Individual Carbon Modeling in Eucalyptus Stands in the Cerrado Region
Abstract
1. Introduction
2. Materials and Methods
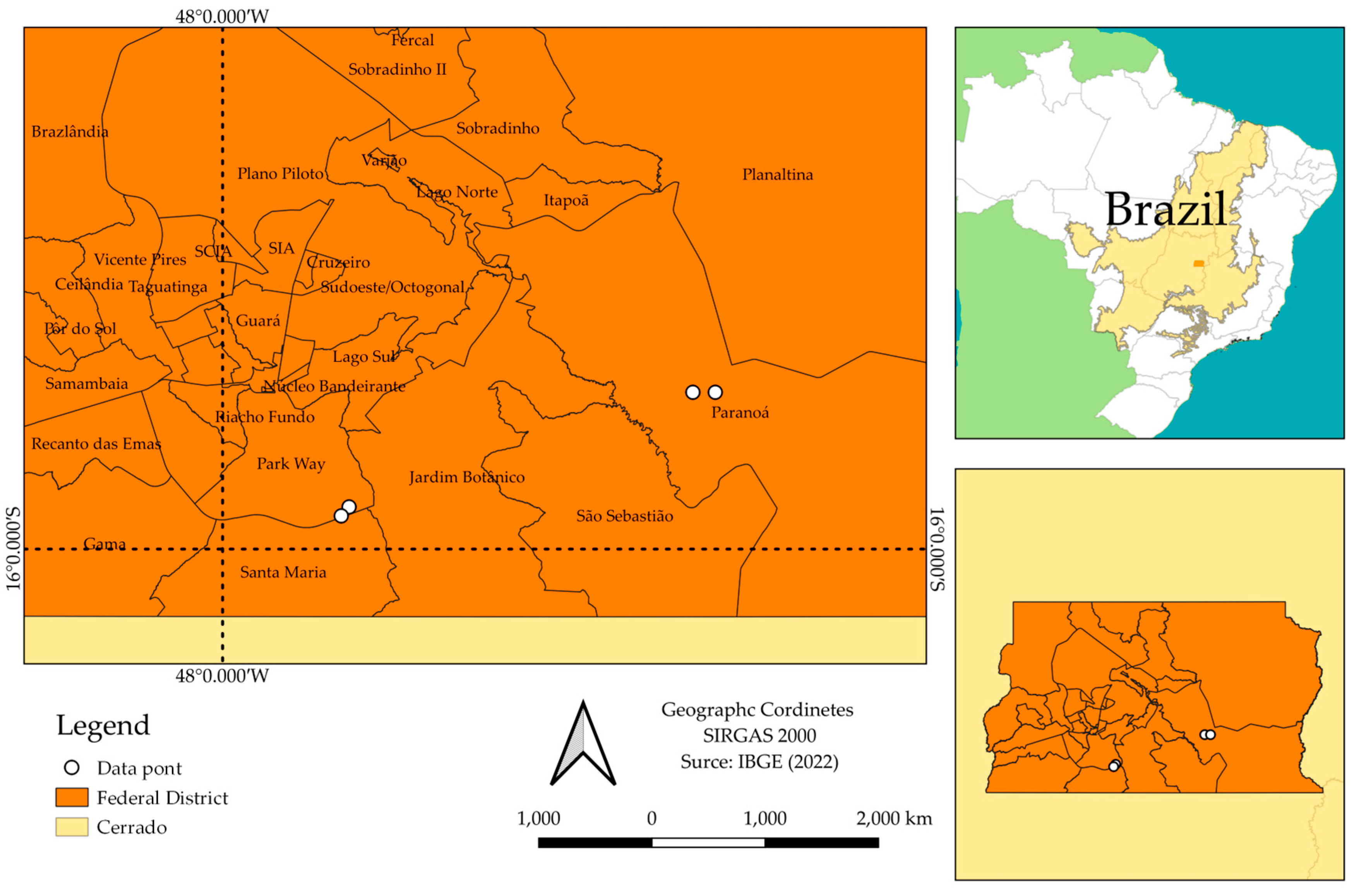
2.1. Localization and Characterization in Experiment
2.2. Tree Cubing
2.3. Root C
2.4. Statistical Analysis
2.5. Model Selection and Validation
3. Results
3.1. Carbon Content and Stock in Eucalyptus urophylla × Eucalyptus grandis Trees
3.2. Individual C Modeling
3.3. Model Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baccini, A.; Goetz, S.J.; Walker, W.S.; Laporte, N.T.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.S.A.; Dubayah, R.; Friedl, M.A.; et al. Estimated Carbon Dioxide Emissions from Tropical Deforestation Improved by Carbon-Density Maps. Nat. Clim. Chang. 2012, 2, 182–185. [Google Scholar] [CrossRef]
- Lian, Y.; Li, H.; Renyang, Q.; Liu, L.; Dong, J.; Liu, X.; Qu, Z.; Lee, L.-C.; Chen, L.; Wang, D.; et al. Mapping the Net Ecosystem Exchange of CO2 of Global Terrestrial Systems. Int. J. Appl. Earth Obs. Geoinf. 2023, 116, 103176. [Google Scholar] [CrossRef]
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Core Writing Team, Lee, H., Romero, J., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2023. [Google Scholar]
- Leal Filho, W.; Matandirotya, N.R.; Lütz, J.M.; Alemu, E.A.; Brearley, F.Q.; Baidoo, A.A.; Kateka, A.; Ogendi, G.M.; Adane, G.B.; Emiru, N.; et al. Impacts of Climate Change to African Indigenous Communities and Examples of Adaptation Responses. Nat. Commun. 2021, 12, 6224. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, D.; Recha, J.; Ambaw, G.; MacSween, K.; Solomon, D.; Wollenberg, E. Assessment of Agricultural Emissions, Climate Change Mitigation and Adaptation Practices in Ethiopia. Clim. Policy 2022, 22, 427–444. [Google Scholar] [CrossRef]
- de Carvalho, A.M.; de Jesus, D.R.; de Sousa, T.R.; Ramos, M.L.G.; de Figueiredo, C.C.; de Oliveira, A.D.; Marchão, R.L.; Ribeiro, F.P.; Dantas, R.d.A.; Borges, L.d.A.B. Soil Carbon Stocks and Greenhouse Gas Mitigation of Agriculture in the Brazilian Cerrado—A Review. Plants 2023, 12, 2449. [Google Scholar] [CrossRef] [PubMed]
