Assessing the Flight Potential of the Four-Eyed Fir Bark Beetle Polygraphus proximus Blandford in Natural Conditions
Abstract
1. Introduction
2. Materials and Methods
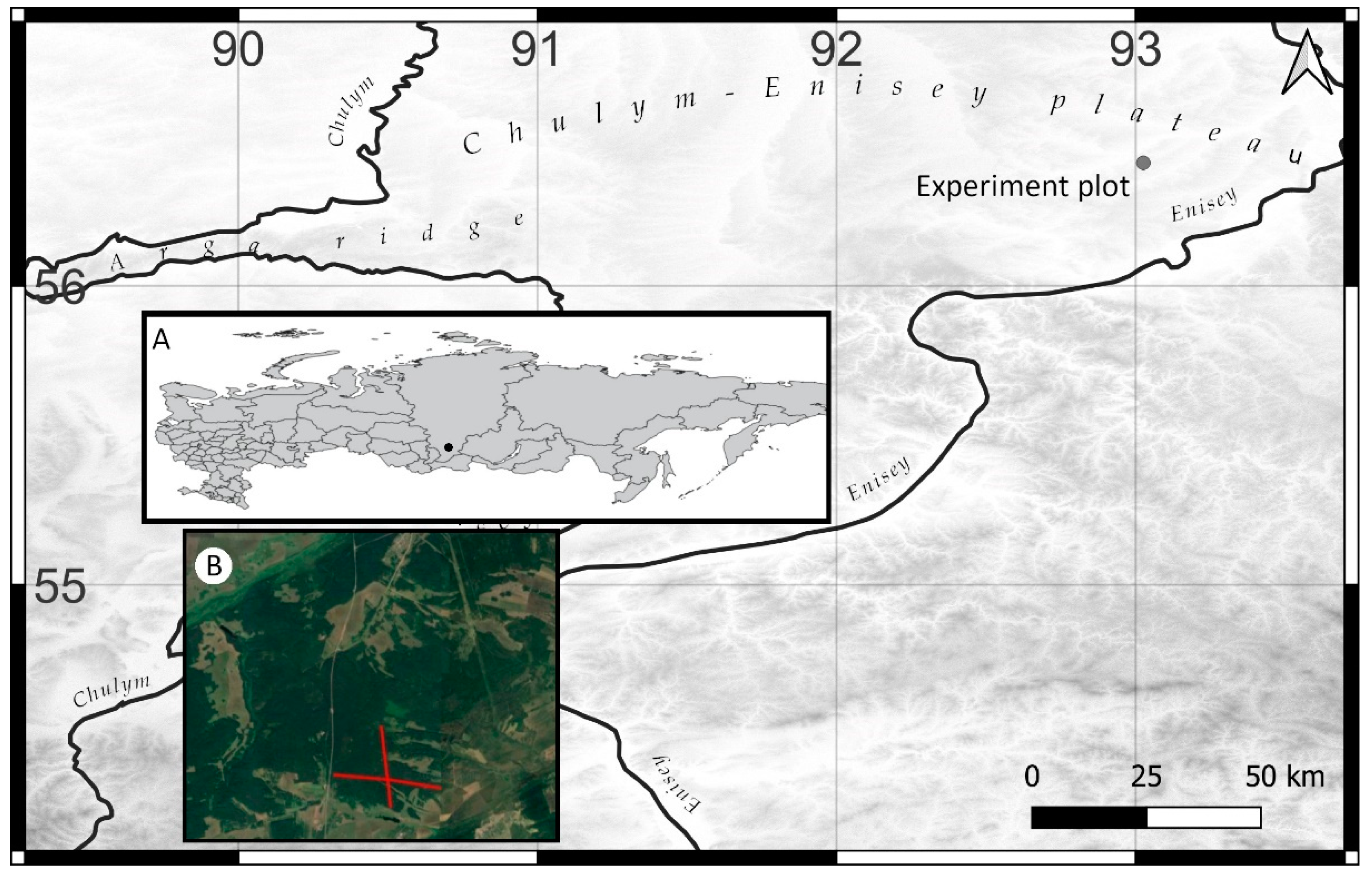
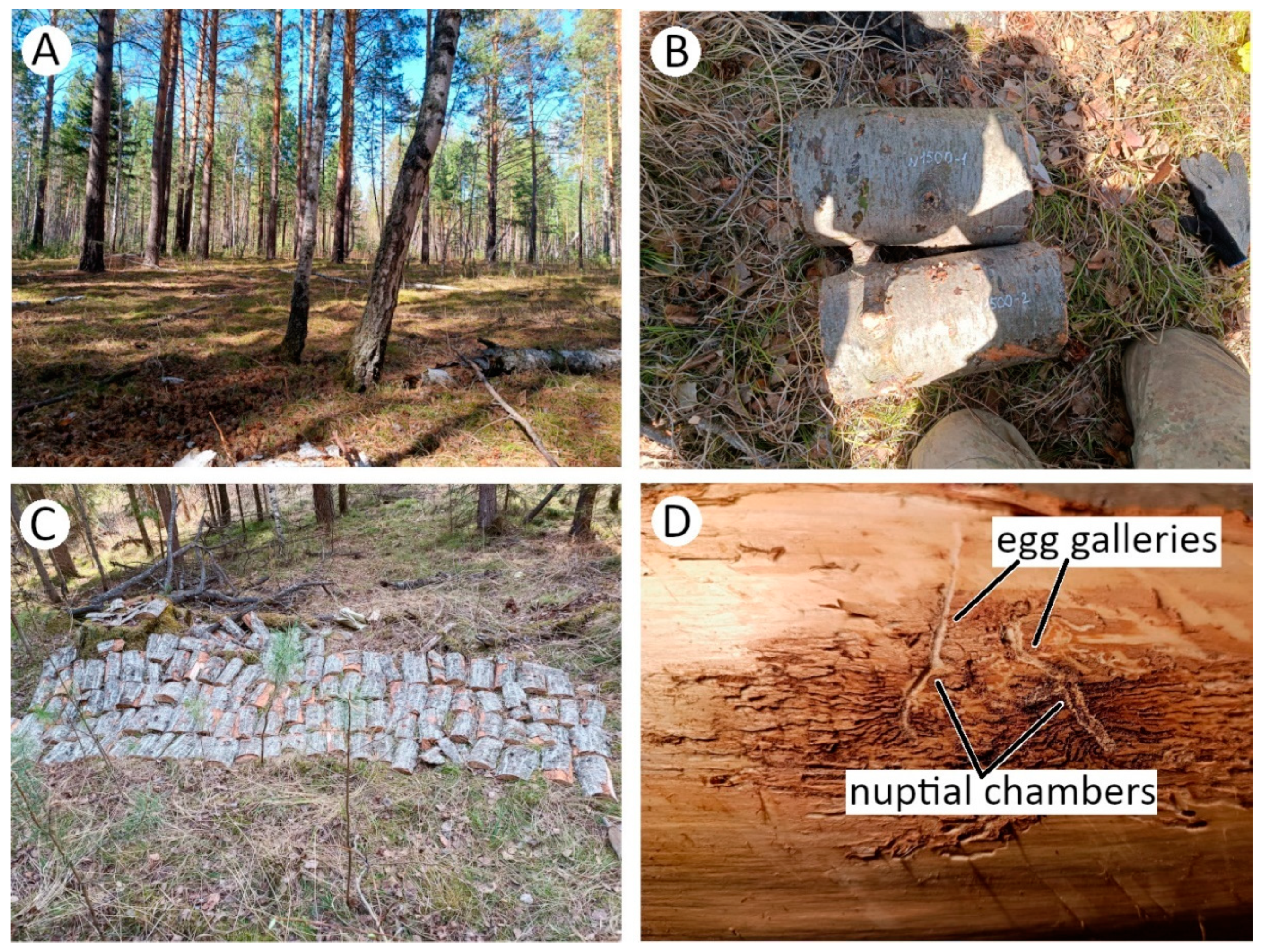
2.1. Study Site and Experimental Set Up
2.2. Statistical Processing and Modeling
3. Results
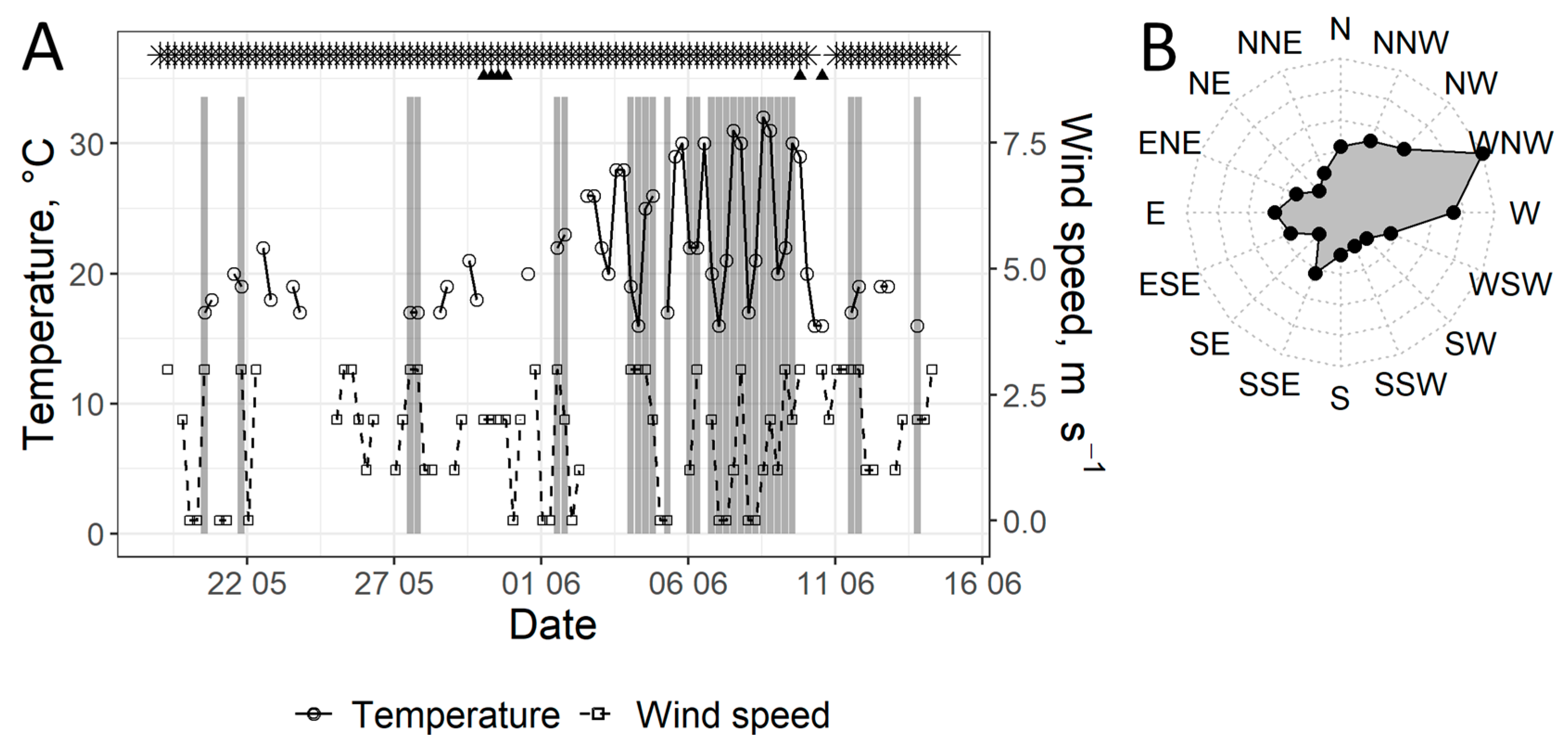
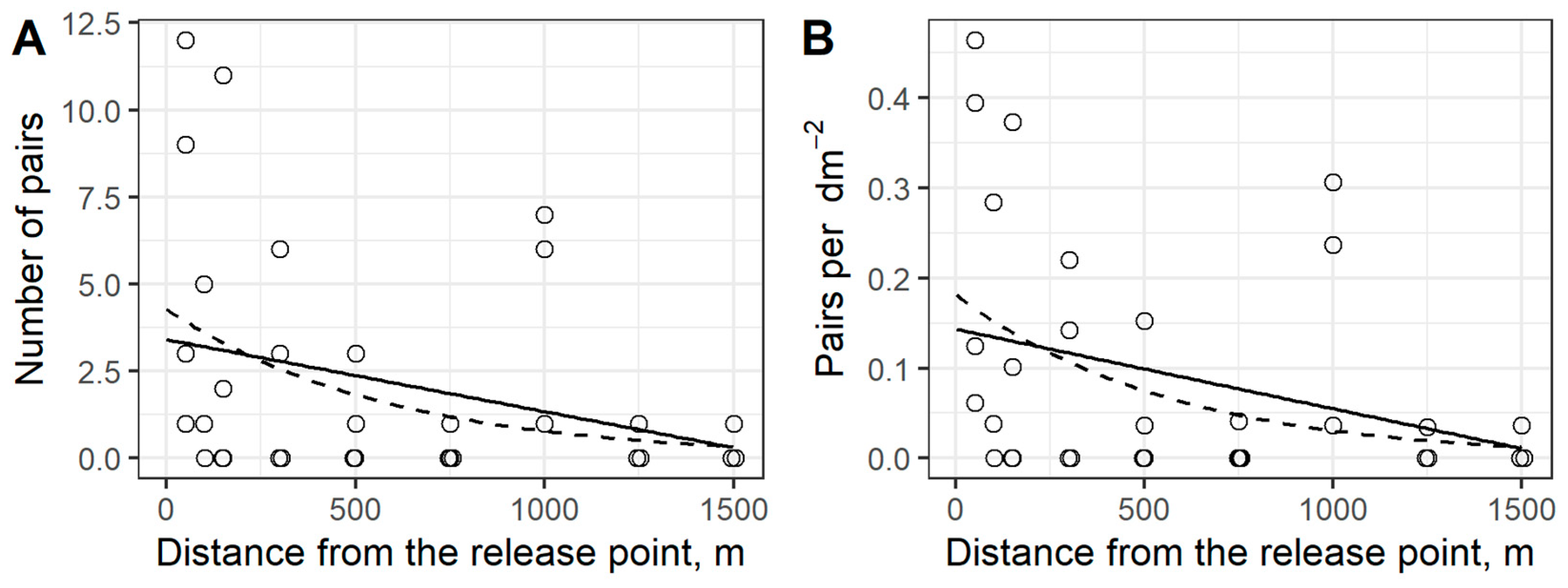
3.1. Analysis of Trap Logs
3.2. Modeling
4. Discussion
4.1. Eliminating Possible Sources of Result Distortion
4.2. The Impact of Wind Direction on the Flight of the Four-Eyed Fir Bark Beetle
4.3. Effectiveness of Attracting the Four-Eyed Fir Bark Beetle with Trap Logs
4.4. Flight Range of the Four-Eyed Fir Bark Beetle and Its Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aukema, J.; Mccullough, D.; Von Holle, B.; Liebhold, A.; Britton, K.; Frankel, S. Historical Accumulation of Nonindigenous Forest Pests in the Continental United States. BioScience 2010, 60, 886–897. [Google Scholar] [CrossRef]
- Izhevskiy, S.S.; Mozolevskaya, E.G. Agrilus planipennis Fairmaire on Moscow ash trees. Russ. J. Biol. Invasions 2008, 1, 20–25. [Google Scholar] [CrossRef]
- Santini, A.; Faccoli, M. Dutch Elm Disease and Elm Bark Beetles: A Century of Association. Ifor. Biogeosci. For. 2015, 8, 126. [Google Scholar] [CrossRef]
- Kerchev, I.A. Ecology of Four-Eyed Fir Bark Beetle Polygraphus Proximus Blandford (Coleoptera; Curculionidae, Scolytinae) in the West Siberian Region of Invasion. Russ. J. Biol. Invasions 2014, 5, 176–185. [Google Scholar] [CrossRef]
