Altitudinal Difference of Growth–Climate Response Models in the Coniferous Forests of Southeastern Tibetan Plateau, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Dendrochronological Analyses
2.3. Climate Data
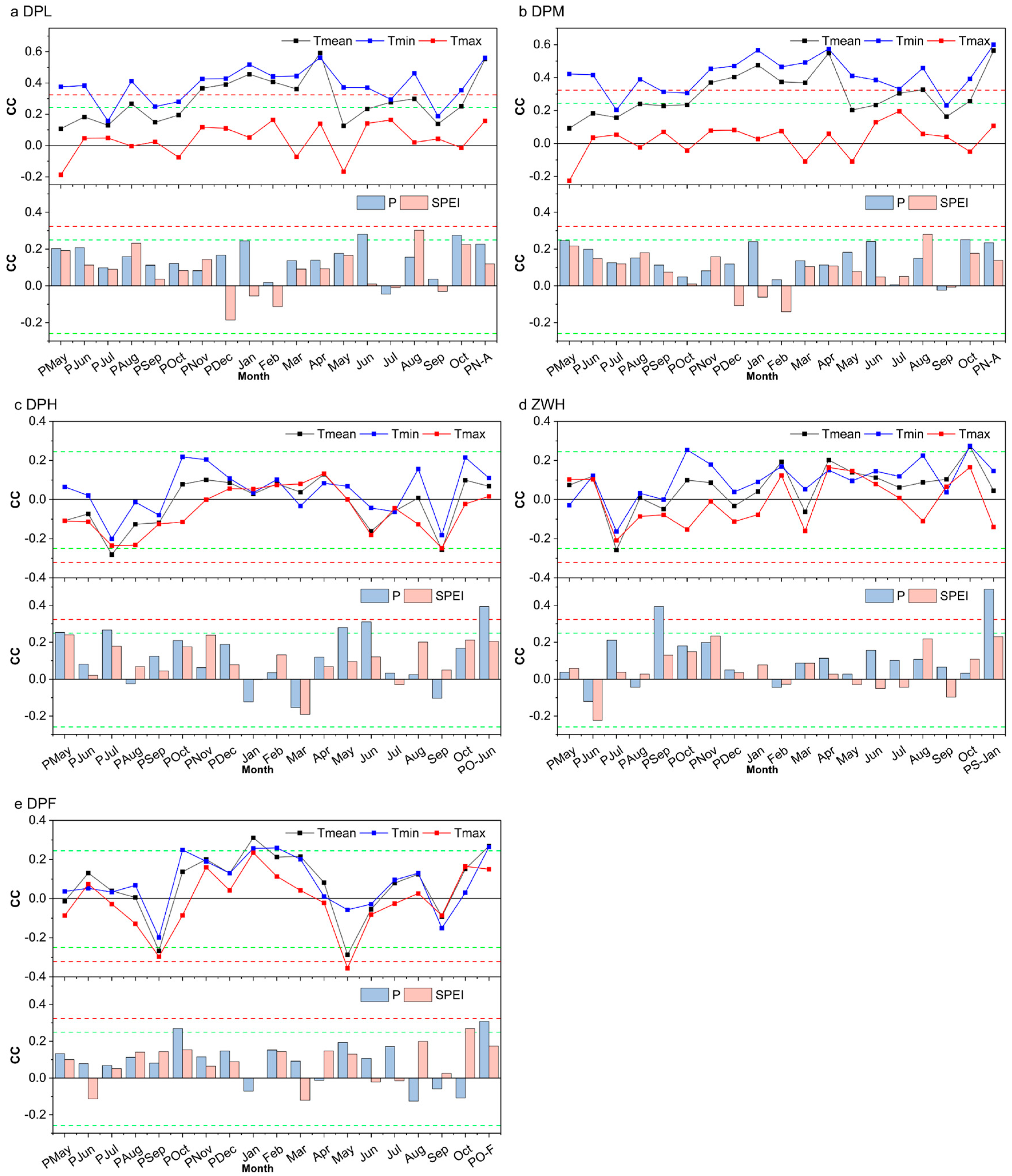
3. Results
3.1. Tree-Ring Width Chronologies and Statistics
3.2. Response to Climatic Condition
4. Discussion
4.1. Climate–Growth Relationships along the Altitudinal Gradient
4.2. Factors May Influence Growth Response to Climate Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pepin, N.; Bradley, R.S.; Diaz, F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowle, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D.; et al. Mountain Research Initiative EDW Working Group. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef]
- Guerrero-Hernández, R.; Muñiz-Castro, M.Á.; Villanueva-Díaz, J.; Hernández-Vera, G.; Vázquez-García, J.A.; Ruiz-Corral, J.A. Tree-Ring Patterns and Growth Response of Abies jaliscana to Climate along Elevational Gradients in the Mountains of Western Jalisco, Mexico. Forests 2022, 13, 981. [Google Scholar] [CrossRef]
- LaMarche, V.C. Frequency-dependent relationships between tree-ring series along an ecological gradient and some dendro- climatic implications. Tree-Ring Bull. J. Ecol. 1974, 90, 68–77. [Google Scholar]
- Thomas, S.C. Genetic vs. phenotypic responses of trees to altitude. Tree Physiol. 2011, 31, 1161–1163. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Breshears, D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, art129. [Google Scholar] [CrossRef]
- Yang, B.; He, M.; Shishov, V.; Tychkov, I.; Vaganov, E.; Rossi, S.; Ljungqvist, F.C.; Bräuning, A.; Grießinger, J. New perspective on spring vegetation phenology and global climate change based on Tibetan Plateau tree-ring data. Proc. Natl. Acad. Sci. USA 2017, 114, 6966–6971. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change 2021; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Salzer, M.W.; Larso, E.R.; Bunn, A.G.; Hughes, M.K. Changing climate response in near-treeline bristlecone pine with elevation and aspect. Environ. Res. Lett. 2014, 9, 114007. [Google Scholar] [CrossRef]
- Fritts, H.C. The statistics of ring-width and climatic data. In Tree Rings and Climate; Academic Press: London, UK, 1976; pp. 246–311. [Google Scholar]
- Arias, N.S.; Scholz, F.G.; Goldstein, G.; Bucci, S.J. Low-temperature acclimation and legacy effects of summer water deficits in olive freezing resistance. Tree Physiol. 2021, 41, 1836–1847. [Google Scholar] [CrossRef]