- Amiraslani, F.; Dragovich, D.; Henry, B.; Rumpel, C. The ‘Net Zero’ Carbon Needs ‘Billions of Zeros’ Capital. But What about Cheaper Solutions? Urban Gov. 2022, 2, 282–284. [Google Scholar] [CrossRef]
- Alam, S.A.; Kivinen, S.; Kujala, H.; Tanhuanpää, T.; Forsius, M. Integrating Carbon Sequestration and Biodiversity Impacts in Forested Ecosystems: Concepts, Cases, and Policies. Ambio 2023, 52, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Hari, M.; Tyagi, B. Terrestrial Carbon Cycle: Tipping Edge of Climate Change between the Atmosphere and Biosphere Ecosystems. Environ. Sci. Atmos. 2022, 2, 867–890. [Google Scholar] [CrossRef]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon Pools and Flux of Global Forest Ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef]
- Herrero, C.; Juez, L.; Tejedor, C.; Pando, V.; Bravo, F. Importance of Root System in Total Biomass for Eucalyptus Globulus in Northern Spain. Biomass Bioenergy 2014, 67, 212–222. [Google Scholar] [CrossRef]
- Ribeiro, F.P.; Gatto, A.; de Oliveira, A.D.; Pulrolnik, K.; Valadão, M.B.X.; Araújo, J.B.C.N.; de Carvalho, A.M.; Ferreira, E.A.B. Carbon Storage in Different Compartments in Eucalyptus Stands and Native Cerrado Vegetation. Plants 2023, 12, 2751. [Google Scholar] [CrossRef] [PubMed]
- Wanlong, S.; Yowhan, S.; Baishuo, H.; Xuehua, L. An Individual Tree-Based Model for Estimating Regional and Temporal Carbon Storage of Abies Chensiensis Forest Ecosystem in the Qinling Mountains, China. Ecol. Model. 2023, 479, 110305. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Geographical Spatial Distribution and Productivity Dynamic Change of Eucalyptus Plantations in China. Sci. Rep. 2021, 11, 19764. [Google Scholar] [CrossRef] [PubMed]
- Viera, M.; Rodríguez-Soalleiro, R. A Complete Assessment of Carbon Stocks in Above and Belowground Biomass Components of a Hybrid Eucalyptus Plantation in Southern Brazil. Forests 2019, 10, 536. [Google Scholar] [CrossRef]
