Abstract
MYB transcription factors containing an R2R3 DNA-binding domain are involved in the regulation of various physiological processes, such as responses to abiotic stresses, including salt stress. In poplar, various MYB transcription factors regulate stress responses. In this study, we aimed to identify the correlation between the transcription factor MYB148 and salt stress responses in the hybrid poplar (Populus alba × P. glandulosa). We induced a mutation in the MYB-binding domain of PagMYB148 using CRISPR/Cas9-mediated editing of the PagMYB148 gene, which induced a more suppressed expression of PagMYB148 than that in the wild-type. Although salt and drought treatments enhance the expression of PagMYB148, the pagmyb148-transgenic plants exhibited more sensitive phenotypes than the wild-type plants under salt stress. After exposure to salinity stress, the chlorophyll content was lower in pagmyb148-transgenic plants than in wild-type plants, whereas the mutation increased ion leakage from cells. Additionally, the expression of genes involved in the salt stress response was higher in pagmyb148-transgenic plants than in the wild-type. After salt treatment, pagmyb148-transgenic plants exhibited an increased level of H2O2 and reduced activity of antioxidant enzymes. In summary, the MYB148 transcription factor is involved in the regulation of salt stress resistance in hybrid poplar trees. This report contributes to providing a basis for further investigating the molecular mechanisms of the poplar PagMYB148 transcription factor under abiotic stress.
1. Introduction
Hybrid poplar (Populus alba × P. glandulosa) is generated by artificially crossing female P. alba and male P. glandulosa trees belonging to the willow family [1]. Poplars grow faster than other tree species and exhibit excellent sprouting potential, which facilitates mass proliferation through vegetative propagation. Owing to these traits, they are considered essential species for global afforestation [2]. Previous reports have suggested poplars, with their well-established transformation and regeneration methods and small genome size, as model species for forest genetic engineering research [3,4,5].
As each plant lives in a distinct area throughout its lifespan, adaptability to environmental changes is crucial for its survival. Abrupt climate change, such as high temperatures and drought, and changes in the optimal growth environment, such as soil salinity altered by excessive use of fertilizers and emission of pollutants, serve as stressors to plants, causing damage to cells. Salt stress is known to induce ionic, osmotic, and oxidative stresses [6,7]. Plants cope with salinity stress using specific adaptation mechanisms, acting via a complicated network of transcriptional and hormonal components, and several factors involved in this mechanism have been identified. Transcriptional elements may involve various transcription factors (TFs), such as MYB (myeloblastosis viral oncogene homolog), WRKY, AP2 (APETALA2)/ERF (ethylene-responsive factor), and NAC (no apical meristem [NAM], Arabidopsis thaliana transcription activation factor [ATAF1/2], and cup-shaped cotyledons [CUC2]) [8].
Among these, the MYB transcription factors represent a large family of transcription factors associated with 209 genes of poplar, and are characterized by an N-terminal MYB-DNA-binding domain [7,9]. This domain has three α-helices, each comprising 52 amino acids, and each repeat is divided into R1, R2, and R3, according to similarity with the c-MYB protein. Most MYB transcription factors found in plants belong to the R2R3-MYB group, have well-conserved amino acid sequences among different species, and regulate various physiological responses in plants [9]. For instance, Zhang et al. [10] reported that AtMYB49 is involved in suberin synthesis in A. thaliana and positively regulates salt stress tolerance through cuticle formation and the regulation of antioxidant responses. Moreover, MYB52 and MYB108 act as negative regulators that weaken salt stress resistance in Arabidopsis [11,12]. In poplar, MYB acts as an important regulator of secondary cell wall synthesis and resistance to environmental stresses [13,14]. However, the role of the MYB transcription factor in the salt stress response in poplars remains unclear.
Recently developed CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9) technology, which applies the bacterial immune system against viral infection, is widely used in genome editing. It involves the Cas9 nuclease and a single-stranded guide RNA specific to the target sequences [15]. In a CRISPR/Cas9 system, a guide RNA binds to the target gene, and Cas9 severs the double-stranded DNA at the target sequence near the protospacer adjacent motif (PAM). The part cut off by the intracellular DNA repair mechanism is repaired during the repair process, and bases are lost or inserted (non-homologous end joining, NHEJ), resulting in a mutation [16]. CRISPR/Cas9-mediated genome editing is common in both animals and plants. Many studies have reported the effectiveness of this genome editing technology in various plant groups, ranging from crops to perennial trees, using vegetatively propagated gray poplars aged 6–13 months [17,18]. This technology supports the advances in research on producing plants with useful traits, such as increased yield, enhanced growth, improved stress resistance, and essential nutrient accumulation [19,20].
Considering the need for further research to elucidate the role of the MYB transcription factor in the salt stress response in poplars, in this study, we isolated and identified the PagMYB148 gene from hybrid poplars and generated transgenic plants by knocking out PagMYB148 using the CRISPR/Cas9 system. We investigated the role of PagMYB148 in abiotic stress response pathways. The transcript levels of PagMYB148 increased under salt and drought stresses; however, the transgenic plants were sensitive to only salt stress. The results related to the experiments evaluating the degree of cell damage and antioxidant enzyme activity revealed that PagMYB148 is a positive regulator of salt stress resistance.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Hybrid poplars (Populus alba × P. glandulosa, clone BongHwa; BH) were used as both the wild type (WT) and transgenic plants in this study. Control and transgenic plants were propagated in vitro according to the previous study [21] before acclimatization in pots (17 × 20 cm [diameter× height]) using commercial horticultural potting soil. The plants were then cultivated in growth chambers (16 h light/8 h dark cycle; light intensity, 150 μM m−2 s−1; 24 °C), followed by acclimatization in soil and further growth for 6 weeks until they achieved a height of approximately 15 cm. For tissue-specific gene expression analysis, shoot apical meristem (SAM) tissue was collected from 6-week-old plants grown in soil, whereas leaf, stem, and root tissues were collected from 1-year-old poplar plants.
2.2. PagMYB148-CRISPR/Cas9 Vector Construction and Plant Transformation
Single guide RNAs (sgRNAs) targeting PagMYB148 were designed by Cas-Designer in CRISPR RGEN Tools (http://www.rgenome.net/cas-designer, accessed on 14 July 2024) [22] using the full-length genomic DNA sequences of the PagMYB148 and Populus alba × P. tremula var. glandulosa (Poplar 84 K) genomes as the reference sequence. Cas-OFFinder (www.regnome.net/cas-offinder, accessed on 14 July 2024) was used to select target sequences with a low expected number of mismatches and a high out-of-frame score [23]. Finally, considering the number of OFF targets and the out-of-frame score, three single guide RNAs (sg1–sg3), each with a length of 20 bp, were selected for knockout of PagMYB148; the protospacer adjacent motif (PAM) sequence was excluded. The binary vector pHAtC (GenBank: KU213971.1) and AarI-mediated sgRNA cloning system [24] were used for the Agrobacterium-mediated transformation of the hybrid poplar. For this experiment, after AarI-digestion of the vector, the annealed target sgRNA sequence was inserted between the AtU6 promoter and the sgRNA scaffold, followed by circularization using T4 DNA ligase (New England Biolabs, Ipswich, MA, USA). Next, the vector construct was introduced into Agrobacterium tumefaciens strain GV3101, which was used to transform poplars via the stem node transformation-regeneration [25]. All constructs used in this study were verified by DNA sequencing (Macrogen, Seoul, Republic of Korea, http://dna.macrogen.com/kor/, accessed on 14 July 2024).
2.3. Genotyping of Regenerated Transgenic Hybrid Poplars by Targeted Deep Sequencing
Genotyping of the mutated sequences in transgenic hybrid poplars was performed using the Illumina MiSeq platform (KAIST Biocore Center, Daejeon, Republic of Korea). Briefly, genomic DNA was extracted from the leaves of regenerated transgenic hybrid poplars using a DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The target region was amplified using nested PCR primer pairs containing the adapter sequences. Amplicons were then labeled with an index sequence (Illumina Korea, Seoul, Republic of Korea) using index PCR primer pairs, and targeted deep sequencing was performed using Illumina Mi-Seq (KAIST Biocore Center). The resulting deep-sequencing data were analyzed using the Cas-Analyzer (www.rgenome.net/cas-analyzer, accessed on 14 July 2024) [26]. The primer pairs used in this study are listed in Table S1.