- Krivets, S.A.; Bisirova, E.M.; Debkov, N.M.; Kerchev, I.A. Organization and results of monitoring the state of Siberian fir in the area of invasion of four-eyed fir bark beetle in Tomsk oblast. In Proceedings of the Forest Ecosystems of Boreal Zone: Biodiversity, Bioeconomy, Ecological Risks; IF SB RAS: Krasnoyarsk, Russia, 2019; pp. 214–216. [Google Scholar]
- Bisirova, E.M.; Krivets, S.A. Dynamics of the state of Siberian fir tree stands damaged by the four-eyed fir bark beetle Polygraphus proximus Blandf. in Tomsk Oblast. Tomsk State Univ. J. Biol. 2018, 44, 118–140. [Google Scholar] [CrossRef]
- Yanovskij, V.M. Annotated list of scolytids (Coleoptera, Scolytidae) of North Asia. Entomol. Rev. 1999, 78, 327–362. [Google Scholar]
- Takagi, E.; Masaki, D.; Kanai, R.; Sato, M.; Iguchi, K. Mass Mortality of Abies veitchii Caused by Polygraphus proximus Associated with Tree Trunk Diameter in Japan. For. Ecol. Manag. 2018, 428, 14–19. [Google Scholar] [CrossRef]
- Baranchikov, Y.N.; Pet’ko, V.M.; Astapenko, S.A.; Akulov, E.N.; Krivets, S.A. For-eyed fir bark beetle—The new aggressive fir pest in Siberia. For. Bull. 2011, 12, 78–81. [Google Scholar]
- Krivets, S.A.; Kerchev, I.A.; Bisirova, E.M.; Demidko, D.A.; Pet’ko, V.M.; Baranchikov, Y.N. Distribution of four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera, Curculionidae: Scolytinae) in Siberia. Izv. St. Peterbg. Lesoteh. Akad. 2015, 211, 33–45. [Google Scholar]
- Baranchikov, Y.N.; Demidko, D.A.; Pet’ko, V.M.; Laptev, A.V. Dynamics of Siberian fir dieback in the outbreak area of the four-eyed fir bark beetle. For. Bull. 2014, 18, 132–138. [Google Scholar]
- Kononov, A.; Ustyantsev, K.; Blinov, A.; Fet, V.; Baranchikov, Y.N. Genetic Diversity of Aboriginal and Invasive Populations of Four-Eyed Fir Bark Beetle Polygraphus proximus Blandford (Coleoptera, Curculionidae, Scolytinae). Agric. For. Entomol. 2016, 18, 294–301. [Google Scholar] [CrossRef]
