Evaluating the Impact of Climate Change and Human Activities on the Potential Distribution of Pine Wood Nematode (Bursaphelenchus xylophilus) in China
Abstract
:1. Introduction
2. Materials and Methods
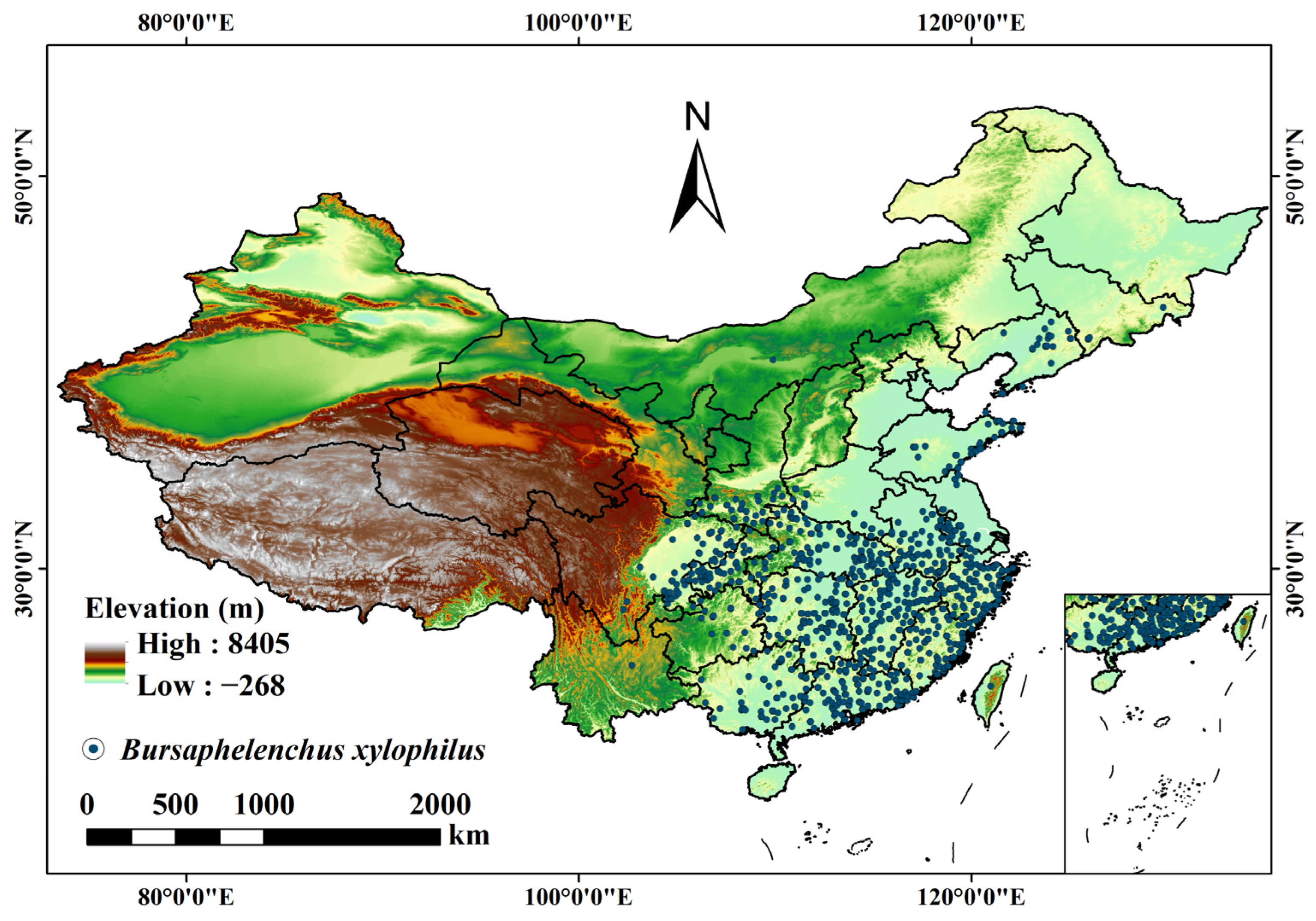
2.1. Data
2.2. Evaluation and Validation of MaxEnt Model
2.3. Changes in the Potential Distribution Areas of B. xylophilus
2.4. Change in Potential Distribution Center Shift under Future Climate Scenarios
2.5. Dynamics of Potential Distribution Areas of B. xylophilus under Future Climate Scenarios
2.6. Niche Dynamics Analyses
3. Results
3.1. Model Accuracy and Evaluation
3.2. Main Environmental Variables Affecting Distribution of B. xylophilus
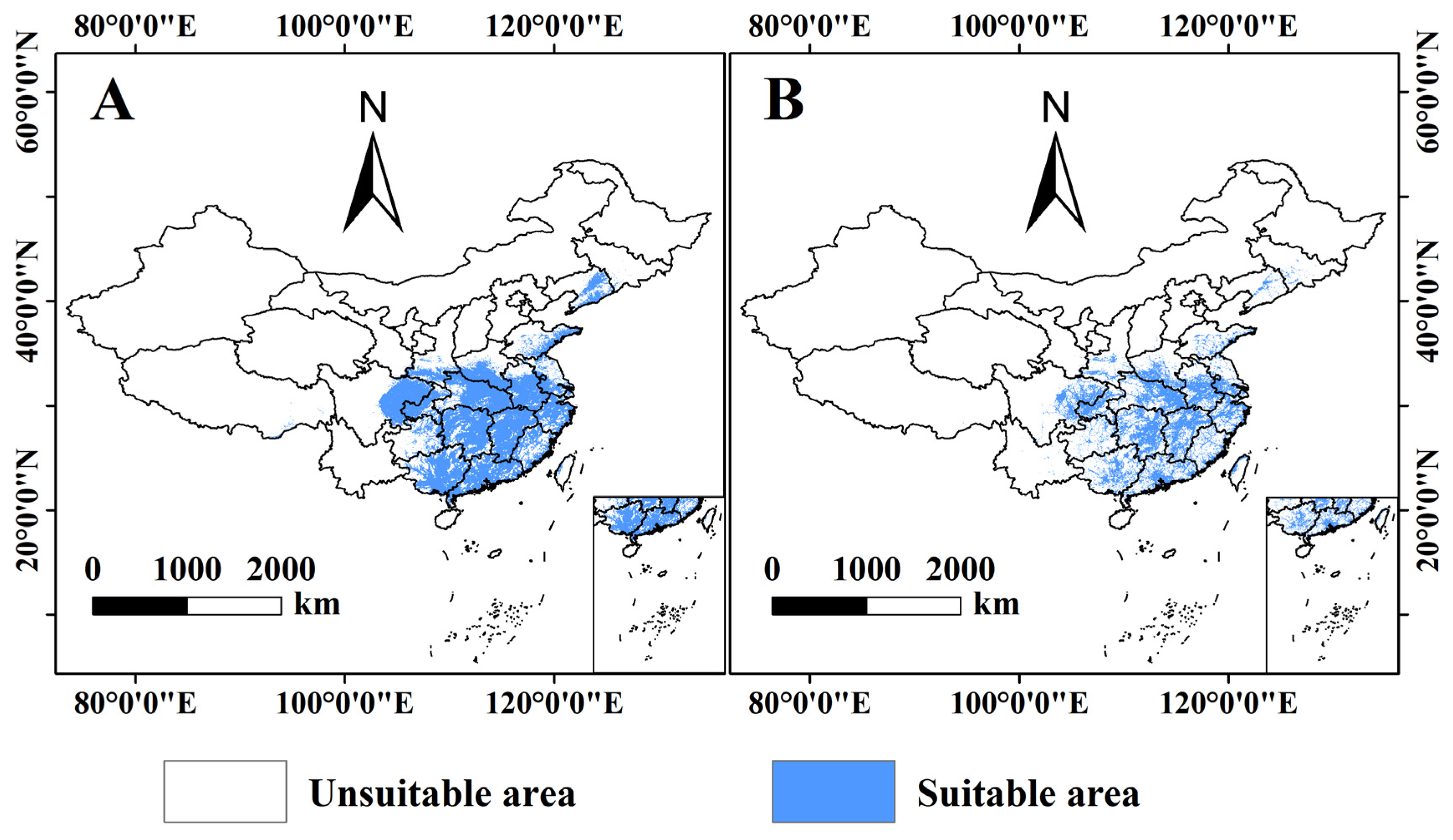
3.3. Prediction of the Potential Distribution of B. xylophilus under Climate and Human Interference in the Current
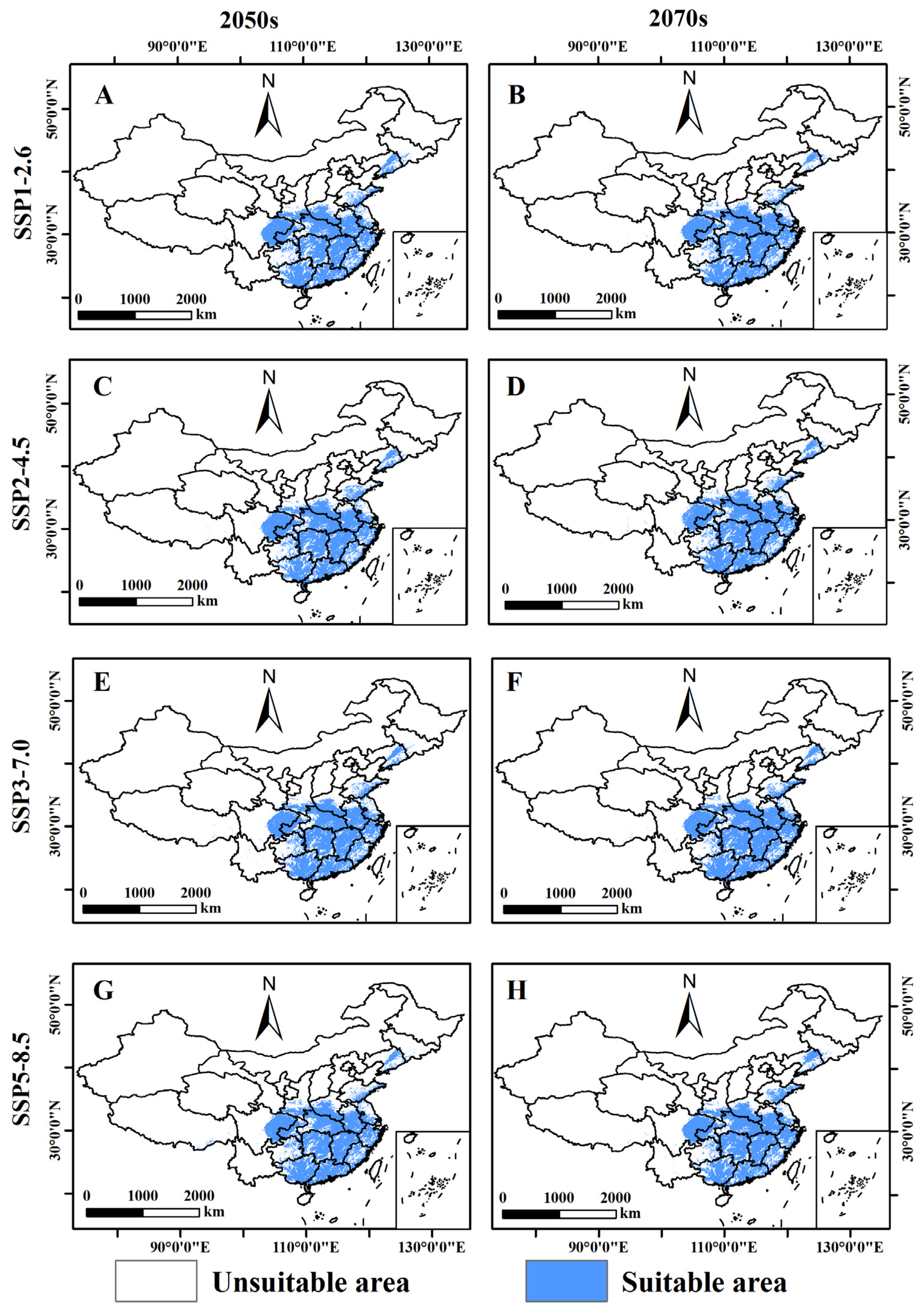
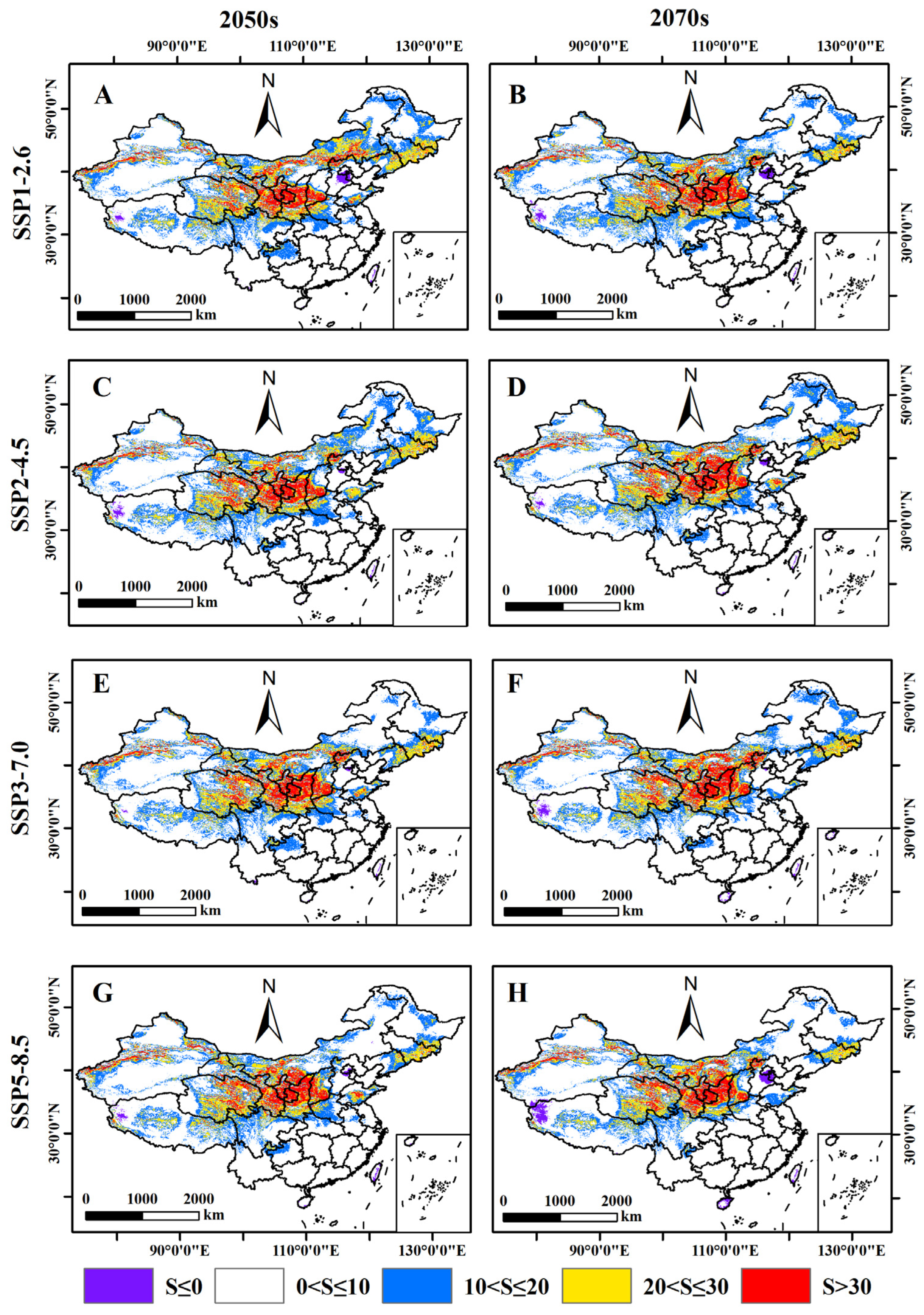
3.4. Prediction of the Potential Distribution of B. xylophilus under Different Climate Scenarios in the Future
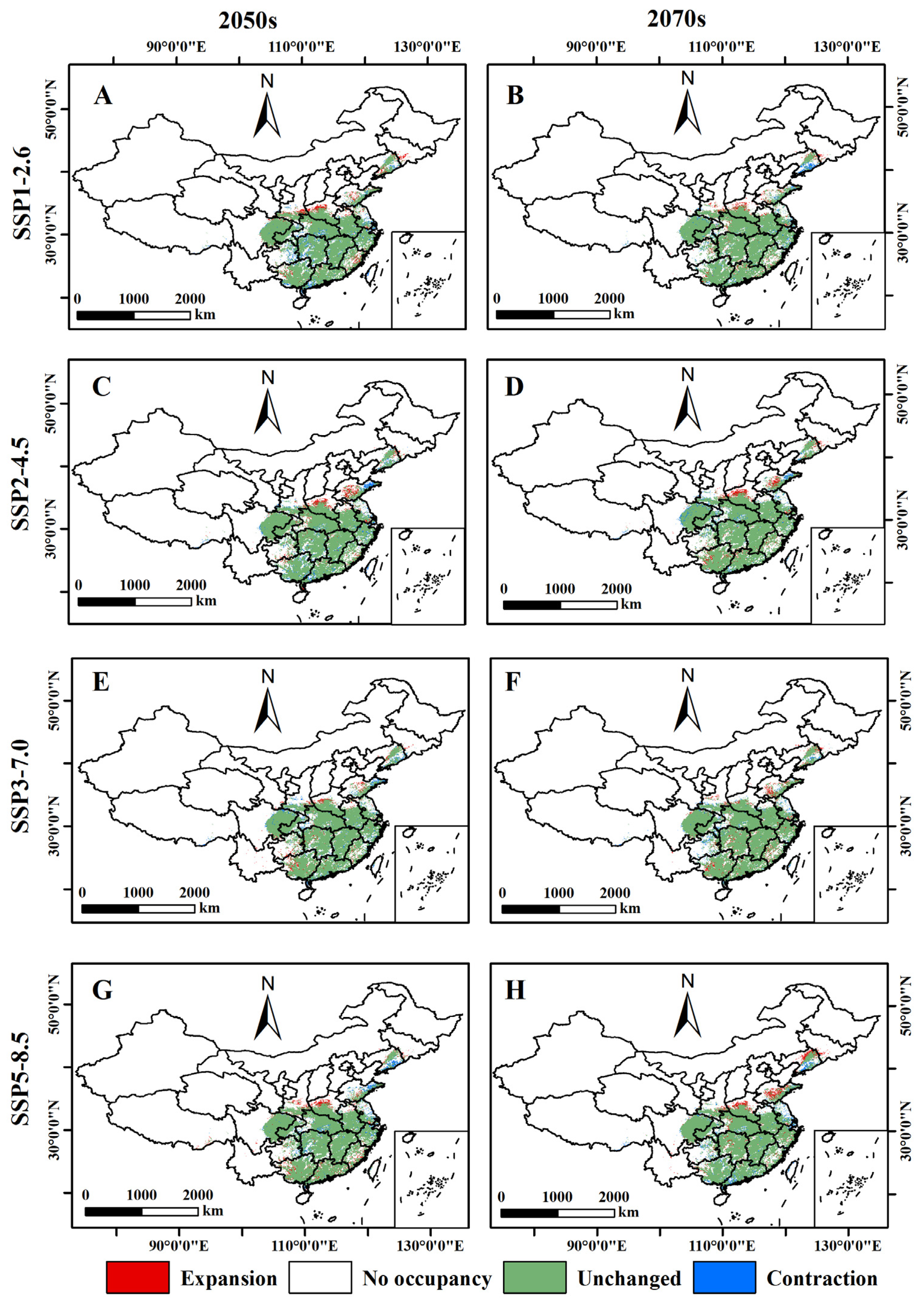
3.5. Relative Changes in the Potential Distribution Area of B. xylophilus under Future Climate Scenarios
3.6. Potential Distribution Center Shifts of B. xylophilus under Different Scenarios in the Future
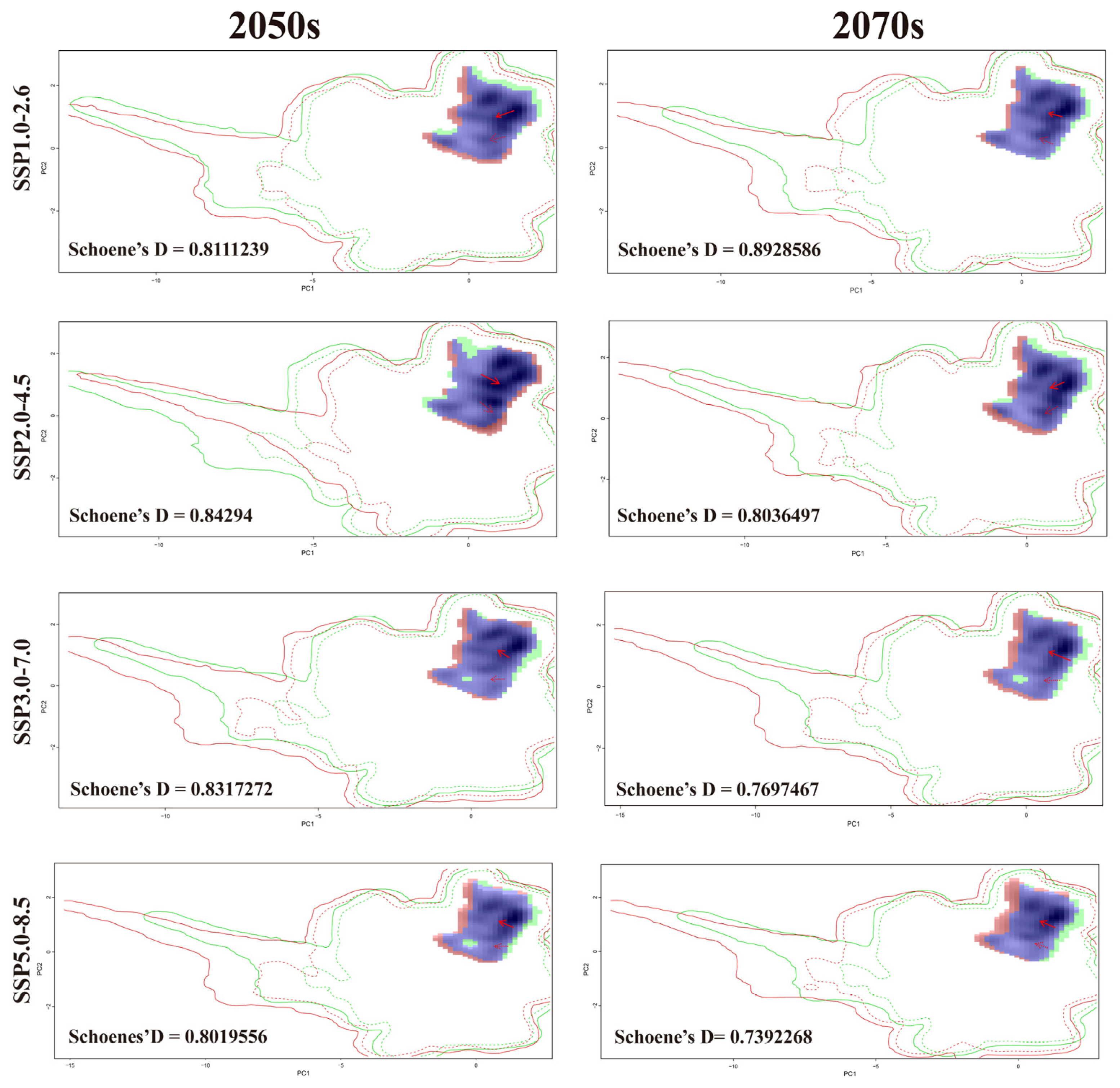
3.7. Niche Dynamics of B. xylophilus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, S.X.; Zhang, Y.; Huang, D.Z.; Wang, H.; Cao, Q.; Fan, P.X.; Yang, N.; Zheng, P.M.; Wang, R.Q. The effect of climate change on the richness distribution pattern of oaks (Quercus L.) in China. Sci. Total Environ. 2020, 744, e140786. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.J.; Xu, D.P.; Zhuo, Z.H. Predicting the impact of climate change on the geographical distribution of Leafhopper, Cicadella viridis in China through the MaxEnt model. Insects 2023, 14, 586. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond predictions: Biodiversity conservation in a changing climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Pound, K.L.; Larson, C.A.; Passy, S.I.; Webb, T. Current distributions and future climate–driven changes in diatoms, insects and fish in U.S. streams. Global Ecol. Biogeogr. 2021, 30, 63–78. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.Y.; Wang, Y.J.; Yang, Y.X. Climate change impacts on the potential distribution pattern of Osphya (Coleoptera: Melandryidae), an old but small beetle group distributed in the Northern Hemisphere. Insects 2023, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Hardy, P.B.; Kinder, P.M.; Sparks, T.H.; Dennis, R.L.H. Elevation and habitats: The potential of sites at different altitudes to provide refuges for phytophagous insects during climatic fluctuations. J. Insect. Conserv. 