2.4. Subcellular Localization of PagMYB148
To determine the subcellular localization of PagMYB148, eGFP was fused in-frame to the C-terminus of PagMYB148. The coding sequence of PagMYB148 was amplified from the cDNA of hybrid poplar (clone BH) using gene-specific primers (Table S1). The PCR products were cloned into the pENTR/dTOPO vector (Invitrogen, Waltham, MA, USA), which was confirmed through sequencing. The PagMYB148 fragment was cloned into the gateway version of the pCsVMV-eGFP-N-999 vector using the LR recombination reaction [27]. Next, the CsVMV:PagMYB148-eGFP recombinant plasmid was introduced into poplar protoplasts using polyethylene glycol [28]. The localization of PagMYB148-eGFP was investigated using a confocal laser scanning microscope (Leica TCS SP5, Wetzlar, Germany).
2.5. RNA Isolation and Reverse-Transcription PCR Analysis
Total RNA used in all experiments was isolated from wild type (P. alba × P. glandulosa, clone BH) or transgenic plants using an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the instructions provided by the manufacturer. The concentration and purity of RNA samples were checked using a Nanodrop spectrophotometer (Thermo-Fisher Scientific, Waltham, MA, USA), and the results were presented in Table S2. Reverse transcription-quantitative PCR (RT-qPCR) primers were designed using Primer3 (http://fokker.wi.mit.edu, accessed on 14 July 2024). Gene expression levels were determined using the 2−∆∆Ct method [29]. The first-strand cDNA was synthesized using EcoDryTM premix (Takara, Kyoto, Japan) and RT-qPCR was performed using the SYBR Green PCR Master Mix (Bio-Rad Laboratories, Hercules, CA, USA) on a CFX96™ Real-Time System (Bio-Rad Laboratories, Hercules, CA, USA). The cycling conditions for amplification were set following the instructions provided by the manufacturer. All primers used for RT-qPCR are listed in Table S1.
2.6. Stress Treatment
To explore the expression profile of PagMYB148 under stress conditions, WT poplar (clone BH) plants were grown for 6 weeks in soil under normal watering conditions; subsequently, they were treated with water (control), 150 mM NaCl, and 10% PEG for 24 h. For heat and cold treatments, 2-month-old WT poplars, grown in soil under normal conditions, were exposed to temperatures of 40 °C and 4 °C, respectively, for 24 h using growth chambers (Vision Scientific, Daejeon, Republic of Korea). For the salt stress treatment, WT and transgenic poplar trees were grown in soil under normal conditions for 6 weeks and then watered using 250 mL of 300 mM NaCl solution in each pot, as performed in a previous study [30]. Afterward, six fully expanded leaves from 6-week-old soil-acclimated WT and transgenic poplar trees were detached and weighed to measure chlorophyll content, following a previous study [31]. Electrolytic leakage was measured following a previously described method with minor modifications [32,33]. For this analysis, five leaf discs (diameter, 1 cm) were acquired from transgenic and WT plants using a stainless-steel borer and soaked in test tubes containing 10 ml of deionized water. The solution was incubated for 2 h at 25 °C, and solution conductivity (C1) was measured with a conductivity meter (455C, iSTEK Inc., Seoul, Republic of Korea). Then, the solution with the leaf discs was boiled for 15 min at 121 °C, cooled to room temperature, and solution conductivity (C2) was measured again. Relative electrical conductivity was calculated as (C1/C2) × 100% [34].
2.7. Determination of H2O2 and Antioxidant Enzyme Activities
To measure the H2O2 content and antioxidant enzyme activities, the leaves of mutant and WT plants were collected after the salt stress treatment and triturated using liquid nitrogen. The powdered frozen leaf tissue (100 mg) was vigorously mixed with 1 mL of phosphate buffer saline (PBS; pH 7.0). The homogenate was centrifuged at 13,000 rpm for 20 min at 4 °C, and the supernatant was collected for subsequent analysis [35,36]. H2O2 content and POD activity were determined using an EZ-Hydrogen Peroxide/Peroxidase assay kit (DoGenBio Co., Daejeon, Republic of Korea). The activity of the antioxidant enzyme superoxide dismutase (SOD) was measured using an EZ-SOD assay kit (DoGenBio Co., Daejeon, Republic of Korea), and catalase activity was estimated using the EZ-Catalase assay kit (DoGenBio Co., Daejeon, Republic of Korea) following the protocol provided by the manufacturer, with minor modifications. For the analysis of H2O2 contents, 50 μL of supernatant was mixed with the same volume of Oxi-probe/HRP working solution and incubated for 30 min at room temperature in the dark. The absorbance at 560 nm was recorded using a microplate reader (SpectraMax M2e, Molecular Devices LLC, San Jose, CA, USA). For analyzing SOD activity, 20 μL of supernatant was mixed with 200 μL of WST working solution and 20 μL of enzyme working solution, followed by incubation for 40 min at 37 °C. Subsequently, the absorbance of the reaction mix was measured at 450 nm using a microplate reader. For the catalase activity assay, 25 μL of supernatant was mixed with the same volume of 40 μM H2O2 and incubated for 30 min at room temperature in the dark. Next, 50 μL of Oxi-probe/HRP working solution was added followed by incubation for 30 min at 37 °C in the dark; next, absorbance at 560 nm was recorded using a microplate reader.
2.8. Statistical Analysis
All data are presented as the mean values ± standard deviation deduced from at least three independent replicates. Statistical analysis was conducted using Student’s t-test (n = 6).