- Krivets, S.A.; Kerchev, I.A.; Bisirova, E.M.; Volkova, E.S.; Astapenko, S.A.; Efremenko, A.A.; Kosilov, A.Y.; Kudryavtsev, P.P.; Kuznetzova, Y.P.; Ponomarev, V.I.; et al. Overwiev of the Current Secondary Range of the Four-Eyed Fir Bark Beetle (Polygraphus proximus Blandford) in the Russian Federation. Russ. J. Biol. Invasions 2024, 17, 49–69. [Google Scholar] [CrossRef]
- Krivolutskaya, G.O. Bark beetles of Sakhalin; USSR Academy of Sciences Publishing: Moskow, Russia; Leningrad, Russia, 1958. [Google Scholar]
- Baranchikov, Y.N.; Pashenova, N.V.; Seraya, L.G. Botanical gardens and “sentinel trees” concept: Experimental evaluation of Far-Eastern pests and pathogens threat for European fir and ash species. In Proceedings of the Phytosanitary Expertise and Plant Protection Management: Modern Systems and Methods; RS-Disayn: Moskow, Russia, 2015; pp. 194–200. [Google Scholar]
- Demidko, D.A.; Pet’ko, V.M.; Pashenova, N.V.; Babichev, N.S.; Efremenko, A.A.; Pertsovaya, A.A.; Baranchikov, Y.N. Interactions of for-eyed fir bark beetle and fir sawyer beetle during Siberian fir colonization. In Proceedings of the Ecology and Evolution: New Challenges; Liberal Arts University—University for Humanities, Ekaterinburg, Russia, 1 April 2019; pp. 508–510. [Google Scholar]
- Liebhold, A.M.; Berec, L.; Brockerhoff, E.G.; Epanchin-Niell, R.S.; Hastings, A.; Herms, D.A.; Kean, J.M.; McCullough, D.G.; Suckling, D.M.; Tobin, P.C.; et al. Eradication of Invading Insect Populations: From Concepts to Applications. Annu. Rev. Entomol. 2016, 61, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Bieńkowski, A.O.; Orlova-Bienkowskaja, M.J. Alien Leaf Beetles (Coleoptera, Chrysomelidae) of European Russia and Some General Tendencies of Leaf Beetle Invasions. PLoS ONE 2018, 13, e0203561. