Abstract
Glycine betaine (GB) serves as a compatible solute crucial for stress tolerance across numerous plant species. Populus trichocarpa grows along coastal regions and often suffers from salt stress. In this study, we explored the effects of foliar spraying of GB on the growth, physicochemical indexes, gene expression, and stress tolerance of poplar under salt stress. To achieve this, poplar plants (Populus deltoides × Populus euramericana “Nanlin 895”) were grown under four NaCl concentrations: 0, 50, 100, and 150 mM. Additionally, they were treated with 0, 5, 15, and 25 mM GB. The degree of effect on potted poplars was observed after 8 weeks. A GB treatment of about 15 mM significantly reduced the impact of salt stress and photosynthetic pigments and caused an improvement in the activity of the antioxidant enzymes peroxidase (POD) and superoxide dismutase (SOD). Moreover, GB-treated poplars had significantly more malonaldehyde (MDA) than the control lines. This study indicated that GB treatment reduces salt stress symptoms in poplars. This research helps to develop safe levels of endogenous GB, which will make poplars more resistant to environmental stresses.
1. Introduction
GB is a glycine-derived amphoteric substance, serves as a compatible solute across numerous plant varieties [1]. Numerous studies indicate that GB contributes to stress resilience in certain plant species. However, no studies on foliar spraying GB on poplar were reported. Salt stress induces an overload of ions and osmotic pressure, which is detrimental to cellular metabolism and impairs the ability of plants to absorb water from the soil [2]. Plants have different mechanisms to deal with stress when they encounter high levels of salt, drought, or low temperature. To increase stress tolerance, plants commonly synthesize compatible solutes through the accumulation of diverse small organic metabolites [3]. As a compatible solute, GB is classified as a quaternary ammonium compound (QAC) that many plant species accumulate in the cytoplasm and chloroplasts under abiotic stress [4,5]. In addition, the external application of GB is an effective way to improve plant growth and tolerance during various stress conditions. Numerous studies indicate that GB can improve stress tolerance in certain plant species. For instance, applying GB externally enhances cold hardiness in tomato plants [6]. The negative effects of salt stress on K+ and Ca2+ uptake and K+/Na+ ratios in shoots and roots of two okra varieties were investigated by foliar spraying with GB [7]. Carapa guianensis showed the ability to absorb exogenous GB through their leaves, leading to enhanced plant tolerance to water stress by positively influencing the activities of ascorbate peroxidase and catalase [8]. In six Turfgrass species, exogenous GB could even improve seed germination amid drought, salinity, or temperature stress [9]. These studies also suggest that plants can transfer exogenous GB to almost all plant parts, especially developing organs.
Genetic engineering has been commonly employed to introduce genes related to the biosynthesis of GB to improve the tolerance of non-synthetic GB plants to diverse environmental stresses. Genes associated with GB biosynthesis have been effectively introduced into a variety of plant species. The choline oxidase gene codA was isolated from a soil bacterium and inserted into Arabidopsis through genetic transformation. By enabling the synthesis of GB, the transgenic plants improved their capacity to withstand different types of stress throughout germination and vegetative growth [10]. Tobacco was genetically modified to produce GB in vivo through the introduction of the betaine aldehyde dehydrogenase (BADH) gene. This altered tobacco exhibited enhanced photosynthesis when subjected to salt stress [11]. In addition, rice [12,13], tomato [14], and potato [15] were transferred by GB biosynthesis-related genes and successfully generated stress-tolerant transgenic plants.
Research in the area of plant physiology, biochemistry, genetics, and molecular biology has indicated that glycerol-3-phosphate (GB) plays a vital role in plant resilience to environmental stresses. However, the specific impact of GB on salt tolerance in forest tree species remains under-explored. Populus, a significant genus globally, is a key renewable resource prized by the pulp and paper industry for its rapid growth and high-quality fibers [16]. Moreover, salinity stress poses a severe challenge to poplar cultivation due to the extensive coastal regions in southeastern China. The hybrid clone Populus deltoides × P. euramericana “Nanlin 895” is widely planted across large areas of southeastern China, yet its photosynthesis and growth are compromised under salt stress conditions. Moreover, in Xu’s study, it was shown that Nanlin 895 poplar has a certain degree of salt tolerance, and 100 mM NaCl is the critical salt tolerance concentration. NaCl mainly affects the growth of Populus deltoides × P. euramericana “Nanlin 895” poplar group seedlings by inhibiting photosynthesis, increasing membrane permeability, etc. The effect on the leaves is relatively large, and the higher the salt concentration, the more serious the yellowing and abscission of the leaves of Nanlin 895 poplar group seedlings [17].