3. Results
3.1. Identification of PagMYB148
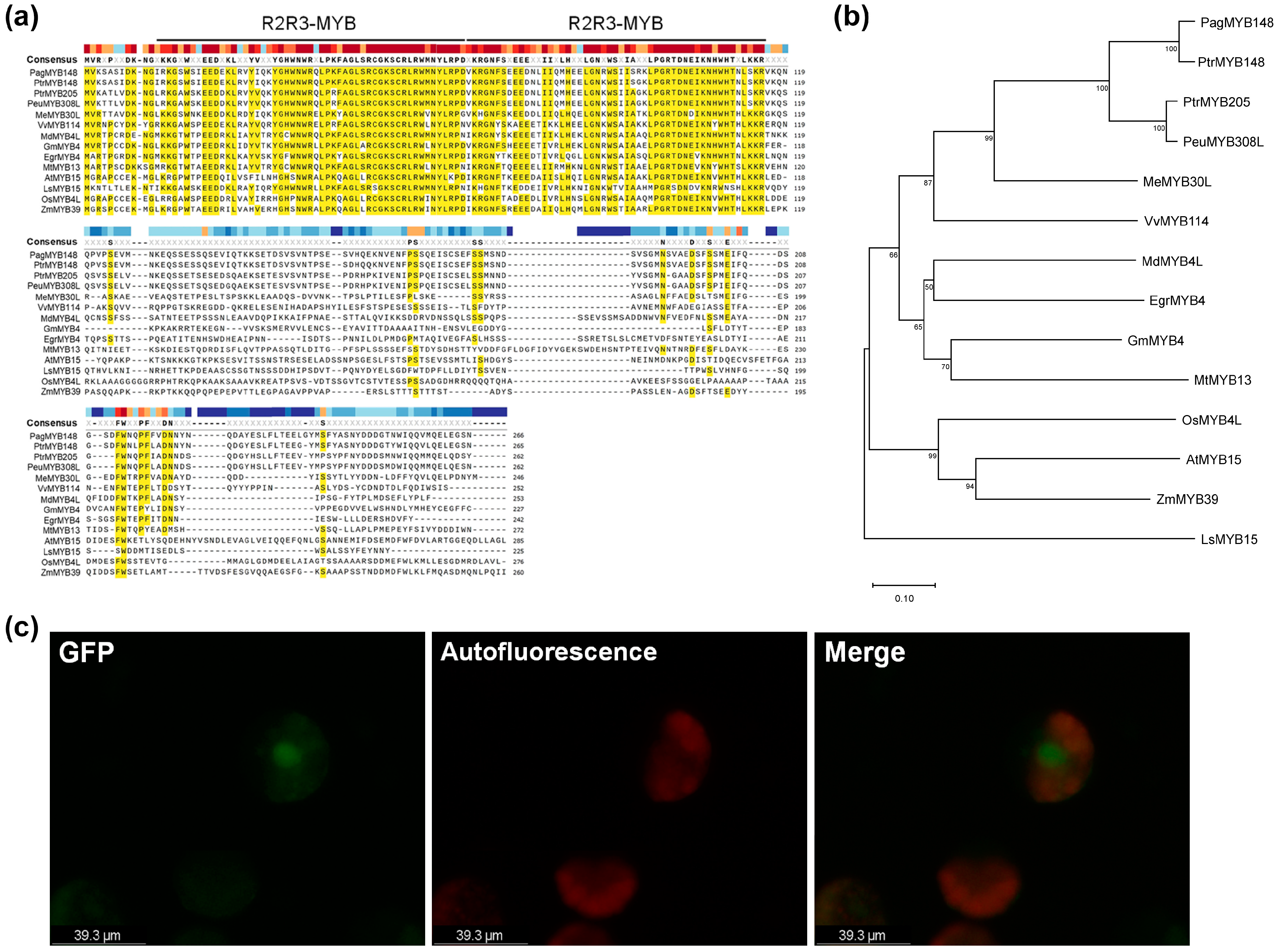
Based on the sequence of Populus trichocarpa (Potri.013G149000), the CDS portion was amplified from the genomic DNA of hybrid poplar (P. alba × P. tremula var. glandulosa, clone BH) and sequenced. The whole genome sequence of P. tremula × P. alba (clone INRA717-B) has already been annotated in a public database [37] (https://phytozome.jgj.doe.gov/, accessed on 14 July 2024). INRA717-B is a hybrid originating from P. alba and P. tremula with the reversed sex in comparison with the hybrid poplar used in this study. Hence, we analyzed the CDS sequences of PagMYB148 and MYB148 of P. tremula × P. alba and identified the same sequence (PtXaAlbH.13G118000); moreover, the comparison of protein sequences with those of the same aspen genus revealed 95% identity with PtrMYB148 (Potri.013G149000) and 76% identity with PtrMYB205 (Potri.019G118700). PeuMYB308L of P. euphratica showed the highest (77%) sequence similarity. Our comparative analysis of proteins with similar sequences in A. thaliana, soybean, grape, and cassava revealed that the analyzed sequence was conserved, and we identified it as an MYB transcription factor with an R2R3 MYB domain (Figure 1a). PagMYB148 was phylogenetically close to that of cassava (Mannihot esculenta) and grape (Vitis vinifera) proteins, except for those in black cottonwood (Figure 1b). In addition, a GFP signal detected in the nucleus confirmed the subcellular localization of this protein (Figure 1c). Therefore, MYB148 has a MYB domain expressed in the nucleus, and it potentially acts as a nuclear transcription factor.
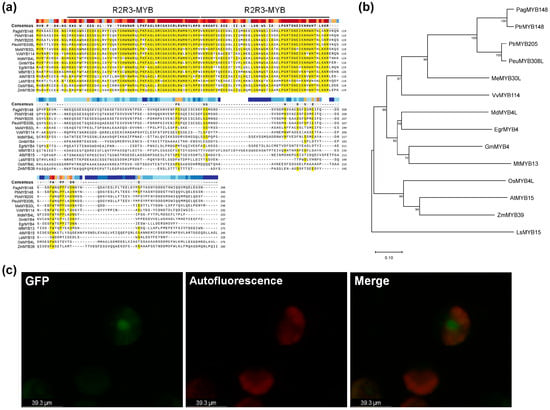
Figure 1.
Identification of PagMYB148. (a) Multiple sequence alignment and (b) phylogenetic tree analysis of PagMYB148 and homologous proteins identified from 12 plant species. The alignment was generated using SnapGene Viewer 6.1. The R2R3-binding domains of MYB protein are indicated indicated by the black bars. The conserved amino acid residues are highlighted in yellow colors. The phylogenetic tree was generated using the maximum likelihood method in MEGA 11.0 software. Numbers at each interior branch indicate the bootstrap values of 1000 replicates. The bar indicates a genetic distance of 0.1. (c) Subcellular localization of PagMYB148 protein.