2010, 14, 297–303. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Wang, B.; Tian, Y.; Quan, Y.; Liu, J. Analysis of factors related to forest fires in different forest ecosystems in China. Forests 2022, 13, 1021. [Google Scholar] [CrossRef]
- Sharma, A.; Jose, S.; Bohn, K.K.; Andreu, M.G. Effects of reproduction methods and overstory species composition on understory light availability in longleaf pine-slash pine ecosystems. For. Ecol. Manag. 2012, 284, 23–33. [Google Scholar] [CrossRef]
- Tuomola, J.; Gruffudd, H.; Ruosteenoja, K.; Hannunen, S. Could pine wood nematode (Bursaphelenchus xylophilus) cause pine wilt disease or even establish inside healthy trees in finland Now-Or ever? Forests 2021, 12, 1679. [Google Scholar] [CrossRef]
- Shi, L.; Wang, L.; Shi, X.; Luo, L.; Ye, J. Negative effects of free-living nematodes on the populations of Bursaphelenchus xylophilus in dead pine trees. Biol. Control 2022, 168, 104858. [Google Scholar] [CrossRef]
- Takai, K.; Soejima, T.; Suzuki, T.; Kawazu, K. Development of a water-soluble preparation of emamectin benzoate and its preventative effect against the wilting of pot-grown pine trees inoculated with the pine wood nematode, Bursaphelenchus xylophilus. Pest. Manag. Sci. 2001, 57, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, X.; Ding, X.; Ye, J. Comparative transcriptomic analysis of candidate effectors to explore the infection and survival strategy of Bursaphelenchus xylophilus during different interaction stages with pine trees. BMC Plant Biol. 2021, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Qin, X.D.; Alvarez, F.; Chen, Z.N.; Wu, Z.J. Potential impact of land–use change on habitat quality in the distribution range of crocodile lizards in China. Ecol. Evol. 2022, 12, 9390. [Google Scholar] [CrossRef]
- Wang, D.; Cui, B.C.; Duan, S.S.; Chen, J.J.; Fan, H.; Lu, B.B.; Zheng, J.H. Moving north in China: The habitat of Pedicularis kansuensis in the context of climate change. Sci. Total Environ. 2019, 697, 133979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, J.Y.; Zhao, H.X.; Li, M.; Han, W.H. Predicting the distribution of the invasive species Leptocybe invasa: Combining MaxEnt and geodetector models. Insects 2021, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Iannella, M.; Console, G.; Cerasoli, F.; De Simone, W.; D’Alessandro, P.; Biondi, M. A step towards SDMs: A “couple-and-weigh” framework based on accessible data for biodiversity conservation and landscape planning. Divers. Distrib. 2021, 27, 2412–2427. [Google Scholar] [CrossRef]