- Du, H.; Zeng, F.; Peng, W.; Wang, K.; Zhang, H.; Liu, L.; Song, T. Carbon Storage in a Eucalyptus Plantation Chronosequence in Southern China. Forests 2015, 6, 1763–1778. [Google Scholar] [CrossRef]
- Pavan, B.E.; Amaral, R.G.; Pupin, S.; da Costa, R.M.L.; Dias, D.d.C.; Scarpinati, E.A.; de Paula, R.C. Competitive Ability among Eucalyptus Spp. Commercial Clones in Mato Grosso Do Sul State. For. Ecol. Manag. 2021, 494, 119297. [Google Scholar] [CrossRef]
- Teixeira, V.; Carneiro, A.; Leite, H.; Trugilho, P.; Carvalho, A.; Castro, R. Selection of Eucalyptus Genotypes for Charcoal Production Based on Using Multivariate Analysis. J. Anal. Appl. Pyrolysis 2024, 179, 106444. [Google Scholar] [CrossRef]
- Rodrigues, G.; Ross, J.; Teixeira, G.; Santiago, O.R.; Franco, C. Eucalipto No Brasil—Expansão Geográfica e Impactos Ambientais; Composer: Uberlândia, Brazil, 2021. [Google Scholar]
- Campoe, O.C.; Alvares, C.A.; Carneiro, R.L.; Binkley, D.; Ryan, M.G.; Hubbard, R.M.; Stahl, J.; Moreira, G.; Moraes, L.F.; Stape, J.L. Influência Do Clima e Genótipos No Fluxo e Partição de Carbono Em Plantios de Eucalipto. For. Ecol. Manag. 2020, 475, 118445. [Google Scholar] [CrossRef]
- de Mattos, E.M.; Binkley, D.; Campoe, O.C.; Alvares, C.A.; Stape, J.L. Variation in Canopy Structure, Leaf Area, Light Interception and Light Use Efficiency among Eucalyptus Clones. For. Ecol. Manag. 2020, 463, 118038. [Google Scholar] [CrossRef]
- da Silva, P.H.M.; Lee, D.J.; Amancio, M.R.; Araujo, M.J. Initiation of Breeding Programs for Three Species of Corymbia: Introduction and Provenances Study. Crop Breed. Appl. Biotechnol. 2022, 22, e40012211. [Google Scholar] [CrossRef]
- Rocha, S.M.G.; Vidaurre, G.B.; Pezzopane, J.E.M.; Almeida, M.N.F.; Carneiro, R.L.; Campoe, O.C.; Scolforo, H.F.; Alvares, C.A.; Neves, J.C.L.; Xavier, A.C.; et al. Influence of Climatic Variations on Production, Biomass and Density of Wood in Eucalyptus Clones of Different Species. For. Ecol. Manag. 2020, 473, 118290. [Google Scholar] [CrossRef]
- IBÁ—Indústria Brasileira de Árvores. Relatório Annual. Available online: http://www.iba.org/ (accessed on 28 May 2024).