3.2. Expression Profile of PagMYB148
PagMYB148 was expressed in the leaves, stems, and apical meristems of the hybrid poplar; however, it exhibited low expression in the roots (Figure S1). It was highly expressed in the stems. Its expression increased under salt and drought stresses (Figure S1). However, it either slightly decreased or did not significantly change under cold and heat stresses (Figure S1). These outcomes indicate the involvement of PagMYB148 in the salt or drought stress responses.
3.3. Generation of PagMYB148 Mutants Using CRISPR/Cas9 System
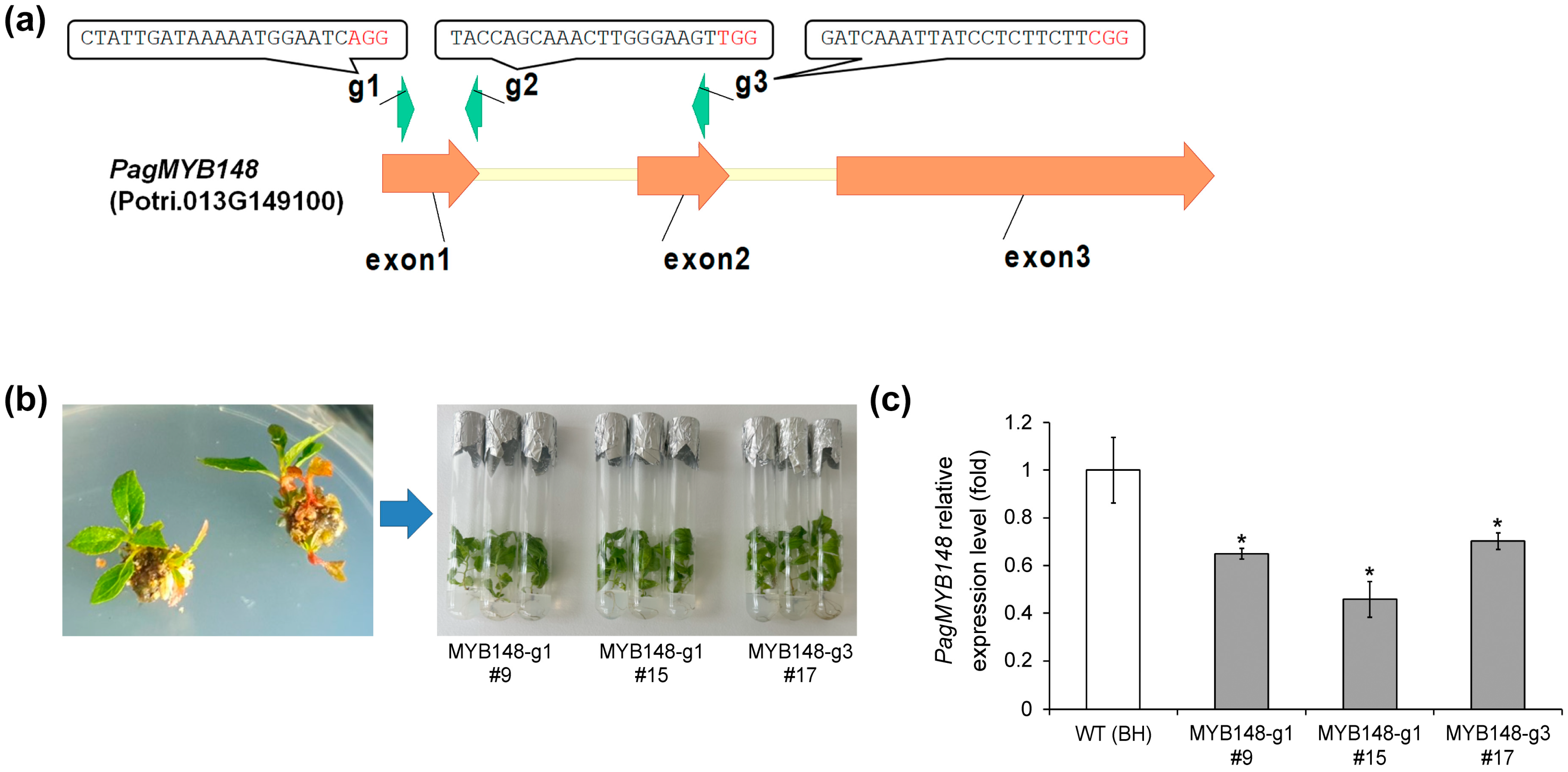
To confirm the role of PagMYB148 in abiotic stress responses, knockout mutants were generated using CRISPR/Cas9 genome-editing technology. We input the genomic DNA sequence of PagMYB148 on the CRISPR RGEN tool website, selected the nuclease to be Cas9, the PAM sequence to be NGG, and the gRNA sequence excluding the PAM sequence to be 20 bp, and used the Poplar 84 K database as a reference to obtain a gRNA targeting PagMYB148. From the results, three gRNAs were selected, considering the number of OFF targets and the out-of-frame score. The selected gRNAs were named gRNA1, 2, and 3 in order of proximity to the start codon (Figure 2). After cloning the gRNA using the AarI site in the pHAtC vector according to a previous report [24], the Agrobacterium transformation method was used to transform the hybrid poplar (clone BH). Transgenic plants were regenerated. The target region was amplified using the genomic DNA of transgenic plants, and the sequence was analyzed by targeted genome sequencing. The expression level of the PagMYB148 gene was investigated in regenerated transgenic plants. Sequencing analysis revealed diverse indel mutations, such as the addition of a base to the upper 3rd to 4th base region of the PAM sequence, deletion of the T base, and deletion of the G base (Table 1). Among them, lines with an indel frequency of 99% or more were selected to investigate the expression level of the PagMYB148 gene, and lines with lower expression compared to that in the wild-type were selected and used in further experiments (Figure 2 and Figure S2).
