Study on the Mechanical Properties of Roots and Friction Characteristics of the Root–Soil Interface of Two Tree Species in the Coastal Region of Southeastern China
Abstract
:1. Introduction
2. Materials and Methods
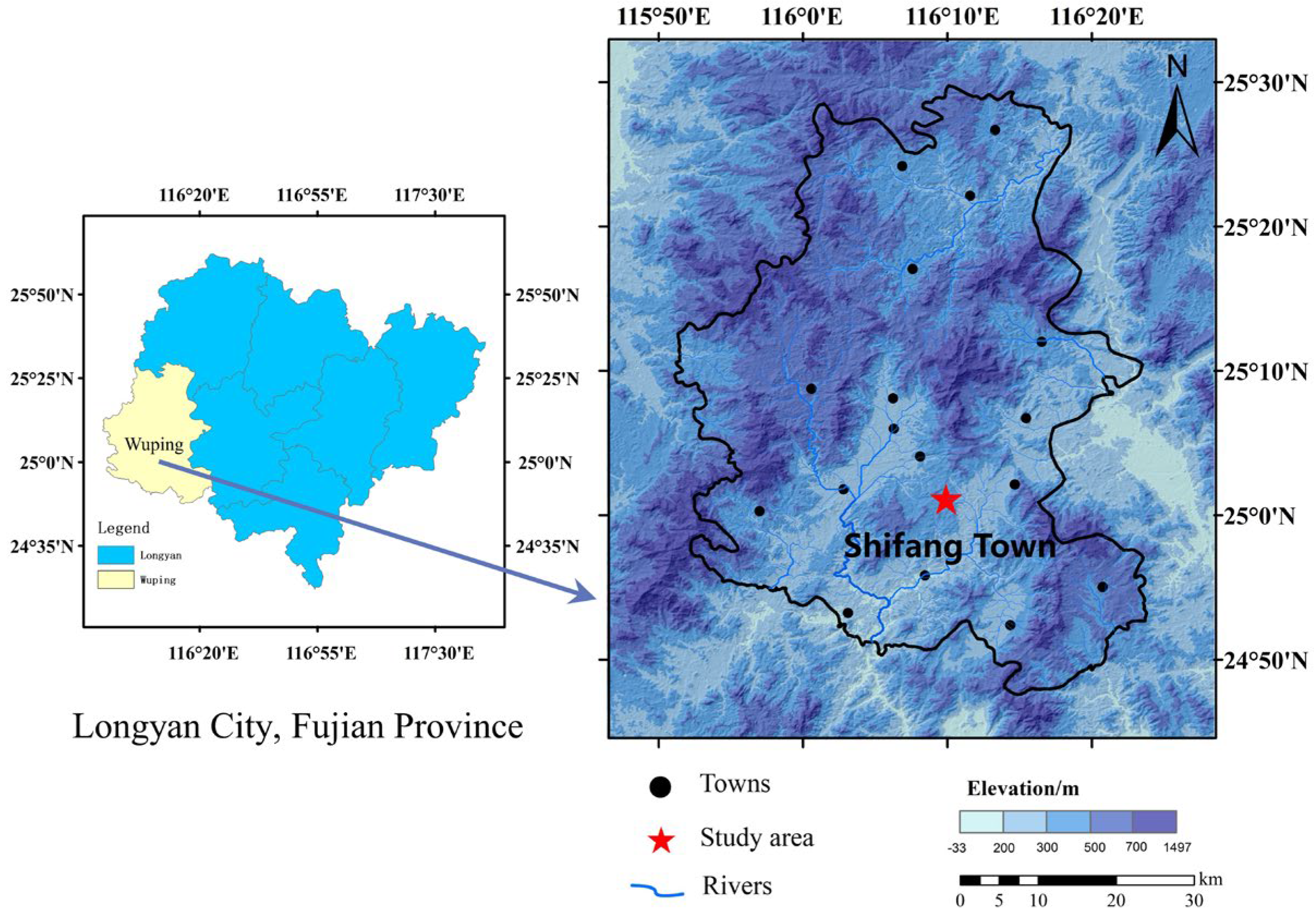
2.1. Study Area
2.2. Root Tensile Tests
2.3. The Analysis of the Structural Chemical Composition of Root Tissues
2.4. Direct Shear Friction Test
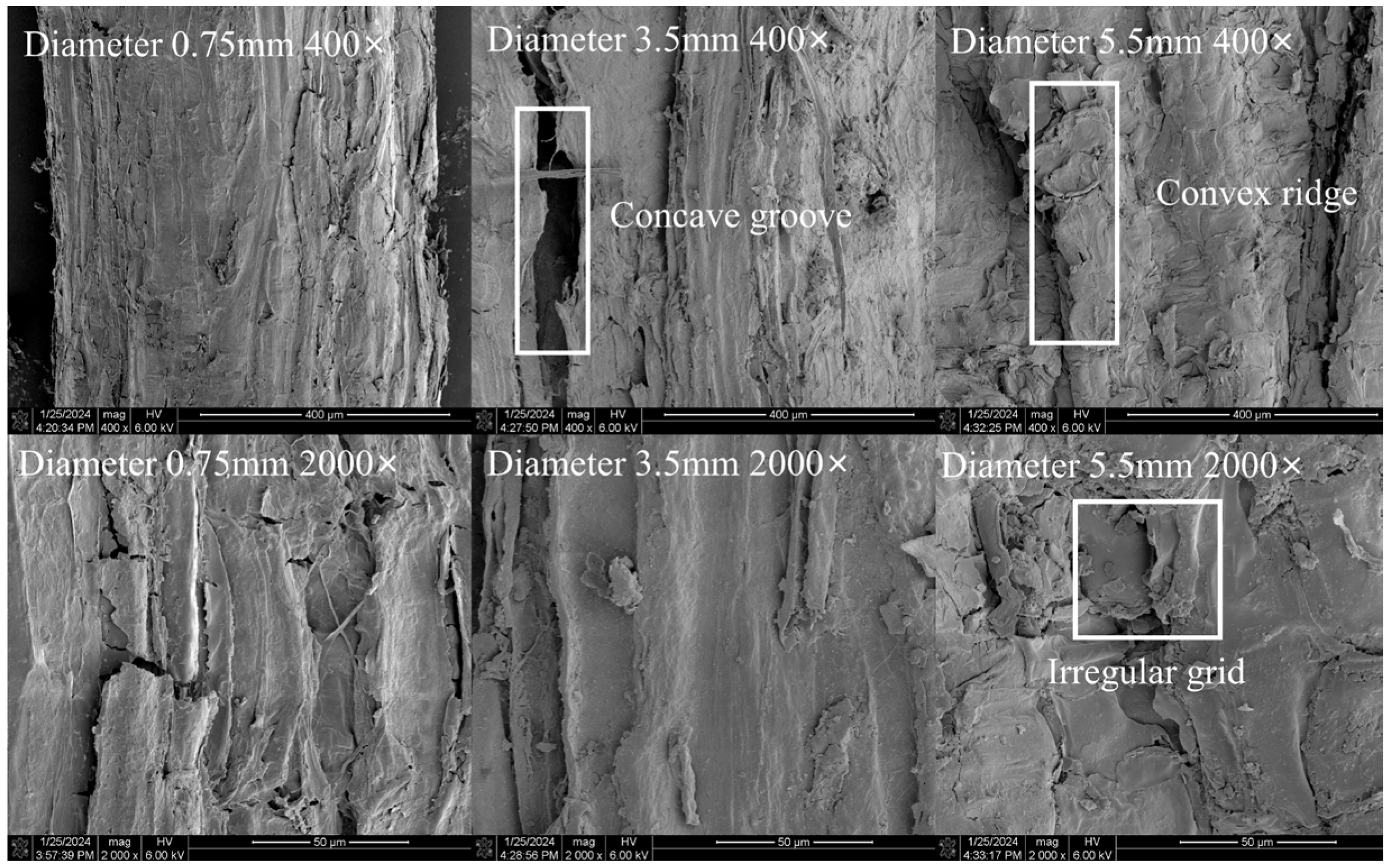
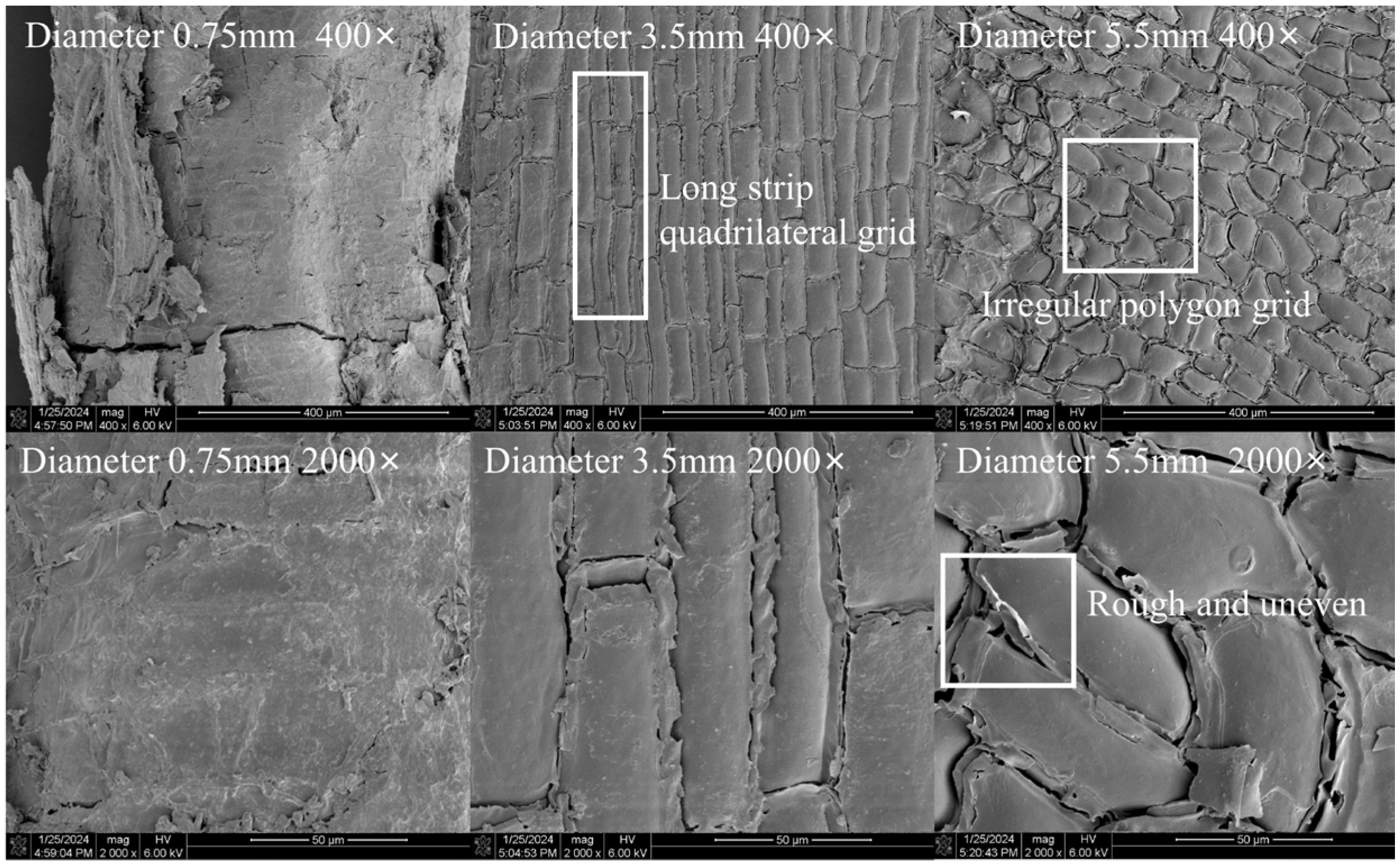
2.5. Scanning Electron Microscopic Evaluation of Roots with Different Diameters
3. Results
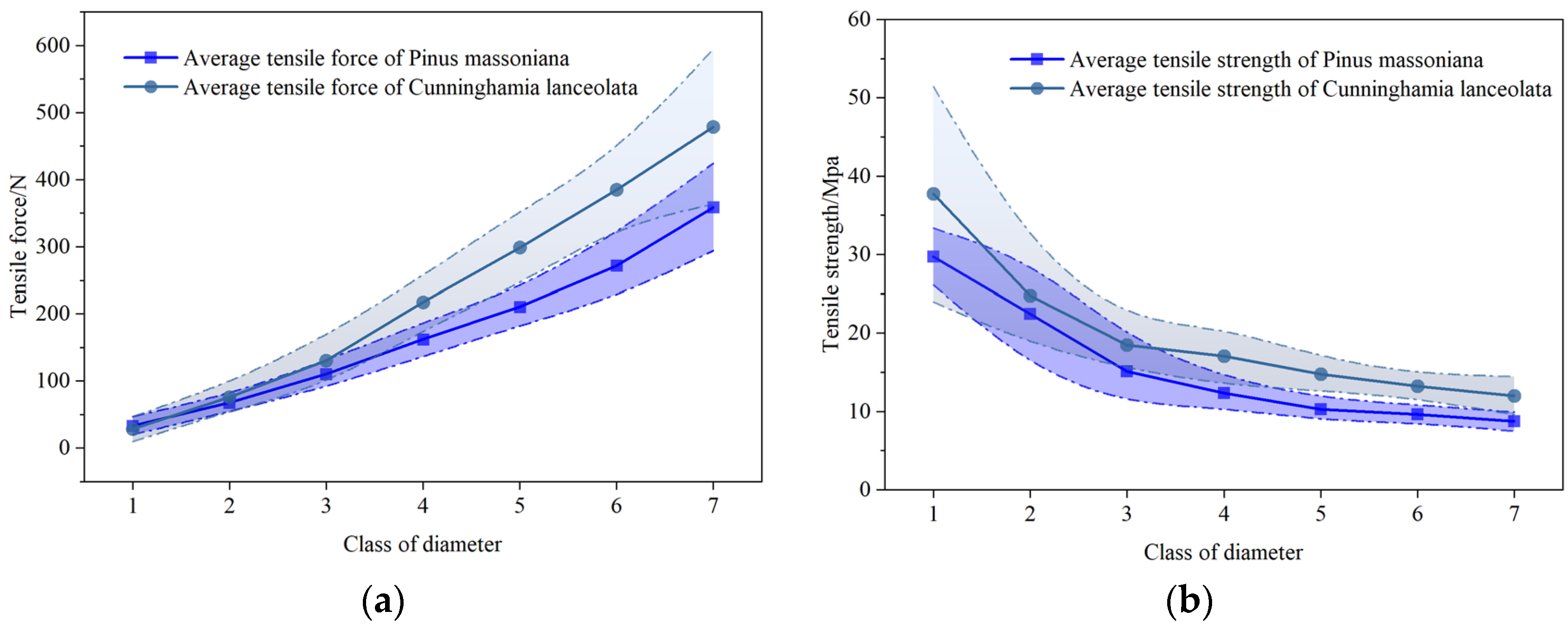
3.1. Root Tensile Strength of the Two Tree Species
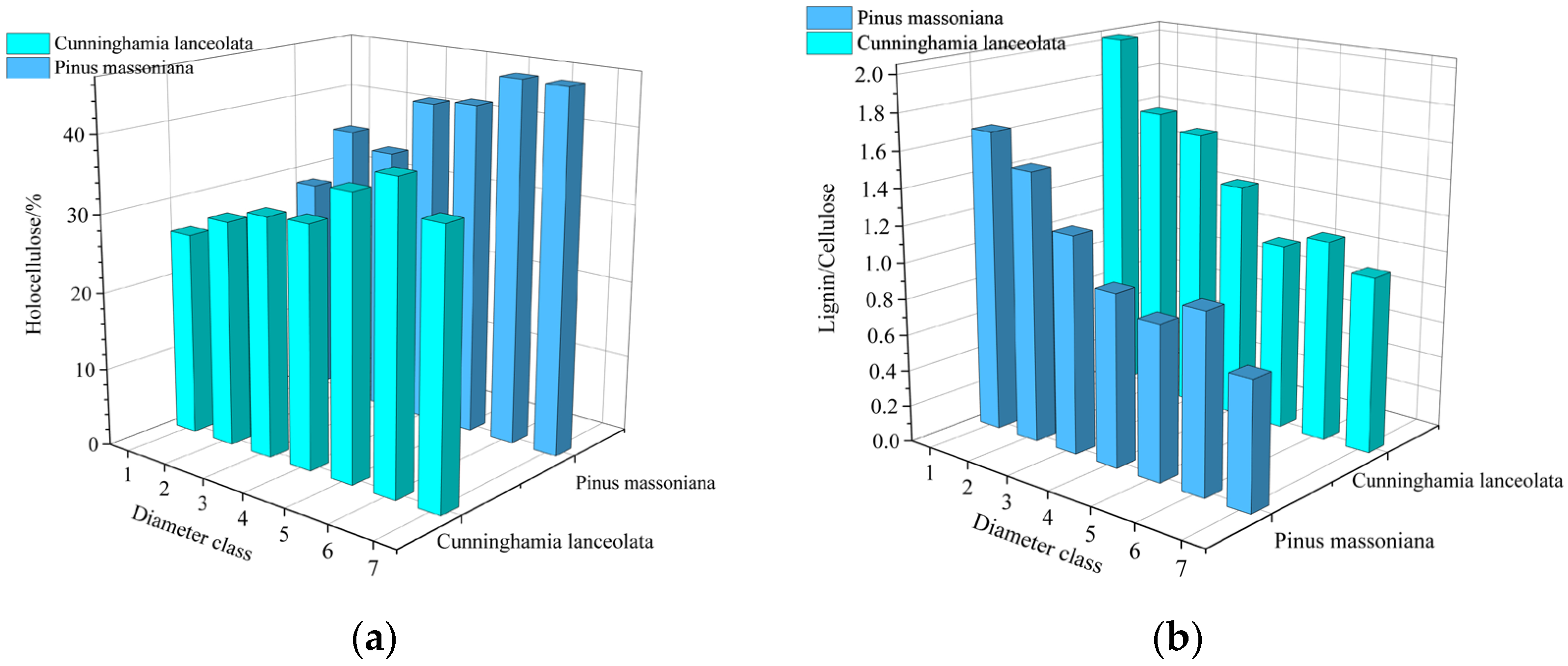
3.2. Variations in Relationships between Root Diameter Classes and Contents of the Structural Chemical Composition in Root Tissues
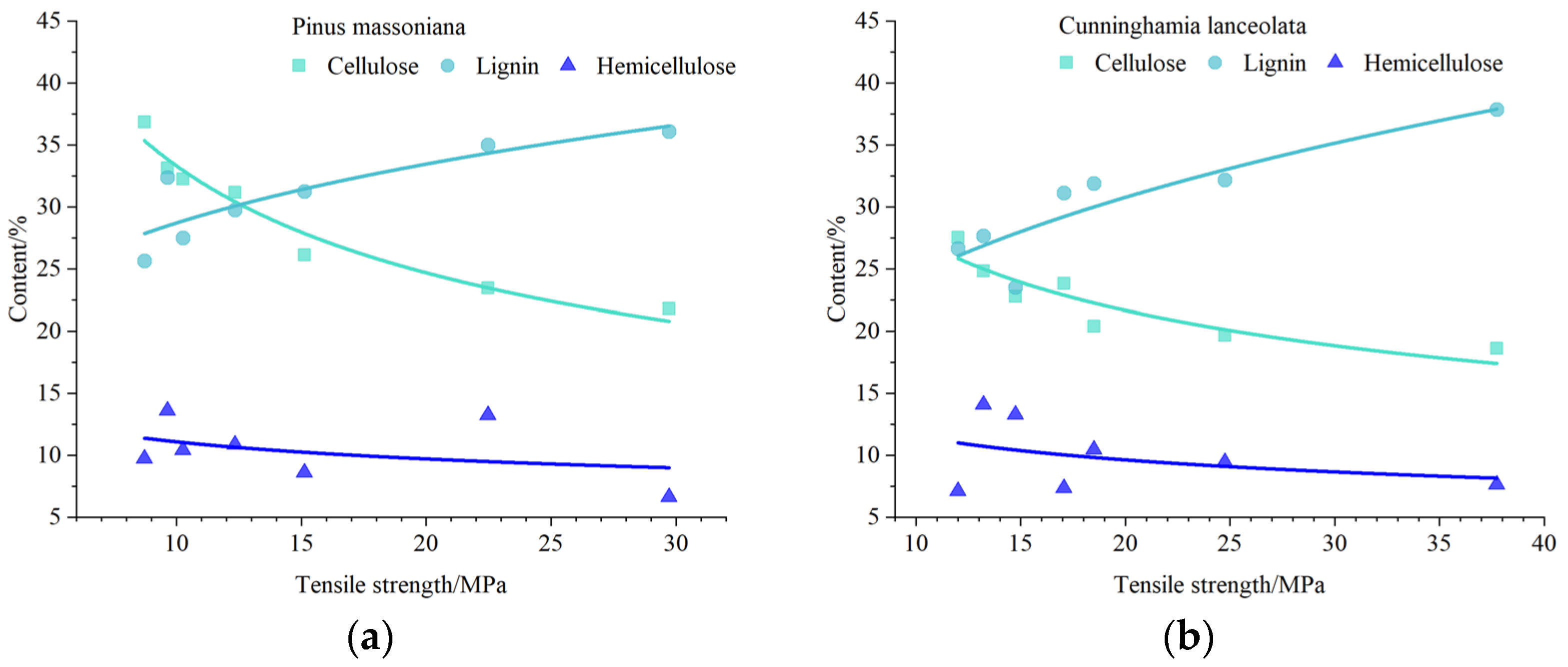
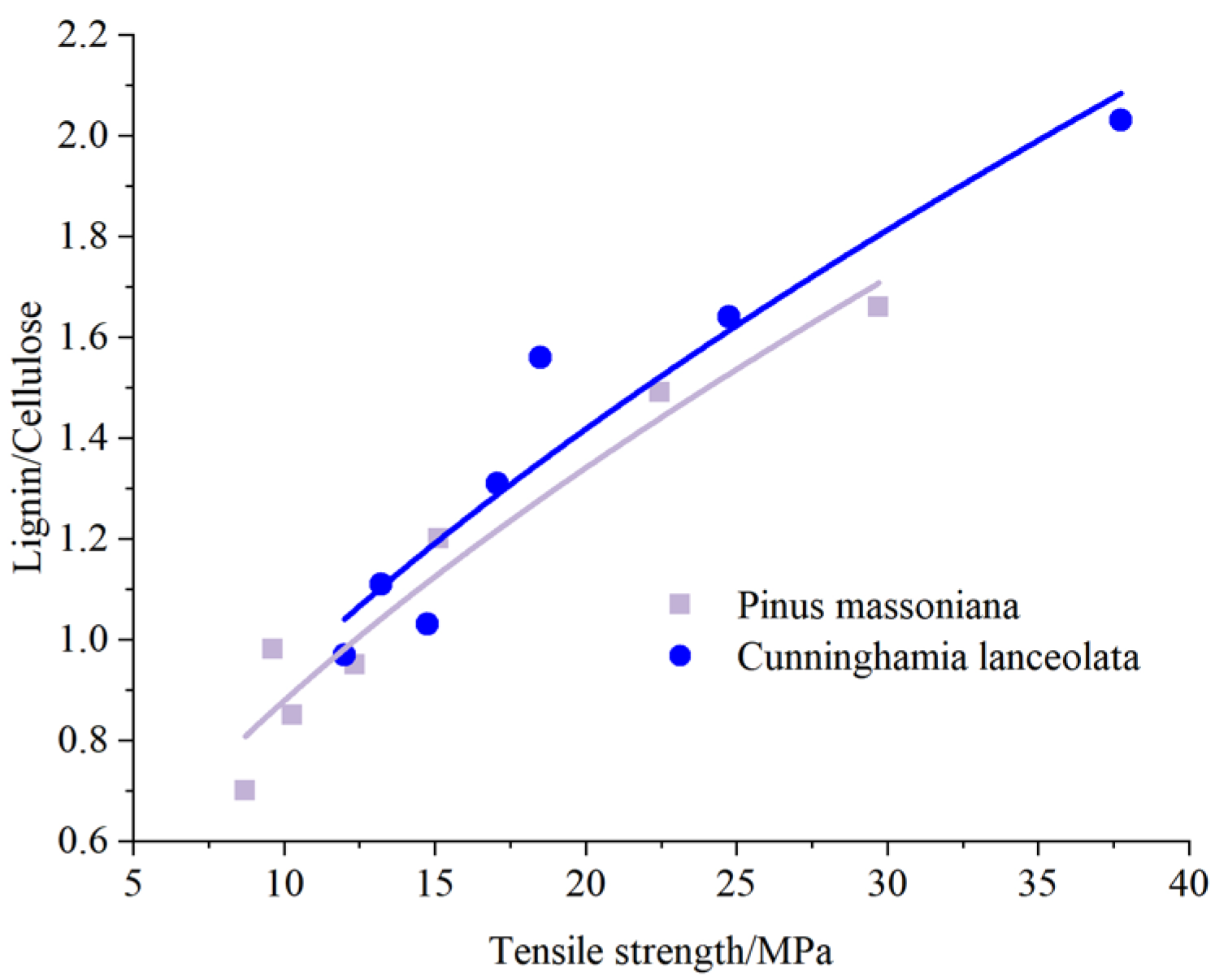
3.3. Variations in Relationships between the Root Tensile Strength and Contents of the Structural Chemical Composition in Root Tissues