- Louthan, A.M.; Doak, D.F.; Angert, A.L. Where and When do Species Interactions Set Range Limits? Trends Ecol. Evol. 2015, 30, 780–792. [Google Scholar] [CrossRef]
- Conlisk, E.; Castanha, C.; Germino, M.J.; Veblen, T.T.; Smith, J.M.; Kueppers, L.M. Declines in low-elevation subalpine tree populations outpace growth in high-elevation populations with warming. J. Ecol. 2017, 105, 1347–1357. [Google Scholar] [CrossRef]
- Gaire, N.P.; Fan, Z.; Bräuning, A.; Panthi, S.; Rana, P.; Shrestha, A.; Bhuju, D.R. Abies spectabilis shows stable growth relations to temperature, but changing response to moisture conditions along an elevation gradient in the central Himalaya. Dendrochronologia 2020, 60, 125675. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree-ring evidence for climatic changes in western North America. Mon. Weather Rev. 1965, 93, 421–443. [Google Scholar] [CrossRef]
- Körner, C. Alpine Treelines: Functional Biology of the Global High Elevation Tree Limits; Springer: Basel, Switzerland, 2012. [Google Scholar]
- Leal, S.; Melvin, T.M.; Grabner, M.; Wimmer, R.; Briffa, K.R. Tree-ring growth variability in the Austrian Alps: The influence of site, altitude, tree species and climate. Boreas 1996, 15, 426–440. [Google Scholar] [CrossRef]
- Dittmar, C.; Zech, W.; Elling, W. Growth variations of Common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe—A dendroecological study. For. Ecol. Manag. 2003, 173, 63–78. [Google Scholar] [CrossRef]
- Savva, Y.; Oleksyn, J.; Reich, P.B.; Tjoelker, M.G.; Vaganov, E.A.; Modrzynski, J. Interannual growth response of Norway spruce to climate along an altitudinal gradient in the Tatra Mountains, Poland. Trees 2006, 20, 735–746. [Google Scholar] [CrossRef]
- Fan, Z.; Bräuning, A.; Cao, K. Annual temperature reconstruction in the central Hengduan Mountains, China, as deduced from tree rings. Dendrochronologia 2008, 26, 97–107. [Google Scholar] [CrossRef]
- Panthi, S.; Fan, Z. Long-term physiological and growth responses of Himalayan fir to environmental change are mediated by mean climate. Glob. Chang. Biol. 2019, 17, 1778–1794. [Google Scholar] [CrossRef]
- Rai, S.; Dawadi, B.; Wang, Y.; Lu, X.; Ru, H.; Sigdel, S.R. Growth response of Abies spectabilis to climate along an elevation gradient of the Manang valley in the central Himalayas. J. For. Res. 2020, 31, 2245–2254. [Google Scholar] [CrossRef]
- He, M.; Yang, B.; Bräuning, A. Tree growth–climate relationships of Juniperus tibetica along an altitudinal gradient on the southern Tibetan Plateau. Trees 2013, 27, 429–439. [Google Scholar] [CrossRef]
