Exon-Enriched Set of Single-Nucleotide Polymorphisms Shows Associations with Climate in European Beech (Fagus sylvatica L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. DNA Extraction and Sequencing
2.3. Data Analysis
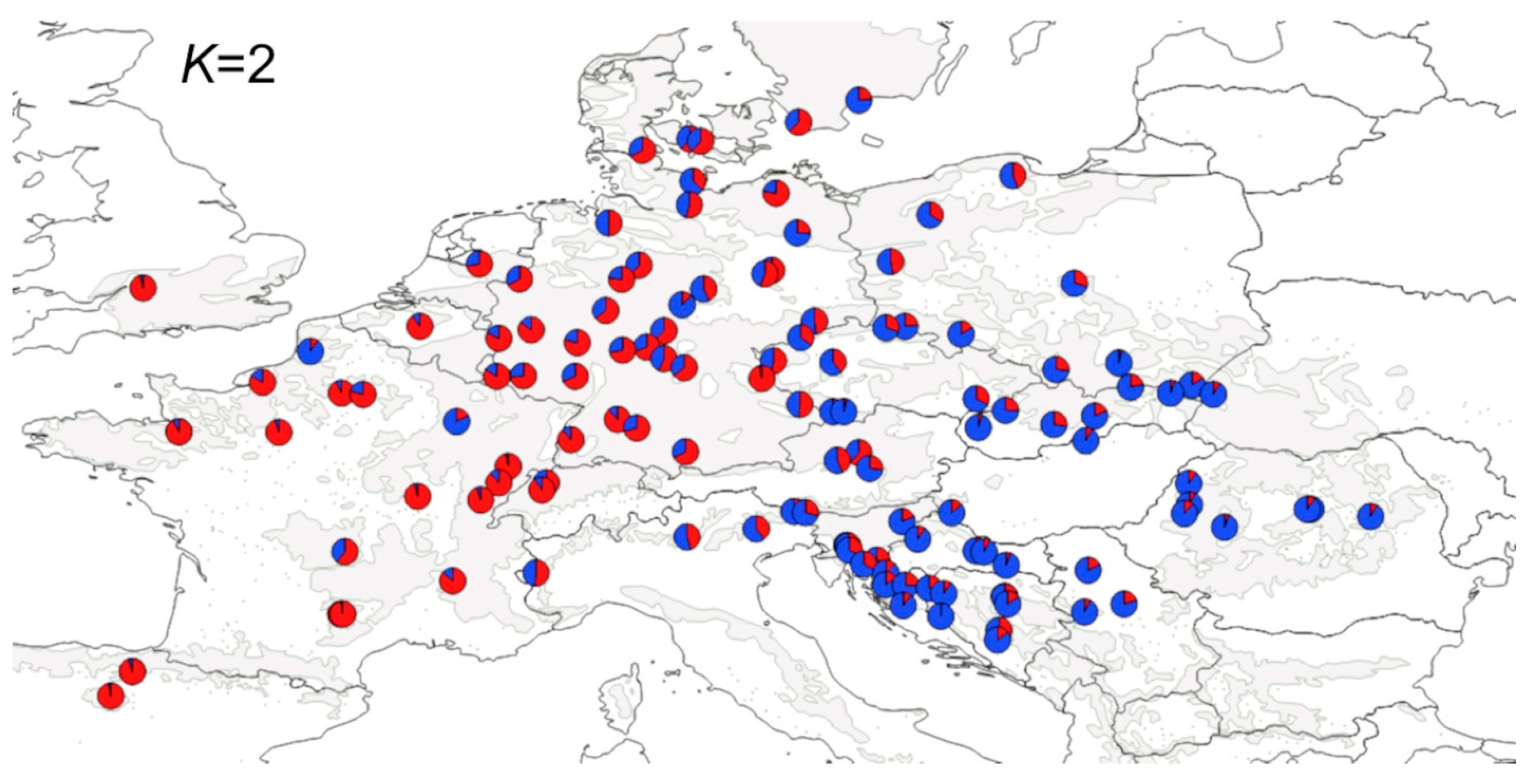
3. Results
3.1. Population Structure
3.2. Climatic Associations
4. Discussion
4.1. Choice of Populations and Climatic Proxies
4.2. Climatic Associations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Fagus sylvatica in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 94–95. [Google Scholar]
- Brunet, J.; Felton, A.; Lindbladh, M. From wooded pasture to timber production—Changes in a European beech (Fagus sylvatica) forest landscape between 1840 and 2010. Scand. J. For. Res. 2012, 27, 245–254. [Google Scholar] [CrossRef]
- Emmer, I.M.; Fanta, J.; Kobus, A.T.; Kooijman, A.; Sevink, J. Reversing borealization as a means to restore biodiversity in Central-European mountain forests—An example from the Krkonose Mountains, Czech Republic. Biodiv. Conserv. 1998, 7, 229–247. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Czúcz, B.; Gálhidy, L.; Mátyás, C. Present and forecasted xeric climatic limits of beech and sessile oak distribution at low altitudes in Central Europe. Ann. For. Sci. 2011, 68, 99–108. [Google Scholar] [CrossRef]
- Czajkowski, T.; Kühling, M.; Bolte, A. Einfluss der Sommertrockenheit im Jahre 2003 auf das Wachstum von Naturverjüngungen der Buche (Fagus sylvatica L.) im nordöstlichen Mitteleuropa. Allg. Forst- U.Jagdztg. 2005, 176, 133–143. [Google Scholar]
- Peñuelas, J.; Ogaya, R.; Boada, M.; Jump, A.S. Migration, invasion and decline: Changes in recruitment and forest structure in a warming-linked shift of European beech forest in Catalonia (NE Spain). Ecography 2007, 30, 829–837. [Google Scholar] [CrossRef]
- Berki, I.; Rasztovits, E.; Móricz, N.; Mátyás, C. Determination of the drought tolerance limit of beech forests and forecasting their future distribution in Hungary. Cereal Res. Comm. 2009, 37, 613–616. [Google Scholar]
- Lakatos, F.; Molnár, M. Mass mortality of beech (Fagus sylvatica L.) in South-West Hungary. Acta Silv. Lign. Hung. 2009, 5, 75–82. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Archambeau, J.; Ruiz-Benito, P.; Ratcliffe, S.; Fréjaville, T.; Changenet, A.; Muñoz Castañeda, J.M.; Lehtonen, A.; Dahlgren, J.; Zavala, M.A.; Benito Garzón, M. Similar patterns of background mortality across Europe are mostly driven by drought in European beech and a combination of drought and competition in Scots pine. Agric. For. Meteorol. 2020, 280, 107772. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2023. [Google Scholar]