“Nanlin 895” is a new fast-growing and productive forest variety cultivated by Nanjing Forestry University, which has excellent biological characteristics and wide ecological adaptability, is more resistant to dryness and early barrenness, is resistant to salinity and alkalinity, grows well on inland saline and alkaline land, is highly resistant to adversity, has a high timber yield, a strong asexual reproduction ability, and a strong genetic stability. Although “Nanlin 895” has been widely introduced throughout the country, and there have been reports on transgenics, ectomycorrhizal mycorrhizae, sucrose metabolism, and transport, etc. [18,19,20,21,22,23], there have been few studies on the biological characteristics and resistance mechanism, which has limited the application and promotion of “Nanlin 895” and the progress of theoretical research. Therefore, research on the salt tolerance and the mechanism of salt tolerance of “Nanlin 895” can help the conventional breeding and promote the large-scale popularisation of the planting of “Nanlin 895” in salinized areas as poplar veneer industrial timber afforestation. The primary goals of this research were to evaluate the impact of GB foliar application on multiple aspects of poplar growth (including fresh and dry weights and lengths of roots and shoots), physiological responses (such as gas exchange and chlorophyll fluorescence), and biochemical markers (involving chlorophyll content and antioxidant enzyme activities) under both saline and non-saline conditions. This investigation has established a basis for subsequent research into the role of external GB in enhancing the stress tolerance of Populus species.
2. Materials and Methods
2.1. Plant Materials and Culture
Seedlings of poplar cultivar Populus deltoides × P. euramericana “Nanlin 895” were developed from the leaf explant cultured in MS medium. Young seedlings in good condition, about 15 cm high, were transferred to grow in a plastic pot (20 cm diameter and 15 cm height) with soil containing pearl stone and roseite. The ratio of soil, pearl stone, and roseite is 7:2:1. Transferred seedlings were grown for three months until used in experiments. An experiment using pots was carried out to investigate the impact of GB foliar spraying on the growth and physiochemical parameters of poplar plants under both normal (control) and salt stress conditions. To ensure that “Nanlin 895” grows under optimal conditions, the experiment took place in a greenhouse with controlled conditions, maintaining temperatures between 18–22 °C during the day and 16–18 °C overnight. Additionally, the relative air humidity was set at 40–60%, with plants receiving 16 h of cool white fluorescent light (~300 μmol·m−2·s−1). A volume of 10 mL GB was sprayed each time for each treatment, once every three days. Control plants were sprayed with water. The growth and physicochemical characteristics of the plants were measured and recorded after 14 days of foliar spraying with GB.
2.2. Endogenous GB Content
The GB levels were quantified using the method outlined by Park et al. [14]. After thoroughly washing with deionized water to remove any remaining GB from their surfaces, all samples were finely ground with liquid nitrogen. The parameters of the quantitative testing method are set as follows: mobile phase was composed of water and methanol with the ratio fixed to 93:7, containing 10 mmol ammonium formate, and the flow rate was 1 mL/min.
2.3. Shoot Growth and Leaf Biomass
The control and GB application plants were treated in a greenhouse with 0, 50, 100, and 150 mM NaCl solutions. The heights of all plants were measured on the 1st day before introducing NaCl stress and measured again on the 28th day after stress was introduced by adding the NaCl solution. The leaves in the middle of the plants with similar growth were selected on the 28th day. The leaves were rinsed with distilled water and dried. The leaves were collected with aluminum foil, and the samples were sterilized in an oven at 105 °C for 30 min. Then, the temperature was adjusted to 80 °C, and the leaves were baked to a constant weight, and the dry weight of the leaves was measured.
2.4. Measurements of Photosynthetic Parameters
Measurements of photosynthetic gas exchange parameters, including net photosynthetic rate (Pn) and stomatal conductance (Gs), were determined with fully expanded leaves using GFS-3000 photosynthesis equipment according to the manufacturer’s instructions (Walz, Effeltrich, Germany). The photosynthetic rate was assessed at a temperature of 20 °C under a relative humidity of 60% and white light (~300 μmol·m−2·s−1). The measurements lasted around 10 min until no significant recovery was observed. All measurements were carried out from 9:00 to 11:00 a.m. The determination and calculation of total chlorophyll content followed the method of Arnon (1949). Leaves were taken at a similar growth stage and were used to measure the chlorophyll content. The leaves were homogenized in liquid nitrogen with 10 mL 80% (v/v) acetone. The homogenate was incubated at 4 °C for 20 min, and then the supernatant was collected after centrifuging at 10,000× g for 10 min at 4 °C. Absorbance readings were taken using a BioDrop spectrophotometer at 663 and 645 nm (BioDrop, Cambridge, UK).