- Liang, E.; Wang, Y.; Xu, Y.; Liu, B.; Shao, X. Growth variation in Abies georgei var. smithii along altitudinal gradients in the Sygera Mountains, southeastern Tibetan Plateau. Trees 2010, 24, 363–373. [Google Scholar] [CrossRef]
- Babushkina, E.; Belokopytova, L.; Zhirnova, D.; Barabantsova, A.; Vaganov, E. Divergent growth trends and climatic response of Picea obovata along elevational gradient in Western Sayan mountains, Siberia. J. Mt. Sci. 2018, 15, 2378–2397. [Google Scholar] [CrossRef]
- Fan, Z.; Bräuning, A.; Thomas, A.; Li, J.; Cao, K. Spatial and temporal temperature trends on the Yunnan Plateau (Southwest China) during 1961–2004. Int. J. Climatol. 2011, 31, 2078–2090. [Google Scholar] [CrossRef]
- Bohner, J. General climatic controls and topoclimatic variations in Central and High Asia. Boreas 2006, 35, 279–295. [Google Scholar] [CrossRef]
- Liang, E.; Shao, X.; Xu, Y. Tree-ring evidence of recent abnormal warming on the southeast Tibetan Plateau. Theor. Appl. Climatol. 2009, 98, 9–18. [Google Scholar] [CrossRef]
- Yang, B.; Kang, X.; Liu, J.; Bräuning, A.; Qin, C. Annual temperature history in Southwest Tibet during the last 400 years recorded by tree rings: Annual Temperature History in Southwest Tibet. Int. J. Climatol. 2010, 30, 962–971. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Qin, C.; Kang, S.; He, M.; Wang, Z. Tree-ring inferred annual mean temperature variations on the southeastern Tibetan Plateau during the last millennium and their relationships with the Atlantic Multidecadal Oscillation. Clim. Dyn. 2014, 43, 627–640. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, Q. Tree-ring based summer minimum temperature reconstruction for the southern edge of the Qinghai-Tibetan Plateau, China. Clim. Res. 2013, 56, 91–101. [Google Scholar] [CrossRef]
- Zou, L.; Xu, S.; Zheng, C. Relationship between the tree ring width of Sabina saltuaria and climate factors in Haizi Mountain, Daocheng, Sichuan. J. Glaciol. Geocryol. 2021, 43, 917–927. (In Chinese) [Google Scholar]
- Li, Z.; Liu, G.; Fu, B.; Hu, C.; Luo, S.; Liu, X.; He, F. Anomalous temperature–growth response of Abies faxoniana to sustained freezing stress along elevational gradients in China’s Western Sichuan Province. Trees 2012, 26, 1373–1388. [Google Scholar] [CrossRef]
- Panthi, S.; Bräuning, A.; Zhou, Z.K.; Fan, Z. Growth response of Abies georgei to climate increases with elevation in the central Hengduan Mountains, southwestern China. Dendrochronologia 2018, 47, 1–9. [Google Scholar] [CrossRef]
- Sun, M.; Li, J.; Cao, R.; Tian, K.; Zhang, W.; Yin, D.; Zhang, Y. Climate-Growth Relations of Abies georgei along an Altitudinal Gradient in Haba Snow Mountain, Southwestern China. Forests 2021, 12, 1569. [Google Scholar] [CrossRef]
