Applying Specific Habitat Indicators to Study Asteraceae Species Diversity Patterns in Mountainous Area of Beijing, China
Abstract
:1. Introduction
2. Materials and Methods
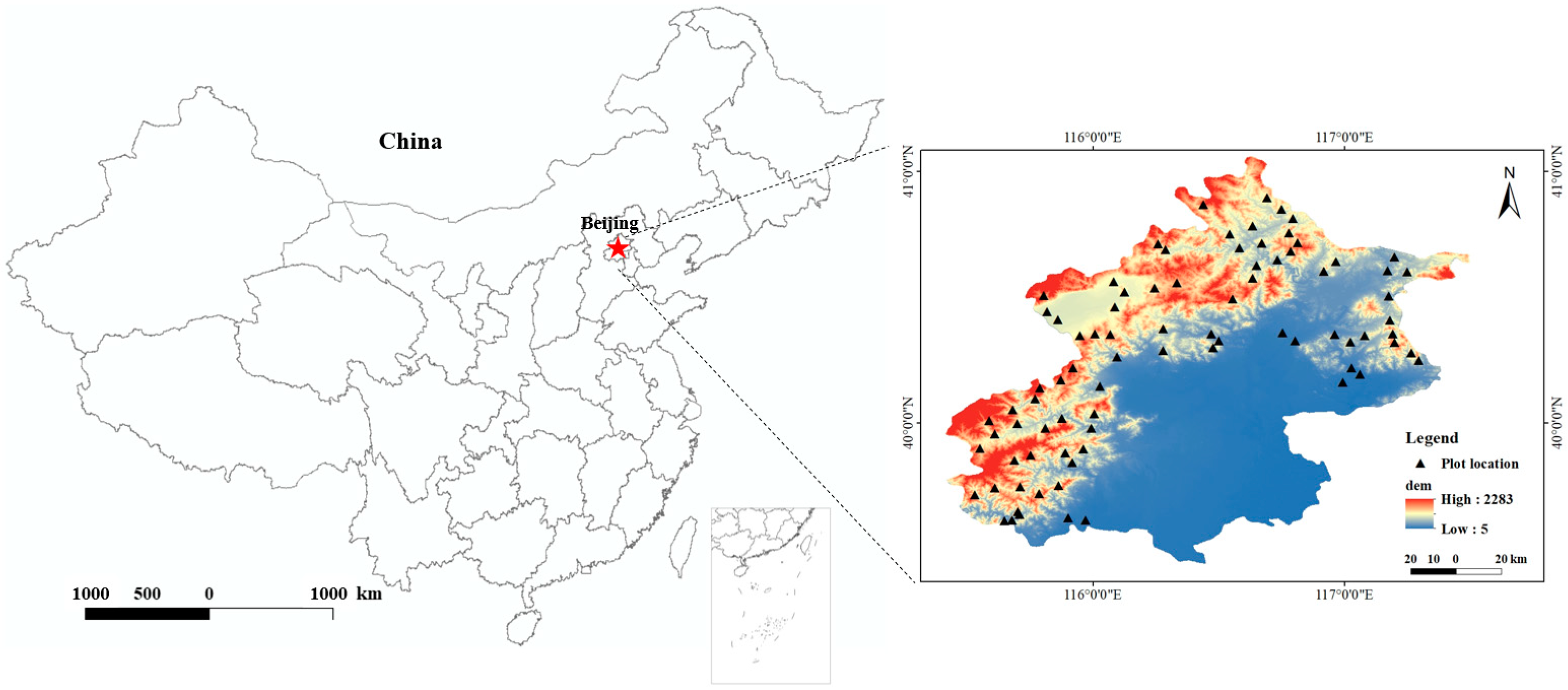
2.1. Study Sites
2.2. Field Investigation and Soil Sampling
2.3. Data Analysis and Modeling
2.3.1. Asteraceae Diversity Index Calculation
2.3.2. Maxent Model
3. Results
3.1. The Relationship between the Asteraceae Species Diversity Indices
3.2. Effects of Environmental Factors on Asteraceae Species Diversity
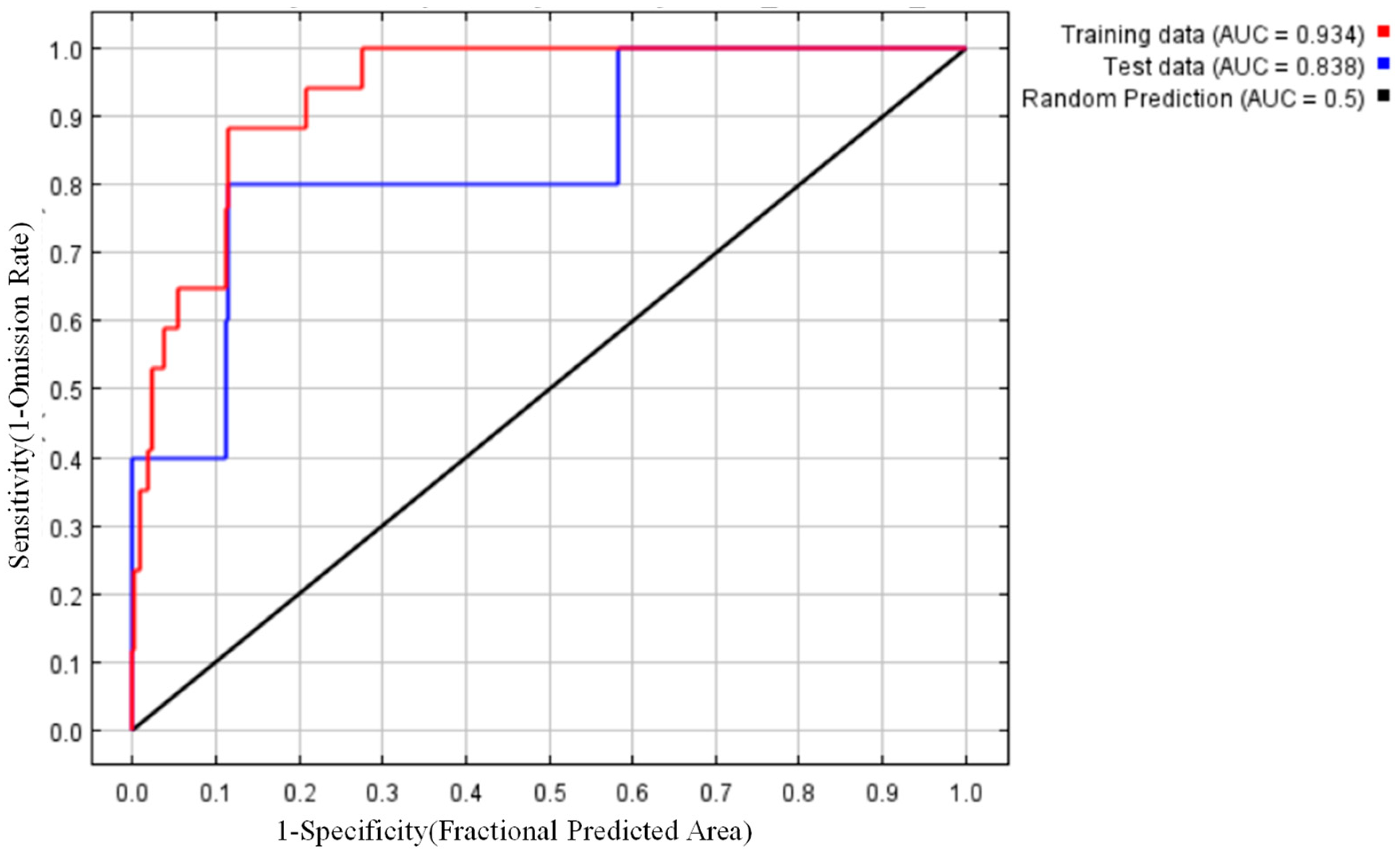
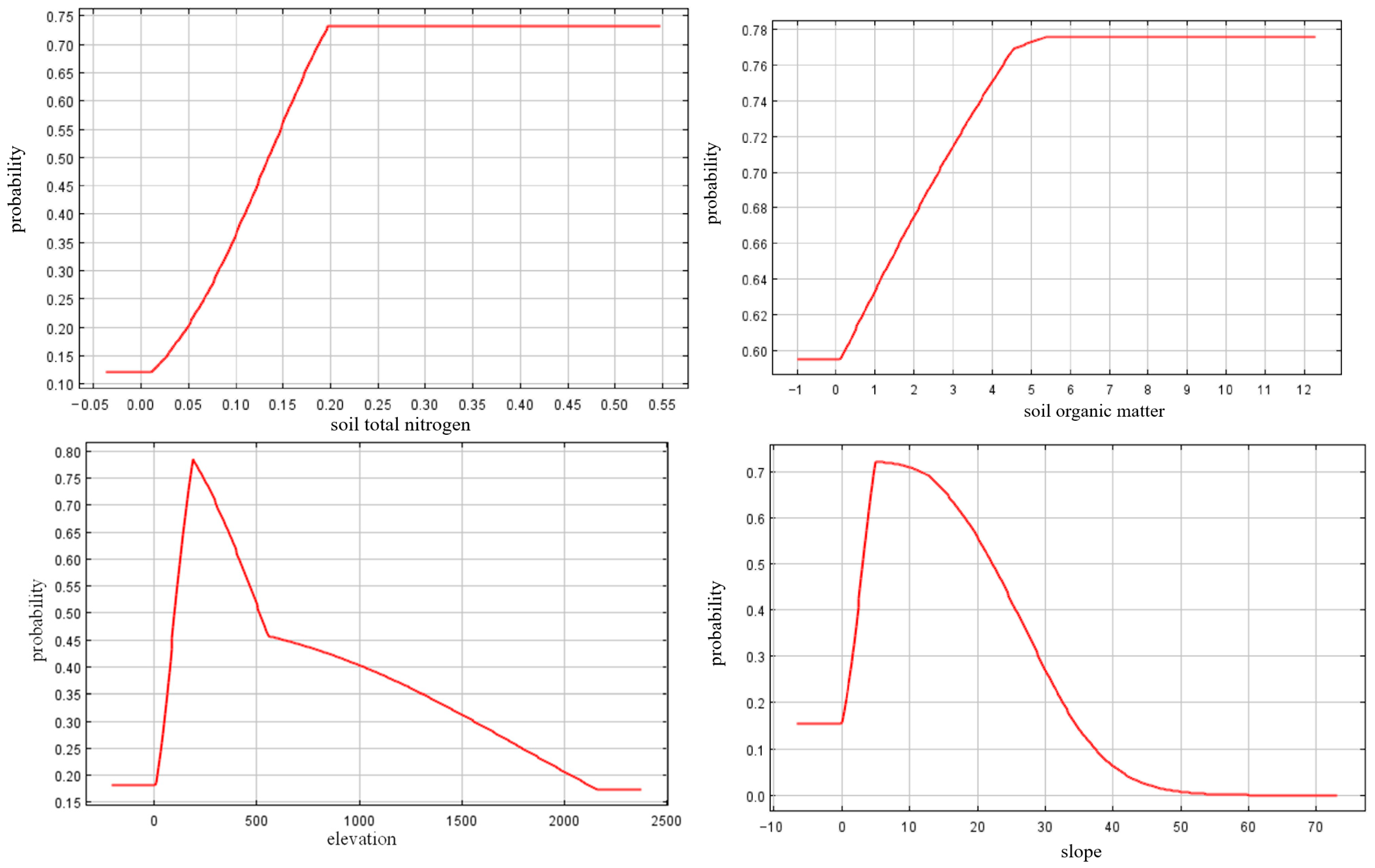
3.3. Maximum Entropy Model Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, C.J. Studies on Plant Diversity in Mountain Areas in Beijing. Master’s Thesis, Beijing Forestry University, Beijing, China, June 2005. [Google Scholar]
- Pan, Y.Z.; Chen, L.; Chen, J.Y.; Zhao, D.; Ye, D.L.; Wang, Z.H.; Wang, H.Y. Effects of Nitrogen and Phosphorus Addition on Medicinal Plant Community Characteristics in the Hulunbuir Meadow Steppe. Chin. J. Grassl. 2024, 6, 117–125. (In Chinese) [Google Scholar]
- Ali, F.; Khan, N.; Rahmonov, O. Ecosystem Services and Linkages of Naturally Managed Monotheca buxifolia (Falc.) A. DC. Forests with Local Communities across Contiguous Mountainous Ranges in Pakistan. Biology 2022, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Bilal, A.; Wang, Y.; Jia, H.; Liu, Y.; Eve, B.; Zhang, K. Optimizing stand structure for trade-offs between overstory timber production and understory plant diversity: A case-study of a larch plantation in northwest China. Land Degrad. Dev. 2018, 29, 2998–3008. [Google Scholar]