2.5. Assays of Antioxidant Enzyme Activities and Non-Enzyme Antioxidants
SOD and POD activities were measured using commercially available assay kits following the provided instructions (Jiancheng Bioengineering Institute, Nanjing, China). The experiment was conducted with three replicates for each treatment. The SOD activity was assessed using the xanthine and xanthine oxidase systems, with absorbance measurements taken at 560 nm. The enzyme activity was defined as a reduction in absorbance reading of samples of 50% compared with tubes without enzymes. The POD activity was determined using a guaiacol reaction catalyzing oxidation in the presence of H2O2, monitored at 420 nm. Following the manual, the MDA content was determined with a botanic MDA assay kit (Jiancheng Bioengineering Institute, Nanjing, China). The MDA content was determined by observing the absorbance at 532 nm, which reflects the conjugation of MDA with TBA.
2.6. Effect of Exogenous Application of GB on Expressions of GB Synthesis-Related Genes
After the treatments, total RNA was isolated using the RNeasy Plant Mini Kit (Qiagen, Beijig, China) from the leaves of control and salt-treated plants. For the assay of BADH expression, the primers were as follows: PtBADH-F (5′-TGGCGATCCATCTACCAATT-3′), PtBADH-R (5′-TTATAGCTTGGCGGGAGACT-3′). Moreover, for the CMO gene, primers were designed as PtCMO-F (5′-TGACTGCGTCTCTGCCTTTT-3′), PtCMO-R (5′-TATAGTGAAGCAATTGGTGGA-3′). The internal control primers, EF1α, were as follows: EF1α-F (5′-AGACCACCAAGTACTACTGCAC-3′), EF1α-R (5′-CCACCAATCTTGTACACATCC-3′). Real time-PCR was performed as follows: 95 °C for 3 min, 40 cycles of 45 s at 94 °C, 45 s at 56 °C, and 45 s at 72 °C. Followed by 10 min at 72°C. Reactions were conducted on an ABI step one plus system (Applied Biosystems, Foster City, CA, USA).
2.7. Statistical Analysis
The experiment consisted of 8 treatments with 10 replications for each treatment. Triplicate measurements were performed for each of these parameters, and each data point represents the mean value. ANOVA was used to compare physiological parameters between plants treated with GB and control plants under varying NaCl concentrations. Duncan’s test in SPSS 15 software was employed for mean separation (SPSS Inc., Chicago, IL, USA). Statistical significance was confirmed when the confidence intervals indicated non-overlapping mean values with a 0.05 error margin. Each experimental replicate consisted of one plant per pot.
3. Results
3.1. Effect of GB Application on Plant Growth
The effect of saline treatment and GB application on the growth of poplar plants was assessed by comparing shoot length obtained during four weeks of treatment (Figure 1). Applications of 5 mM and 15 mM GB helped plants grow faster than CK plants under normal conditions. However, the growth rates were not significantly different due to the GB application. Moreover, the 25 mM GB application ceased the growth of the application plants. The adverse effects were more evident under strengthened NaCl stress. Saline treatment had a strong effect on both control and GB application plants, reducing the growth of plants when treated with 50 mM NaCl, while the leaves of all applications of 15 mM GB plants still produced only a slight increase in shoot length. When treated with 100 mM NaCl, the leaves of all plants turned yellow, and the length of the plants obviously decreased. The shoot length of CK plants decreased by 16.60%, which was more significant than the reduction of 6.74% in the 15 mM GB application plants. At 150 mM NaCl, the shoot length of all plants was significantly reduced. The plants with a 25 mM GB application withered under the 100 and 150 mM NaCl treatments for three weeks.
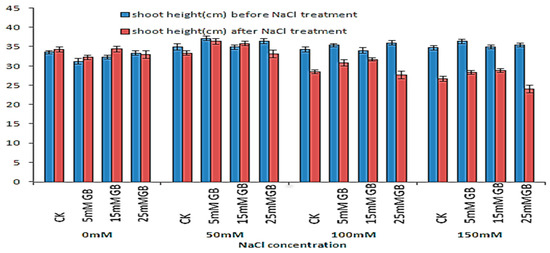
Figure 1.
Shoot growth of GB-applied poplars under NaCl stresses for four weeks. Differences in shoot growth between CK and GB-treated poplars were affected by different concentrations of GB and NaCl. CK, control plants. SE bars are indicated.
Due to the significant damage to leaves and growth differences observed throughout the plant under high concentrations of NaCl and GB application, only leaf growth at low concentrations treatments was measured in later experiments (Figure 2). The results of leaf dry biomass showed no significant difference in the effect of applying 5 mM or 15 mM concentrations of GB on leaf growth under the same concentration of NaCl treatments. However, when comparing the leaf growth under different NaCl treatment conditions, there were significant differences in leaf dry weight between the CK and 5 mM GB application experiments. This difference did not occur in the comparative experiment of 15 mM GB application, indicating that the application of 15 mM GB can effectively alleviate the stress hazards of high salt. Higher concentrations of GB (25 mM) resulted in a complete cessation of growth, which was also observed in leaves.