- Yang, R.; Fan, Z.; Li, Z. Radial growth of Pinus yunnanensis at different elevations and their responses to climatic factors in the Yulong Snow Mountain, Northwest Yunnan, China. Acta Ecol. Sin. 2018, 38, 8983–8991. [Google Scholar]
- Yu, J.; Liu, Q.; Meng, S.; Zhou, G.; Shah, S.; Xu, Z. Summer temperature variability inferred from tree-ring records in the central Hengduan Mountains, southeastern Tibetan Plateau. Dendrochronologia 2018, 51, 92–100. [Google Scholar] [CrossRef]
- Fu, L.K.; Jin, J.M. China Plant Red Data Book: Rare and Endangered Plants; Science Press: Beijing, China, 1992; ISBN 9787030103061. [Google Scholar]
- Zhu, H.; Shao, X.; Yin, Z.; Xu, P.; Xu, Y.; Tian, H. August temperature variability in the southeastern Tibetan Plateau since AD 1385 inferred from tree rings. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 305, 84–92. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, C. Geography of China; Higher Education Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Cook, E.R.; Briffa, K.R. Methods of calibration, verification, and reconstruction. In Methods of Dendrochronology; Cook, E.R., Kairiukstis, L.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990; pp. 104–123. [Google Scholar]
- Wu, X. Tree Annual Rings and Climate Change; Meteorological Press: Beijing, China, 1990. (In Chinese) [Google Scholar]
- Fritts, H.C.; Mosimann, J.E.; Bottorff, C.P. A revised computer program for standardising tree-ring series. Tree Ring Bull. 1969, 29, 15–20. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Cook, E.R. A Time-Series Analysis Approach to TreeRing Standardisation. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1985. [Google Scholar]
- Vicente-Serrano, S.M.; Beguerí, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Li, Z.; Liu, G.; Wu, X.; Wang, X. Tree-ring-based temperature reconstruction for the Wolong Natural Reserve, western Sichuan Plateau of China: A temperature reconstruction in southwestern China from tree rings. Int. J. Clim. 2015, 35, 3296–3307. [Google Scholar] [CrossRef]
- Buckley, B.M.; Cook, E.R.; Peterson, M.J.; Barbetti, M. A Changing Temperature Response with Elevation for Lagarostrobos Franklinii in Tasmania, Australia. In Climatic Change at High Elevation Sites; Diaz, H.F., Beniston, M., Bradley, R.S., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 245–266. [Google Scholar] [CrossRef]
- Takahashi, K.; Tokumitsu, Y.; Yasue, K. Climatic factors affecting the tree-ring width of Betula ermanii at the timberline on Mount Norikura, central Japan. Ecol. Res. 2005, 20, 445–451. [Google Scholar] [CrossRef]