- Makse, H.A.; Lucini, F.A.; Morone, F.; Tomassone, M.S. Diversity increases the stability of ecosystems. PLoS ONE 2020, 15, e0228692. [Google Scholar]
- Du, Z.Z.; Yan, P.; Huang, G.; Ren, S.S. Biodiversity of Asteraceae from Oasis and Desert of Shihezi. J. Northwest Bot. 2013, 33, 1034–1040. (In Chinese) [Google Scholar]
- Wu, B.C.; Zhou, L.J.; Qi, S.; Jin, M.L.; Hu, J.; Lu, J.S. Effect of habitat factors on the understory plant diversity of Platycladus orientalis plantations in Beijing mountainous areas based on maxent model. Ecol. Indic. 2021, 129, 107917. [Google Scholar] [CrossRef]
- Wu, D.H.; Zhao, X.; Liang, S.L.; Zhou, T.; Huang, K.C.; Tang, B.J.; Zhao, W.Q. Time-lag effects of global vegetation responses to climate change. Glob. Chang. Biol. 2015, 21, 3520–3531. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; Kamran, M.; Chang, S.H.; Peng, Z.H.; Wang, Z.F.; Ran, L.J.; Hou, F.J. Climate-soil interactions improve the stability of grassland ecosystem by driving alpine plant diversity. Ecol. Indic. 2022, 141, 109002. [Google Scholar] [CrossRef]
- Mi, J.; Ou, J.; Liu, H.; Shi, J.; Chen, D.M.; Bai, Y.F. The loss of plant species diversity dominated by temperature promotes local productivity in the steppe of eastern Inner Mongolia. Ecol. Indic. 2022, 139, 108953. [Google Scholar] [CrossRef]
- Sabatini, F.; Jiménez-Alfaro, B.; Burrascano, S.; Blasi, C. Drivers of herb-layer species diversity in two unmanaged temperate forests in northern Spain. Community Ecol. 2014, 15, 147–157. [Google Scholar] [CrossRef]
