Factors Driving Unexpected Drought-Induced Nothofagus dombeyi Mortality in a Valdivian Temperate Rainforest, Argentina
Abstract
:1. Introduction
2. Materials and Methods
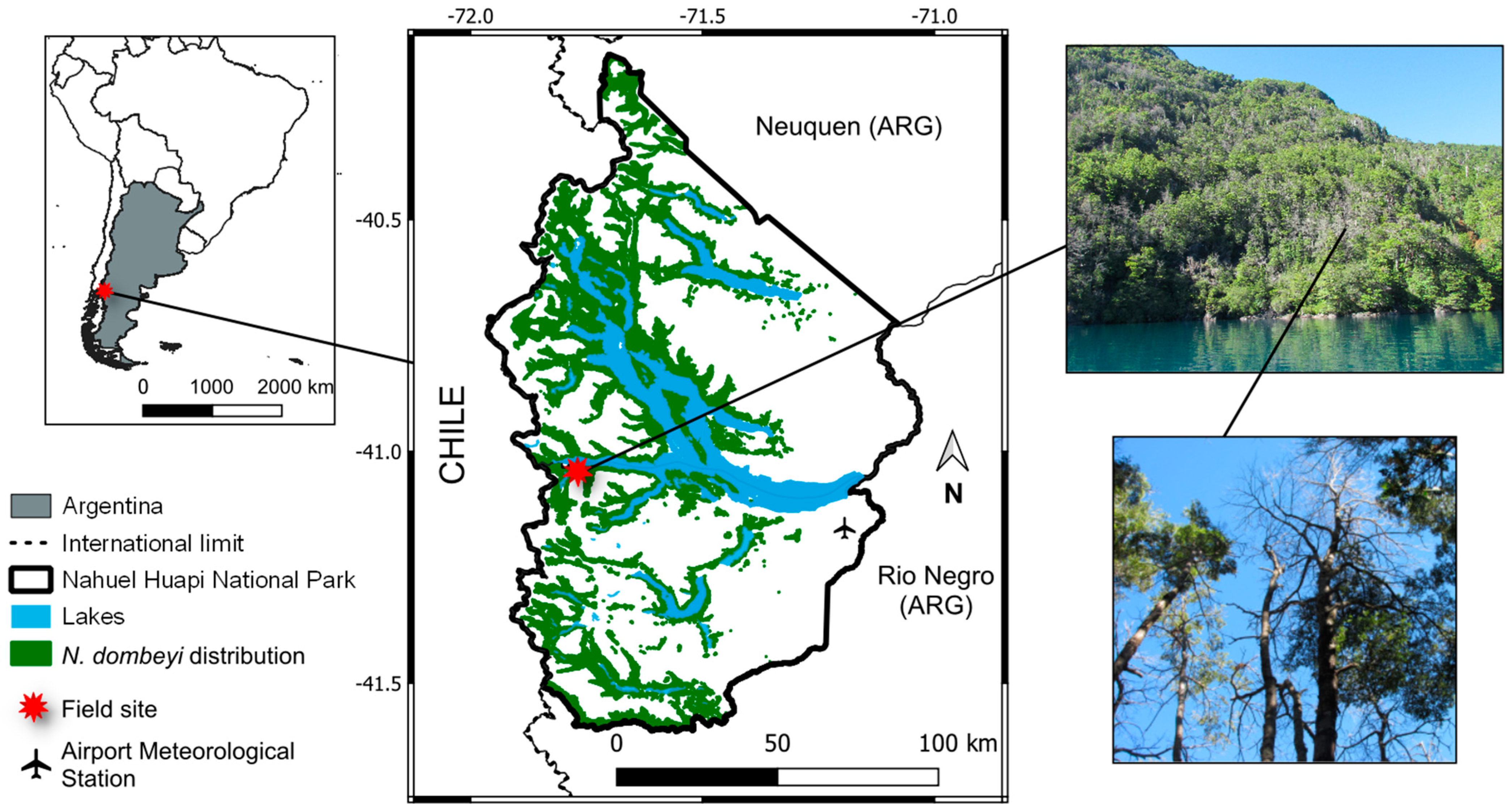
2.1. Study Site and Species
2.2. Climate Data, Drought Index, and Selected Dry Spells
2.3. Field Sampling and Dendrochronological Methods
2.4. Long-Term Growth Trend of Vital and Dead Trees