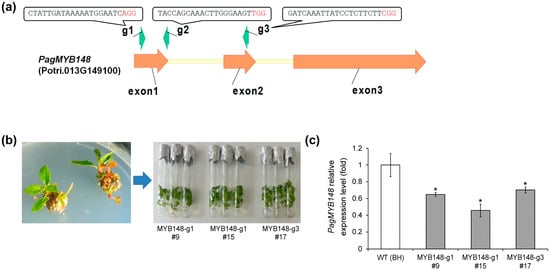
Figure 2.
Characterization of transgenic mutants using CRISPR/Cas9 genome editing systems. (a) Schematic diagram of PagMYB148 gene. The indicated target sites of three types of guide RNA are represented. (b) Regenerated plants. (c) Abundance of PagMYB148 transcripts in distinct regenerated plants. The values represent the means ± standard deviations (SDs) of three biological replicates. Asterisks indicate significant differences as determined by Student’s t-test (*, p < 0.05).

Table 1.
Comparison of the target nucleotides and putative amino acid sequences of the PagMYB148-edited transgenic lines induced by CRISPR/Cas9. The blue letters denote the protospacer adjacent motif (PAM). Within the sequences, red letters indicate inserted nucleotides while the red dashes represent the deleted nucleotides.
3.4. CRISPR/Cas9-Mediated PagMYB148 Mutants Exhibited Reduced Salt Resistance
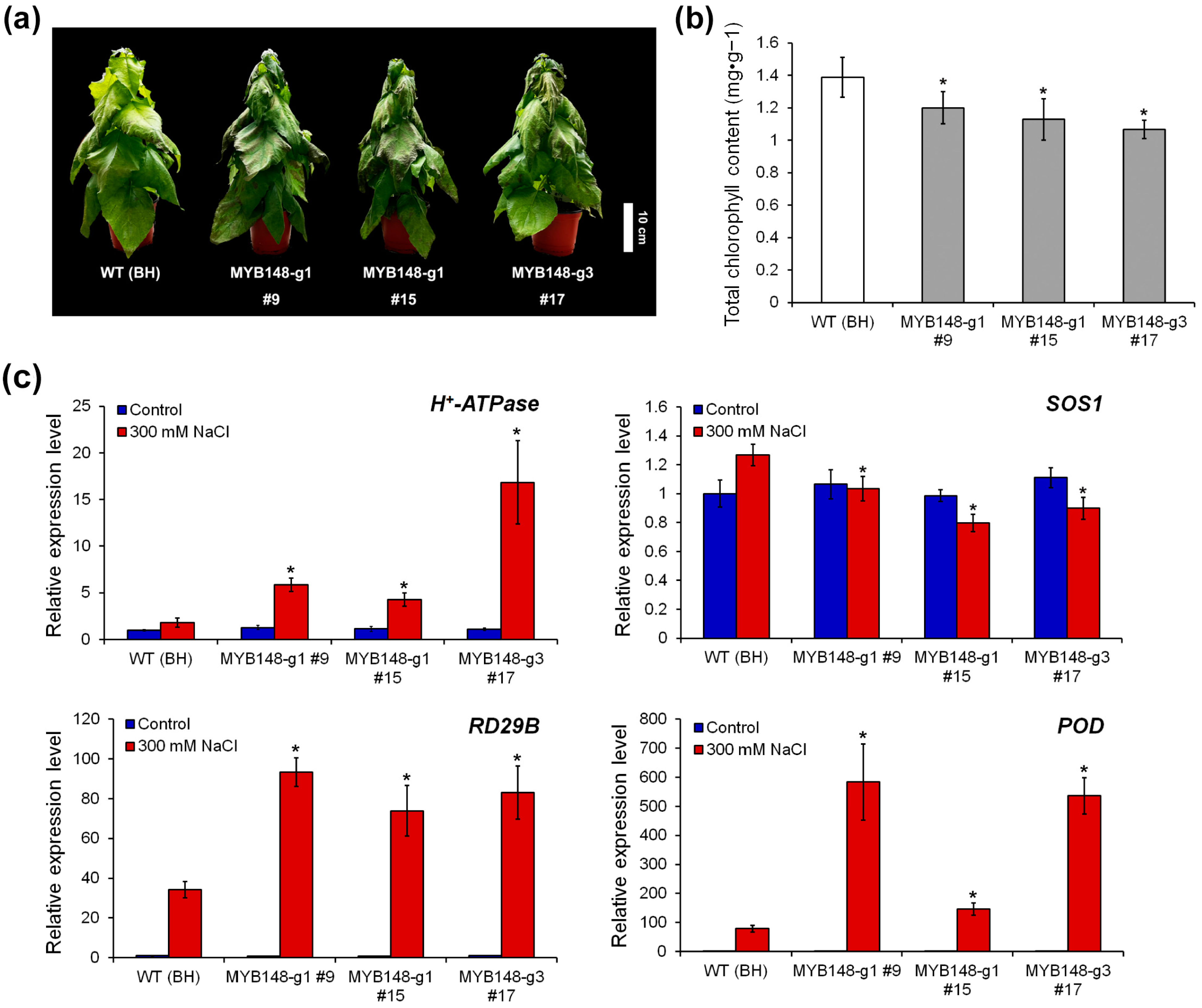
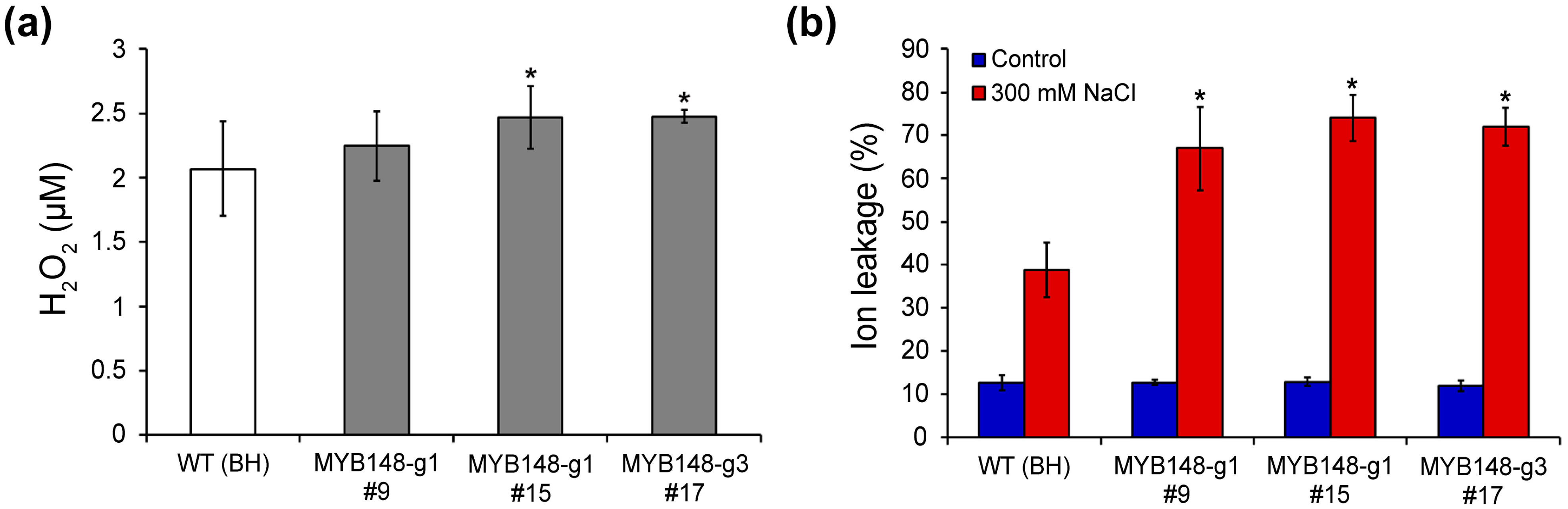
To confirm the role of PagMYB148 in the abiotic stress response, 6-week-old WT (clone BH) and pagmyb148-transgenic poplar plants were exposed to drought and salt stress treatments after watering was stopped. WT and transgenic plants were exposed to natural dehydration under drought stress. Salt stress was induced by irrigation with 300 mM NaCl. Under normal growth conditions, no significant differences in plant height or root diameter were detected between the transgenic and wild-type plants (Figure S3). Five days after drought stress treatment, the phenotypes did not significantly differ between the wild-type and the pagmyb148-transgenic plants (Figure S4). However, pagmyb148 plants exhibited yellowing or necrosis of leaves and a withering phenotype 5 d after salt stress treatment (Figure 3a). After salt stress treatment, the chlorophyll content of pagmyb148 plants was lower than that of the control plants (Figure 3b). Moreover, the enhanced expression levels of genes involved in the salt stress response were demonstrated during salt stress treatment, and their expression levels tended to be significantly higher in pagmyb148 plants than in wild-type plants (Figure 3c). While analyzing the degree of salt stress-induced cell membrane damage in terms of electrolyte leakage and H2O2 contents, we identified little difference in the degree of these properties between pagmyb148 and the control plants before salt stress treatment. However, after salt stress treatment, the pagmyb148 plants exhibited significantly higher electrolyte leakage than that detected in the control group (Figure 4). The H2O2 level was higher in the pagmyb148 plants compared to the control group when treated with salt stress (Figure 4b). Therefore, these outcomes indicate that the inhibition of PagMYB148 function through gene editing weakened the salt stress resistance in the hybrid poplar.
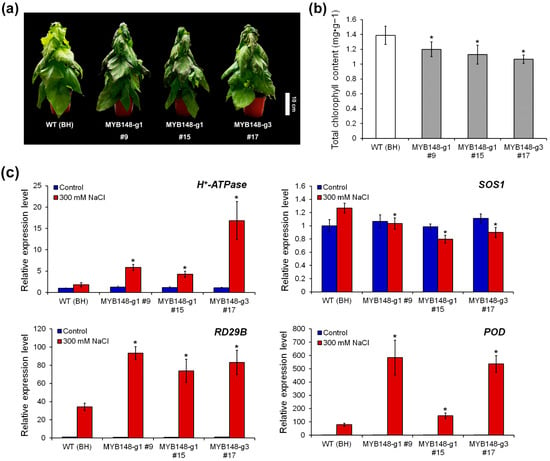
Figure 3.
Knockout of PagMYB15 affects response to salt stress. (a) Phenotype of wild type (WT) (clone BongHwa; BH) and transgenic poplars after being subjected to 300 mM NaCl treatments for 5 days. (b) Chlorophyll contents. (c) Expression changes in stress-related genes in WT and transgenic poplars. The values represent the means ± standard deviations (SDs) of three biological replicates. Asterisks indicate significant differences as determined by Student’s t-test (*, p < 0.05).
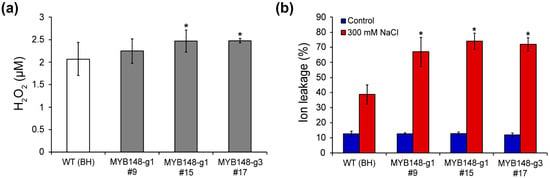
Figure 4.
Characterization of CRISPR/Cas9-mediated mutants based on electrolytic leakage and H2O2 content after high-salinity stress. (a,b) Changes in contents of hydrogen peroxide (H2O2) (a) and electrolytic leakage (b) after salt stress. The error bars indicate the standard deviations of three biological replicates. Asterisks indicate significant differences as determined by Student’s t-test (*, p < 0.05).