- Williams, M.I.; Dumroese, R.K. Preparing for climate change: Forestry and assisted migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Mishra, B.; Ulaszewski, B.; Meger, J.; Aury, J.M.; Bodénès, C.; Lesur-Kupin, I.; Pfenninger, M.; Da Silva, C.; Gupta, D.K.; Guichoux, E.; et al. A chromosome-level genome assembly of the European beech (Fagus sylvatica) reveals anomalies for organelle DNA integration, repeat content and distribution of SNPs. Front. Genet. 2022, 12, 691058. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, S.C.; Krutovsky, K.V.; Neale, D.B. Forest-tree population genomics and adaptive evolution. New Phytol. 2006, 170, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Neale, D.B.; Kremer, A. Forest tree genomics: Growing resources and applications. Nat. Rev. Genet. 2011, 12, 111–122. [Google Scholar] [PubMed]
- Eklöf, H.; Bernhardsson, C.; Ingvarsson, P.K. Comparing the effectiveness of exome capture probes, genotyping by sequencing and whole-genome re-sequencing for assessing genetic diversity in natural and managed stands of Picea abies. Forests 2020, 11, 1185. [Google Scholar] [CrossRef]
- Meger, J.; Ulaszewski, B.; Burczyk, J. Genomic signatures of natural selection at phenology-related genes in a widely dis-tributed tree species Fagus sylvatica L. BMC Genom. 2021, 22, 583. [Google Scholar] [CrossRef]
- Tóth, E.G.; Köbölkuti, Z.A.; Cseke, K.; Kámpel, J.D.; Takács, R.; Tomov, V.T.; Ábrán, P.; Stojnić, S.; Vastag, E.; Mataruga, M.; et al. A genomic dataset of single-nucleotide polymorphisms generated by ddRAD tag sequencing in Q. petraea (Matt.) Liebl. populations from Central-Eastern Europe and Balkan Peninsula. Ann. For. Sci. 2021, 78, 43. [Google Scholar] [CrossRef]
- Ulaszewski, B.; Meger, J.; Burczyk, J. Comparative analysis of SNP discovery and genotyping in Fagus sylvatica L. and Quercus robur L. using RADseq, GBS, and ddRAD methods. Forests 2021, 12, 222. [Google Scholar] [CrossRef]
- Catchen, J.M.; Hohenlohe, P.A.; Bernatchez, L.; Funk, W.C.; Andrews, K.R.; Allendorf, F.W. Unbroken: RADseq remains a powerful tool for understanding the genetics of adaptation in natural populations. Mol. Ecol. Resourc. 2017, 17, 362–365. [Google Scholar] [CrossRef]
- Parchman, T.L.; Jahner, J.P.; Uckele, K.A.; Galland, L.M.; Eckert, A.J. RADseq approaches and applications for forest tree ge-netics. Tree Genet. Genomes 2018, 14, 39. [Google Scholar] [CrossRef]