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.P.W. Modelling the Spread of Invasive Species across Heteroheneous Landscapes. Ph.D. Thesis, Lincoln University, Lincoln, New Zealand, 2008. [Google Scholar]
- Jones, K.L.; Shegelski, V.A.; Marculis, N.G.; Wijerathna, A.N.; Evenden, M.L. Factors Influencing Dispersal by Flight in Bark Beetles (Coleoptera: Curculionidae: Scolytinae): From Genes to Landscapes. Can. J. For. Res. 2019, 49, 1024–1041. [Google Scholar] [CrossRef]
- Smitley, D.; Davis, T.; Rebek, E. Progression of Ash Canopy Thinning and Dieback Outward from the Initial Infestation of Emerald Ash Borer (Coleoptera: Buprestidae) in Southeastern Michigan. J. Econ. Entomol. 2008, 101, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Siegert, N.W.; McCullough, D.G.; Liebhold, A.M.; Telewski, F.W. Dendrochronological Reconstruction of the Epicentre and Early Spread of Emerald Ash Borer in North America. Divers. Distrib. 2014, 20, 847–858. [Google Scholar] [CrossRef]
- Kerchev, I.A.; Torchkova, D.A. UAV-estimation of four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera: Curculionidae: Scolytinae) flight activity in dark-coniferous forests. In Proceedings of the Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems; Insects and Other Invertebrates; Saint Petersburg State Forest Technical University: Saint Petersburg, Russia, 2018; Volume 1, p. 45. [Google Scholar]
- Leung, B.; Cacho, O.J.; Spring, D. Searching for Non-indigenous Species: Rapidly Delimiting the Invasion Boundary. Divers. Distrib. 2010, 16, 451–460. [Google Scholar] [CrossRef]