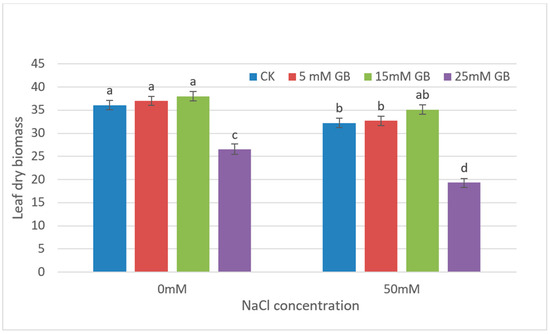
Figure 2.
Leaf dry biomass of GB-applied poplars under NaCl stresses for four weeks. Differences in leaf dry biomass between CK and GB-treated poplars were affected by different concentrations of GB and NaCl. CK, control plants. Different lowercase letters represent significant differences at the 0.05 level (p < 0.05).
3.2. Effects of Exogenous GB on Photosynthetic Parameters
In the conducted experiments, the exogenous application of GB affected the net photosynthetic rate (Pn) and stomatal conductance (Gs) of poplar. Under normal conditions, the photosynthetic rate increased in plants except those sprayed with 25 mM GB, indicating a negative impact on rates under salt stress where Pn significantly decreased compared with the unstressed control group. The application of 15 mM GB could retard the decrease in Pn (Figure 3a). However, the delay effect became weaker. As shown in Figure 3b, salt-stressed poplar plants had significantly reduced stomatal conductance (Gs). Similarly, the application of GB (15 mM) could retard the decline in Gs in poplar plants under salt stress. At the same time, the application of 5 mM GB did not significantly affect the Pn and Gs, and a higher concentration of GB (25 mM) exacerbated the damage to the photosynthesis of poplar plants.
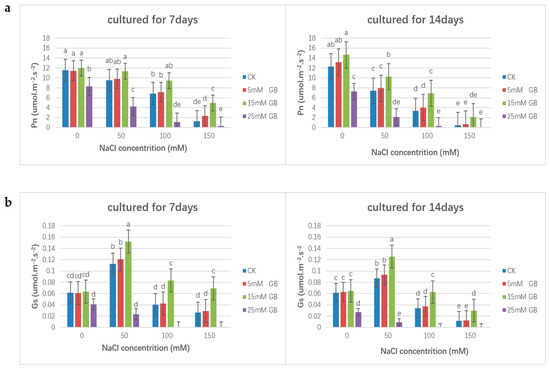
Figure 3.
Comparisons of photosynthetic pigment levels of GB applied poplars under salinity within two weeks. (a) The effect of exogenous application of GB on the net photosynthetic rate (Pn) of poplars. (b) The effect of exogenous application of GB on the stomatal conductance (Gs) of poplars. Different lowercase letters represent significant differences at the 0.05 level (p < 0.05).
3.3. Real-Time qPCR Results of the CMO and BADH Genes
GB is produced through the dual-stage oxidation process of choline, and in higher plants, two key genes, CMO and BADH, catalyze the reactions. The expression pattern of these genes with and without salt stress was analyzed in real-time qPCR. Under normal circumstances, the BADH gene of 5 mM GB application plants showed a slightly higher expression level. With the same treatment, the CMO gene showed a similar expression pattern (Figure 4a,b). In contrast, BADH and CMO gene expression showed big fluctuations under higher GB concentration applications. With 15 mM GB application, the BADH gene showed significant down-regulation after 24 h and then returned to normal. In addition, with 25 mM GB application, the expression of the BADH gene became down-regulating for 24 h after GB application (Figure 4a). For the CMO gene, expression levels showed constant up-regulation 24 h after GB application in both the 15 and 25 mM treatment plants (Figure 4b). Under the 50 mM NaCl treatment (Plants treated with 100 mM and 150 mM NaCl were excluded from measurements due to severe leaf damage post 25 mM GB application), both BADH and CMO genes in CK and plants applied with 5 and 15 mM GB increased at first for 24 h and then declined as time went on (Figure 4d,e).
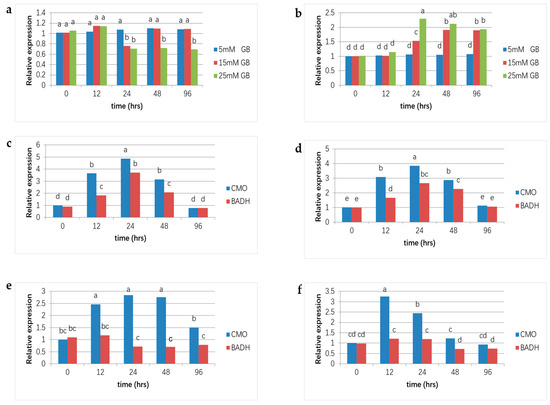
Figure 4.