- Ashwath, M.N.; Lavale, S.A.; Santhoshkumar, A.V.; Mohapatra, S.R.; Bhardwaj, A.; Dash, U.; Shiran, K.; Samantara, K.; Wani, S.H. Genome-wide association studies: An intuitive solution for SNP identification and gene mapping in trees. Funct. Integr. Genom. 2023, 23, 297. [Google Scholar]
- Csilléry, K.; Lalagüe, H.; Vendramin, G.G.; González-Martínez, S.C.; Fady, B.; Oddou-Muratorio, S. Detecting short spatial scale local adaptation and epistatic selection in climate-related candidate genes in European beech (Fagus sylvatica) populations. Mol. Ecol. 2014, 23, 4696–4708. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Alarcon, L.; Arend, M.; Müller, M.; Sperisen, C.; Finkeldey, R.; Krutovsky, K.V. A candidate gene association analysis identifies SNPs potentially involved in drought tolerance in European beech (Fagus sylvatica L.). Sci. Rep. 2021, 11, 2386. [Google Scholar] [CrossRef] [PubMed]
- Pluess, A.R.; Frank, A.; Heiri, C.; Lalagüe, H.; Vendramin, G.G.; Oddou-Muratorio, S. Genome-environment association study suggests local adaptation to climate at the regional scale in Fagus sylvatica. New Phytol. 2016, 210, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Jolivet, C.; Mader, M.; Liesebach, H.; Kersten, B.; Degen, B. A set of nuclear SNP loci derived from single sample double digest RAD and from pool sequencing for large-scale genetic studies in the European beech Fagus sylvatica. Cons. Genet. Resour. 2022, 14, 151–153. [Google Scholar] [CrossRef]
- Capblancq, T.; Morin, X.; Gueguen, M.; Renaud, J.; Lobreaux, S.; Bazin, E. Climate-associated genetic variation in Fagus sylvatica and potential responses to climate change in the French Alps. J. Evol. Biol. 2020, 33, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, A.; Silverj, A.; Torre, S.; Rota-Stabelli, O.; Girardi, M.; Passeri, I.; Fracasso, I.; Sebastiani, F.; Vernesi, C. First genome-wide data from Italian European beech (Fagus sylvatica L.): Strong and ancient differentiation between Alps and Apennines. PLoS ONE 2023, 18, e0288986. [Google Scholar] [CrossRef]
- Krajmerová, D.; Hrivnák, M.; Ditmarová, Ľ.; Jamnická, G.; Kmeť, J.; Kurjak, D.; Gömöry, D. Nucleotide polymorphisms associated with climate, phenology and physiological traits in European beech (Fagus sylvatica L.). New For. 2017, 48, 463–477. [Google Scholar] [CrossRef]
- Konôpková, A.; Krajmerová, D.; Kurjak, D.; Kmeť, J.; Pšidová, E.; Kučerová, J.; Hrivnák, M.; Longauer, R.; Ditmarová, Ľ.; Gömöry, D. Nucleotide polymorphisms associated with climate and physiological traits in silver fir (Abies alba Mill.) provenances. Flora 2019, 250, 7–43. [Google Scholar] [CrossRef]