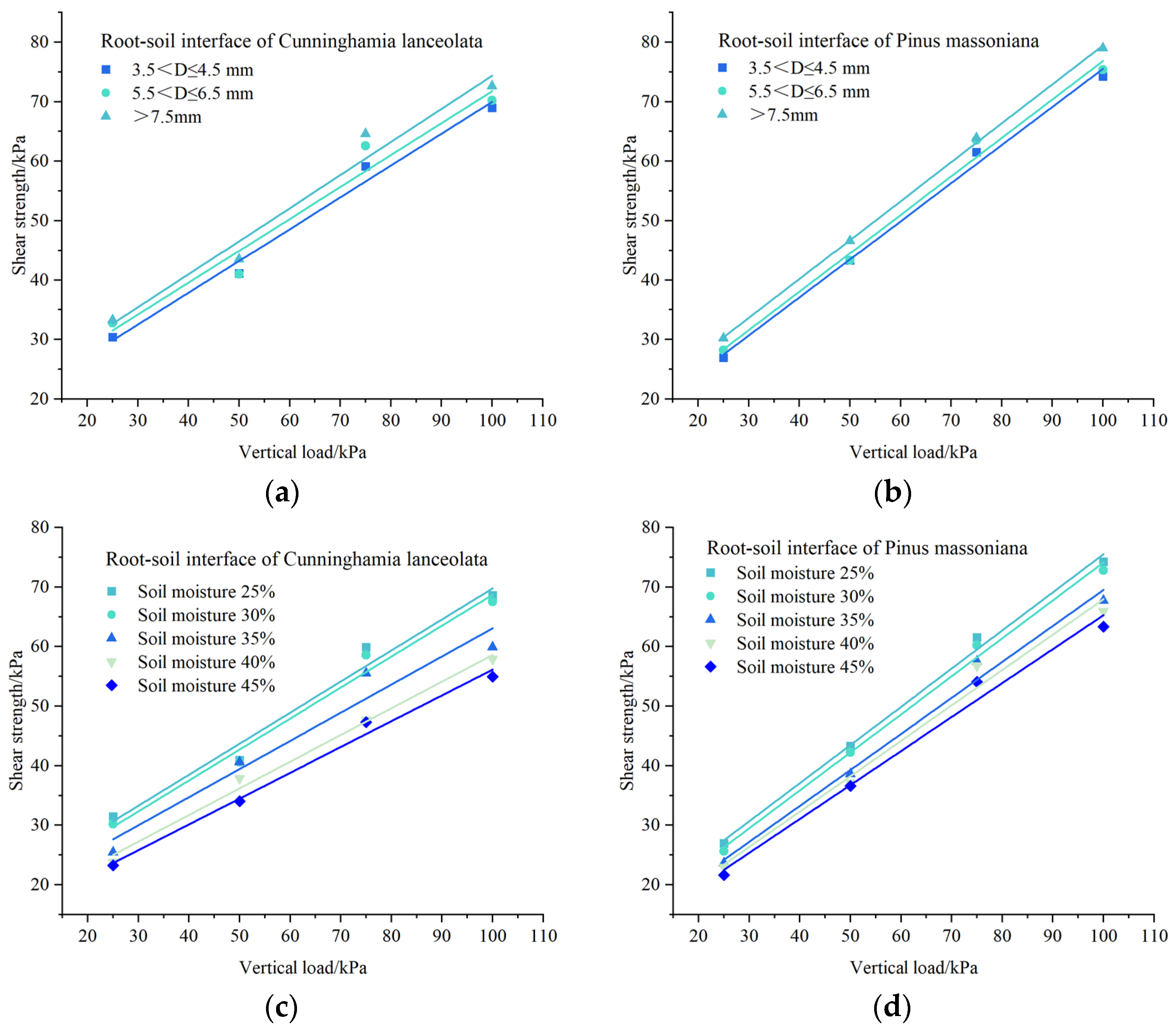
3.4. Relationships between the Vertical Load and Shear Strength of the Root–Soil Interface
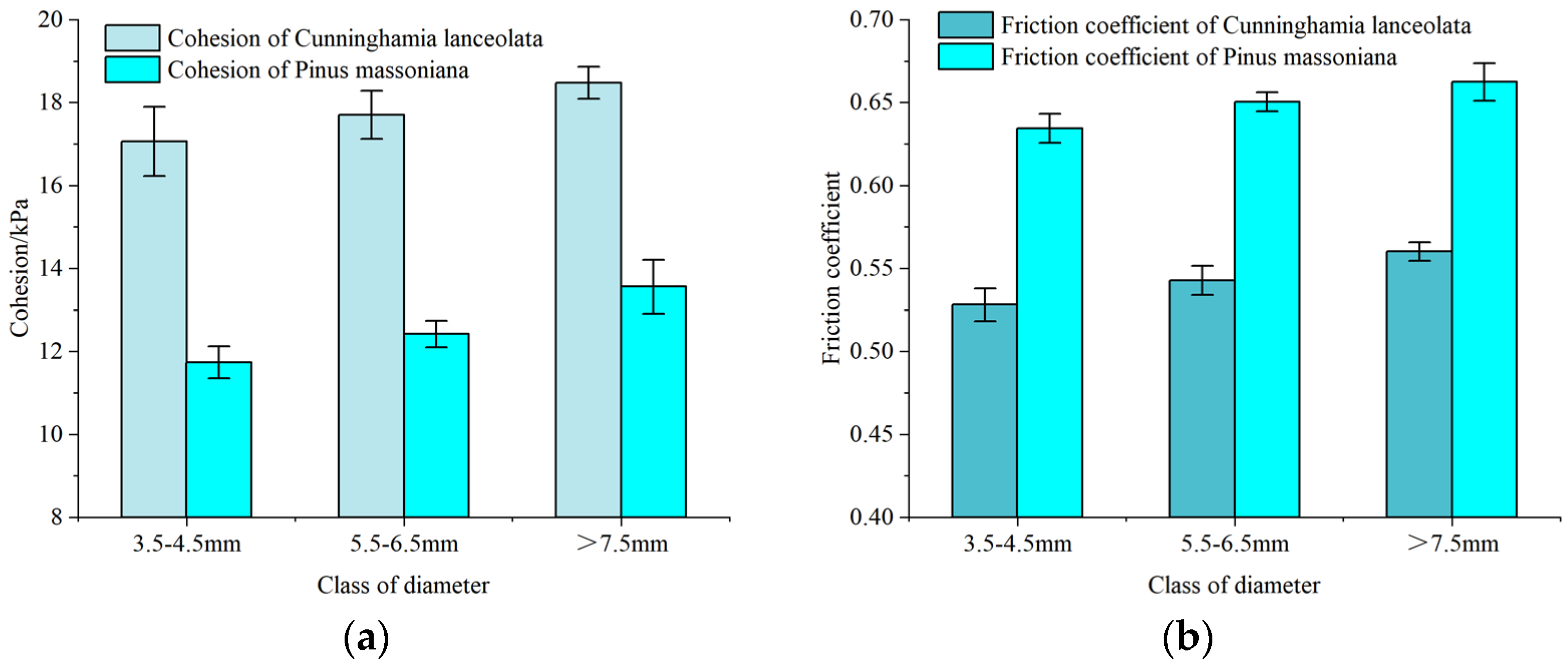
3.5. Friction Characteristics of the Root–Soil Interface Obtained from the Direct Shear Test of the Tree Species Roots with Different Diameter Size Classes
3.6. Friction Characteristics of the Root–Soil Interface Obtained from the Direct Shear Test at Different Soil Moisture Contents
4. Discussion
4.1. Effects of the Structural Chemical Composition of Root Tissues on Parameters of the Root Tensile Strength
4.2. The Influence of the Microstructural Characteristics of the Root Surface of the Two Tree Species on the Frictional Performance of the Root–Soil Interface
4.3. Mechanism of the Influence of Soil Moisture on the Friction Characteristics of the Root–Soil Interface and Landslide Occurrence
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boldrin, D.; Leung, A.K.; Bengough, A.G. Hydro-Mechanical Reinforcement of Contrasting Woody Species: A Full-Scale Investigation of a Field Slope. Géotechnique 2021, 71, 970–984. [Google Scholar] [CrossRef]