- Hadi Ahmad, M.; Abubakar, A.; Yusoff Ishak, M.; Shehu Danhassan, S.; Zhang, J.H.; Aiatalo, J.M. Modeling the influence of daily temperature and precipitation extreme indices on vegetation dynamics in Katsina State using statistical downscaling model (SDM). Ecol. Indic. 2023, 155, 110979. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, D.P.; Liao, W.K.; Xu, Y.; Zhuo, Z.H. Predicting the current and future distributions of Frankliniella occidentalis (Pergande) based on the MaxEnt species distribution model. Insects 2023, 14, 458. [Google Scholar] [CrossRef]
- Harte, J.; Umemura, K.; Brush, M.; Chase, J. DynaMETE: A hybrid MaxEnt–plus–mechanism theory of dynamic macroecology. Ecol. Lett. 2021, 24, 935–949. [Google Scholar] [CrossRef]
- Guga, S.; Xu, J.; Riao, D.; Li, K.W.; Han, A.; Zhang, J.Q. Combining MaxEnt model and landscape pattern theory for analyzing interdecadal variation of sugarcane climate suitability in Guangxi, China. Ecol. Indic. 2021, 131, 108152. [Google Scholar] [CrossRef]
- Soliman, M.M.; Al-Khalaf, A.A.; El-Hawagry, M.S.A. Effects of climatic change on potential distribution of Spogostylum ocyale (Diptera: Bombyliidae) in the middle east using Maxent modelling. Insects 2023, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Lee, D.S.; Kwon, T.S.; Athar, M.; Park, Y.S. Predicting the global distribution of Solenopsis geminata (Hymenoptera: Formicidae) under climate change using the MaxEnt model. Insects 2021, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.X.; Chao, Y.; Matsushita, N.; Lian, C.L.; Geng, Q.F. Analysis of the distribution pattern of the ectomycorrhizal fungus Cenococcum geophilum under climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, 10565. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Chen, A.; Li, Y.; Han, X.; Lin, H. Predicting the potential distribution of pine wilt disease in china under climate change. Insects 2022, 13, 1147. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Guo, Q.; Xie, N.; Yuan, G.; Liao, M.; Gui, Q.; Ding, G. Predicting the global potential distribution of Bursaphelenchus xylophilus using an ecological niche model: Expansion trend and the main driving factors. BMC Ecol Evol. 2024, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simoes, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R package for comparative ecological biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Wu, T.W.; Lu, Y.X.; Fang, Y.J.; Xin, X.G.; Li, L.; Li, W.P.; Jie, W.H.; Zhang, J.; Liu, Y.M.; Zhang, L.; et al. The Beijing climate center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Li, D.X.; Li, Z.X.; Liu, Z.W.; Yang, Y.J.; Khoso, A.G.; Wang, L.; Liu, D.G. Climate change simulations revealed potentially drastic shifts in insect community structure and crop yields in China’s farmland. J. Pest Sci. 2022, 96, 1–15. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Kaky, E.; Nolan, V.; Alatawi, A.; Gilbert, F. A comparison between ensemble and MaxEnt species distribution modelling approaches for conservation: A case study with Egyptian medicinal plants. Ecol. Inf. 2020, 60, 101150. [Google Scholar] [CrossRef]
- Maruthadurai, R.; Das, B.; Ramesh, R. Predicting the invasion risk of rugose spiraling Whitefly, Aleurodicus rugioperculatus, in India based on CMIP6 projections by MaxEnt. Pest Manag. Sci. 2023, 79, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, W.H. Assessing potential European areas of Pierce’s disease mediated by insect vectors by using spatial ensemble model. Front. Plant Sci. 2023, 14, 1209694. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Souza, P.G.C.; Silva, R.S.; Santana, P.A.; Picanço, M.C.; Kyerematen, R.; Sètamou, M.; Ekesi, S.; Borgemeister, C. Climate-induced range shifts of invasive species (Diaphorina citri Kuwayama). Pest Manag. Sci. 2022, 78, 2534–2549. [Google Scholar] [CrossRef]
- Yang, S.L.; Wang, H.M.; Tong, J.P.; Bai, Y.; Alatalo, J.M.; Liu, G.; Fang, Z.; Zhang, F. Impacts of environment and human activity on grid-scale land cropping suitability and optimization of planting structure, measured based on the MaxEnt model. Sci. Total Environ. 2022, 836, 155356. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.H.; Liu, L.; Zhao, L.J.; Cui, S.P. Potentially suitable geographical area for Monochamus alternatus under current and future climatic scenarios based on optimized MaxEnt model. Insects 2023, 14, 182. [Google Scholar] [CrossRef]