- Dey, T.; Islam, M.; Jubair, S.M. Biomass and Carbon Accumulation in Northern Bangladesh Eucalyptus Plantations: Effects of Stand Structure and Age. Asian J. For. 2022, 6, 126–132. [Google Scholar] [CrossRef]
- Bispo, G.B.S.; Santos, R.F.; Pompeo, M.L.M.; Ferraz, S.F.B.; Rodrigues, C.B.; Brentan, B.M. The Effects of Natural Forest and Eucalyptus Plantations on Seven Water-Related Ecosystem Services in Cerrado Landscapes. Perspect. Ecol. Conserv. 2023, 21, 41–51. [Google Scholar] [CrossRef]
- Teodoro, P.E.; Rossi, F.S.; Teodoro, L.P.R.; Santana, D.C.; Ratke, R.F.; de Oliveira, I.C.; Silva, J.L.D.; de Oliveira, J.L.G.; da Silva, N.P.; Baio, F.H.R.; et al. Soil CO2 Emissions under Different Land-Use Managements in Mato Grosso Do Sul, Brazil. J. Clean. Prod. 2024, 434, 139983. [Google Scholar] [CrossRef]
- Rodríguez-Soalleiro, R.; Eimil-Fraga, C.; Gómez-García, E.; García-Villabrille, J.D.; Rojo-Alboreca, A.; Muñoz, F.; Oliveira, N.; Sixto, H.; Pérez-Cruzado, C. Exploring the Factors Affecting Carbon and Nutrient Concentrations in Tree Biomass Components in Natural Forests, Forest Plantations and Short Rotation Forestry. For. Ecosyst. 2018, 5, 35. [Google Scholar] [CrossRef]
- Tonini, H.; Magalhães, C.A.d.S.; Faria Neto, A.L. de Technical Thinning Age of Eucalyptus Trees Grown in Crop-Forest Integration Systems. Pesq. Agropec. Bras. 2019, 54, e00648. [Google Scholar] [CrossRef]
- Silva, S.; de Oliveira Neto, S.N.; Leite, H.G.; de Alcântara, A.E.M.; de Oliveira Neto, R.R.; de Souza, G.S.A. Productivity Estimate Using Regression and Artificial Neural Networks in Small Familiar Areas with Agrosilvopastoral Systems. Agroforest. Syst. 2020, 94, 2081–2097. [Google Scholar] [CrossRef]
- Carrijo, J.V.N.; Ferreira, A.B.d.F.; Ferreira, M.C.; de Aguiar, M.C.; Miguel, E.P.; Matricardi, E.A.T.; Rezende, A.V. The Growth and Production Modeling of Individual Trees of Eucalyptus Urophylla Plantations. J. For. Res. 2020, 31, 1663–1672. [Google Scholar] [CrossRef]
- Tavares Júnior, I.d.S.; Torres, C.M.M.E.; Leite, H.G.; de Castro, N.L.M.; Soares, C.P.B.; Castro, R.V.O.; Farias, A.A. Machine Learning: Modeling Increment in Diameter of Individual Trees on Atlantic Forest Fragments. Ecol. Indic. 2020, 117, 106685. [Google Scholar] [CrossRef]
- Huang, H.; Liu, C.; Wang, X.; Zhou, X.; Gong, P. Integration of Multi-Resource Remotely Sensed Data and Allometric Models for Forest Aboveground Biomass Estimation in China. Remote Sens. Environ. 2019, 221, 225–234. [Google Scholar] [CrossRef]
- Ogana, F.N.; Corral-Rivas, S.; Gorgoso-Varela, J.J. Nonlinear Mixed-Effect Height-Diameter Model For Pinus Pinaster Ait. And Pinus Radiata D. Don. Cerne 2020, 26, 150–161. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, D.; Jiang, X.; Li, Y.; Li, D. Forest Structure Simulation of Eucalyptus Plantation Using Remote-Sensing-Based Forest Age Data and 3-PG Model. Remote Sens. 2023, 15, 183. [Google Scholar] [CrossRef]