- Hrivnák, M.; Krajmerová, D.; Kurjak, D.; Konôpková, A.; Magni, F.; Scaglione, D.; Ditmarová, Ľ.; Jamnická, G.; Marešová, J.; Gömöry, D. Differential associations between nucleotide polymorphisms and physiological traits in Norway spruce (Picea abies Karst.) plants under contrasting water regimes. Forestry 2022, 95, 686–697. [Google Scholar]
- Magri, D.; Vendramin, G.G.; Comps, B.; Dupanloup, I.; Geburek, T.; Gömöry, D.; Latalowa, M.; Litt, T.; Paule, L.; Roure, J.M.; et al. A new scenario for the Quaternary history of European beech populations: Palaeobotanical evidence and genetic consequences. New Phytol. 2006, 171, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Marchi, M.; Castellanos-Acuna, D.; Hamann, A.; Wang, T.; Ray, D.; Menzel, A.; Climate, E.U. Scale-free climate normals, historical time series, and future projections for Europe. Sci. Data 2020, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double digest RADseq: An inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 2012, 7, e37135. [Google Scholar] [CrossRef] [PubMed]
- Catchen, J.; Hohenlohe, P.A.; Bassham, S.; Amores, A.; Cresko, W.A. Stacks: An analysis tool set for population genomics. Mol. Ecol. 2013, 22, 3124–3140. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure from multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software Structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef]
- Conrad, V.; Pollak, L.W. Methods in Climatology; Harvard University Press: Cambridge, MA, USA, 1950. [Google Scholar]
- Bradbury, P.J.; Zhang, Z.W.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Frichot, E.; Schoville, S.D.; Bouchard, G.; François, O. Testing for associations between loci and environmental gradients using latent factor mixed models. Mol. Biol. Evol. 2013, 30, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Frichot, E.; François, O. LEA: An R package for landscape and ecological association studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Storey, J.D.; Bass, A.J.; Dabney, A.; Robinson, D. Qvalue: Q-Value Estimation for False Discovery Rate Control. R Package Version 2.24.0. Available online: http://github.com/jdstorey/qvalue (accessed on 21 March 2022).
- Capblancq, T.; Luu, K.; Blum, M.G.B.; Bazin, E. Evaluation of redundancy analysis to identify signatures of local adaptation. Mol. Ecol. Resour. 2018, 18, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Available online: https://CRAN.R-project.org/package=vegan (accessed on 5 May 2019).
- Agostinelli, C.; Lund, U. R Package ‘Circular’: Circular Statistics (Version 0.4–95). Available online: https://r-forge.r-project.org/projects/circular) (accessed on 2 February 2022).