- Zhang HCai, Q.; Liu, Y. Altitudinal difference of growth-climate response models in the north subtropical forests of China. Dendrochronologia 2022, 72, 125935. [Google Scholar] [CrossRef]
- Affolter, P.; Büntgen, U.; Esper, J.; Rigling, A.; Weber, P.; Luterbacher, J.; Frank, D. Inner Alpine conifer response to 20th century drought swings. Eur. J. For. Res. 2010, 129, 289–298. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Ma, K. Tree-ring reconstruction of summer temperature for A.D. 1475–2003 in the central Hengduan Mountains, Northwestern Yunnan, China. Clim. Chan. 2012, 110, 455–467. [Google Scholar] [CrossRef]
- Fan, Z.; Bräuning, A.; Tian, Q.; Yang, B.; Cao, K. Tree ring recorded May–August temperature variations since A.D. 1585 in the Gaoligong Mountains, southeastern Tibetan Plateau. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 296, 94–102. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, H.; Liang, E.; Liu, B.; Shi, J.; Zhang, R.; Yuan, Y.; Grießinger, J. A tree ring-based winter temperature reconstruction for the southeastern Tibetan Plateau since 1340 CE. Clim. Dyn. 2019, 53, 3221–3233. [Google Scholar] [CrossRef]
- Keyimu, M.; Li, Z.; Liu, G.; Fu, B.; Fan, Z.; Wang, X.; Wu, X.; Zhang, Y.; Halik, U. Tree-ring based minimum temperature reconstruction on the southeastern Tibetan Plateau. Quat. Sci. Rev. 2021, 251, 106712. [Google Scholar] [CrossRef]
- Takahashi, K.; Okuhara, I.; Tokumitsu, Y.; Yasue, K. Responses to climate by tree-ring widths and maximum latewood densities of two Abies species at upper and lower altitudinal distribution limits in central Japan. Trees 2011, 25, 745–753. [Google Scholar] [CrossRef]
- Hosoo, Y.; Yoshida, M.; Imai, T.; Okuyama, T. Diurnal difference in the amount of immunogold-labeled glucomannans detected with field emission scanning electron microscopy at the innermost surface of developing secondary walls of differentiating conifer tracheids. Planta 2002, 215, 1006–1012. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Anfodillo, T.; Saracino, A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008, 28, 863–871. [Google Scholar] [CrossRef]
- Shao, X.; Fan, J. Past climate on west Sichuan Plateau as reconstructed from ring widths of Dragon Spruce. Quat. Sci. 1999, 19, 81–89. (In Chinese) [Google Scholar]
- Bräuning, A. Tree-ring evidence of ‘Little Ice Age’ glacier advances in southern Tibet. Holocene 2006, 16, 369–380. [Google Scholar] [CrossRef]