- Huang, M.Y.; Ge, X.Z.; Shi, H.L.; Tong, Y.G.; Shi, J. Prediction of current and future potential distributions of the Eucalyptus pest Leptocybe invasa (Hymenoptera: Eulophidae) in China using the CLIMEX model. Pest Manag. Sci. 2019, 75, 2958–2968. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.X.; Hof, A.R.; Matson, K.D.; Langevelde, F.; Ma, C.S. Climate change, host plant availability, and irrigation shape future region–specific distributions of the Sitobion grain aphid complex. Pest Manag. Sci. 2023, 79, 2311–2324. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.; Li, Y.; Yang, Y. Staying on the current niche: Consensus model reveals the habitat loss of a critically endangered dragonfly Libellula angelina under climate changes. J Insect Conserv. 2024, 28, 483–499. [Google Scholar] [CrossRef]
- Ge, X.Z.; He, S.Y.; Zhu, C.Y.; Wang, T.; Xu, Z.C.; Zong, S.X. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest Manag. Sci. 2019, 75, 160–169. [Google Scholar] [CrossRef]
- Gao, H.; Qian, Q.Q.; Liu, L.J.; Xu, D.P. Predicting the distribution of Sclerodermus sichuanensis (Hymenoptera: Bethylidae) under climate change in China. Insects 2023, 14, 475. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Z.; Duan, Q.; Shen, X.Y.; Zhang, S.Y. Climate change influences the population density and suitable area of Hippotiscus dorsalis (Hemiptera: Pentatomidae) in China. Insects 2023, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.A.; Kumar, L.; Da Silva, R.S.; Picanço, M.C. Global geographic distribution of Tuta absoluta as affected by climate change. J. Pest Sci. 2019, 92, 1373–1385. [Google Scholar] [CrossRef]
- Kumar, A.V.; Zimova, M.; Martin, T.E.; Mills, L.S. Contrasting seasonal effects of climate change influence density in a cold–adapted species. Global Change Biol. 2022, 28, 6228–6238. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.A.; Kumar, L.; Da Silva, R.S.; Pereira, J.L.; Picanço, M.C. Assessing the impact of climate change on the worldwide distribution of Dalbulus maidis (DeLong) using MaxEnt. Pest Manag. Sci. 2019, 10, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, A.; Lundström, J.; Forsell, N.; Moor, H.; Eggers, J.; Subramanian, N.; Aquilué, N.; Morán–Ordóñez, A.; Brotons, L.; Snäll, T. More future synergies and less trade–offs between forest ecosystem services with natural climate solutions instead of bioeconomy solutions. Global Change Biol. 2022, 28, 6333–6348. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yang, J.; Beckett, S.; Ren, Y. Preliminary trials of the ethanedinitrile fumigation of logs for eradication of Bursaphelenchus xylophilus and its vector insect Monochamus alternatus: C 2 N 2 fumigation of logs to eradicate B. xylophilus and its vector insect M. alternatus. Pest. Manag. Sci. 2017, 73, 1446–1452. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Zheng, Y. Feeding preferences and responses of Monochamus saltuarius to volatile components of host pine trees. Insects 2022, 13, 888. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Liu, Z.; Li, Y.; Zhang, X. Genes encoding potential molecular mimicry proteins as the specific targets for detecting Bursaphelenchus xylophilus in PCR and loop-mediated isothermal amplification assays. Front. Plant Sci. 2022, 13, 890949. [Google Scholar] [CrossRef]
- Wedegärtner, R.E.M.; Lembrechts, J.J.; Van der Wal, R.; Barros, A.; Chauvin, A.; Janssens, I.; Graae, B.J. Hiking trails shift plant species’ realized climatic niches and locally increase species richness. Divers. Distrib. 2022, 28, 1416–1429. [Google Scholar] [CrossRef]
- Haran, J.; Roques, A.; Bernard, A.; Robinet, C.; Roux, G. Altitudinal barrier to the spread of an invasive species: Could the pyrenean chain slow the natural spread of the pinewood nematode? PLoS ONE. 2015, 10, e0134126. [Google Scholar] [CrossRef] [PubMed]
- Spaak, J.W.; Carpentier, C.; De Laender, F.; Mayfield, M. Species richness increases fitness differences, but does not affect niche differences. Ecol. Lett. 2021, 24, 2611–2623. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Jarvie, S.; Guo, W.; Deng, T.; Mao, L.; Zhang, M.; Chu, C.; Qian, H.; Svenning, J.; He, F.; et al. Niche overlap and divergence times support niche conservatism in eastern Asia-eastern north America disjunct plants. Global Ecol. Biogeogr. 2021, 30, 1990–2003. [Google Scholar] [CrossRef]
- Ling, P.Y.; Aguilar-Amuchastegui, N.; Baldwin-Cantello, W.; Rayden, T.; Gordon, J.; Dainton, S.; Bagwill, A.L.; Pacheco, P. Mapping global forest regeneration–an untapped potential to mitigate climate change and biodiversity loss. Environ. Res. Lett. 2023, 18, 54025. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Kass, J.M.; Mammola, S.; Koizumi, I.; Li, X.C.; Tanaka, K.; Ikeda, K.; Suzuki, T.; Yokota, M.; Usio, N. Lineage-level distribution models lead to more realistic climate change predictions for a threatened crayfish. Divers. Distrib. 2021, 27, 684–695. [Google Scholar] [CrossRef]
- Jiang, K.; Teuling, A.J.; Chen, X.; Huang, N.; Wang, J.; Zhang, Z.; Gao, R.; Men, J.; Zhang, Z.; Wu, Y.; et al. Global land degradation hotspots based on multiple methods and indicators. Ecol. Indic. 2024, 158, 111462. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, S.; Lu, F.; Guo, K.; Huang, L.; Su, X.; Chen, Y. Nematotoxicity of a Cyt–like protein toxin from Conidiobolus obscurus (entomophthoromycotina) on the pine wood nematode Bursaphelenchus xylophilus. Pest. Manag. Sci. 2021, 77, 686–692. [Google Scholar] [CrossRef]