- Magri, D. Patterns of post-glacial spread and the extent of glacial refugia of European beech (Fagus sylvatica). J. Biogeogr. 2008, 35, 450–463. [Google Scholar] [CrossRef]
- Demesure, B.; Comps, B.; Petit, R.J. Chloroplast DNA phylogeography of the Common Beech (Fagus sylvatica L.) in Europe. Evolution 1996, 50, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Comps, B.; Thiébaut, B.; Paule, L.; Merzeau, D.; Letouzey, J. Allozymic variability in beechwoods (Fagus sylvatica L.) over Central Europe—spatial differentiation among and within populations. Heredity 1990, 65, 407–417. [Google Scholar] [CrossRef]
- Comps, B.; Gömöry, D.; Letouzey, J.; Thiébaut, B.; Petit, R.J. Diverging trends between heterozygosity and allelic richness during postglacial colonization in the European beech. Genetics 2001, 157, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Gömöry, D.; Vyšný, J.; Comps, B.; Thiebaut, B. Geographical patterns of genetic differentiation and diversity in European beech (Fagus sylvatica L.) populations in France. Biologia 1992, 47, 571–579. [Google Scholar]
- Gömöry, D.; Paule, L.; Vyšný, J. Patterns of allozyme variation in western-Eurasian beeches. Bot. J. Linn. Soc. 2007, 154, 165–174. [Google Scholar] [CrossRef]
- Hrivnák, M.; Krajmerová, D.; Paule, L.; Zhelev, P.; Sevik, H.; Ivanković, M.; Goginashvili, N.; Paule, J.; Gömöry, D. Are there hybrid zones in Fagus sylvatica L. sensu lato? Eur. J. For. Res. 2024, 143, 451–464. [Google Scholar] [CrossRef]
- Huntley, B. Reconstructing palaeoclimates from biological proxies: Some often overlooked sources of uncertainty. Quat. Sci. Rev. 2012, 31, 1–16. [Google Scholar] [CrossRef]
- Höhn, M.; Major, E.; Avdagić, A.; Bielak, K.; Bošeľa, M.; Coll, L.; Dinca, L.; Giammarchi, F.; Ibrahimspahić, A.; Mataruga, M.; et al. Local characteristics of the standing genetic diversity of European beech with high within-region differentiation at the eastern part of the range. Can. J. For. Res. 2021, 51, 1791–1798. [Google Scholar] [CrossRef]
- Kirk, H.; Freeland, J.R. Applications and implications of neutral versus non-neutral markers in molecular ecology. Int. J. Mol. Sci. 2011, 12, 3966–3988. [Google Scholar] [CrossRef] [PubMed]
- Skibinski, D.O.; Woodwark, M.; Ward, R.D. A quantitative test of the neutral theory using pooled allozyme data. Genetics 1993, 135, 233–248. [Google Scholar] [CrossRef]
- Comps, B.; Barrière, G.; Merzeau, D.; Letouzey, J. La variabilité alloenzymatique des hêtraies dans les sousdomaines médio- et eu-atlantiques d’Europe. Can. J. For. Res. 1987, 17, 1043–1049. [Google Scholar] [CrossRef]
- Fang, J.; Lechowicz, M.J. Climatic limits for the present distribution of beech (Fagus L.) species in the world. J. Biogeogr. 2006, 33, 1804–1819. [Google Scholar] [CrossRef]