- Yang, Y.F.; Xu, B.; Yu, Q.Q.; Fan, L.K.; Guo, T.T.; Fu, D.S.; Chen, H.; Yan, H.; Shao, F.; Li, X.P. Distribution Pattern and Factors Influencing Spontaneous Plant Diversity in Different Wetland Habitats. Forests 2022, 13, 1678. [Google Scholar] [CrossRef]
- Zilliox, C.; Gosselin, F. Tree species diversity and abundance as indicators of understory diversity in French mountain forests: Variations of the relationship in geographical and ecological space. For. Ecol. Manag. 2014, 321, 105–116. [Google Scholar] [CrossRef]
- Zhang, Q.P.; Fang, R.Y.; Deng, C.Y.; Zhao, H.J.; Shen, M.H.; Wang, Q. Slope aspect effects on plant community characteristics and soil properties of alpine meadows on Eastern Qinghai-Tibetan plateau. Ecol. Indic. 2022, 143, 109400. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, S.; Zhou, P.; Wu, B.C.; Zhang, D.; Cui, R.R.; Huang, X.; Ma, N. Influencing factors on herb diversity of Platycladus platycladus understory in Beijing Mountain area. J. Grassl. Sci. 2022, 30, 1794–1801. (In Chinese) [Google Scholar]
- Zhang, L.; Qi, S.; Li, P.; Zhou, P. Influence of stand and environmental factors on forest productivity of Platycladus orientalis plantations in Beijing’s mountainous areas. Ecol. Indic. 2024, 158, 111385. [Google Scholar] [CrossRef]
- He, K.J.; Huang, Y.M.; Qi, Y.; Sheng, Z.L.; Chen, H.Y. Effects of nitrogen addition on vegetation and soil and its linkages to plant diversity and productivity in a semi-arid steppe. Sci. Total Environ. 2021, 778, 146299. [Google Scholar] [CrossRef]
