle from the Qinghai-Tibet Plateau of China. J. Basic Microbiol. 2015, 54, 1331–1341. [Google Scholar] [CrossRef]
- Ge, Z.W.; Du, H.J.; Gao, Y.L.; Qiu, W.F. Analysis on Metabolic Functions of Stored Rice Microbial Communities by BIOLOG ECO Microplates. Front. Microbiol. 2018, 9, 1375. [Google Scholar] [CrossRef]
- Jiang, L.L.; Han, G.M.; Lan, Y.; Liu, S.N.; Gao, J.P.; Yang, X.; Meng, J.; Chen, W.F. Corn cob biochar increases soil culturable bacterial abundance without enhancing their capacities in utilizing carbon sources in Biolog Eco-plates. J. Integr. Agric. 2017, 16, 713–724. [Google Scholar] [CrossRef]
- Weng, X.H.; Li, J.Y.; Sui, X.; Li, M.S.; Yin, W.P.; Ma, W.C.; Yang, L.B.; Mu, L.Q. Soil microbial functional diversity responses to different vegetation types in the Heilongjiang Zhongyangzhan Black-billed Capercaillie Nature Reserve. Ann. Microbiol. 2021, 71, 26. [Google Scholar] [CrossRef]
- Jin, Z.; Lei, J.; Li, S.; Xu, X. Metabolic characteristics of microbial communities of Aeolian sandy soils induced by saline water drip irrigation in shelter forests. Eur. J. Soil Sci. 2015, 66, 476–484. [Google Scholar] [CrossRef]
- Li, D.L.; Chen, J.W.; Zhang, H.; Li, J.J. Effects of Pb and Cd on forest soil bacterial functional diversity and community structure. Acta Ecol. Sin. 2021, 41, 8472–8483. [Google Scholar] [CrossRef]
- Jin, Z.; Ji, F.Y.; Xu, X.; Xu, X.Y.; Chen, Q.K.; Li, Q. Microbial and metabolic characterization of a denitrifying phosphorus-uptake/side stream phosphorus removal system for treating domestic sewage. Biodegradation 2014, 25, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.H.; Xu, T.L.; Wang, W.; Sun, S.M.; Zhang, M.M.; Song, F.Q. Nitrogen addition changed soil fungal community structure and increased the biomass of functional fungi in Korean pine plantations in temperate northeast China. Sci. Total Environ. 2024, 927, 172349. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.P.; Guo, F.L.; Jiao, Z.B.; Zhou, J.; Qiu, Z.M. The effect of monoculture and rotation planting on soil bacterial community structure at different elevation in Hubei. Soil Use Manag. 2021, 37, 667–676. [Google Scholar] [CrossRef]
- Fazekasová, D.; Fazekas, J. Functional diversity of soil microorganisms in the conditions of an ecological farming system. Folia Oecologica 2019, 46, 146–152. [Google Scholar] [CrossRef]
- Jones, D.L.; Hill, P.W.; Smith, A.R.; Farrell, M.; Ge, T.; Banning, N.C.; Murphy, D.V. Role of substrate supply on microbial carbon use efficiency and its role in interpreting soil microbial community-level physiological profiles (CLPP). Soil Biol. Biochem. 2018, 123, 1–6. [Google Scholar] [CrossRef]
- Sun, M.M.; Luo, Y.M.; Christie, P.; Jia, Z.J.; Li, Z.G.; Teng, Y. Methyl-β-cyclodextrin enhanced biodegradation of polycyclic aromatic hydrocarbons and associated microbial activity in contaminated soil. J. Environ. Sci. 2012, 24, 926–933. [Google Scholar] [CrossRef]
- Zheng, W.N.; Fan, X.P.; Chen, H.; Ye, M.J.; Yin, C.; Wu, C.Y.; Liang, Y.C. The response patterns of r- and K-strategist bacteria to long-term organic and inorganic fertilization regimes within the microbial food web are closely linked to rice production. Sci. Total Environ. 2024, 942, 173681. [Google Scholar] [CrossRef]
- Furuta, K.; Byrne, J.; Luat, K.; Cheung, C.; Carter, D.O.; Tipton, L.; Uptmor, K.A.P. Volatile organic compounds produced during postmortem processes can be linked via chromatographic profiles to individual postmortem bacterial species. J. Chromatogr. A 2024, 1728, 465017. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.T.; Zi, H.B.; Hu, L.; Yang, X.Z.; Yang, Y.F. Effects of fire disturbance on the functional diversity of soil microbial Effects of fi re disturbance on the functional diversity of soil microbial community in alpine meadows community in alpine meadows. Chin. J. Appl. Environ. Biol. 2016, 22, 263–270. [Google Scholar] [CrossRef]
- Sun, H.R.; Liu, J.Y.; Wu, J.H.; Hu, H.Y.; Chen, Q.B.; Fang, H.Y.; Tao, K. Effects of alpine grassland degradation on soil microbial community structure and metabolic activity in the Qinghai-Tibet Plateau. Appl. Soil Ecol. 2024, 200, 105458. [Google Scholar] [CrossRef]
- Frossard, A.; De Maeyer, L.; Adamczyk, M.; Svenning, M.; Verleyen, E.; Frey, B. Microbial carbon use and associated changes in microbial community structure in high-Arctic tundra soils under elevated temperature. Soil Biol. Biochem. 2021, 162, 108419. [Google Scholar] [CrossRef]