- Stolle, L.; Corte, A.P.D.; Sanquetta, C.R.; Behling, A.; Hentz, Â.M.K.; Eisfeld, R.d.L. Predicting Stand Volume by Number of Trees Automatically Detected in UAV Images: An Alternative Method for Forest Inventory. Forests 2021, 12, 1508. [Google Scholar] [CrossRef]
- Valadão, M.B.X.; Carneiro, K.M.S.; Ribeiro, F.P.; Inkotte, J.; Rodrigues, M.I.; Mendes, T.R.S.; Vieira, D.A.; Matias, R.A.M.; Lima, M.B.O.; Miguel, E.P.; et al. Modeling Biomass and Nutrients in a Eucalyptus Stand in the Cerrado. Forests 2020, 11, 1097. [Google Scholar] [CrossRef]
- Gatto, A.; Bussinguer, A.P.; Ribeiro, F.C.; de Azevedo, G.B.; Bueno, M.C.; Monteiro, M.M.; de Souza, P.F. Ciclagem e balanço de nutrientes no sistema solo-planta em um plantio de Eucalyptus sp., no Distrito Federal. Rev. Bras. Ciênc. Solo 2014, 38, 879–887. [Google Scholar] [CrossRef]
- Péllico, S.; Behling, A. Rational Volumetric Models: Tested In Forest Stands Of Acacia Mearnsii De Wild In Southern Brazil. Cerne 2020, 25, 491–505. [Google Scholar] [CrossRef]
- Attia, A.; Nouvellon, Y.; Cuadra, S.; Cabral, O.; Laclau, J.-P.; Guillemot, J.; Campoe, O.; Stape, J.-L.; Galdos, M.; Lamparelli, R.; et al. Modelagem Do Balanço Hídrico e de Carbono de Plantios de Eucalipto Em Escala Regional: Efeito Do Clima, Solo e Genótipos. For. Ecol. Manag. 2019, 449, 117460. [Google Scholar] [CrossRef]
- Salekin, S.; Mason, E.G.; Morgenroth, J.; Bloomberg, M.; Meason, D.F. Hybrid Height Growth and Survival Model for Juvenile Eucalyptus Globoidea (Blakely) and E. Bosistoana (F. Muell) in New Zealand. For. Ecol. Manag. 2021, 490, 119074. [Google Scholar] [CrossRef]
- Xu, N.; Cai, D.; Zhao, X. Constructing a Eucalyptus Identification Model Based on NDVI Time-Series Remote Sensing Images. Catena 2024, 238, 107846. [Google Scholar] [CrossRef]
- Santos, J.S.; de Mendonça, A.R.; Gonçalves, F.G.; da Silva, G.F.; de Almeida, A.Q.; Carvalho, S.d.P.C.; Silva, J.P.M.; Carvalho, R.C.; da Silva, E.F.; Aguiar, M.O. Predicting Eucalyptus Plantation Growth and Yield Using Landsat Imagery in Minas Gerais, Brazil. Ecol. Inform. 2023, 75, 102120. [Google Scholar] [CrossRef]
- Schumacher, F.X.; Hall, F.S. Logarithmic Expression of Timber-Tree Volume. J. Agric. Res. 1933, 47, 719–734. [Google Scholar]
- Spurr, S.H. Forest Inventory; Ronald Press Company: New York, NY, USA, 1952. [Google Scholar]
- Parresol, B.R. Assessing Tree and Stand Biomass: A Review with Examples and Critical Comparisons. For. Sci. 1999, 45, 573–593. [Google Scholar] [CrossRef]
- Akaike, H. On the Likelihood of a Time Series Model. J. R. Stat. Soc. Ser. D 1978, 27, 217–235. [Google Scholar] [CrossRef]
- Bergmeir, C.; Hyndman, R.J.; Koo, B. A Note on the Validity of Cross-Validation for Evaluating Autoregressive Time Series Prediction. Comput. Stat. Data Anal. 2018, 120, 70–83. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing—ScienceOpen. Available online: https://www.scienceopen.com/book?vid=b164ea90-95d2-43bf-9710-99753c479112 (accessed on 19 June 2024).