- Shi, F.; Ge, S.; Hou, Z.; Xu, Y.; Tao, J.; Wu, H.; Zong, S. Species–specific primers for rapid detection of Monochamus saltuarius, an effective vector of Bursaphelenchus xylophilus in China. J. Appl. Entomol. 2022, 146, 636–647. [Google Scholar] [CrossRef]
- Park, C.G.; Son, J.; Lee, B.; Cho, J.H.; Ren, Y. Comparison of ethanedinitrile (C2N2) and metam sodium for control of Bursaphelenchus xylophilus (Nematoda: Aphelenchidae) and Monochamus alternatus (Coleoptera: Cerambycidae) in naturally infested logs at low temperatures. J. Econ. Entomol. 2014, 107, 2055–2060. [Google Scholar] [CrossRef]
- Gruffudd, H.R.; Schröder, T.; Jenkins, T.A.R.; Evans, H.F. Modelling pine wilt disease (PWD) for current and future climate scenarios as part of a pest risk analysis for pine wood nematode Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle in Germany. J. Plant Dis. Prot. 2019, 126, 129–144. [Google Scholar] [CrossRef]


| Abbreviation | Environmental Variables | Operation (|r| > 0.9) |
|---|---|---|
| Bio1 | Annual mean temperature (°C) | Retain |
| Bio2 | Mean diurnal range (°C) | Retain |
| Bio3 | Isothermality | Retain |
| Bio4 | Temperature seasonality | Retain |
| Bio5 | Maximum temp of warmest month (°C) | Eliminate |
| Bio6 | Minimum temp of coldest month (°C) | Eliminate |
| Bio7 | Temperature annual range (°C) | Eliminate |
| Bio8 | Mean temp of wettest quarter (°C) | Eliminate |
| Bio9 | Mean temp of driest quarter (°C) | Eliminate |
| Bio10 | Mean temp of warmest quarter (°C) | Eliminate |
| Bio11 | Mean temp of coldest quarter (°C) | Eliminate |
| Bio12 | Annual precipitation (mm) | Retain |
| Bio13 | Precipitation of wettest month (mm) | Eliminate |
| Bio14 | Precipitation of driest month (mm) | Retain |
| Bio15 | Precipitation seasonality (mm) | Retain |
| Bio16 | Precipitation of wettest quarter (mm) | Eliminate |
| Bio17 | Precipitation of driest quarter (mm) | Eliminate |
| Bio18 | Precipitation of warmest quarter (mm) | Eliminate |
| Bio19 | Precipitation of coldest quarter (mm) | Eliminate |
| Bio20 | Elevation (m) | Retain |
| Bio21 | NDVI | Retain |
| Bio22 | Slope | Retain |
| Bio23 | Aspect | Retain |
| Bio24 | Annual_mean_UV-B | Eliminate |
| Bio25 | UV-B_seasonality | Eliminate |
| Bio26 | Mean_UV-B_of_highest_month | Eliminate |
| Bio27 | Mean_UV-B_of_lowest_month | Eliminate |