- Dong, D.; Ashfaq, A.; Huang, X.; Teng, M.J.; Wu, C.G.; Zhou, Z.X.; Liu, Y. Soil Available Phosphorus Loss Caused by Periodical Understory Management Reduce Understory Plant Diversity in a Northern Subtropical Pinus massoniana Plantation Chronosequence. Forests 2020, 11, 231. [Google Scholar] [CrossRef]
- Cui, R.R.; Qi, S.; Wu, B.C.; Zhang, D.; Zhang, L.; Zhou, P.; Ma, N.; Huang, X. The Influence of Stand Structure on Understory Herbaceous Plants Species Diversity of Platycladus orientalis Plantations in Beijing, China. Forests 2022, 13, 1921. [Google Scholar] [CrossRef]
- Wei, L.; Archaux, F.; Hu, F.L.; Bilger, I.; Gosselin, F. Stand attributes or soil micro-environment exert greater influence than management type on understory plant diversity in even-aged oak high forests. For. Ecol. Manag. 2020, 460, 117897. [Google Scholar] [CrossRef]
- Reza, E.; Moslem, Y.; Mosleh, A.A. Effect of different shrub species on their sub-canopy soil and vegetation properties in semiarid regions. Land Degrad. Dev. 2021, 32, 3236–3247. [Google Scholar]
- Zhang, L.; Ning, C.D.; Qi, S.; Hu, Y.S.; Li, P.; Wei, X.Y.; Wang, X.Y. Understory specific species appearances indicate the soil improvement of low-function Platycladus orientalis forest in Beijing’s mountainous areas. Ecol. Indic. 2024, 162, 111996. [Google Scholar] [CrossRef]
- Vanhellemont, M.; Baeten, L.; Verheyen, K. Relating changes in understory diversity to environmental drivers in an ancient forest in northern Belgium. Plant Ecol. Evol. 2014, 147, 22–32. [Google Scholar] [CrossRef]