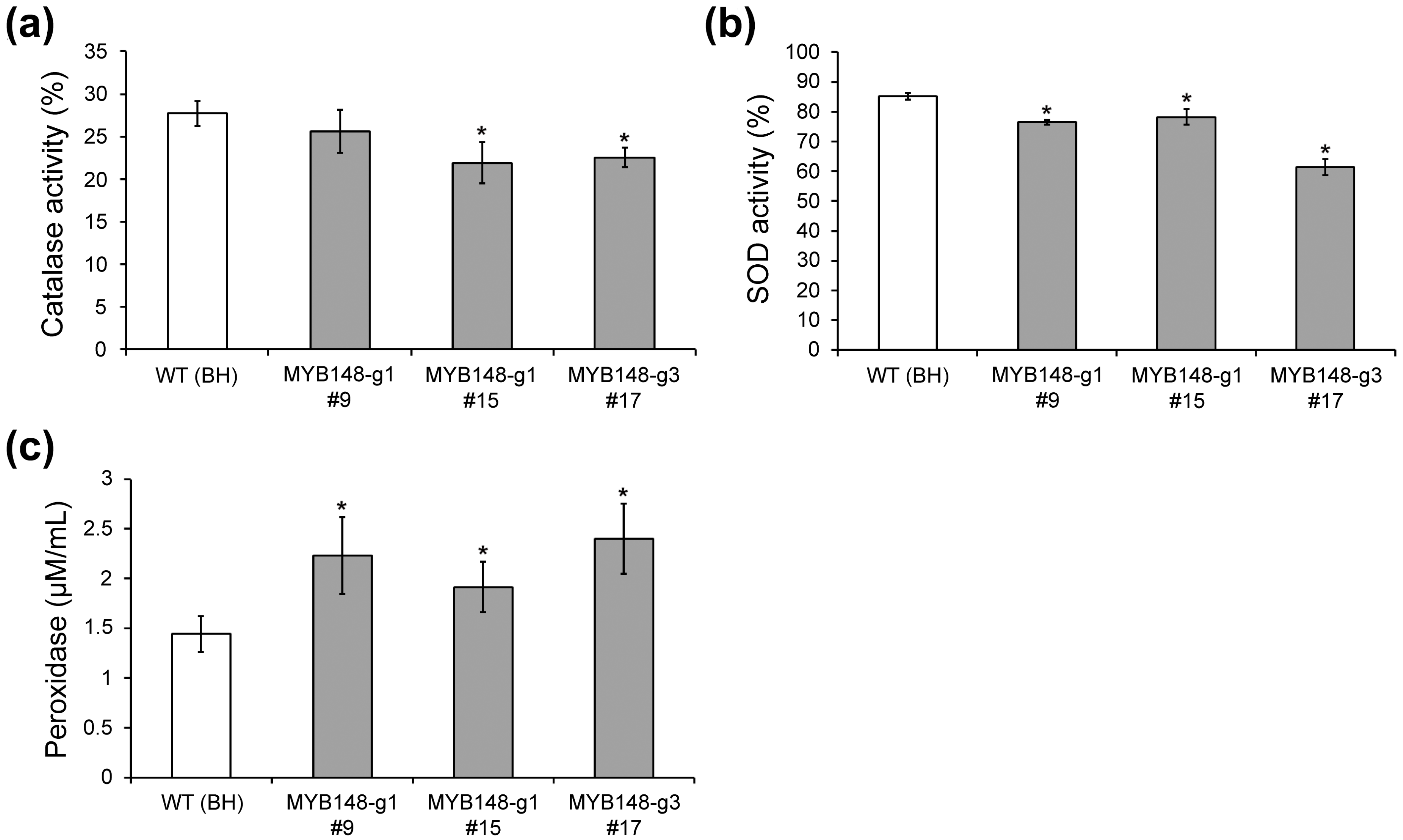
3.5. SOD, CAT, and POD Activities in CRISPR/Cas9-Mediated Mutants Exposed to Salt Stress
We demonstrated reduced catalase, superoxide dismutase (SOD), and peroxidase (POD) activities in pagmyb148 plants as compared to wild-type plants, which further validated the role of PagMYB148 in the salt stress response. The production of reactive oxygen species (ROS) increases in plants exposed to environmental stress, and antioxidant enzymes such as catalase, SOD, and POD are activated through specific defense mechanisms to reduce cellular damage [38,39]. The relative activity of catalase and SOD was decreased in pagmyb148 plants compared to wild-type plants (Figure 5a,b). However, the peroxidase level was higher in pagmyb148 plants than in the wild-type (Figure 5c), which could potentially be attributed to the salt stress-mediated increase in the transcript levels of the POD gene in pagmyb148 plants as compared to the control. These results were comparable to the increase in electrolyte leakage and H2O2 content, indicating that CRISPR/Cas9-mediated mutagenesis of PagMYB148 leads to reduced tolerance to salt stress.
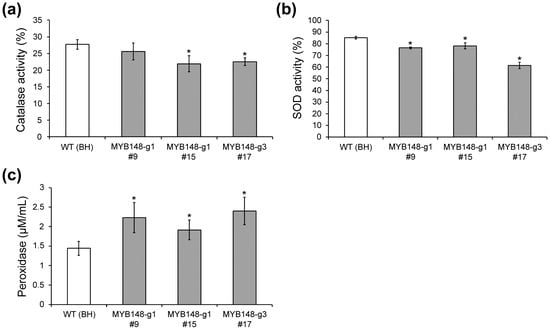
Figure 5.
Characterization of CRISPR/Cas9-mediated mutants based on antioxidant enzyme activities after salt stress. Relative enzyme activities of catalase (a) and superoxide dismutase (SOD, (b)) after salt stress. (c) Peroxidase activity in salt-treated leaves. The error bars indicate the standard deviations of three biological replicates. Asterisks indicate significant differences as determined by Student’s t-test (*, p < 0.05).
4. Discussion
4.1. Identification and Generation of CRISPR/Cas9-Mediated Mutagenesis of PagMYB148
Recent extreme changes in global climate and increased human activity has increased the need for engineered woody plants with enhanced resistance to stresses which can survive under extreme environmental conditions [40]. Therefore, the discovery of factors that affect the environmental stress adaptation process and the identification of their functions are crucial [7,41]. With advances in gene editing technology, efforts are being made to cultivate varieties that are well-adapted to environmental stress by editing factors that regulate environmental stress through the application of gene editing technology [20]. In this study, PagMYB148, a MYB transcription factor with well-conserved protein sequences among plant species which regulates various physiological responses, was identified in hybrid poplar (Populus alba × P. glandulosa) using CRISPR/Cas9-mediated genome editing.
PagMYB148 is an ortholog of Arabidopsis AtMYB15 (Figure 1), which was initially demonstrated to be involved in the cold stress response in Arabidopsis. AtMYB15 interacts with ICE1 and binds to the CBF promoter to suppress its expression. AtMYB15-OX is sensitive to cold stress, whereas knockout mutants exhibit an increased tolerance to cold stress [42]. A previous study reported that AtMYB15 increases the expression of genes related to ABA synthesis, signal transduction, and response in A. thaliana. AtMYB15-OX is sensitive to ABA and resistant to drought and salt stress [43]. Additionally, AtMYB15 significantly contributes to disease resistance in Arabidopsis. Transcript levels of genes related to lignin synthesis were reduced in atmyb15 mutants. Furthermore, atmyb15 mutants exhibited sensitivity to inoculation with avirulent pathogens, and the HR response did not occur properly. AtMYB15 was reported to play an important role in inducing disease resistance through secondary cell wall formation [13]. LsMYB15 and BpMYB15 are also involved in heat stress resistance and flavonoid biosynthesis in lettuce and birch; therefore, they are potentially related to stress responses [44,45]. Moreover, OsMYB4 and ZmMYB39, which are closely related to PagMYB148, are involved in cold stress resistance in rice and maize, respectively [46,47]. VvMYB114, another ortholog of PagMYB148, is associated with anthocyanin biosynthesis in grapes [48]. However, the functions of the other orthologs of PagMYB148 have not been fully explored. MYB transcription factors comprise one of the largest protein families in plants, but they need to be further characterized [7]. Nonetheless, we detected the nuclear expression of PagMYB148, a transcription factor mainly expressed in the aboveground parts of the plant; moreover, we found that its expression increased under drought and salt stresses (Figure 1c and Figure S1). These results indicate that PagMYB148 potentially plays a role in the abiotic stress response.
4.2. CRISPR/Cas9-Mediated Genome Editing of PagMYB148 Diminished Resistance to Salt Stress
We generated pagmyb148 knockout plants using the CRISPR/Cas9 genome editing system (Figure 2). Sequence analysis validated that pagmyb148 knockout mutants harbored mutations in the MYB148 protein sequence (Table 1). A reduced level of PagMYB148 expression was detected in the pagmyb148 plants (Figure 2). Interestingly, the morphology of pagmyb148 knockout plants did not significantly differ from that of wild-type plants acclimatized in the soil (Figure S3). The phenotype of plants exposed to drought and salt stress conditions at the 6th week of acclimatization was analyzed. No significant difference in phenotype was identified between wild-type and pagmyb148 knockout plants exposed to drought stress (Figure S4). However, under salt stress, pagmyb148 knockout plants more rapidly withered compared to the wild-type plants (Figure 3a). The measurement of the chlorophyll content, which is considered an indicator of stress-induced yellowing of leaves, revealed a lower concentration of chlorophyll in the pagmyb148 knockout mutants than in the wild-type plants (Figure 3b). In addition, the expression of genes such as H+-ATPase, RD29B, and POD, which are involved in the salt stress response, increased during salt stress treatment in the wild-type, but the stress-induced enhancement of the expression of these genes was more evident in the pagmyb148 knockout mutants than in the wild-type (Figure 3c). However, the abundance of SOS1 transcripts decreased in pagmyb148 knockout plants compared to the wild-type after the salt stress treatment (Figure 3c). H+-ATPase and SOS1 encode cation transporters present in plant cell membranes and play important roles in controlling the concentration gradient of cations using energy and maintaining intracellular osmotic pressure [49,50]. The RD29B gene codes for dehydrin and is considered a salt stress marker [51], and POD is a gene that codes for peroxidase, which breaks down H2O2. Considering the altered expression profiles of genes involved in the stress response, we speculate that the stress response appears quickly in pagmyb148 knockout mutants, resulting in enhanced susceptibility to salt stress. Shoot-expressed genes are involved in reducing Na+ toxicity and preserving leaf turgor under salt stress conditions [52]. PagMYB148 is highly expressed in shoots of hybrid poplar (Figure S1). The disruption of PagMYB148 was associated with a decline in the transcript levels of the SOS1 gene, which contributed to the attenuation of pagmyb148 knockout plants under salt stress.