- Liang, E.; Shao, X.; Qin, N. Tree-ring based summer temperature reconstruction for the source region of the Yangtze River on the Tibetan Plateau. Glob. Planet. Chang. 2008, 61, 313–320. [Google Scholar] [CrossRef]
- Liang, H.; Lyu, L.; Wahab, M. A 382-year reconstruction of August mean minimum temperature from tree-ring maximum latewood density on the southeastern Tibetan Plateau, China. Dendrochronologia 2016, 37, 1–8. [Google Scholar] [CrossRef]
- Misson, L.; Rathgeber, C.; Guiot, J. Dendroecological analysis of climatic effects on Quercus petraea and Pinus halepensis radial growth using the process-based MAIDEN model. Can. J. For. Res. 2004, 34, 888–898. [Google Scholar] [CrossRef]
- Dang, H.; Zhang, Y.; Zhang, K.; Jiang, M.; Zhang, Q. Climate-growth relationships of subalpine fir (Abies fargesii) across the altitudinal range in the Shennongjia Mountains, central China. Clim. Chang. 2013, 117, 903–917. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, Y.; Wei, W.; Gou, X.H.; Yu, S.L.; Shang, H.M.; Chen, F.; Zhang, T.W.; Qin, L. Dendroclimatic reconstruction of autumn–winter mean minimum temperature in the eastern Tibetan Plateau since 1600 AD. Dendrochronologia 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Shah, S.K.; Pandey, U.; Mehrotra, N.; Wiles, G.C.; Chandra, R. A winter temperature reconstruction for the Lidder Valley, Kashmir, Northwest Himalaya based on tree-rings of Pinus wallichiana. Clim. Dyn. 2019, 53, 4059–4075. [Google Scholar] [CrossRef]
- Fan, Z.; Bräuning, A.; Cao, K.; Zhu, S. Growth–climate responses of high-elevation conifers in the central Hengduan Mountains, southwestern China. For. Ecol. Manag. 2009, 258, 306–313. [Google Scholar] [CrossRef]
- Gou, X.; Yang, T.; Gao, L.; Deng, Y.; Yang, M.; Chen, F. A 457-year reconstruction of precipitation in the southeastern Qinghai-Tibet Plateau, China using tree-ring records. Chin. Sci. Bull. 2013, 58, 1107–1114. [Google Scholar] [CrossRef]
- Liang, E.; Dawadi, B.; Pederson, N.; Eckstein, D. Is the growth of birch at the upper timberline in the Himalayas limited by moisture or by temperature? Ecology 2014, 95, 2453–2465. [Google Scholar] [CrossRef]
- Liu, J.; Deng, X.; Lv, L.X. Relationship of tree growth and climatic factors at treeline of Picea likiangensis var. balfouriana forest in Basu County, Xizang. Chin. J. Plant Ecol. 2015, 39, 442–452. (In Chinese) [Google Scholar] [CrossRef]
- Devi, N.M.; Kukarskih, V.V.; Galimova, A.A.; Mazepa, V.S.; Grigoriev, A.A. Climate change evidence in tree growth and stand productivity at the upper treeline ecotone in the Polar Ural Mountains. Forest Ecosyst. 2020, 7, 7. [Google Scholar] [CrossRef]