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C. Köppen’s Climate Classification Map for Brazil. Meteorol. Z 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Schaefer, C.E.R. Brazilian Latosols and Their B Horizon Microstructure as Long-Term Biotic Constructs. Soil Res. 2001, 39, 909–926. [Google Scholar] [CrossRef]
- Oliveira, D.M.S.; Santos, R.S.; Chizzotti, F.H.M.; Bretas, I.L.; Franco, A.L.C.; Lima, R.P.; Freitas, D.A.F.; Cherubin, M.R.; Cerri, C.E.P. Crop, Livestock, and Forestry Integration to Reconcile Soil Health, Food Production, and Climate Change Mitigation in the Brazilian Cerrado: A Review. Geoderma Reg. 2024, 37, e00796. [Google Scholar] [CrossRef]
- Lima, L.; Miranda, I.; Knapic, S.; Quilhó, T.; Pereira, H. Chemical and Anatomical Characterization, and Antioxidant Properties of Barks from 11 Eucalyptus Species. Eur. J. Wood Prod. 2018, 76, 783–792. [Google Scholar] [CrossRef]
- Balboni, B.M.; Batista, A.S.; Garcia, J.N. Evaluating the Potential for Timber Production of Young Forests of Eucalyptus Spp. Clones Used for Bioenergy: Wood Density and Mechanical Properties. Aust. For. 2021, 84, 122–132. [Google Scholar] [CrossRef]
- Kumar, T.; Bhardwaj, K.K.; Kaushik, P. Effect of Soil Properties and Nutrient Status on Eucalyptus Tereticornis Based Agroforestry System in India. agriRxiv 2020, agriRxiv:20203280761. [Google Scholar] [CrossRef]
- Laclau, J.-P.; Silva, E.A.; Rodrigues Lambais, G.; Bernoux, M.; le Maire, G.; Stape, J.L.; Bouillet, J.-P.; Leonardo, M. Gonçalves, J.; Jourdan, C.; Nouvellon, Y. Dynamics of Soil Exploration by Fine Roots down to a Depth of 10 m throughout the Entire Rotation in Eucalyptus Grandis Plantations. Front. Plant Sci. 2013, 4, 243. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, Y.; Goodale, U.M.; Zuo, H.; Zhu, H.; Li, X.; You, Y.; Yan, L.; Su, Y.; Huang, X. Optimal Rotation Length for Carbon Sequestration in Eucalyptus Plantations in Subtropical China. New For. 2017, 48, 609–627. [Google Scholar] [CrossRef]
- Morales, M.M.; Tonini, H.; Behling, M.; Hoshide, A.K. Eucalyptus Carbon Stock Research in an Integrated Livestock-Forestry System in Brazil. Sustainability 2023, 15, 7750. [Google Scholar] [CrossRef]
- Gatto, A.; de Barros, N.F.; de Novais, R.F.; da Silva, I.R.; Leite, H.G.; Villani, E.M.d.L. Estoque de carbono na biomassa de plantações de eucalipto na região centro-leste do estado de Minas Gerais. Rev. Árvore 2011, 35, 895–905. [Google Scholar] [CrossRef]
- Babu, K.N.; Mandyam, S.; Jetty, S.; Dar, A.A.; Ayushi, K.; Narayanan, A.; Somaiah, S.; Narayanaswamy, P. Carbon Stocks of Tree Plantations in a Western Ghats Landscape, India: Influencing Factors and Management Implications. Environ. Monit Assess. 2023, 195, 404. [Google Scholar] [CrossRef]