2.5. Effect of Species Diversity on Tree Growth and Drought Performance
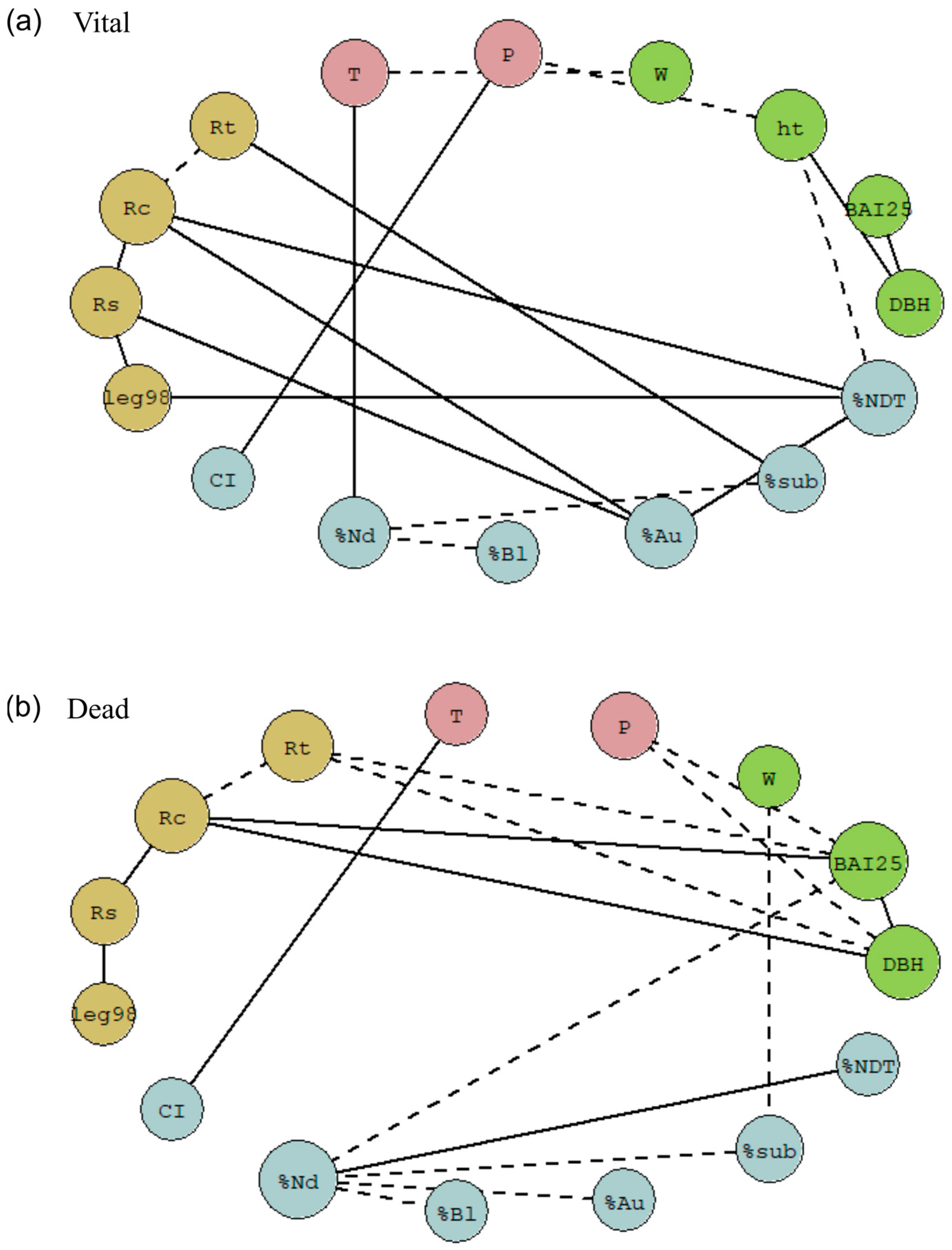
2.6. Network Analysis for Vital and Dead Trees
3. Results
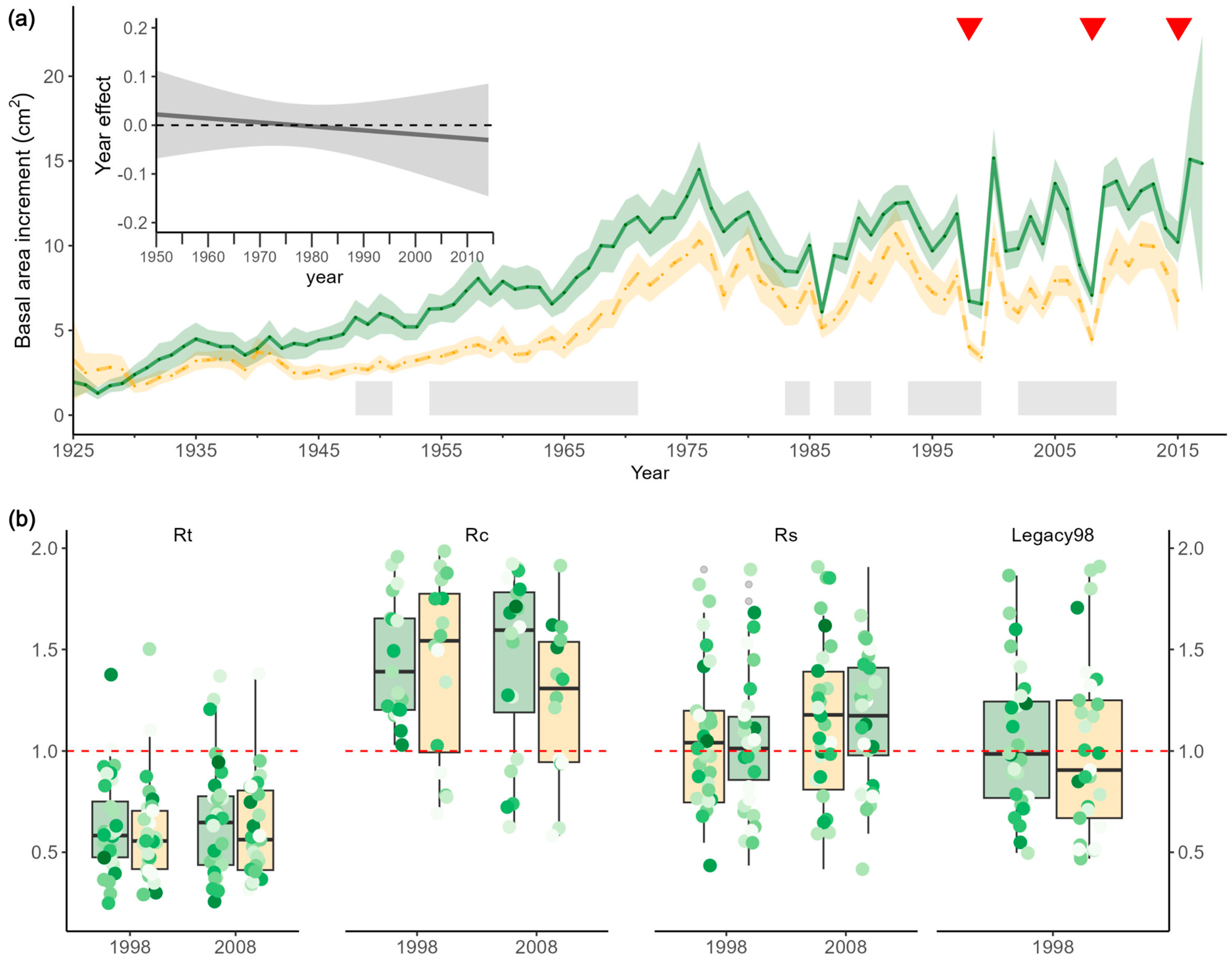
3.1. Long-Term Growth Trend Differed between Vital and Dead Trees
3.2. Role Played by Neighbourhood in Growth and Resilience
3.3. Integrating Predictors of Tree Mortality