- Seifert, S.; Vornam, B.; Finkeldey, R. DNA sequence variation and development of SNP markers in beech (Fagus sylvatica L.). Eur. J. For. Res. 2012, 131, 1761–1770. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Kompa, T. The north-eastern distribution range of European beech—A review. Forestry, 2007; 80, 413–429. [Google Scholar]
- Charra-Vaskou, K.; Charrier, G.; Wortemann, R.; Beikircher, B.; Cochard, H.; Ameglio, T.; Mayr, S. Drought and frost resistance of trees: A comparison of four species at different sites and altitudes. Ann. For. Sci. 2012, 69, 325–333. [Google Scholar] [CrossRef]
- Weigel, R.; Muffler, L.; Klisz, M.; Kreyling, J.; Maaten-Theunissen, M.v.d.; Wilmking, M.; van der Maaten, E. Winter matters: Sensitivity to winter climate and cold events increases towards the cold distribution margin of European beech (Fagus sylvatica L.). J. Biogeogr. 2018, 45, 2779–2790. [Google Scholar] [CrossRef]
- Cushman, S.A.; Landguth, E.L. Spurious correlations and inference in landscape genetics. Mol. Ecol. 2010, 19, 3592–3602. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, C. Drought response of European beech (Fagus sylvatica L.)—A review. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125576. [Google Scholar] [CrossRef]
- Rennenberg, H.; Loreto, F.; Polle, A.; Brilli, F.; Fares, S.; Beniwal, R.; Gessler, A. Physiological responses of forest trees to heat and drought. Plant Biol. 2006, 8, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Stojnić, S.; Suchocka, M.; Benito-Garzón, M.; Torres-Ruiz, J.M.; Cochard, H.; Bolte, A.; Cocozza, C.; Cvjetković, B.; de Luis, M.; Martinez-Vilalta, J.; et al. Variation in xylem vulnerability to embolism in European beech from geographically marginal populations. Tree Physiol. 2017, 38, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Húdoková, H.; Petrík, P.; Petek-Petrík, A.; Konôpková, A.; Leštianska, A.; Střelcová, K.; Kmeť, J.; Kurjak, D. Heat-stress response of photosystem II in five ecologically important tree species of European temperate forests. Biologia 2022, 77, 671–680. [Google Scholar] [CrossRef]
- Kurjak, D.; Konôpková, A.; Kmeť, J.; Macková, M.; Frýdl, J.; Živčák, M.; Palmroth, S.; Ditmarová, Ľ.; Gömöry, D. Variation in the performance and thermostability of photosystem II in European beech (Fagus sylvatica L.) provenances is influenced more by acclimation than by adaption. Eur. J. For. Res. 2019, 138, 79–92. [Google Scholar] [CrossRef]
- Vitasse, Y.; Basler, D. What role for photoperiod in the bud burst phenology of European beech. Eur. J. For. Res. 2013, 132, 1–8. [Google Scholar] [CrossRef]