- Pires Coelho, A.J.; Ribeiro Matos, F.A.; Villa, P.M.; Heringer, G.; Pontara, V.; de Paula Almado, R.; Alves Meira-Neto, J.A. Multiple Drivers Influence Tree Species Diversity and Above-Ground Carbon Stock in Second-Growth Atlantic Forests: Implications for Passive Restoration. J. Environ. Manag. 2022, 318, 115588. [Google Scholar] [CrossRef]
- da Silva, M.L.M.; Binoti, D.H.B.; Gleriani, J.M.; Leite, H.G. Ajuste do modelo de Schumacher e Hall e aplicação de redes neurais artificiais para estimar volume de árvores de eucalipto. Rev. Árvore 2009, 33, 1133–1139. [Google Scholar] [CrossRef]
- Hakamada, R.; Binkley, D.; Cegatta, I.; Alvares, C.; Campoe, O.; Stape, J.L. Stocking Response of Eucalyptus Growth Depends on Site Water Deficit across a 2100-Km Gradient in Brazil. For. Ecol. Manag. 2023, 546, 121325. [Google Scholar] [CrossRef]
- Romero, F.M.B.; Jacovine, L.A.G.; Torres, C.M.M.E.; Ribeiro, S.C.; da Rocha, S.J.S.S.; Novais, T.d.O.; Gaspar, R.d.O.; da Silva, L.F.; Vidal, E.; Leite, H.G.; et al. Aboveground Biomass Allometric Models for Large Trees in Southwestern Amazonia. Trees For. People 2022, 9, 100317. [Google Scholar] [CrossRef]
- Schettini, B.L.S.; Oliveira Neto, S.N.D.; Jacovine, L.A.G.; Torres, C.M.M.E.; Villanova, P.H.; Rocha, S.J.S.S.D. Viabilidade econômica de um sistema silvipastoril na zona da mata de Minas Gerais. Sci. For. 2021, 49, e3463. [Google Scholar] [CrossRef]


| Experimental Area | Description |
|---|---|
| Area 1 (A1): Eucalyptus urophylla × Eucalyptus grandis | 12 ha planted in December 2011. Previous native Cerrado vegetation. The 48-month-old EAC1528 clone was planted at a spacing of 3.5 × 1.7 m. Before planting, the soil was tilled with a disk harrow in 15 cm strips and subsoiled in the planting line to a depth of 90 cm. |
| Area 2 (A2): Eucalyptus urophylla × Eucalyptus grandis | 1.2 ha planted in November 2009. The 60-month-old Urograndis clone was planted at a spacing of 3 × 2 m. The soil was subsoiled to a depth of 70 cm and received 500 kg of simple superphosphate/ha, 280 g/plant of NPK 20-5-20, and B and Zn after 15 days and at the beginning of the rainy season. |
| Area 3 (A3): Eucalyptus urophylla × Eucalyptus grandis | 3.29 ha area planted in January 2010. The 72-month-old I224 clone was planted at a spacing of 3 × 2 m. The soil was tilled to a depth of 40 cm. Fertilizer was applied along the planting line, with 100 g of simple phosphate + 100 g of NPK (4-30-16). |
| Area 4 (A4): Eucalyptus urophylla × Eucalyptus grandis | 19 ha area planted in December 2009. The area was already used for agriculture. The 72-month-old GG100 clone was planted at a spacing of 3.5 × 1.7 m. The soil was tilled with a disk harrow in 15 cm strips and a furrow opener was used along the planting line to a depth of 90 cm. |
| Model | Classification | References | |
|---|---|---|---|
| 1 | C = β0 × DBH β1 × hβ2 × Ageβ3 | Linear | Schumacher–Hall [44] * |
| 2 | C = β0 × DBHβ1 × h^β2 | Linear | Schumacher–Hall [44] |