- Chen, H.; Du, S.; Huang, H.; Tian, L.Y.; Zhou, H.; Wu, J.Q.; Yu, X.B. Plant diversity in the understory of Eucalyptus plantations on Hainan Island and its response to environmental factors. Front. Ecol. Evol. 2024, 12, 1366094. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Peng, J.; Gu, Y.J.; Guo, H.Y.; Jiang, T.Y.; Yang, H.B. The Early Effect of Plant Density on Soil Physicochemical Attributes and Bacterial and Understory Plant Diversity in Phoebe zhennan Plantations. Forests 2023, 14, 1612. [Google Scholar] [CrossRef]
- Zhao, X.N.; Zheng, Y.T.; Wei, W. Habitat Suitability Evaluation of Different Forest Species in Lvliang Mountain by Combining Prior Knowledge and maxent Model. Forests 2023, 14, 438. [Google Scholar] [CrossRef]
- Shi, X.D.; Wang, J.W.; Zhang, L.; Chen, S.X.; Zhao, A.L.; Ning, X.D.; Fan, G.R.; Wu, N.S.; Zhang, L.; Wang., Z.D. Prediction of the potentially suitable areas of Litsea cubeba in China based on future climate change using the optimized Maxent model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Liu, J.M.; Xu, Y.Y.; Sun, C.W.; Wang, X.; Zheng, Y.L.; Shi, S.L.; Jia, L.M. Distinct ecological habits and habitat responses to future climate change in three east and southeast Asian Sapindus species. For. Ecol. Manag. 2022, 507, 119982. [Google Scholar] [CrossRef]
- LY/T 2250-2014; China National Forestry and Grassland Administration. Technical Regulations for Forest Soil Survey; Standards Press of China: Beijing, China, 2014.