Gene expression analyses of CMO and BADH genes in Nanlin 895 poplars. The fold change in gene expression is shown as the ratio of the relative gene expression of each sample to that in untreated leaves, and values are the mean and standard error of three biological replicates. Different lowercase letters represent significant differences at the 0.05 level (p < 0.05). (a) CMO gene expression under normal conditions with different concentrations of GB applications; (b) BADH gene expression under normal conditions with concentrations of GB applications; (c–f) expressions of CMO and BADH genes under 50 mM NaCl stress with 0, 5, 15, and 25 mM GB applications, respectively.
Nevertheless, for plants treated with 25 mM GB, the decline in BADH and CMO appeared 12 h after the GB application (Figure 4f). In addition, the CMO gene had significantly higher expression values than the BADH gene. The normal conditions showed that the GB application regulated expressions of BADH and CMO. Moreover, data obtained under NaCl stress showed that the variety of expressions of BADH and CMO were approximative between CK and 15 mM GB application plants (Figure 4c,e), which suggested GB application at a suitable concentration could develop salt tolerance in poplar plants.
3.4. Chlorophyll Content
As shown in Figure 5, from 0 to 150 mM NaCl, the chlorophyll contained sustained losses in CK and GB-applying plants. Compared with the non-stressed control, chlorophyll content was decreased by 42.3% at 150 mM in CK plants, and the decrease was smaller in plants with foliar-applied 5 mM and 15 mM GB (31.0% and 22.1%, respectively). With the same decreasing trend, the chlorophyll content measured by foliar-applied 25 mM GB showed a significantly lower level (59.1%).
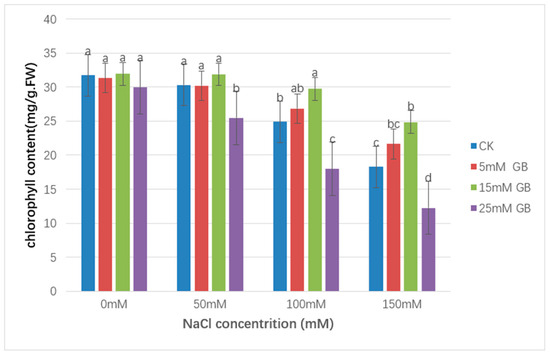
Figure 5.
Effect of GB on the chlorophyll content in poplar leaves under NaCl stress. NaCl treatment decreased chlorophyll content in poplar plants under saline treatment. Chlorophyll was higher in the 15 mM GB application plants than in other lines. 25 mM of GB accumulation intensified the negative effect of salt stress. Different lowercase letters represent significant differences at the 0.05 level (p < 0.05).
3.5. MDA Content Keeps Increasing
Under salt stress, a continuous increase in MDA content was observed. Compared with CK plants, foliar application of GB with 5 and 15 mM concentrations was able to retard the increase, and no significant difference in the effect existed between the two concentrations (Figure 6a). Contrary to the lower concentration application, the 25 mM GB application made the plants suffer a higher MDA content.
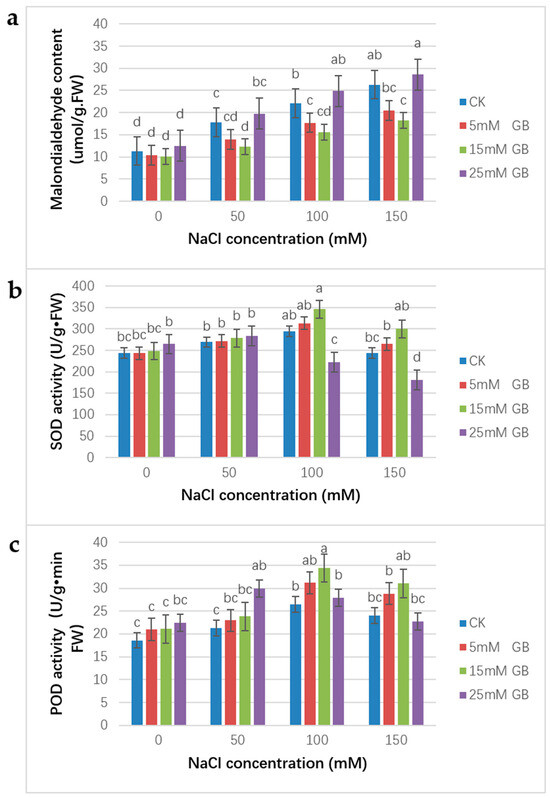
Figure 6.
Effect of GB on the MDA, SOD, and POD content in poplar leaves under NaCl stress. Poplar plants were foliar sprayed with 0, 5, 15, and 25 mM GB. Plants were grown in pots in the greenhouse, supplemented with different concentrations of NaCl for 15 days. (a) MDA activity; (b) SOD activity; (c) POD content. Different lowercase letters represent significant differences at the 0.05 level (p < 0.05).