- Haack, R.A.; Petrice, T.R. Emerald Ash Borer Adult Dispersal. In Proceedings of the Emerald Ash Borer, Research and Technology Development Meeting, Morgantown, WV, USA, 30 September 2003; Forest Health Technology Enterprise Team: Morgantown, WV, USA; p. 10. [Google Scholar]
- McCullough, D.G.; Poland, T.M.; Cappaert, D. Dispersal of Emerald Ash Borer: A Case Study at Tipton, Michigan. In Proceedings of the Emerald Ash Borer, Research and Technology Development Meeting, Morgantown, WV, USA, 30 October 2003; Forest Health Technology Enterprise Team: Morgantown, WV, USA; pp. 6–7. [Google Scholar]
- Taylor, R.A.J.; Bauer, L.S.; Poland, T.M.; Windell, K.N. Flight Performance of Agrilus planipennis (Coleoptera: Buprestidae) on a Flight Mill and in Free Flight. J. Insect Behav. 2010, 23, 128–148. [Google Scholar] [CrossRef]
- Siegert, N.W.; Mercader, R.J.; McCullough, D.G. Spread and Dispersal of Emerald Ash Borer (Coleoptera: Buprestidae): Estimating the Spatial Dynamics of a Difficult-to-Detect Invasive Forest Pest. Can. Entomol. 2015, 147, 338–348. [Google Scholar] [CrossRef]
- Weslien, J.; Lindelöw, Å. Recapture of Marked Spruce Bark Beetles (Ips Typographus) in Pheromone Traps Using Area-Wide Mass Trapping. Can. J. For. Res. 1990, 20, 1786–1790. [Google Scholar] [CrossRef]
- Zumr, V. Dispersal of the Spruce Bark Beetle Ips typographus (L.) (Col., Scolytidae) in Spruce Woods1. J. Appl. Entomol. 1992, 114, 348–352. [Google Scholar] [CrossRef]
- Turchin, P.; Thoeny, W.T. Quantifying Dispersal of Southern Pine Beetles with Mark-Recapture Experiments and a Diffusion Model. Ecol. Appl. 1993, 3, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Holsten, E.H. Dispersal of the Spruce Beetle, Dendroctonus Rufipennis, and the Engraver Beetle, Ips Perturbatus, in Alaska; Pacific Northwest Research Station: Portland, OR, USA, 1997.