- González de Andrés, E. Interactions between Climate and Nutrient Cycles on Forest Response to Global Change: The Role of Mixed Forests. Forests 2019, 10, 609. [Google Scholar] [CrossRef]
- Diallo, A.; Agbangba, E.C.; Ndiaye, O.; Guisse, A. Ecological Structure and Prediction Equations for Estimating Tree Age, and Dendometric Parameters of Acacia senegal in the Senegalese Semi-Arid Zone—Ferlo. Am. J. Plant Sci. 2013, 4, 1046–1053. [Google Scholar] [CrossRef]
- Martin-Benito, D.; Kint, V.; del Río, M.; Muys, B.; Cañellas, I. Growth responses of West-Mediterranean Pinus nigra to climate change are modulated by competition and productivity: Past trends and future perspectives. For. Ecol. Manag. 2011, 262, 1030–1040. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; McRae, D.J. Spatial and age-dependent tree-ring growth responses of Larix gmelinii to climate in northeastern China. Trees 2009, 23, 875–885. [Google Scholar] [CrossRef]
- Vlam, M.; Van Der Sleen, P.; Groenendijk, P.; Zuidema, P.A. Tree Age Distributions Reveal Large-Scale Disturbance-Recovery Cycles in Three Tropical Forests. Front. Plant Sci. 2017, 7, 1984. [Google Scholar] [CrossRef]
- Rajsnerová, P.; Klem, K.; Holub, P.; Novotná, K.; Večeřová, K.; Kozáčiková, M.; Rivas-Ubach, A.; Sardans, J.; Marek, M.V.; Peñuelas, J.; et al. Morphological, biochemical and physiological traits of upper and lower canopy leaves of European beech tend to converge with increasing altitude. Tree Physiol. 2015, 35, 47–60. [Google Scholar] [CrossRef]
- Qiu, T.; Aravena, M.-C.; Andrus, R.; Ascoli, D.; Bergeron, Y.; Berretti, R.; Bogdziewicz, M.; Boivin, T.; Bonal, R.; Caignard, T.; et al. Is there tree senescence? The fecundity evidence. Proc. Natl. Acad. Sci. USA 2021, 118, e2106130118. [Google Scholar] [CrossRef]
- Cullen, L.E.; Grierson, P.F. Multi-decadal scale variability in autumn-winter rainfall in south-western Australia since 1655 AD as reconstructed from tree rings of Callitris columellaris. Clim. Dyn. 2009, 33, 433–444. [Google Scholar] [CrossRef]
- Liang, P.; Wang, X.; Sun, H.; Fan, Y.; Wu, Y.; Lin, X.; Chang, J. Forest type and height are important in shaping the altitudinal change of radial growth response to climate change. Sci. Rep. 2019, 9, 1336. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gou, X.; Chen, F.; Li, J.; Liu, P.; Zhang, Y. Altitudinal variability of climate–tree growth relationships along a consistent slope of Anyemaqen Mountains, northeastern Tibetan Plateau. Dendrochronologia 2008, 26, 87–96. [Google Scholar] [CrossRef]