- Qin, M.; Cui, P.; Jiang, Y.; Guo, J.; Zhang, G.; Ramzan, M. Occurrence of Shallow Landslides Triggered by Increased Hydraulic Conductivity Due to Tree Roots. Landslides 2022, 19, 2593–2604. [Google Scholar] [CrossRef]
- Docker, B.B.; Hubble, T.C.T. Quantifying Root-Reinforcement of River Bank Soils by Four Australian Tree Species. Geomorphology 2008, 100, 401–418. [Google Scholar] [CrossRef]
- Zhang, C.-B.; Chen, L.-H.; Jiang, J. Why Fine Tree Roots Are Stronger than Thicker Roots: The Role of Cellulose and Lignin in Relation to Slope Stability. Geomorphology 2014, 206, 196–202. [Google Scholar] [CrossRef]
- Meijer, G.J.; Bengough, A.G.; Knappett, J.A.; Loades, K.W.; Nicoll, B.C. New in Situ Techniques for Measuring the Properties of Root-Reinforced Soil—Laboratory Evaluation. Géotechnique 2016, 66, 27–40. [Google Scholar] [CrossRef]
- Burylo, M.; Hudek, C.; Rey, F. Soil Reinforcement by the Roots of Six Dominant Species on Eroded Mountainous Marly Slopes (Southern Alps, France). CATENA 2011, 84, 70–78. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Jiang, J.; Wei, Y.; Ma, J.; Hallett, P.D. Root Moisture Content Influence on Root Tensile Tests of Herbaceous Plants. CATENA 2019, 172, 140–147. [Google Scholar] [CrossRef]
- Su, X.; Zhou, Z.; Liu, J.; Wang, P.; Liu, J.; Li, Q.; Zhao, F. The Role of Roots Traits of Climax Community Species to Shear Strength in the Loess Hilly Region, China. Soil Tillage Res. 2022, 221, 105417. [Google Scholar] [CrossRef]
- Kamchoom, V.; Leung, A.K.; Boldrin, D.; Sakolpanya, T.; Wu, Z.; Likitlersuang, S. Shearing Behaviour of Vegetated Soils with Growing and Decaying Roots. Can. Geotech. J. 2022, 59, 2067–2084. [Google Scholar] [CrossRef]
- Lv, C.; Chen, L. Relationship between root tensile mechanical properties and its main chemical components of tipical tree species in North China. Trans. Chin. Soc. Agric. Eng. 2013, 29, 69–78. [Google Scholar]