- von Wuehlisch, G.; Krusche, D.; Muhs, H.J. Variation in temperature sum requirement for flushing of beech provenances. Silvae Genet. 1995, 44, 343–347. [Google Scholar]
- Gömöry, D.; Paule, L. Trade-off between height growth and spring flushing in common beech (Fagus sylvatica L.). Ann. For. Sci. 2011, 68, 975–984. [Google Scholar] [CrossRef]
- Schenck, C.A.; Maeda, H.A. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 2018, 149, 82–102. [Google Scholar] [CrossRef]
- Maeda, H.; Song, W.; Sage, T.L.; DellaPenna, D. Tocopherols play a crucial role in low-temperature adaptation and phloem loading in Arabidopsis. Plant Cell 2006, 18, 2710–2732. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.N.; Bemmels, J.B. Time to get moving: Assisted gene flow of forest trees. Evol. Appl. 2015, 9, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Schueler, S.; Lexer, M.J.; Wang, T. Genetic trials improve the transfer of Douglas-fir distribution models across continents. Ecography 2019, 42, 88–101. [Google Scholar] [CrossRef]
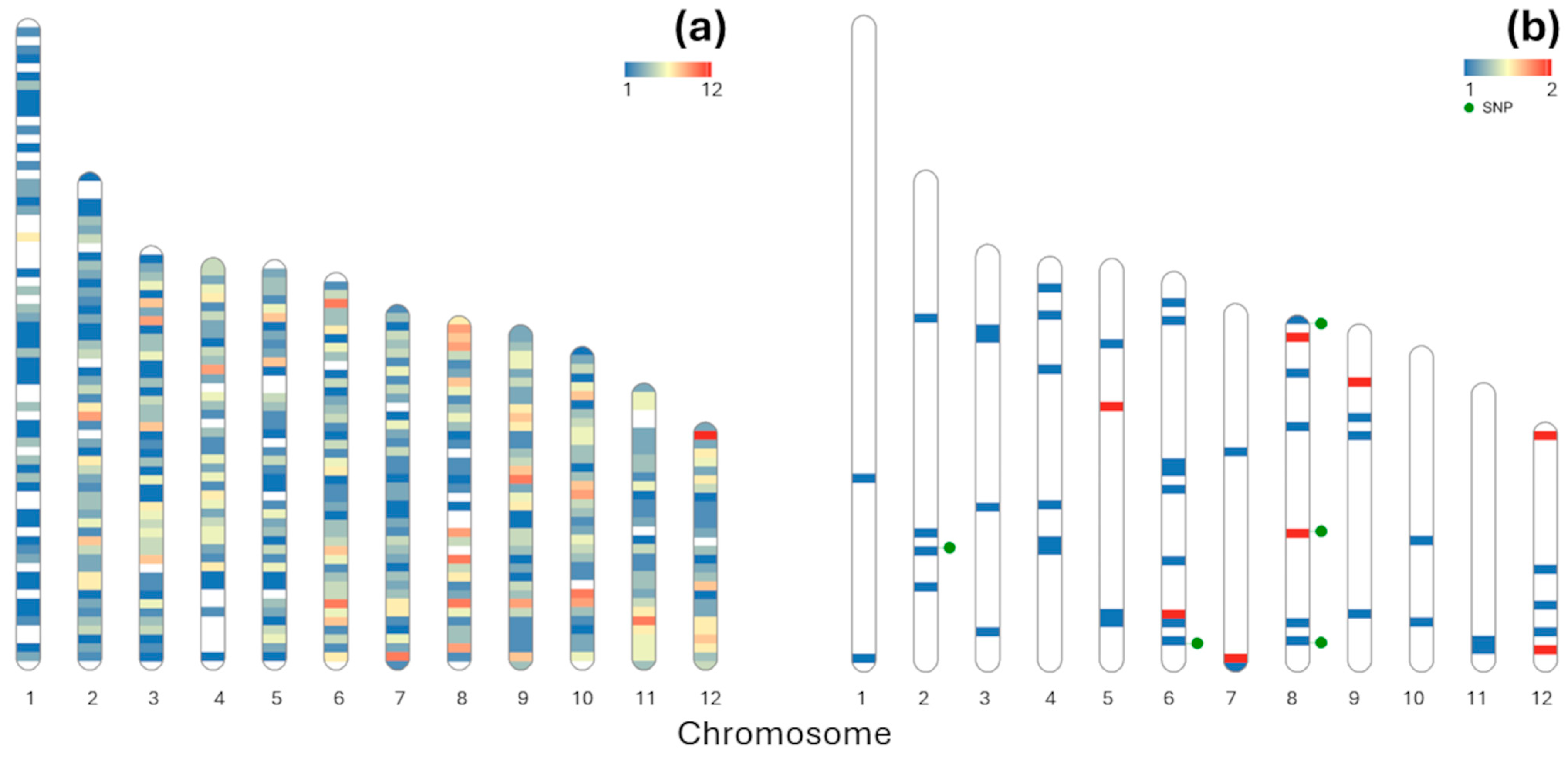

| Reference Chromosome | SNP Position | SNP | Climatic Variable | r 1 | Annotation [12] |
|---|---|---|---|---|---|
| 2 | 42540393 | G/A | BIO2 | −0.809 | >Bhaga_2.g4653 XP_018816688.1 probable aminotransferase TAT2 isoform X1|transferase activity|metabolic process |
| 8 | 947644 | G/A | AHM | −0.695 | >Bhaga_8.g118 VVA10054.1 AT2G17540 |
| 8 | 24354137 | A/G | bFFP | 0.914 | >Bhaga_8.g2937 XP_030953202.1 SUPPRESSOR OF ABI3-5 isoform X1|nucleus|RNA binding|metal ion binding|mRNA splicing, via spliceosome |
| 8 | 36882971 | T/A | BIO3 | 0.682 | >Bhaga_8.g4420 XP_030954219.1 ABC transporter B family member 19|integral component of membrane|ATP binding|ATPase activity|ATPase-coupled transmembrane transporter activity|transmembrane transport |
| 6 | 41874431 | C/T | AHM 2 DD > 18 3 | −0.695 | >Bhaga_6.g4735 XP_030953127.1 pentatricopeptide repeat-containing protein At4g39530-like |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajmerová, D.; Hrivnák, M.; Gömöry, D. Exon-Enriched Set of Single-Nucleotide Polymorphisms Shows Associations with Climate in European Beech (Fagus sylvatica L.). Forests 2024, 15, 1229. https://doi.org/10.3390/f15071229
Krajmerová D, Hrivnák M, Gömöry D. Exon-Enriched Set of Single-Nucleotide Polymorphisms Shows Associations with Climate in European Beech (Fagus sylvatica L.). Forests. 2024; 15(7):1229. https://doi.org/10.3390/f15071229
Chicago/Turabian StyleKrajmerová, Diana, Matúš Hrivnák, and Dušan Gömöry. 2024. "Exon-Enriched Set of Single-Nucleotide Polymorphisms Shows Associations with Climate in European Beech (Fagus sylvatica L.)" Forests 15, no. 7: 1229. https://doi.org/10.3390/f15071229