3.6. SOD and POD Activities
All plants had similar SOD activities under normal conditions. Moreover, POD activities in GB application plants were slightly higher than in CK plants under normal conditions. Then, as shown in Figure 6, the SOD and POD activities experienced an increased period from the 0 mM to 100 mM NaCl concentration. Under the 100 mM NaCl treatment, the SOD activities were 1.07- and 1.18-fold higher in the 5 and 15 mM GB application plants compared with CK (Figure 6b). With the same treatment, the POD activities were 1.18- and 1.30-fold higher in the 5 and 15 mM GB application plants compared with CK (Figure 6c). Then, both the SOD and POD activities declined under the 150 mM NaCl treatment. Even though higher NaCl concentrations resulted in lower SOD and POD activities, SOD and POD activities would still be higher in the 5 and 15 mM GB applied plants compared with the control plants, however, than in the CK plants. The SOD activities were 1.23-fold higher, and the POD activities were 1.29-fold higher in the 25 mM GB application plants compared with CK plants. These results demonstrate that salt tolerance was enhanced in plants treated with 5 and 15 mM GB in comparison to the control plants. Unlike plants with lower concentrations of GB application, the SOD activity of plants with a 25 mM GB application began to decline at a 50 mM NaCl concentration. The same situation was observed with POD activity in the 25 mM GB application plants. This result means that higher concentrations of GB might be toxic to the plants.
4. Discussion
Stress conditions trigger diverse biochemical and physiological reactions in plants, impacting biomass production and growth negatively. Abiotic stressors such as high salt, low temperature, and drought can trigger oxidative bursts in plant cells. When plants experience unfavorable environmental conditions, plant growth is a reliable and easy parameter to measure how they respond to these conditions. Our experiments revealed that the exogenous application of GB affected the growth of Nanlin 895 plants to some extent after experiencing salt stress.
Plants sprayed with 5 mM and 15 mM GB showed slightly increased growth rates under non-stress conditions, with no significant differences observed between the control and GB-treated plants. In addition, shoot length showed cessation with a 25 mM GB application. Applying GB at concentrations higher than 25 mM causes a growth reduction in Nanlin 895 poplars. The same result was reported in field-grown grapevines with 25 mM GB [24]. Higher concentrations of GB (100 mM and 200 mM) halted growth entirely, leading to severe leaf damage, as noted by Mickelbart et al. [25]. These different concentration levels may be due to cultivar differences and specific condition effects. This suggested that other poplar cultivars could bear higher concentrations in GB applications. Under normal conditions, the application of 5 and 15 mM GB had no significant effect on the above-ground growth of Nanlin 895, while the application of GB on the foliage under salt stress conditions helped to improve the salt tolerance of Nanlin 895. Although plant growth began to decrease under the other salinity treatments, the application of 15 mM GB still increased branch length under 50 mM NaCl stress. When treated with 100 mM NaCl, the shoot length of the 15 mM GB application plants decreased by 6.74%, significantly lower than the reduction of 16.60% in CK plants. However, the growth of all plants was obviously damaged by the strengthened NaCl stress. Because plant tolerance is related to low Na+ uptake [2], the results suggested that exogenously applied GB did not effectively reduce the absorption of sodium ions in poplar leaves under high NaCl stress. Reactive oxygen species (ROS) are produced even when plants grow under normal conditions.
Abiotic stresses such as high salinity, low temperature, and drought may all lead to an oxidative burst in plant cells. It is understood that GB can help prevent ROS accumulation and protect plants from oxidative damage. Externally applied GB notably reduced H2O2 production induced by chilling in tomato plants. Furthermore, the medium contains 5 mM of GB, which aided Arabidopsis in mitigating oxidative stress effects [26]. In our study, foliar applications of GB at 5 and 15 mM concentrations could retard this increase and maintain a lower MDA content. MDA levels can serve as an indicator to assess plant tolerance to oxidative stress [27]. The lower MDA content suggested that GB-applied plants possess increased oxidative stress tolerance.
Moreover, 15 mM GB applied significantly improved the activity of SOD and POD under 100 mM NaCl stress. The activities of SOD and POD are crucial in scavenging ROS, contributing to improved salt-induced oxidative stress tolerance. The result showed that GB mitigated the adverse effects of salinity by modulating the activities of antioxidant enzymes.
Prolonged exposure to NaCl stress decreases chlorophyll contents and photosynthetic rate [28]. In this study, salt stress, especially at 100 and 150 mM NaCl concentrations, significantly lowered gas exchange parameters such as Pn and Gs. However, foliar spraying with GB increased Pn and Gs under normal conditions. In addition, these two parameters could maintain a higher level due to a 15 mM GB application under NaCl stress. Similarly to our result, Studies have indicated that external application of GB led to increased stomatal conductance, enhanced photosynthetic rate, and improved salt stress tolerance in field-grown tomato plants [29]. Raza et al. [30] reported that the exogenous application of GB to wheat increased the photosynthetic rate under NaCl stress. Moreover, research indicates that externally applying GB enhances all gas exchange characteristics of maize under NaCl-induced stress [31]. Our study also showed that applying 5 and 15 mM GB was more effective than CK in preventing chlorophyll loss with increasing NaCl concentration. All these above data demonstrate the effective role of GB in stabilizing photosynthesis.