- Hebbar, K.B.; Abhin, P.S.; Sanjo, J.V.; Neethu, P.; Santhosh, A.; Shil, S.; Prasad, P.V.V. Predicting the potential suitable climate for coconut (Cocos nucifera L.) cultivation in India under climate change scenarios using the maxent model. Plants 2022, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.H.; Cui, X.Y.; Sun, J.T.; Li, T.T.; Wang, Q.; Ye, X.Z.; Fan, B.G. Analysis of the distribution pattern of Chinese ziziphus jujuba under climate change based on optimized biomed and maxent models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Zhou, H.T.; Na, X.D.; Li, L.; Ning, X.L.; Bai, Y.R.; Wu, X.D.; Zang, S.Y. Suitability evaluation of the rural settlements in a farming-pastoral ecotone area based on machine learning maximum entropy. Ecol. Indic. 2023, 154, 110794. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, S.T.; Gao, Y.; Yang, L.; Yu, H. Prediction of global potential suitable habitats of nicotiana alata link et otto based on Maxent model. Sci. Rep. 2023, 13, 4851. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Wang, H.M.; Tong, J.P.; Bai, Y.; Alatalo, J.M.; Liu, G.; Fang, Z.; Zhang, F. Impacts of environment and human activity on grid-scale land cropping suitability and optimization of planting structure, measured based on the Maxent model. Sci. Total Environ. 2022, 836, 155356. [Google Scholar] [CrossRef]
- Homeier, J.; Leuschner, C. Factors controlling the productivity of tropical and an forests: Climate and soil are more important than tree diversity. Biogeosciences 2021, 18, 1525–1541. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Li, Y.H.; Chen, Q.; Wang, Q.L.; He, K.N. Influence of tree size, local forest structure, topography, and soil Resource availability on plantation growth in Qinghai Province, China. Ecol. Indic. 2021, 120, 106957. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Ji, X.D.; Zhang, Z.Q.; Zhang, H.S.; Zha, T.G.; Li, J. Elevation and total nitrogen are the critical factors that control the spatial distribution of soil organic carbon content in the shrubland on the Bashang Plateau, China. Catena 2021, 204, 105415. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhang, Y.M.; Wang, L.J.; Jing, X.; Yu, L.; Liu, P. Response of leaf biomass, leaf and soil C: N:P stoichiometry characteristics to different site conditions and forest ages: A case of Pinus Tabuliformis Plantations in the temperate mountainous area of China. Front. Plant Sci. 2022, 13, 1060406. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.G.; Qiang, S. Inventory Invasive Alien Species in China; China Environmental Science Press: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Tang, S.C.; Wei, C.Q.; Pan, Y.M.; Pu, G.Z. Reproductive adaptability of the invasive weed Parthenium hysterophorus L. under different nitrogen and phosphorus levels. J. Wuhan Bot. Res. 2010, 28, 213–217. (In Chinese) [Google Scholar]
- Jia, Y.Y.; Zhang, X.Y.; Yan, J.; Yin, J.L.; Zhang, F.J. Effects of three Asteraceae invasive plants on soil fertility of invaded domain. J. Hebei Univ. 2015, 35, 494–502. (In Chinese) [Google Scholar]