- Guo, W.-Z.; Chen, Z.-X.; Wang, W.-L.; Gao, W.-W.; Guo, M.-M.; Kang, H.-L.; Li, P.-F.; Wang, W.-X.; Zhao, M. Telling a Different Story: The Promote Role of Vegetation in the Initiation of Shallow Landslides during Rainfall on the Chinese Loess Plateau. Geomorphology 2020, 350, 106879. [Google Scholar] [CrossRef]
- Li, M.; Ma, C.; Du, C.; Yang, W.; Lyu, L.; Wang, X. Landslide Response to Vegetation by Example of July 25–26, 2013, Extreme Rainstorm, Tianshui, Gansu Province, China. Bull. Eng. Geol. Environ. 2021, 80, 751–764. [Google Scholar] [CrossRef]
- Fan, C.-C.; Su, C.-F. Role of Roots in the Shear Strength of Root-Reinforced Soils with High Moisture Content. Ecol. Eng. 2008, 33, 157–166. [Google Scholar] [CrossRef]
- Zhu, J.; Mao, Z.; Wang, Y.; Wang, Y.; Li, T.; Wang, K.; Langendoen, E.J.; Zheng, B. Soil Moisture and Hysteresis Affect Both Magnitude and Efficiency of Root Reinforcement. CATENA 2022, 219, 106574. [Google Scholar] [CrossRef]
- Schwarz, M.; Cohen, D.; Or, D. Pullout Tests of Root Analogs and Natural Root Bundles in Soil: Experiments and Modeling. J. Geophys. Res. 2011, 116, 2010JF001753. [Google Scholar] [CrossRef]