4.3. Disruption of PagMYB148 Reduced Cell Retentivity
Stress-induced cellular damage is associated with increased levels of H2O2 and ion leakage owing to damage in cell membranes. To prevent cell damage induced by reactive oxygen species generated by H2O2, plant cells modulate antioxidant enzyme activity and protect the cells through a series of processes [40]. Our analyses of these indicators revealed that salt stress treatment significantly increased the intracellular H2O2 amount and ion leakage in pagmyb148 transgenic plants, moreso than in the wild-type (Figure 4). Hence, the salt stress treatment caused more severe cell damage in pagmyb148 knockout plants, indicating that the loss of function of MYB148 made the plant more vulnerable to salt stress-associated cell damage. Additionally, after salt stress treatment, antioxidant enzymes, including catalase and SOD, exhibited lower activity in pagmyb148 knockout plants than in wild-type plants (Figure 5a,b). Similar to the changes in H2O2 and ion leakage, these results indicate that the inhibition of MYB148 function increases susceptibility to stress by suppressing the activity of antioxidant enzymes crucial for salt stress tolerance. However, the post-salt treatment peroxidase level was slightly higher in the pagmyb148 transgenic plants than in the wild-type plants (Figure 5c), which could potentially be attributed to the enhanced expression of the POD gene in myb148 transgenic plants.
Similar to AtMYB15, which binds to the ICE promoter in Arabidopsis [53], PagMYB148 has a binding motif; however, genes presumed to be involved in the salt stress response by binding were not found. Further research can elucidate the detailed regulatory mechanisms at the molecular level.
5. Conclusions
In conclusion, PagMYB148 positively modulates the salt stress tolerance in hybrid poplars. Changes in chlorophyll content and ion leakage indicated enhanced damage in pagmyb148 knockout plants as compared to wild-type plants under salt stress. Moreover, changes in H2O2 content, gene expression, and antioxidant enzyme activity validated that the inhibition of PagMYB148 affects adaptation to salt stress conditions. These results suggest the potential involvement of the PagMYB148 transcription factor in salt stress responses and its crucial role in the protection of cells against stress by regulating osmotic pressure through a cation concentration gradient and decomposition of H2O2 in hybrid poplar trees. This and further studies will provide new ideas for PagMYB148-mediated regulatory mechanism of salt resistance and deliver better knowledge regarding the functions of PagMYB148 in plant growth and in response to abiotic stress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15081344/s1, Figure S1: Expression of PagMYB148 gene in response to different abiotic stress and tissues. Figure S2: Relative transcript levels of PagMYB148 in MYB148-g2 lines. Figure S3: Growth indexes and phenotypes of transgenic and wild-type poplars. (a) Photographic image of 6-week-old transgenic and wild-type plants grown in soil; (b) plant height; (c) diameter at root. Figure S4: Phenotypic changes in WT and transgenic poplars under drought stress. Table S1: List of primers used in this study. Table S2. Concentration and purity of RNA samples used in this study.
Author Contributions
S.J.P. and H.C. designed the experiments; S.J.P. and H.-A.J. performed the experiments and analyzed the data; S.J.P., H.L. and H.C. wrote, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the project of National Institute of Forest Science (FG0702-2023-01-2024).
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Materials published online.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| MYB | Myeloblastosis viral oncogene homolog |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| Cas9 | CRISPR-associated protein 9 |
| gRNA | single guide RNA |
| BH | BongHwa |
| WT | Wild type |
| GFP | Green Fluorescence Protein |
| SOD | Superoxide Dismutase |
| POD | Peroxidase |
References
- Hyun, S.K.; Hong, S.C. Inter- and Intra-species hybridization in poplar. Res. Rep. Inst. For. Gen. Korea 1959, 1, 61–73. [Google Scholar]
- Zhang, X.; Liu, L.; Chen, B.; Qin, Z.; Xiao, Y.; Zhang, Y.; Yao, R.; Liu, H.; Yang, H. Progress in understanding the physiological and molecular responses of Populus to salt stress. Int. J. Mol. Sci. 2019, 20, 1312. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.; Yeo, J. The status and prospect of poplar research in Korea. J. Korea For. Energy 2003, 22, 1–17. [Google Scholar]
- Qiu, D.; Bai, S.; Ma, J.; Zhang, L.; Shao, F.; Zhang, K.; Yang, Y.; Sun, Y.; Huang, J.; Zhou, Y.; et al. The genome of Populus alba × Populus tremula var. glandulosa clone 84K. DNA. Res. 2019, 26, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Bae, E.; Kim, M.; Park, S.J.; Choi, N.; Pyo, S.; Lee, C.; Jeong, H.; Lee, H.; Choi, Y.; et al. CRISPR-knockout of CSE gene improves saccharification efficiency by reducing lignin content in hybrid poplar. Int. J. Mol. Sci. 2021, 22, 9750. [Google Scholar] [CrossRef] [PubMed]
- Ynag, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 69, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, J.; Wang, X.; Fan, Y.; Liu, Q.; Han, Y. PagERF16 of Populus promotes lateral root proliferation and sensitizes to salt stress. Front. Plant Sci. 2021, 12, 669143. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, X.; Jiang, M. CRISPR/Cas9-mediated mutagenesis of WRKY3 and WRKY4 function decreases salt and Me-JA stress tolerance. Mol. Biol. Rep. 2021, 48, 5821–5832. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zou, S.; Huang, J.; Huan, X.; Jin, X.; Zhou, L.; Zhao, K.; Han, Y.; Wang, S. PagMYB151 facilitates proline accumulation to enhance salt tolerance of poplar. BMC Genom. 2023, 24, 345. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, R.; Yang, X.; Ju, Q.; Li, W.; Lu, S.; Tran, L.-S.P.; Xu, J. The R2R3-MYB transcription factor AtMYB49 modulates salt tolerance in Arabidopsis by modulating the cuticle formation and antioxidant defence. Plant Cell Environ. 2020, 43, 1925–1943. [Google Scholar] [CrossRef]
- Mengiste, T.; Chen, X.; Salmeron, J.; Dietrich, R. The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3 MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 2003, 15, 2551–2565. [Google Scholar] [CrossRef]
- Park, M.Y.; Kang, J.Y.; Kim, S.Y. Overexpression of AtMYB52 confers ABA hypersensitivity and drought tolerance. Mol. Cells 2011, 31, 447–454. [Google Scholar] [CrossRef]
- Kim, S.H.; Lam, P.Y.; Lee, M.; Jeon, H.S.; Tobimatsu, Y.; Park, O.K. The Arabidopsis R2R3 MYB transcription factor MYB15 is a key regulator of lignin biosynthesis in effector-triggered immunity. Front. Plant Sci. 2020, 11, 583153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zhang, C.; Guo, X.; Li, H.; Lu, H. MYB transcription factors and its regulation in secondary cell wall formation and lignin biosynthesis during xylem development. Int. J. Mol. Sci. 2021, 22, 3560. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.; Cox, D.; Lin, S.; Barrretto, R.; Habib, N.; Hsu, P.; Wu, X.; Jiang, W.; Marraffini, L.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, Q.; Chen, Y.; Liu, Y.G. CRISPR/Cas9 platforms for genome editing in plants: Developments and applications. Mol. Plant 2016, 9, 961–974. [Google Scholar] [CrossRef]