- Souza, C.R.; Mariano, R.F.; Pompeu, P.V.; Santos, R.M.; Fontes, M.A. Carbon stock and uptake in the high-elevation tropical montane forests of the threatened Atlantic Forest hotspot: Ecosystem function and effects of elevation variation. Sci. Total Environ. 2023, 882, 163503. [Google Scholar] [CrossRef]
- Zu, K.L.; Zhang, C.C.; Chen, F.S.; Shahid, A.; Ghulam, N. Latitudinal gradients of angiosperm plant diversity and phylogenetic structure in China’s nature reserves. Glob. Ecol. Conserv. 2023, 42, e02403. [Google Scholar] [CrossRef]
- Chen, S.; Ma, M.H.; Wu, S.J.; Tang, Q.Q.; Wen, Z.F. Topography intensifies variations in the effect of human activities on forest NPP across altitude and slope gradients. Environ. Dev. 2023, 45, 100826. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.Z.; Wang, L. The resource distribution and growth status for Playcladus orientalis Plantations in lower mountains of Beijing. For. Resour. Manag. 2011, 6, 73–78. (In Chinese) [Google Scholar]
- Wagner, S.; Fischer, H.; Huth, F. Canopy effects on vegetation caused by harvesting and regeneration treatments. Eur. J. For. Res. 2011, 130, 17–40. [Google Scholar] [CrossRef]



| Environmental and Stand Factors | Ranges | Mean | Standard Deviation |
|---|---|---|---|
| Elevation (m) | 114–770 | 359.69 | 116.12 |
| Slope (°) | 3–39 | 18.81 | 10.02 |
| Soil organic matte content (g/kg) | 13.84–116.58 | 44.68 | 21.35 |
| Soil total nitrogen content (g/kg) | 0.98–6.58 | 2.97 | 1.25 |
| Soil total phosphorus content (g/kg) | 0.24–1.12 | 0.57 | 0.15 |
| Soil total potassium content (g/kg) | 9.25–40.72 | 20.25 | 6.06 |
| Soil available nitrogen content (mg/kg) | 91.57–415.24 | 135.39 | 80.62 |
| Soil available phosphorus content (mg/kg) | 0.09–4.005 | 6.91 | 1.59 |
| Soil available potassium content (mg/kg) | 37.533–444.82 | 1.01 | 0.34 |
| Stand density (plant·hm−2) | 500–1975 | 1035.18 | 286.71 |
| Canopy closure | 0.5–0.95 | 0.76 | 0.11 |
| Impact Factors | Direct Effect | Indirect Effect | Total Effect |
|---|---|---|---|
| Elevation | −0.17 * | −0.06 | −0.24 * |
| Slope | −0.21 * | −0.02 | −0.23 * |
| Soil nutrients | 0.54 ** | 0.54 ** |
| Variable Factors | Contribution Rate (%) | Cumulative Contribution Rate (%) |
|---|---|---|
| Soil total nitrogen | 29.7 | 29.7 |
| Soil organic matter | 28.5 | 58.2 |
| Slope | 8.5 | 66.7 |
| Elevation | 8.1 | 74.8 |
| Soil available nitrogen | 4.9 | 79.7 |
| Aspect | 4.7 | 84.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Qi, S.; Zhao, T.; Li, P.; Wang, X. Applying Specific Habitat Indicators to Study Asteraceae Species Diversity Patterns in Mountainous Area of Beijing, China. Forests 2024, 15, 1348. https://doi.org/10.3390/f15081348
Zhang L, Qi S, Zhao T, Li P, Wang X. Applying Specific Habitat Indicators to Study Asteraceae Species Diversity Patterns in Mountainous Area of Beijing, China. Forests. 2024; 15(8):1348. https://doi.org/10.3390/f15081348
Chicago/Turabian StyleZhang, Lin, Shi Qi, Tianheng Zhao, Peng Li, and Xiangyu Wang. 2024. "Applying Specific Habitat Indicators to Study Asteraceae Species Diversity Patterns in Mountainous Area of Beijing, China" Forests 15, no. 8: 1348. https://doi.org/10.3390/f15081348