- Yu, S.; Yuan, Y.; Qin, L. Tree-ring-width Growth Responses of Picea schrenkiana to Climate Change for Different Elevations in the Central Tianshan Mountains. Desert Oasis Meteorol. 2016, 10, 30–38. (In Chinese) [Google Scholar]
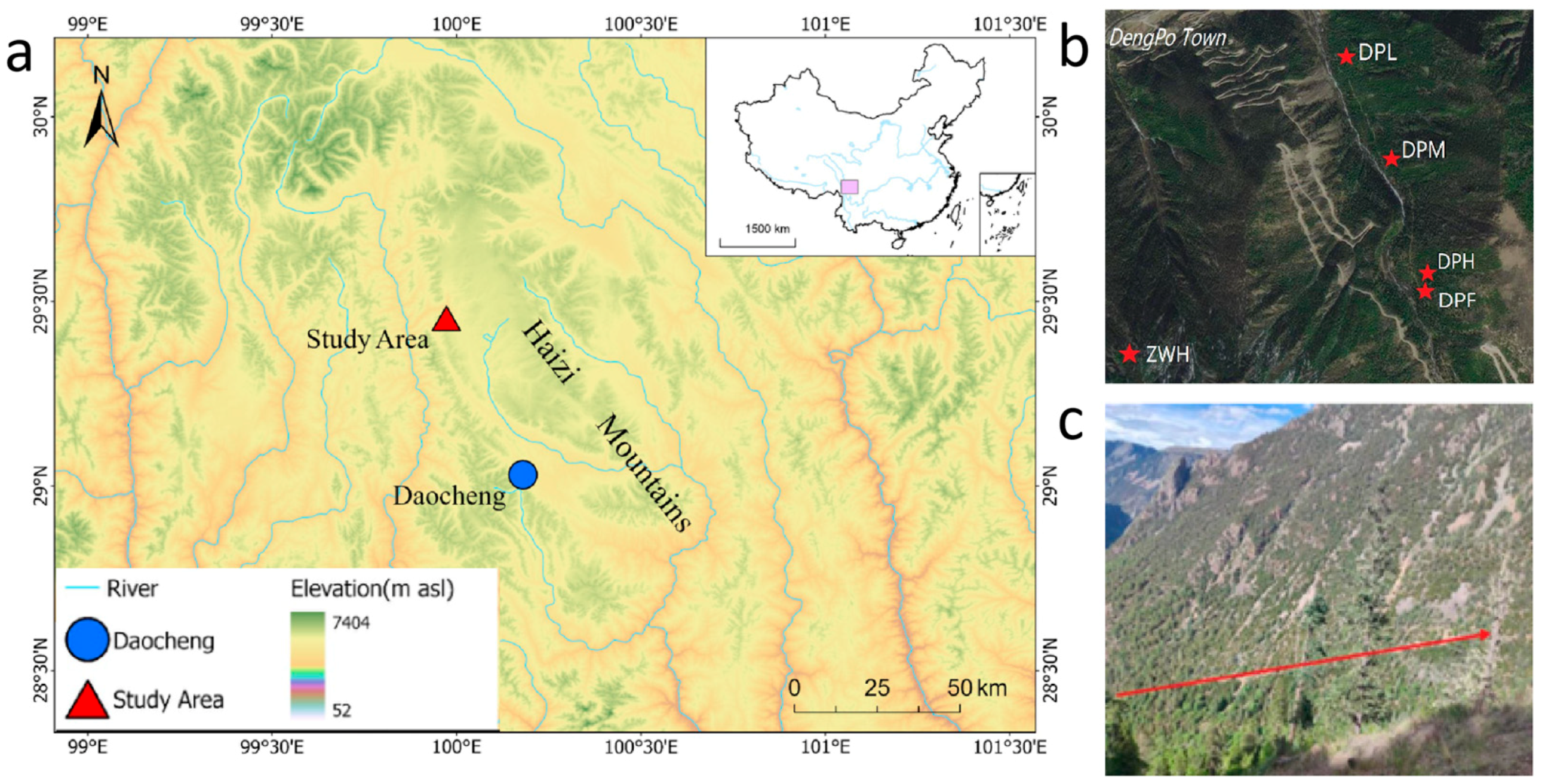
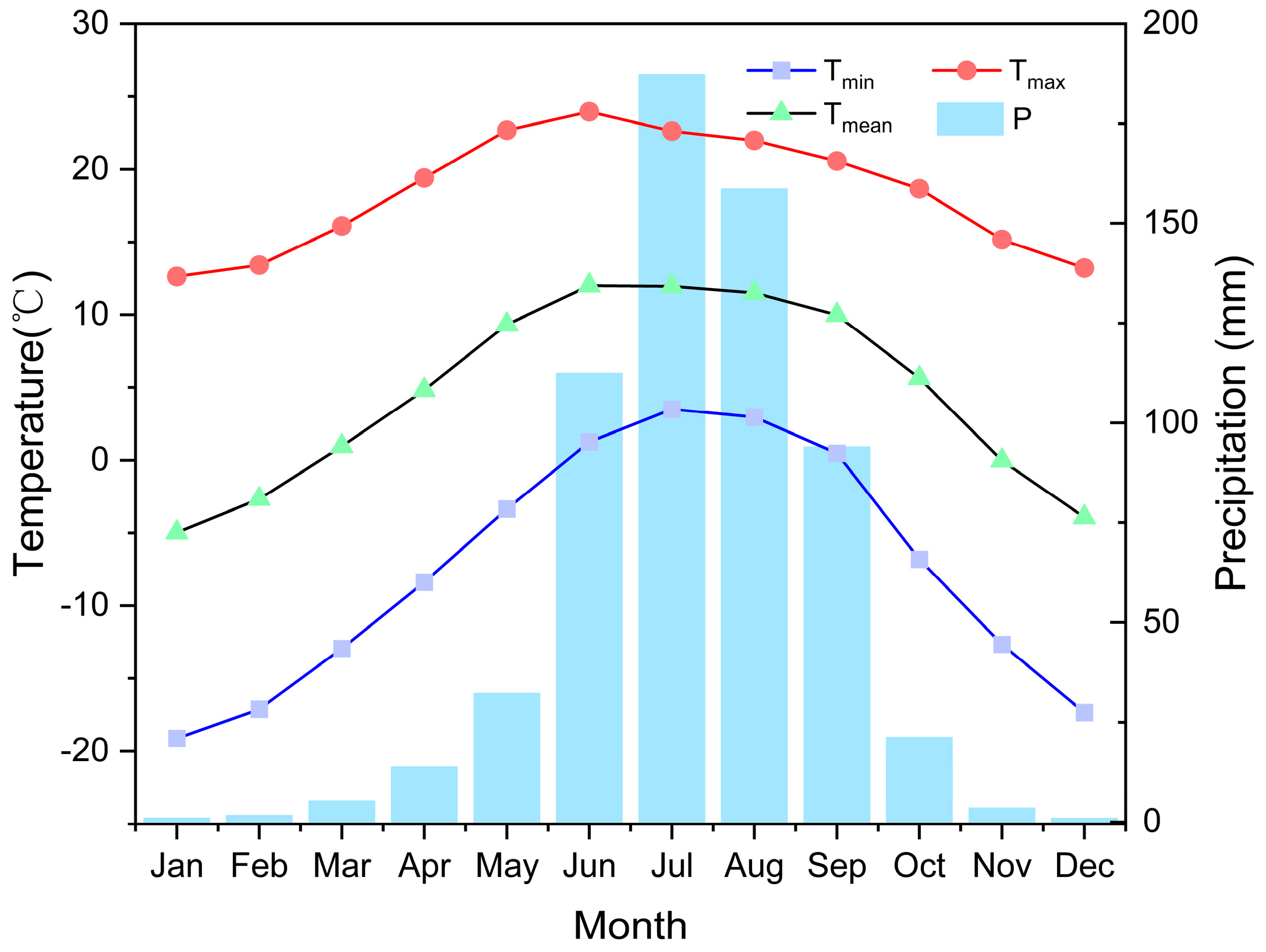
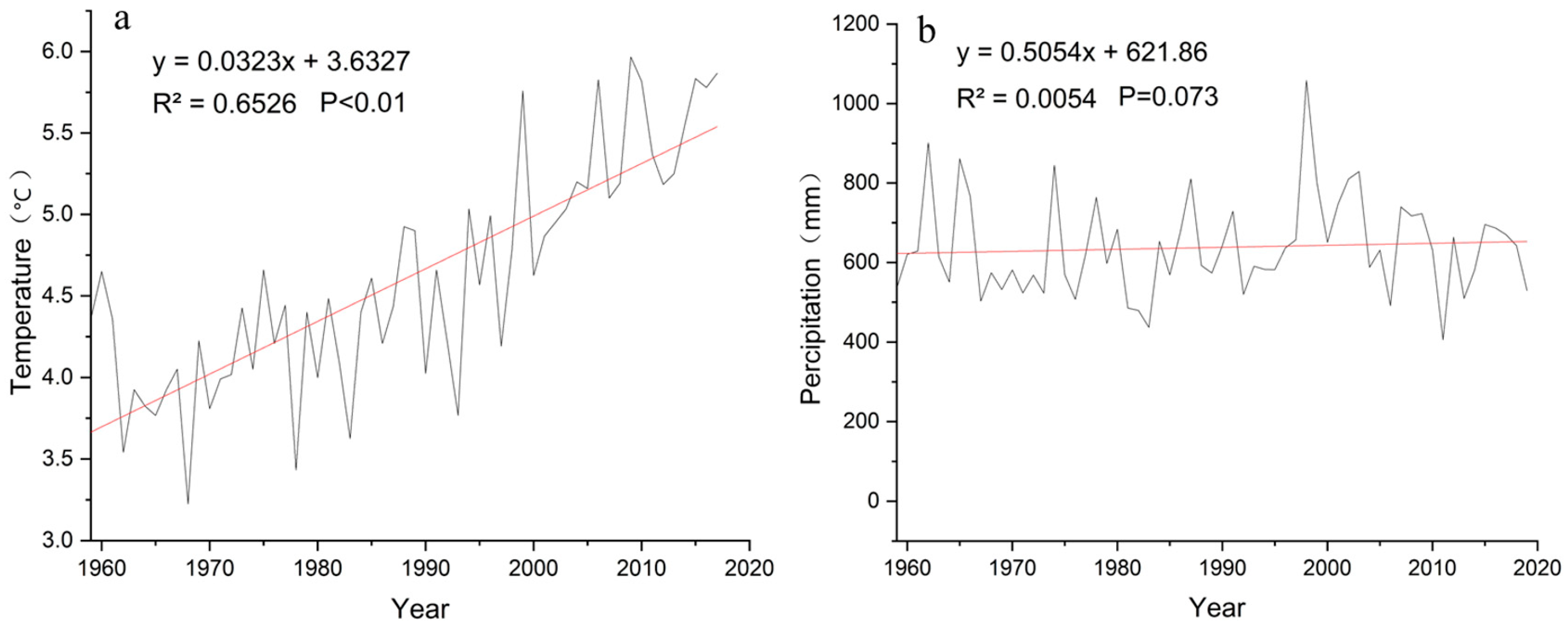
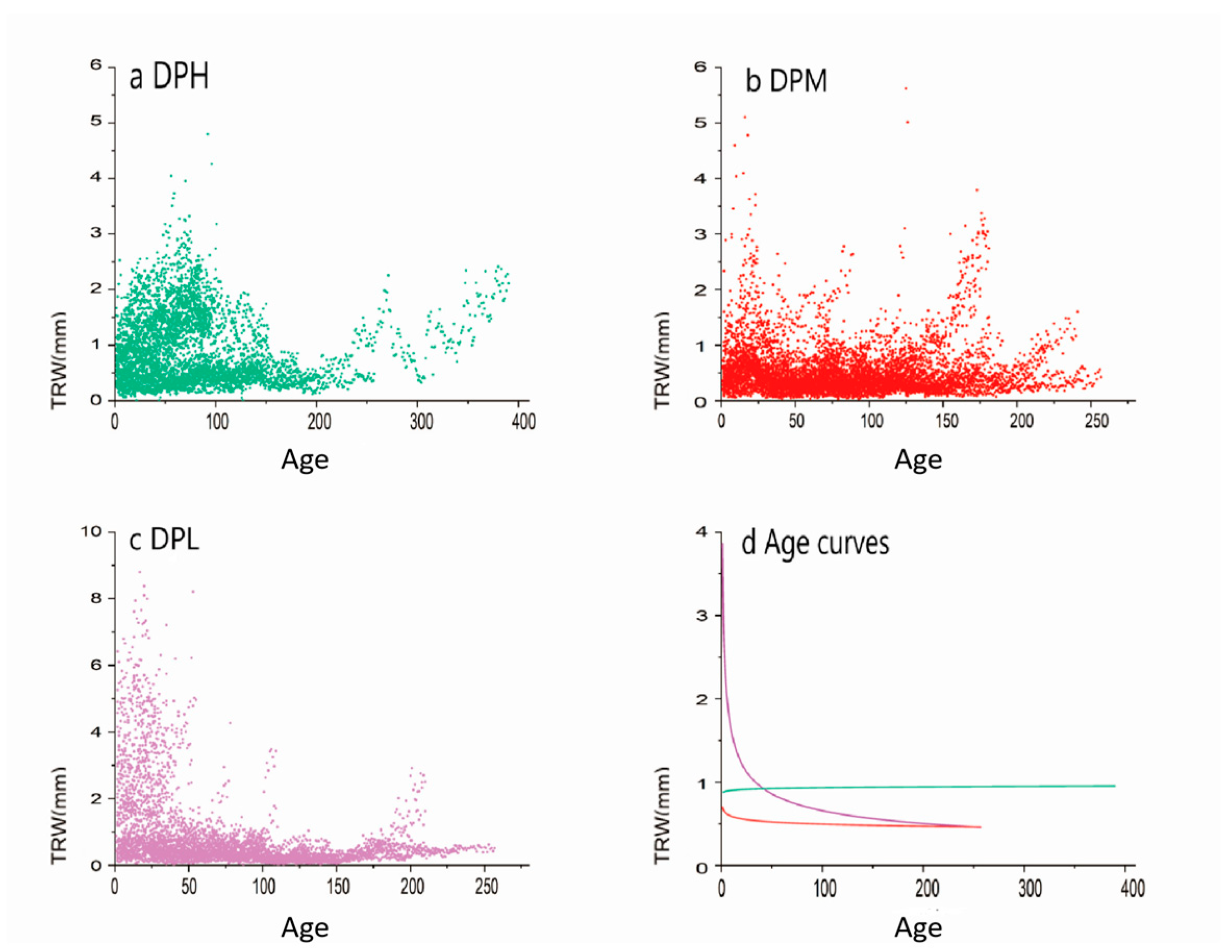


| Site | Date | Specie | No. T/C | Mean Series Length | Time Span | Elevation (m) | Latitude | Longitude |
|---|---|---|---|---|---|---|---|---|
| DPL | 8 July 2022 | P. likiangensis | 21/47 | 108 | AD1766–2021 | 3678–3774 | 29°28′ N | 99°57′ E |
| DPM | 9 July 2022 | P. likiangensis | 26/56 | 145 | AD1766–2021 | 3874–3897 | 29°28′ N | 99°58′ E |
| DPH | 10 July 2022 | P. likiangensis | 24/50 | 127 | AD1633–2021 | 4254–4305 | 29°27′ N | 99°58′ E |
| DPF | 6 December 2022 | S. saltuaria | 20/44 | 146 | AD1773–2022 | 4170–4283 | 29°27′ N | 99°58′ E |
| ZWH | 6 July 2022 | A. squamata | 20/42 | 57 | AD1918–2021 | 4355–4398 | 29°26′ N | 99°56′ E |
| Num. | T/C | Common Period | M.S. | SD | SNR | AC1 | EPS | WTR | BTR | Rbar |
|---|---|---|---|---|---|---|---|---|---|---|
| DPL | 19/42 | 1950–2020 | 0.222 | 0.106 | 5.621 | 0.753 | 0.861 | 0.392 | 0.210 | 0.333 |
| DPM | 26/51 | 1950–2020 | 0.239 | 0.140 | 23.392 | 0.663 | 0.959 | 0.641 | 0.334 | 0.342 |
| DPH | 20/34 | 1950–2020 | 0.163 | 0.162 | 12.376 | 0.563 | 0.925 | 0.462 | 0.273 | 0.279 |
| DPF | 18/32 | 1930–2000 | 0.186 | 0.142 | 4.385 | 0.745 | 0.814 | 0.262 | 0.132 | 0.135 |
| ZWH | 20/41 | 1950–2020 | 0.122 | 0.173 | 1.540 | 0.540 | 0.606 | 0.528 | 0.098 | 0.146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Zheng, C.; Zhang, Z.; Shang, Z.; Kong, X.; Zhao, Z. Altitudinal Difference of Growth–Climate Response Models in the Coniferous Forests of Southeastern Tibetan Plateau, China. Forests 2024, 15, 1265. https://doi.org/10.3390/f15071265
Xu S, Zheng C, Zhang Z, Shang Z, Kong X, Zhao Z. Altitudinal Difference of Growth–Climate Response Models in the Coniferous Forests of Southeastern Tibetan Plateau, China. Forests. 2024; 15(7):1265. https://doi.org/10.3390/f15071265
Chicago/Turabian StyleXu, Shanshan, Chaogang Zheng, Zhigang Zhang, Zhiyuan Shang, Xinggong Kong, and Zhijun Zhao. 2024. "Altitudinal Difference of Growth–Climate Response Models in the Coniferous Forests of Southeastern Tibetan Plateau, China" Forests 15, no. 7: 1265. https://doi.org/10.3390/f15071265
APA StyleXu, S., Zheng, C., Zhang, Z., Shang, Z., Kong, X., & Zhao, Z. (2024). Altitudinal Difference of Growth–Climate Response Models in the Coniferous Forests of Southeastern Tibetan Plateau, China. Forests, 15(7), 1265. https://doi.org/10.3390/f15071265