- Barclay, H.; Safranyik, L.; Linton, D.A. Trapping Mountain Pine Beetles Dendroctonus ponderosae (Coleoptera: Scolytidae) Using Pheromone-Baited Traps: Effects of Trapping Distance. J. Entomol. Soc. Br. Columbia 1998, 25, 25–32. [Google Scholar]
- Franklin, A.J.; Debruyne, C.; Grégoire, J.-C. Recapture of Ips typographus L. (Col., Scolytidae) with Attractants of Low Release Rates: Localized Dispersion and Environmental Influences. Agric. For. Entomol. 2000, 2, 259–270. [Google Scholar] [CrossRef]
- Dodds, K.J.; Ross, D.W. Sampling Range and Range of Attraction of Dendroctonus pseudotsugae Pheromone-Baited Traps. Can. Entomol. 2002, 134, 343–355. [Google Scholar] [CrossRef]
- Doležal, P.; Okrouhlík, J.; Davídková, M. Fine Fluorescent Powder Marking Study of Dispersal in the Spruce Bark Beetle, Ips typographus (Coleoptera: Scolytidae). Eur. J. Entomol. 2016, 113, 1–8. [Google Scholar] [CrossRef]
- Meurisse, N.; Pawson, S. Quantifying Dispersal of a Non-Aggressive Saprophytic Bark Beetle. PLoS ONE 2017, 12, e0174111. [Google Scholar] [CrossRef] [PubMed]
- Safranyik, L.; Linton, D.A.; Silversides, R.; McMullen, L.H. Dispersal of Released Mountain Pine Beetles under the Canopy of a Mature Lodgepole Pine Stand. J. Appl. Entomol. 1992, 113, 441–450. [Google Scholar] [CrossRef]
- Duelli, P.; Zahradnik, P.; Knizek, M.; Kalinova, B. Migration in Spruce Bark Beetles (Ips typographis L.) and the Efficiency of Pheromone Traps. J. Appl. Entomol. 1997, 121, 297–303. [Google Scholar] [CrossRef]
- Franklin, A.J.; Grégoire, J.-C. Flight Behaviour of Ips typographus L. (Col., Scolytidae) in an Environment without Pheromones. Ann. For. Sci. 1999, 56, 591–598. [Google Scholar] [CrossRef]
- Linton, D.A.; Safranyik, L.; McMullen, L.H.; Betts, R. Field Techniques for Rearing and Marking Mountain Pine Beetle for Use in Dispersal Studies. J. Entomol. Soc. Br. Columbia 1987, 84, 53–57. [Google Scholar]
- Safranyik, L.; Silversides, R.; McMullen, L.H.; Linton, D.A. An Empirical Approach to Modeling the Local Dispersal of the Mountain Pine Beetle (Dendroctonus ponderosae Hopk.) (Col., Scolytidae) in Relation to Sources of Attraction, Wind Direction and Speed. J. Appl. Entomol. 1989, 108, 498–511. [Google Scholar] [CrossRef]
- Evenden, M.L.; Whitehouse, C.M.; Sykes, J. Factors Influencing Flight Capacity of the Mountain Pine Beetle (Coleoptera: Curculionidae: Scolytinae). Environ. Entomol. 2014, 43, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Jactel, H. Dispersal and Flight Behaviour of Lps sexdentatus (Coleoptera: Scolytidae) in Pine Forest. Ann. For. Sci. 1991, 48, 417–428. [Google Scholar] [CrossRef]
- Krivets, S.A.; Kerchev, I.A.; Bisirova, E.M.; Pashenova, N.V.; Demidko, D.A.; Pet’ko, V.M.; Baranchikov, Y.N. Four-Eyed Fir Bark Beetle in Siberian Forests (Distribution, Biology, Ecology, Detection and Survey of Damaged Stands); UMIUM: Krasnoyarsk, Russia, 2015; ISBN 978-5-9907381-0-2. [Google Scholar]
- Efremenko, A.A.; Demidko, D.A. Optimal density of the nests of four-eyed fir bark beetle in open-air and laboratory conditions. In Proceedings of the Ecology of Southern Siberia and Adjacent Regions; Khakassian State University: Abakan, Russia, 2018; Volume 1, pp. 58–59. [Google Scholar]
- Takagi, E.; Aochi, M. Cutting into Short Logs Reduces Infestation by the Bark Beetle Polygraphus proximus. J. Appl. Entomol. 2024, 148, 723–726. [Google Scholar] [CrossRef]
- Baranchikov, Y.N.; Demidko, D.A.; Pet’ko, V.M. The moisture of host tree’s bark and the probability of spring-summer four-eyed fir bark beetle flight. In Proceedings of the Dendrobiotic Invertebrates and Fungi and Their Role in Forest Ecosystems; Insects and Other Invertebrates; Saint Petersburg State Forest Technical University: Saint Petersburg, Russia, 2016; p. 5. [Google Scholar]
- Weather Schedule. Available online: https://rp5.ru/ (accessed on 15 November 2023).