- Fan, C.-C. A Displacement-Based Model for Estimating the Shear Resistance of Root-Permeated Soils. Plant Soil 2012, 355, 103–119. [Google Scholar] [CrossRef]
- Gan, F.; Wei, J.; Li, S. Root–Soil Friction Mechanism of Typical Grasses on Purple Soil Bunds in the Three Gorges Reservoir Area, China. J. Soil Sci. Plant Nutr. 2023, 23, 3381–3392. [Google Scholar] [CrossRef]
- Ning, P.; Xia, X.; Jiang, Y. An Estimation Model of the Ultimate Shear Strength of Root-Permeated Soil, Fully Considering Interface Bonding. Forests 2023, 14, 819. [Google Scholar] [CrossRef]
- Zhou, Y.; Watts, D.; Li, Y.; Cheng, X. A Case Study of Effect of Lateral Roots of Pinus Yunnanensis on Shallow Soil Reinforcement. For. Ecol. Manag. 1998, 103, 107–120. [Google Scholar] [CrossRef]
- Ge, R.; Liu, Y.; Zuo, Z.; A, R.; Na, R. Effect of Soil Moisture on the Characteristics of Root-soil Interface Interaction. J. Soil Water Conserv. 2018, 32, 135–140. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Yu, D.; Li, S. Microstructural features and friction characteristics of the interface of shrub roots and soil in loess area of Xining Basin. Chin. J. Rock Mech. Eng. 2018, 37, 1270–1280. [Google Scholar] [CrossRef]
- Zhao, D.; Ji, X.; Zhang, X.; Li, X.; Zhang, H. Friction performance of root-soil interface of Betula platyphylla in Northwestern Hebei Province, China. Trans. Chin. Soc. Agric. Eng. 2021, 37, 124–131. [Google Scholar]
- Ouyang, M.; Lan, R.; Zhang, H.; Wang, G.; Tan, G.; Guo, O.; Li, C. Shear Strength Model of Root-Soil Interface Considering Interfacial Suction and Its Influencing Factors. China J. Highw. Transp. 2024, 1, 1–17. [Google Scholar]
- Zhuang, Y.; Xing, A.; Jiang, Y.; Sun, Q.; Yan, J.; Zhang, Y. Typhoon, Rainfall and Trees Jointly Cause Landslides in Coastal Regions. Eng. Geol. 2022, 298, 106561. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Standard for geotechnical Test Methods. Water Conservancy and Hydropower Engineering Construction Quality Monitoring Station: Zhengzhou, China, 2019.