| Bio28 | Sum_of_UV-B_radiation_of_highest_quarter | Eliminate |
| Bio29 | Sum_of_UV-B_radiation_of_lowest_quarter | Eliminate |
| Bio30 | Global human footprint | Retain |
| Bio31 | Global human influence index | Retain |
| Shared Socioeconomic Pathways | Train AUC (Avg) | Test AUC (Avg) |
|---|---|---|
| Current-Environmental variables | 0.9338 | 0.9285 |
| Current-Environmental variables + Human activity | 0.9500 | 0.9465 |
| Future-SSP1.0-2.6 2040–2060 | 0.9345 | 0.9277 |
| Future-SSP1.0-2.6 2060–2080 | 0.9338 | 0.9284 |
| Future-SSP2.0-4.5 2040–2060 | 0.9349 | 0.9287 |
| Future-SSP2.0-4.5 2060–2080 | 0.9342 | 0.9291 |
| Future-SSP3.0-7.0 2040–2060 | 0.9357 | 0.9302 |
| Future-SSP3.0-7.0 2060–2080 | 0.9324 | 0.9266 |
| Future-SSP5.0-8.5 2040–2060 | 0.9351 | 0.9301 |
| Future-SSP5.0-8.5 2060–2080 | 0.9351 | 0.9286 |
| Shared Socioeconomic Pathways | Longitude (°E) | Latitude (°N) | Center Migration Distance (km) |
|---|---|---|---|
| Current | 113.18 | 31.60 | - |
| Future-SSP1.0-2.6 2040–2060 | 113.49 | 31.53 | 30.52 |
| Future-SSP1.0-2.6 2060–2080 | 114.13 | 32.35 | 122.58 |
| Future-SSP2.0-4.5 2040–2060 | 113.63 | 31.71 | 43.98 |
| Future-SSP2.0-4.5 2060–2080 | 113.05 | 31.75 | 20.86 |
| Future-SSP3.0-7.0 2040–2060 | 113.30 | 31.63 | 11.93 |
| Future-SSP3.0-7.0 2060–2080 | 113.13 | 31.55 | 7.20 |
| Future-SSP5.0-8.5 2040–2060 | 113.36 | 31.87 | 34.76 |
| Future-SSP5.0-8.5 2060–2080 | 113.40 | 31.55 | 21.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, P.; Xie, G.; Wang, W. Evaluating the Impact of Climate Change and Human Activities on the Potential Distribution of Pine Wood Nematode (Bursaphelenchus xylophilus) in China. Forests 2024, 15, 1253. https://doi.org/10.3390/f15071253
Zhang L, Wang P, Xie G, Wang W. Evaluating the Impact of Climate Change and Human Activities on the Potential Distribution of Pine Wood Nematode (Bursaphelenchus xylophilus) in China. Forests. 2024; 15(7):1253. https://doi.org/10.3390/f15071253
Chicago/Turabian StyleZhang, Liang, Ping Wang, Guanglin Xie, and Wenkai Wang. 2024. "Evaluating the Impact of Climate Change and Human Activities on the Potential Distribution of Pine Wood Nematode (Bursaphelenchus xylophilus) in China" Forests 15, no. 7: 1253. https://doi.org/10.3390/f15071253
APA StyleZhang, L., Wang, P., Xie, G., & Wang, W. (2024). Evaluating the Impact of Climate Change and Human Activities on the Potential Distribution of Pine Wood Nematode (Bursaphelenchus xylophilus) in China. Forests, 15(7), 1253. https://doi.org/10.3390/f15071253