- Bacchi, B.; Kottegoda, N.T. Identification and Calibration of Spatial Correlation Patterns of Rainfall. J. Hydrol. 1995, 165, 311–348. [Google Scholar] [CrossRef]
- Isaev, A.S.; Palnikova, E.N.; Soukhovolsky, V.G.; Tarasova, O.V. Population Dynamics of Forest Insects: Models and Predictions; KMK Publishing House: Moskow, Russia, 2015; ISBN 978-5-9907157-6-9. [Google Scholar]
- Kerchev, I.A. On Monogyny of the Four-Eyed Fir Bark Beetle Polygraphus proximus Blandf. (Coleoptera, Curculionidae: Scolytinae) and Its Reproductive Behavior. Entomol. Rev. 2014, 94, 1059–1066. [Google Scholar] [CrossRef]
- Köbayashi, K.; Takagi, E. Mating Systems of the Tree-Killing Bark Beetle Polygraphus proximus (Coleoptera: Curculionidae: Scolytinae). J. Insect Sci. 2020, 20, 38. [Google Scholar] [CrossRef]
- Takagi, E.; Masaki, D.; Köbayashi, K.; Takei, S. Trunk Diameter Influences Attack by Polygraphus Proximus and Subsequent Mortality of Abies veitchii. For. Ecol. Manag. 2021, 479, 118617. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Muirhead, J.R.; Leung, B.; van Overdijk, C.; Kelly, D.W.; Nandakumar, K.; Marchant, K.R.; MacIsaac, H.J. Modelling Local and Long-Distance Dispersal of Invasive Emerald Ash Borer Agrilus planipennis (Coleoptera) in North America. Divers. Distrib. 2006, 12, 71–79. [Google Scholar] [CrossRef]
- Kovacs, K.F.; Haight, R.G.; McCullough, D.G.; Mercader, R.J.; Siegert, N.W.; Liebhold, A.M. Cost of Potential Emerald Ash Borer Damage in U.S. Communities, 2009–2019. Ecol. Econ. 2010, 69, 569–578. [Google Scholar] [CrossRef]
- Grothendieck, G. Nls2: Non-Linear Regression with Brute Force. Available online: https://cran.r-project.org/web/packages/nls2/nls2.pdf (accessed on 24 July 2024).
- Elzhov, T.V.; Mullen, K.M.; Spiess, A.-N.; Bolker, B. Minpack.Lm: R Interface to the Levenberg-Marquardt Nonlinear Least-Squares Algorithm Found in MINPACK, Plus Support for Bounds. Available online: https://cran.r-project.org/web/packages/minpack.lm/minpack.lm.pdf (accessed on 24 July 2024).
- Viklund, L.; Baranchikov, Y.; Schroeder, M.; Efremenko, A.; Demidko, D.; Hedenström, E. Identification of Sex-Specific Compounds in the Invasive Four-Eyed Fir Bark Beetle Polygraphus proximus. Chemoecology 2022, 32, 183–195. [Google Scholar] [CrossRef]
- Byers, J.A. Behavioral Mechanisms Involved in Reducing Competition in Bark Beetles. Ecography 1989, 12, 466–476. [Google Scholar] [CrossRef]
- Krivets, S.A. Notes on the ecology of the fir bark beetle Polygraphus proximus Blandf. (Coleoptera, Scolytidae) in West Siberia. Izv. St. Peterbg. Lesoteh. Akad. 2012, 200, 94–105. [Google Scholar]
- Krivets, S.A.; Bisirova, E.M.; Debkov, N.M.; Kerchev, I.A.; Mel’nik, M.A.; Nikiforov, A.N.; Chernova, N.A. The Technology of Four-Eyed Fir Bark Beetle Monitoring in the Invasion Zone in Siberia; UMIUM: Tomsk, Russia, 2018. [Google Scholar]
- Krivets, S.A.; Bisirova, E.M.; Kerchev, I.A.; Pats, E.N.; Simonova, G.V. The State of Four-Eyed Fir Bark Beetle Population and Its Role in the Forests of the Northeastern Part of the “Kuznetsk Alatau” Reserve (Kemerovo Oblast). Izv. St. Peterbg. Lesoteh. Akad. 2019, 228, 7–28. [Google Scholar]
- Mizeeva, A.S.; Titova, K.G.; Krivets, S.A. The Four-Eyed Fir Bark Beetle Polygraphus proximus Bladford (Coleoptera, Scolytidae) Influence on Stands of Siberian Fir Vitality in Tomsk Sity. In Proceedings of the Ecological and Economical Consequences of Dendrophilc Insects Invasions; IF SB RAS: Krasnoyarsk, Russia, 2012; pp. 65–68. [Google Scholar]