| 3 | C = β0 + β1 × DBH2 × h | Non-linear | Spurr [45] |
| Areas | Leaf | Branches | Wood | Bark | Roots | CV | Overall Mean * |
|---|---|---|---|---|---|---|---|
| ---------------------------------- dag kg−1 ------------------------------------- | |||||||
| 1 | 45.15 a | 44.86 a | 43.77 a | 45.46 a | 45.46 a | 4.51 | 44.84 B |
| 2 | 43.01 a | 43.84 a | 42.92 a | 42.81 a | 42.81 a | 6.13 | 43.10 B |
| 3 | 56.25 c | 56.94 b | 57.93 a | 56.10 c | 56.10 c | 0.41 | 55.40 A |
| 4 | 42.50 a | 44.19 a | 43.22 a | 41.60 a | 41.60 a | 7.56 | 42.80 B |
| Areas | Shoot | Root | Total |
|---|---|---|---|
| --------------------- kg ---------------------- | |||
| 1 | 37.61 c | 4.14 b | 41.75 c |
| 2 | 27.22 d | 2.78 c | 30.01 d |
| 3 | 76.13 a | 6.45 a | 82.58 a |
| 4 | 47.09 b | 4.24 b | 51.34 b |
| CV (%) | 9.30 | 8.73 | 9.24 |
| Model | β0 | β1 | β2 | β3 | r | RMSE (%) | AIC |
|---|---|---|---|---|---|---|---|
| 1 | 0.00113 | 2.14718 | 1.15236 | 0.83763 | 0.99 | 9.65 | 258 |
| 2 | 0.00174 | 2.19539 | 1.43419 | 0.98 | 12.86 | 279 | |
| 3 | 0.00288 | 1.16254 | 0.98 | 12.83 | 278 |
| Model | Validation | r | RMSE (%) | AIC |
|---|---|---|---|---|
| 1 | A, B, C, D | 0.992 | 10.01 | 258,460 |
| 1 | A, B, C, E | 0.996 | 9.42 | 264,580 |
| 1 | A, B, D, E | 0.994 | 11.07 | 267,830 |
| 1 | A, C, D, E | 0.985 | 12.02 | 262,400 |
| 1 | B, C, D, E | 0.980 | 12.26 | 259,430 |
| Mean | 0.989 | 10.96 | 262,540 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, F.P.; de Sousa, T.R.; Silva, F.R.d.C.; da Fonseca, A.C.P.; dos Santos, M.G.B.; dos Santos, J.R.; de Jesus, D.R.; Lozada, C.M.C.; Valadão, M.B.X.; Miguel, E.P.; et al. Individual Carbon Modeling in Eucalyptus Stands in the Cerrado Region. Forests 2024, 15, 1332. https://doi.org/10.3390/f15081332
Ribeiro FP, de Sousa TR, Silva FRdC, da Fonseca ACP, dos Santos MGB, dos Santos JR, de Jesus DR, Lozada CMC, Valadão MBX, Miguel EP, et al. Individual Carbon Modeling in Eucalyptus Stands in the Cerrado Region. Forests. 2024; 15(8):1332. https://doi.org/10.3390/f15081332
Chicago/Turabian StyleRibeiro, Fabiana Piontekowski, Thais Rodrigues de Sousa, Fernanda Rodrigues da Costa Silva, Ana Caroline Pereira da Fonseca, Marcela Granato Barbosa dos Santos, Jane Ribeiro dos Santos, Douglas Rodrigues de Jesus, Clara Milena Concha Lozada, Marco Bruno Xavier Valadão, Eder Pereira Miguel, and et al. 2024. "Individual Carbon Modeling in Eucalyptus Stands in the Cerrado Region" Forests 15, no. 8: 1332. https://doi.org/10.3390/f15081332
APA StyleRibeiro, F. P., de Sousa, T. R., Silva, F. R. d. C., da Fonseca, A. C. P., dos Santos, M. G. B., dos Santos, J. R., de Jesus, D. R., Lozada, C. M. C., Valadão, M. B. X., Miguel, E. P., de Oliveira, A. D., Carvalho, A. M. d., & Gatto, A. (2024). Individual Carbon Modeling in Eucalyptus Stands in the Cerrado Region. Forests, 15(8), 1332. https://doi.org/10.3390/f15081332