The previous studies indicated that exogenous GB regulates the expression of specific genes, and the products of these genes are involved in the development of resistance. When a foliar spray of 250 mM GB was applied to wheat plants, an accumulation of transcripts for two cold-responsive proteins was observed [32]. The application of exogenous GB in tomato plants resulted in heightened catalase activity and elevated levels of cat1 gene transcripts under chilling stress conditions. GB is produced from choline through a two-step enzymatic reaction. In poplar plants, the reaction is catalyzed by CMO and BADH genes.
Moreover, in the current study, expressions of the CMO and BADH genes were affected by exogenous GB under normal conditions, whereas the application of 25 mM GB enhanced the expression of CMO and reduced the expression of BADH. Moreover, expression of the CMO gene was also up-regulated by 15 mM GB under normal conditions. With the same treatment, BADH gene expression decreased at the 24 h point and recovered to normal. GB was certified to take part in the process that regulated the expression of the CMO and BADH genes. Research has demonstrated that a concentration of 15 mM GB is effective in enhancing salinity stress tolerance in Nanlin 895 poplar trees. The expression of the CMO and BADH genes under 50 mM NaCl stress maintained a similar level to that under normal conditions for a 15 mM GB application.
5. Conclusions
In summary, foliar spray GB plays a protective role in shoot growth, photosynthetic pigments such as chlorophyll content, Pn, and Gs, and activities of POD and SOD. Of various GB levels, 15 mM showed more effectiveness in enhancing the growth and tolerance of poplar plants under NaCl stress. Involved in GB-mediated mechanisms of salt stress tolerance, which include protecting photosynthetic machinery, scavenging reactive oxygen species, and inducing specific gene expression. However, high salinity (150 mM NaCl) conditions reduced growth in Nanlin 895 poplars, and no significant improvement could be observed with the foliar application of GB. Moreover, on the other side, a higher concentration of GB (25 mM) could cause an adverse effect on Nanlin 895 poplars. This study provides information that may be useful for improving the tolerance of wood plants to salt stress. Furthermore, the external application of GB may have additional benefits in enhancing the stress tolerance of poplar trees. Further work on the exogenous GB-regulated expression of special genes is needed to establish transgenic poplars that accumulate GB at varying levels.
Author Contributions
F.C. designed the experiments, drafted the manuscript, and funded this study. A.M. reviewed and commented. F.C. and H.W. performed experiments. W.S. and Q.Z. supervised this research. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Innovation Foundation of Nanjing Forestry University (CX2019030) and the State Forestry Bureau Science and Technology Development Center Program of China (KY201800618).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salt tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to Environmental Stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Murata, N. Regulation of the desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr. Opin. Microbiol. 2002, 5, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.H.; Murata, N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011, 34, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Jeknic, Z.; Chen, T.H.H. Exogenous application of glycinebetaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006, 47, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Ashraf, M.; Ali, Q.; Perveen, R. Response of salt stressed okra (Abelmoschus esculentus Moench) plants to foliar-applied glycine betaine and glycine betaine containing sugarbeet extract. S. Afr. J. Bot. 2012, 83, 151–158. [Google Scholar] [CrossRef]
- Cruz, F.J.; Castro, G.L.; Silva, J.D.D.; Festucci-Buselli, R.A.; Pinheiro, H.A. Exogenous glycine betaine modulates ascorbate peroxidase and catalase activities and prevent lipid peroxidation in mild water-stressed Carapa guianensis plants. Photosynthetica 2013, 51, 102–108. [Google Scholar] [CrossRef]
- Zhang, Q.; Rue, K.; Mueller, J. The Effect of Glycinebetaine Priming on Seed Germination of Six Turfgrass Species under Drought, Salinity, or Temperature Stress. Hortscience 2014, 49, 1454–1460. [Google Scholar] [CrossRef]
- Sulpice, R.; Tsukaya, H.; Nonaka, H.; Mustardy, L.; Chen, T.H.H.; Murata, N. Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the synthesis of glycine betaine. Plant J. 2003, 36, 165–176. [Google Scholar] [CrossRef]