- Ji, L.; Yang, Y.C.; Yang, L.X. Seasonal variations in soil fungal communities and co-occurrence networks along an altitudinal gradient in the cold temperate zone of China: A case study on Oakley Mountain. Catena 2021, 204, 105448. [Google Scholar] [CrossRef]
- Brown, A.L.; Koo, E.; Reisner, J. Estimating stratospheric carbon from fires during a regional nuclear exchange. Fire Saf. J. 2023, 141, 103877. [Google Scholar] [CrossRef]
- Castrillo, G.; Teixeira, P.J.P.L.; Paredes, S.H.; Law, T.F.; de Lorenzo, L.; Feltcher, M.E.; Finkel, O.M.; Breakfield, N.W.; Mieczkowski, P.; Jones, C.D.; et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature 2017, 543, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Negri, S.; Giannetta, B.; Till, J.; de Souza, D.O.; Said-Pullicino, D.; Bonifacio, E. Fire simulation effects on the transformation of iron minerals in alpine soils. Geoderma 2024, 444, 116858. [Google Scholar] [CrossRef]
- De Baets, S.; van de Weg, M.J.; Lewis, R.; Steinberg, N.; Meersmans, J.; Quine, T.A.; Shaver, G.R.; Hartley, I.P. Investigating the controls on soil organic matter decomposition in tussock tundra soil and permafrost after fire. Soil Biol. Biochem. 2016, 99, 108–116. [Google Scholar] [CrossRef]
- Huang, X.L.; Tang, Z.Y.; Liu, J.M.; Tong, B.L. Rhizosphere microbial community structure and its correlation with soil nutrients in Cinnamomum migao. J. Northeast For. Univ. 2023, 51, 92–97+105. [Google Scholar] [CrossRef]
- Yang, G.S.; Song, C.C.; Guo, Y.D.; Lu, Y.Z.; Song, Y.Y. Influence of fire on soil microbial activity of restoration marsh. J. Soil Water Conserv. 2010, 06, 218–221. [Google Scholar] [CrossRef]
- Li, B.Y. Effects of Different Fire Intensities on Soil Organic Carbon and Soil Nutrients in Pinus Tabulaeformis Forests in Northern China. Master’s Thesis, Beijing Forestry University, Beijing, China, 2018. [Google Scholar]
- Escalas, A.; Hale, L.; Voordeckers, J.W.; Yang, Y.F.; Firestone, M.K.; Alvarez-Cohen, L.; Zhou, J.Z. Microbial functional diversity: From concepts to applications. Ecol. Evol. 2019, 9, 12000–12016. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Choung, S.; Oh, J.; Jeong, J. Groundwater and Soil Pollution Caused by Forest Fires, and Its Effects on the Distribution and Transport of Radionuclides in Subsurface Environments: Review. Econ. Environ. Geol. 2023, 56, 501–514. [Google Scholar] [CrossRef]
- Ascoli, D.; Hacket-Pain, A.; LaMontagne, J.M.; Cardil, A.; Conedera, M.; Maringer, J.; Motta, R.; Pearse, I.S.; Vacchiano, G. Climate teleconnections synchronize Picea glauca masting and fire disturbance: Evidence for a fire-related form of environmental prediction. J. Ecol. 2020, 108, 1186–1198. [Google Scholar] [CrossRef]
- Tran, D.V.; Tominaga, A.; Pham, L.T.; Nishikawa, K. Ecological niche modeling shed light on new insights of the speciation processes and historical distribution of Japanese fire-bellied newt Cynops pyrrhogaster (Amphibia: Urodela). Ecol. Inform. 2024, 79, 102443. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, Y.T.; Zhang, J.H.; Chai, X.; Zhou, S.S.; Tong, Z.K. Effects of Chinese fir plantations with different densities on understory vegetation and soil microbial community structure. Chin. J. Plant Ecol. 2021, 45, 62–73. [Google Scholar] [CrossRef]
- Song, M.Y.; Li, Z.P.; Liu, M.; Liu, M.Q.; Jiang, C.Y. Effeets of mixture of forest litter on nutrient contents and functional diversity of microbial community in soil. Chin. J. Ecol. 2014, 33, 2454–2461. [Google Scholar] [CrossRef]
- Su, S.F.; Wang, X.Y.; Lin, Z.P.; Jin, Y.H.; Xue, Y. The characteristics of soil microbial functional diversity of six types of vegetation in tropical regions. J. Yunnan Agric. Univ. Nat. Sci. 2022, 37, 505–514. [Google Scholar] [CrossRef]
- Li, Y.N.; Qian, Z.Y.; Li, D.J. Effects of tree diversity on soil microbial community in a subtropical forest in Southwest China. Eur. J. Soil Biol. 2023, 116, 103490. [Google Scholar] [CrossRef]
- Xiao, T.; Li, P.; Fei, W.B.; Wang, J.D. Effects of vegetation roots on the structure and hydraulic properties of soils: A perspective review. Sci. Total Environ. 2023, 906, 167524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).