- Tan, H.; Chen, F.; Chen, J.; Gao, Y. Direct Shear Tests of Shear Strength of Soils Reinforced by Geomats and Plant Roots. Geotext. Geomembr. 2019, 47, 780–791. [Google Scholar] [CrossRef]
- Crawford, M.M.; Bryson, L.S.; Woolery, E.W.; Wang, Z. Long-Term Landslide Monitoring Using Soil-Water Relationships and Electrical Data to Estimate Suction Stress. Eng. Geol. 2019, 251, 146–157. [Google Scholar] [CrossRef]
- Liu, Q.; Jian, W.; Nie, W. Rainstorm-Induced Landslides Early Warning System in Mountainous Cities Based on Groundwater Level Change Fast Prediction. Sustain. Cities Soc. 2021, 69, 102817. [Google Scholar] [CrossRef]
- Freschet, G.T.; Roumet, C. Sampling Roots to Capture Plant and Soil Functions. Funct. Ecol. 2017, 31, 1506–1518. [Google Scholar] [CrossRef]
- Fattet, M.; Fu, Y.; Ghestem, M.; Ma, W.; Foulonneau, M.; Nespoulous, J.; Le Bissonnais, Y.; Stokes, A. Effects of Vegetation Type on Soil Resistance to Erosion: Relationship between Aggregate Stability and Shear Strength. CATENA 2011, 87, 60–69. [Google Scholar] [CrossRef]
- Ghestem, M.; Veylon, G.; Bernard, A.; Vanel, Q.; Stokes, A. Influence of Plant Root System Morphology and Architectural Traits on Soil Shear Resistance. Plant Soil 2014, 377, 43–61. [Google Scholar] [CrossRef]
- Lian, B.; Peng, J.; Zhan, H.; Wang, X. Mechanical Response of Root-Reinforced Loess with Various Water Contents. Soil Tillage Res. 2019, 193, 85–94. [Google Scholar] [CrossRef]
- Hales, T.C.; Ford, C.R.; Hwang, T.; Vose, J.M.; Band, L.E. Topographic and Ecologic Controls on Root Reinforcement. J. Geophys. Res. 2009, 114, 2008JF001168. [Google Scholar] [CrossRef]
- Jiang, K.; Chen, L.; Gai, X. Relationship between tensile properties and microstructures of three different broadleaf tree roots in North China. Trans. Chin. Soc. Agric. Eng. 2013, 29, 115–123. [Google Scholar]
- Zhang, Q.; Tang, L.; Ran, J. Frictional Characteristics of Root-Soil Interface of Indigofera amblyantha and Cassia bicapsularis. J. Chang. River Sci. Res. 2023, 40, 161–164. [Google Scholar]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable Plant Root Traits for Protecting Natural and Engi-neered Slopes against Landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, L.; Liu, X. The Influence Factors of Plant Root-Soil Interface Friction. Tribology 2014, 34, 482–488. [Google Scholar]
- Jemberu, W.; Baartman, J.E.M.; Fleskens, L.; Selassie, Y.G.; Ritsema, C.J. Assessing the Variation in Bund Structure Dimensions and Its Impact on Soil Physical Properties and Hydrology in Koga Catchment, Highlands of Ethiopia. CATENA 2017, 157, 195–204. [Google Scholar] [CrossRef]
- Wang, L.; Ma, A.; Zhang, H.; Zhang, J.; Dong, Q.; Fu, G. Effects of Long-Term Vegetation Restoration on Distribution of Deep Soil Moisture in Semi-Arid Northwest of China. J. Soil Sci. Plant Nutr. 2020, 20, 2123–2132. [Google Scholar] [CrossRef]
- Rai, R.; Shrivastva, B.K. Biological Stabilization of Mine Dumps: Shear Strength and Numerical Simulation Approach with Special Reference to Sisam Tree. Environ. Earth Sci. 2011, 63, 177–188. [Google Scholar] [CrossRef]
- Lotfalian, M.; Nasiri, M.; Modarres, A.; Wu, W. Slope Stability Analysis Considering Weight of Trees and Root Reinforcement. J. Environ. Eng. Landsc. Manag. 2019, 27, 201–208. [Google Scholar] [CrossRef]
- Ghestem, M.; Sidle, R.C.; Stokes, A. The Influence of Plant Root Systems on Subsurface Flow: Implications for Slope Stability. BioScience 2011, 61, 869–879. [Google Scholar] [CrossRef]






| Soil Type | Density/g·cm−3 | Dry Density/g·cm−3 | Saturated Hydraulic Conductivity/m·s−1 |
|---|---|---|---|
| Residual sandy clay | 1.63 | 1.38 | 1.75 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Jian, W.; Zhu, Z.; Wu, Y.; Wang, H.; Fan, X. Study on the Mechanical Properties of Roots and Friction Characteristics of the Root–Soil Interface of Two Tree Species in the Coastal Region of Southeastern China. Forests 2024, 15, 1285. https://doi.org/10.3390/f15081285
Lin Y, Jian W, Zhu Z, Wu Y, Wang H, Fan X. Study on the Mechanical Properties of Roots and Friction Characteristics of the Root–Soil Interface of Two Tree Species in the Coastal Region of Southeastern China. Forests. 2024; 15(8):1285. https://doi.org/10.3390/f15081285
Chicago/Turabian StyleLin, Yunzhao, Wenbin Jian, Zuteng Zhu, Yilong Wu, Hao Wang, and Xiufeng Fan. 2024. "Study on the Mechanical Properties of Roots and Friction Characteristics of the Root–Soil Interface of Two Tree Species in the Coastal Region of Southeastern China" Forests 15, no. 8: 1285. https://doi.org/10.3390/f15081285