- Yang, X.; Liang, Z.; Wen, X.; Lu, C. Genetic engineering of the biosynthesis of glycinebetaine leads to increased tolerance of photosynthesis to salt stress in transgenic tobacco plants. Plant Mol. Biol. 2008, 66, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Kathuria, H.; Ferjani, A.; Sakamoto, A.; Mohanty, P.; Murata, N.; Tyagi, A.K. Transgenics of an elite indica rice variety Pusa Basmati 1 harbouring the codA gene are highly tolerant to salt stress. Theor. Appl. Genet. 2002, 106, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Hirji, R.; Zhang, L.; He, C.; Selvaraj, G.; Wu, R. Evaluation of the stress-inducible production of choline oxidase in transgenic rice as a strategy for producing the stress-protectant glycine betaine. J. Exp. Bot. 2006, 57, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Jeknić, Z.; Sakamoto, A.; DeNoma, J.; Yuwansiri, R.; Murata, N.; Chen, T.H.H. Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J. 2004, 40, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Kim, M.D.; Back, K.-H.; Kim, H.-S.; Lee, H.-S.; Kwon, S.-Y.; Murata, N.; Chung, W.-I.; Kwak, S.-S. Stress-induced expression of choline oxidase in potato plant chloroplasts confers enhanced tolerance to oxidative, salt, and drought stresses. Plant Cell Rep. 2008, 27, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, R.H.; Holmqvist, B.H.; Cowling, S.A.; Sykes, M.T. The effects of climate change on the distribution and management of Picea abies in southern Scandinavia. Can. J. For. Res. 2000, 30, 1992–1998. [Google Scholar] [CrossRef]
- Xu, C. Study on salt-tolerance and mechanism for salt-resistance of ‘Nanlin 895′ tissue culture plantlets. Nanjing For. Univ. China 2012, 55, 17. [Google Scholar]
- Zhang, K. New fast-growing high-quality poplar varieties—‘Nanlin 95’ and ‘Nanlin 895’. Agric. Knowl. 2004, 1–16. [Google Scholar]
- Zhuge, Q.; Fang, D.; Li, Q.; Sun, W.; Huang, M.; Wang, M. Transformation of Populus×euramericana cv. ‘Nanlin 895’ Using Bt and CpTI Insect-resistant Genes. Mol. Plant Breed. 2006, 6, 819–824. [Google Scholar]
- Xiao, X.; Wu, L.; Tang, Y.; Wu, M.; Bian, G.; Luo, P. The compare experiments on 18 strains of Populus in lake area. Hunan For. Sci. E Technol. 2008, 3, 20–22. [Google Scholar]
- Yang, C.; Li, H.; Cheng, Q.; Chen, Y. Transformation of Drought and Salt Resistant Gene (DREB1C) in Populus × euramericana cv.’Nanlin 895’. Sci. Silvae Sin. 2009, 45, 17–21. [Google Scholar]
- Wang, L.; Yin, Z.; Ma, Q.; Xu, M. Effects of exogenous Ca2+ on the photosynthesis and the changes of growth in Populus×euramericana Nanlin895 cuttings. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2010, 34, 23–27. [Google Scholar]
- Song, W.; Wu, X. Effect of Ectomycorrhizal Fungi on Photosynthesis of Poplar NL-895. Acta Bot. Boreali-Occident. Sin. 2011, 31, 1474–1478. [Google Scholar]
- Wilson, S. Frost Management in Cool Climate Vineyards; Final Report to Grape and Wine; University of Tasmania: Tasmania, Australia, 2001. [Google Scholar]
- Mickelbart, M.V.; Chapman, P.; Collier-Christian, L. Endogenous levels and exogenous application of glycinebetaine to grapevines. Sci. Hortic. 2006, 3, 7–16. [Google Scholar] [CrossRef]
- Cuin, T.A.; Shabala, S. Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ. 2007, 30, 875–885. [Google Scholar] [CrossRef]
- Jain, M.; Mathur, G.; Koul, S.; Sarin, N.B. Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogea L.). Plant Cell Rep. 2001, 20, 463–468. [Google Scholar] [CrossRef]
- Cramer, G.R.; Nowak, R.S. Supplemental manganese improves the relative growth, net assimilation and photosynthetic rates of salt-stressed barley. Physiol. Plant. 1992, 84, 600–605. [Google Scholar] [CrossRef]
- Mäkelä, M.; Larjava, H.; Pirilä, E.; Maisi, P.; Salo, T.; Sorsa, T.; Uitto, V.J. Matrix metalloproteinase 2 (gelatinase A) is related to migration of keratinocytes. Exp. Cell Res. 1999, 251, 67–78. [Google Scholar] [CrossRef]
- Raza, S.H.; Athar, H.R.; Ashraf, M. Influence of exogenously applied glycinebetaine on the photosynthetic capacity of two differently adapted wheat cultivars under salt stress. Pak. J. Bot. 2006, 38, 341–351. [Google Scholar]
- Kausar, N.; Nawaz, K.; Hussain, K.; Bhatti, K.H.; Siddiqi, E.H.; Tallat, A. Effect of exogenous applications of glycine betaine on growth and gaseous exchange attributes of two maize (Zea mays L.) cultivars under saline conditions. World Appl. Sci. J. 2014, 29, 1559–1565. [Google Scholar]
- Allard, F.; Sarhan, F.; Houde, M. Betaine improves freezing tolerance in wheat. Plant Cell Physiol. 2000, 39, 1194–1202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).