- Bruegmann, T.; Deecke, K.; Fladung, M. Evaluating the efficiency of gRNAs in CRISPR/Cas9 mediated genome editing in poplars. Int. J. Mol. Sci. 2019, 20, 3623. [Google Scholar] [CrossRef]
- Park, S.; Joung, Y.; Kim, K.; Kim, J.; Koh, H. Gene-edited crops: Present status and their future. Korean J. Breed. Sci. 2019, 51, 175–183. [Google Scholar] [CrossRef]
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef]
- Bae, E.; Choi, H.; Choi, J.; Lee, H.; Kim, S.; Ko, J.; Choi, Y. Efficient knockout of the phytoene desaturase gene in a hybrid poplar (Populus alba × Populus glandulosa) using the CRISPR/Cas9 system with a single gRNA. Transgenic Res. 2021, 30, 837–849. [Google Scholar] [CrossRef]
- Park, J.; Bae, S.; Kim, J. Cas-Designer: A web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef]
- Bae, S.; Park, J.; Kim, J. Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9- RNA-guided endonucleases. Bioinformatics 2014, 30, 1473–1475. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Ryu, J.; Choi, M.K.; Kweon, J.; Kang, B.; Ahn, H.; Bae, S.; Kim, J.; Kim, J.; et al. A simple, flexible and high-throughput cloning system for plant genome editing via CRISPR-Cas system. J. Int. Plant Biol. 2016, 58, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Noh, E.; Han, M.; Lee, J.; Choi, K. An efficient and novel plant selectable marker based on organomercurial resistance. J. Plant Biol. 2005, 48, 351–355. [Google Scholar] [CrossRef]
- Park, J.; Kim, K.; Kim, J.; Bae, S. Cas-Analyzer: An online tool for assessing genome editing results using NGS data. Bioinformatics 2017, 33, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.; Yeom, M.; Kim, J.; Nam, H. FIONA1 is essential for regulating period length in the Arabidopsis circadian clock. Plant Cell 2008, 20, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Theologis, A. Transient gene expression in protoplasts of Arabidopsis thaliana. Methods Mol. Biol. 1998, 82, 209–217. [Google Scholar]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tong, S.; Wang, Y.; Chen, N.; Wang, D.; Liu, B.; Wang, W.; Chen, Y.; Liu, J.; Ma, T.; Jiang, Y. PtoNF-YC9-SRMT-PtoRD26 module regulates the high saline tolerance of a triploid poplar. Genome Biol. 2022, 23, 148. [Google Scholar] [CrossRef]
- Lee, I.H.; Lee, I.C.; Kim, J.; Kim, J.H.; Chung, E.; Kim, H.J.; Park, S.J.; Kim, Y.M.; Kang, S.K.; Nam, H.G.; et al. NORE1/SAUL1 integrates temperature-dependent defense programs involving SGT1b and PAD4 pathways and leaf senescence in Arabidopsis. Physiol. Plant. 2016, 158, 180–199. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Song, J.; Yu, D.; Kim, M.; Jung, Y.; Kang, K.K.; Park, S.; Cho, Y. Overexpression of and oligopeptide transporter gene enhances heat tolerance in transgenic rice. J. Plant Biotechnol. 2017, 44, 296–302. [Google Scholar] [CrossRef]
- Lee, K.C.; Han, S.K.; Yoon, K.K.; Lee, H.B.; Song, J.M. Effects of NaCl on the growth and physiological characteristics of Crepidiastrum sonchifolium (Maxim.) Pak & Kawano. Korean J. Med. Crop Sci. 2020, 28, 1–8. [Google Scholar]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Wi, S.; Yu, I.; Yeo, K.; An, S.; Jang, Y.; Jang, S. Enhancement of growth and antioxidant enzyme activities on Kimchi cabbage by melatonin foliar application under high temperature and drought stress conditions. Hortic. Sci. Technol. 2021, 39, 583–592. [Google Scholar] [CrossRef]
- Park, A.; Lee, Y.; Kang, N.; Kang, D.; Heo, S. Antioxidant efficacy of Jeju crop extracts using Jeju lava seawater as a solvent. J. Plant Biotechnol. 2022, 49, 347–355. [Google Scholar] [CrossRef]
- Mader, M.; Paslier, M.; Bounon, R.; Bérard, A.; Rampant, P.; Fladung, M.; Liplé, J.; Kersten, B. Whole-genome draft assembly of Populus tremula × P. alba clone INRA 717-1B4. Silvae Genet. 2017, 65, 74–79. [Google Scholar] [CrossRef]
- Berta, M.; Giovannelli, A.; Caparrini, S.; Racchi, M.L. Expression of antioxidant genes in relation to water deficit in cambium and leaves of poplar. J. Plant Interact. 2005, 1, 223–227. [Google Scholar] [CrossRef]
- Zhao, Y.; Xin, D.; Lu, W.; Zong, X.; Niu, Y.; Guo, X.; Ma, Y.; Qiang, W.; Su, H.; Zhang, S.; et al. PeMPK7 is induced in an ROS-dependent manner and confers poplar para-hydroxybenzoic acid stress resistance through the removal of ROS. Ind. Crops Prod. 2022, 182, 114861. [Google Scholar] [CrossRef]
- Harfouche, A.; Meilan, R.; Altman, A. Molecular and physiological responses to abiotic stress in forest trees and their relevance to tree improvement. Tree Physiol. 2014, 34, 1181–1198. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Agarwal, M.; Hao, Y.; Kapoor, A.; Dong, C.; Fujii, H.; Zheng, X.; Zhu, J. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 2006, 281, 37636–37645. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, S.; An, X.; Liu, X.; Qin, H.; Wang, D. Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. J. Genet. Genom. 2008, 36, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, M.; Liu, C.; Hao, J.; Fan, S.; Han, Y. LsMYB15 regulates bolting in leaf lettuce (Lactuca sativa L.) under high-temperature stress. Front. Plant Sci. 2022, 13, 921021. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, Z.; Fan, G.; Zheng, B. Genome-level analysis of BpR2R3-MYB family genes transcribed in seedlings of Betula platyphylla and BpR2R3-MYB15 enhanced flavonoid production. Chem. Biol. Technol. Agric. 2022, 9, 37. [Google Scholar] [CrossRef]
- Vannini, C.; Locatelli, F.; Bracale, M.; Magnani, E.; Marsoni, M.; Osnato, M.; Mattana, M.; Baldoni, E.; Coraggio, I. Overexpression of the rice Osmyb4 gene increases chilling and freezing tolerance of Arabidopsis thaliana plants. Plant J. 2004, 37, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shi, Y.; Liu, J.; Li, Z.; Fu, D.; Wu, S.; Li, M.; Yang, Z.; Shi, Y.; Lai, J.; et al. Natural polymorphism of ZmICE1 contributes to amino acid metabolism that impacts cold tolerance in maize. Nat. Plants 2022, 8, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Tirumalai, V.; Swetha, C.; Nair, A.; Pandit, A.; Shivaprasad, P.V. miR828 and miR858 regulate VvMYB114 to promote anthocyanin and flavonol accumulation in grapes. J. Exp. Bot. 2019, 70, 4775–4791. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Isayenkov, S.V. Evolution of plant Na+-P-type ATPases: From saline environments to land colonization. Plants 2021, 10, 221. [Google Scholar] [CrossRef]
- Ali, A.; Petrov, V.; Yun, D.; Gechev, T. Revisiting plant salt tolerance: Novel components of the SOS pathway. Trends Plant Sci. 2023, 28, 1060–1069. [Google Scholar] [CrossRef]
- Galović, V.; Joseph, M.P.; Pekeč, S.; Vasić, V.; Vasić, S.; Szabados, L. Characterization of abiotic stress-responsive RD29B and RD17 genes in different poplar clones. Topola/Poplar 2020, 206, 13–20. [Google Scholar] [CrossRef]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.; Liu, Q.; Ahammed, G.J.; Lin, R.; Wang, L.; Shao, S.; Yu, J.; Zhou, Y. The HY5 and MYB15 transcription factors positively regulate cold tolerance in tomato via the CBF pathway. Plant Cell Environ. 2020, 43, 2712–2726. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).