- Kobzar, V.F.; Kolesova, N.I.; Petrik, A.A. Polygraphus proximus Blandford, 1894 in the fir forests of the “Lakes on Snezhnaya” Ecopark (Irkutsk Oblast). Plant Health Quar. 2023, 1, 59–71. [Google Scholar]
- Baranchikov, Y.N.; Demidko, D.A.; Efremenko, A.A. Males of the Invasive Bark Beetle Polygraphus Proximus Blandford (Coleoptera, Curculionidae, Scolytinae) as a Source of Aggregation Pheromone: Results of a Field Experiment. Izv. St. Peterbg. Lesoteh. Akad. 2023, 244, 200–212. [Google Scholar]
- Taylor, R.A.J. The Relationship between Density and Distance of Dispersing Insects. Ecol. Entomol. 1978, 3, 63–70. [Google Scholar] [CrossRef]
- Mercader, R.J.; Siegert, N.W.; Liebhold, A.M.; McCullough, D.G. Dispersal of the Emerald Ash Borer, Agrilus planipennis, in Newly-Colonized Sites. Agric. For. Entomol. 2009, 11, 421–424. [Google Scholar] [CrossRef]
- David, G.; Giffard, B.; Piou, D.; Jactel, H. Dispersal Capacity of Monochamus galloprovincialis, the European Vector of the Pine Wood Nematode, on Flight Mills. J. Appl. Entomol. 2014, 138, 566–576. [Google Scholar] [CrossRef]
- Lopez, V.M.; Hoddle, M.S.; Francese, J.A.; Lance, D.R.; Ray, A.M. Assessing Flight Potential of the Invasive Asian Longhorned Beetle (Coleoptera: Cerambycidae) With Computerized Flight Mills. J. Econ. Entomol. 2017, 110, 1070–1077. [Google Scholar] [CrossRef] [PubMed]

| Model | Dist | S | Cdir |
|---|---|---|---|
| 1 | + | ||
| 2 | + | + | |
| 3 | + | + | |
| 4 | + | + | + |
| Distance | N | E | S | W |
|---|---|---|---|---|
| 50 | 3 (0.12) | 9 (0.39) | 12 (0.46) | 1 (0.06) |
| 100 | 1 (0.04) | 5 (0.28) | ||
| 150 | ○ | 11 (0.37) | 2 (0.10) | |
| 300 | 3 (0.14) | 6 (0.22) | ||
| 500 | 1 (0.04) | 3 (0.15) | ||
| 750 | ○ | 1 (0.04) | ||
| 1000 | 6 (0.24) | 1 (0.04) | 7 (0.31) | |
| 1250 | ○ | 1 (0.03) | ||
| 1500 | 1 (0.04) |
| Distance | N | E | S | W |
|---|---|---|---|---|
| 50 | L (3), P (3) | L (2) | L (4) | |
| 100 | ||||
| 150 | L (1) | |||
| 300 | L (1), P (1) | L (3) | L (4) | |
| 500 | ||||
| 750 | L (1), P (1) | |||
| 1000 | L (1) | L (3), P (3), A (2) | ||
| 1250 | L (1), P (1) | |||
| 1500 |
| Dependent Variable | Predictors and Coefficients | AIC | r | |
|---|---|---|---|---|
| p | dist, –0.0021 | 175.77 | 0.061 (0.090) 1 | 0.300 |
| b | dist, –5.21 × 10−4 | 142.20 | −0.015 (0.470) | 0.130 |
| pS | dist, –8.83 × 10−5 | −37.18 | 0.074 (0.069) | 0.320 |
| bS | dist, −2.07 × 10−5 | −67.80 | −0.016 (0.489) | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demidko, D.A.; Kulakov, S.S.; Efremenko, A.A.; Babichev, N.S.; Barchenkov, A.P.; Mikhailov, P.V. Assessing the Flight Potential of the Four-Eyed Fir Bark Beetle Polygraphus proximus Blandford in Natural Conditions. Forests 2024, 15, 1316. https://doi.org/10.3390/f15081316
Demidko DA, Kulakov SS, Efremenko AA, Babichev NS, Barchenkov AP, Mikhailov PV. Assessing the Flight Potential of the Four-Eyed Fir Bark Beetle Polygraphus proximus Blandford in Natural Conditions. Forests. 2024; 15(8):1316. https://doi.org/10.3390/f15081316
Chicago/Turabian StyleDemidko, Denis A., Sergey S. Kulakov, Anton A. Efremenko, Nikita S. Babichev, Alexey P. Barchenkov, and Pavel V. Mikhailov. 2024. "Assessing the Flight Potential of the Four-Eyed Fir Bark Beetle Polygraphus proximus Blandford in Natural Conditions" Forests 15, no. 8: 1316. https://doi.org/10.3390/f15081316
APA StyleDemidko, D. A., Kulakov, S. S., Efremenko, A. A., Babichev, N. S., Barchenkov, A. P., & Mikhailov, P. V. (2024). Assessing the Flight Potential of the Four-Eyed Fir Bark Beetle Polygraphus proximus Blandford in Natural Conditions. Forests, 15(8), 1316. https://doi.org/10.3390/f15081316