4. Discussion
4.1. Tree Size and Long-Term Growth in Relation to Drought Response
4.2. Neighbour Effect on Tree Growth and Drought Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
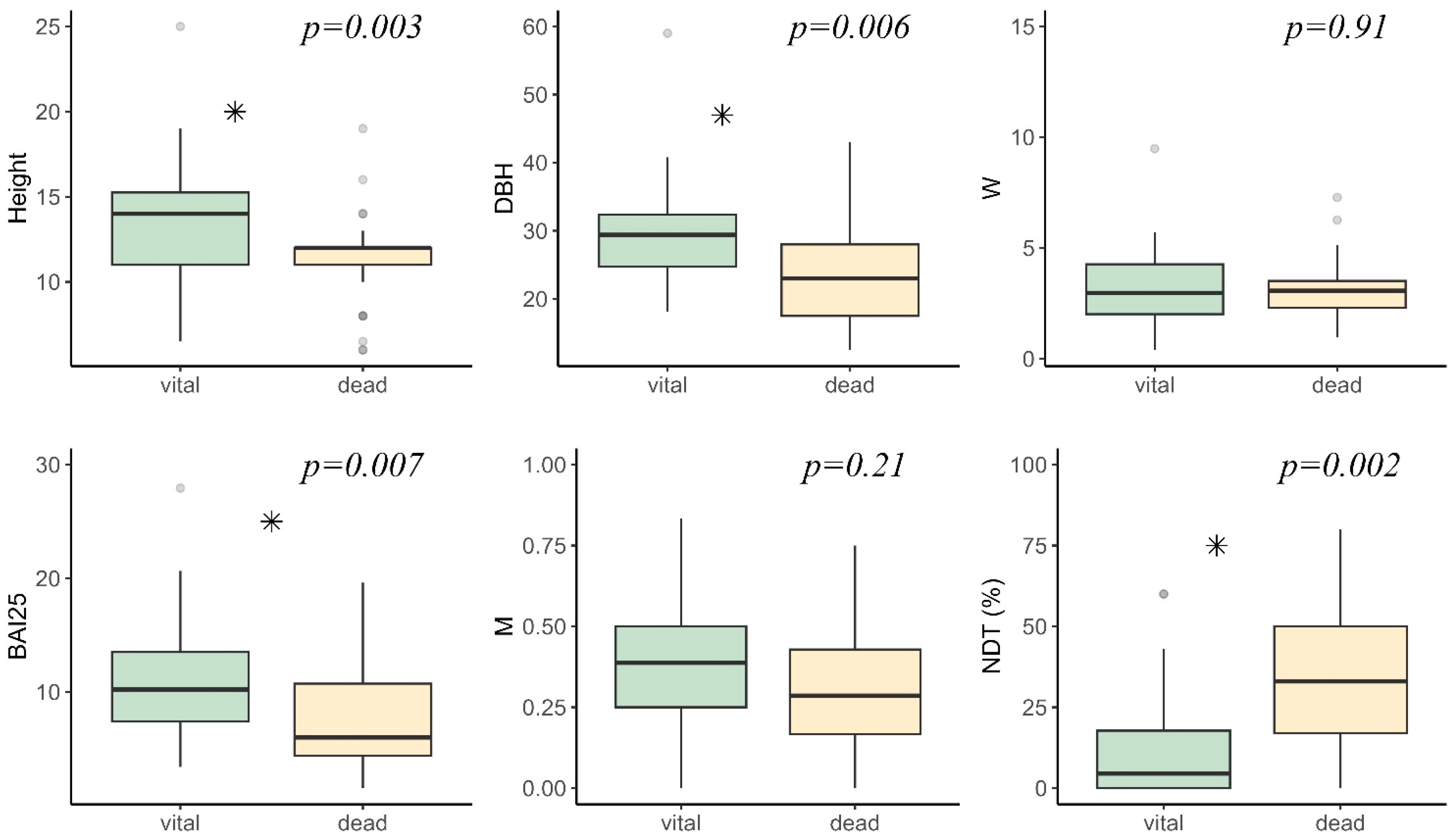
Appendix A. Vital and Dead Tree Characteristics
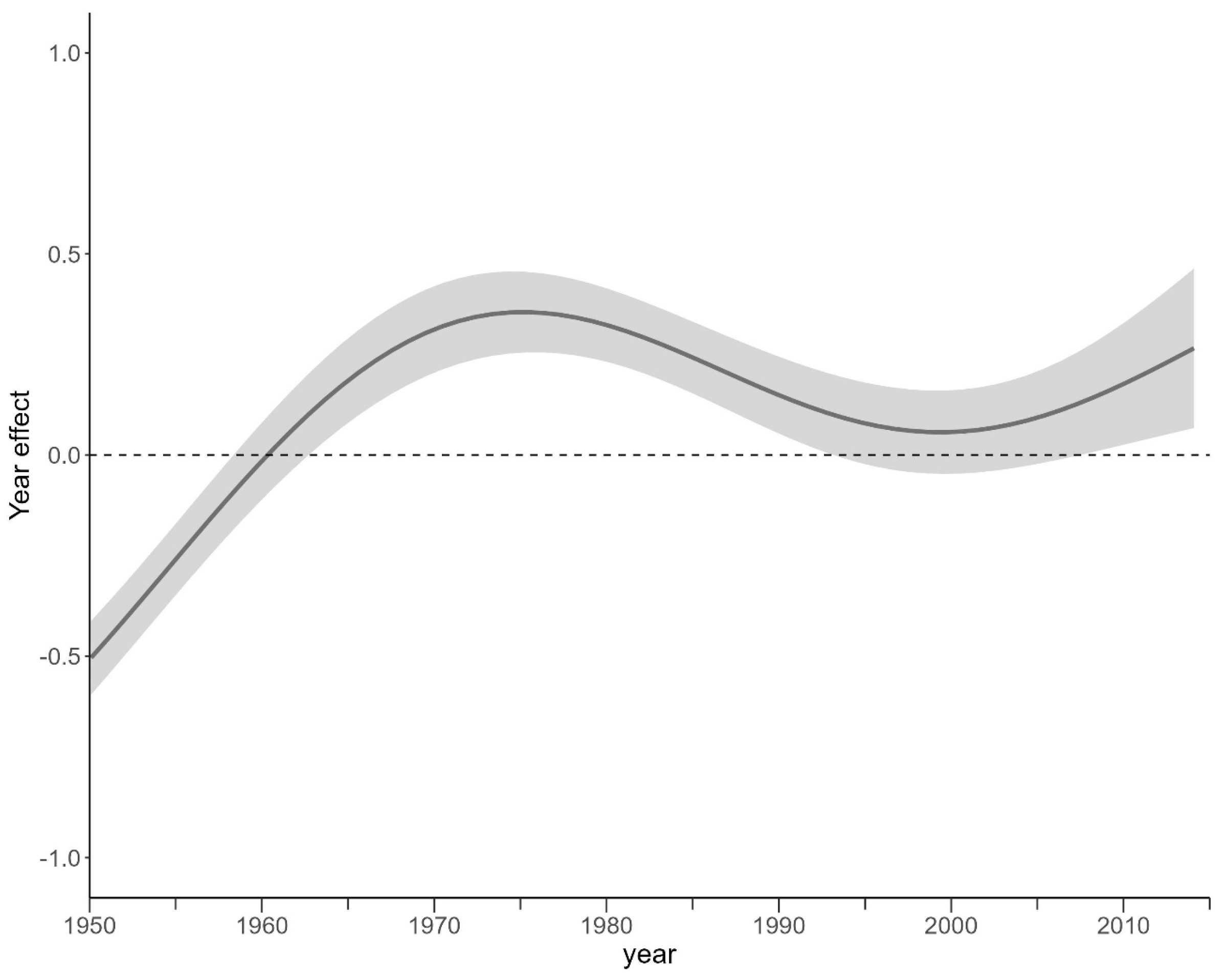
Appendix B. Partial Effect of the General Additive Linear Model

References
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barret, K. IPCC, 2023: Climate Change 2023: Synthesis Report, Summary for Policymakers. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Aguayo, R.; León-Muñoz, J.; Garreaud, R.; Montecinos, A. Hydrological Droughts in the Southern Andes (40–45 S) from an Ensemble Experiment Using CMIP5 and CMIP6 Models. Sci. Rep. 2021, 11, 5530. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Jalabert, R.; Barichivich, J.; Rozas, V.; Lara, A.; Rojas, Y.; Bahamondez, C.; Rojas-Badilla, M.; Gipoulou-Zuñiga, T.; Cuq, E. Climate Response and Drought Resilience of Nothofagus Obliqua Secondary Forests across a Latitudinal Gradient in South-Central Chile. For. Ecol. Manag. 2021, 485, 118962. [Google Scholar] [CrossRef]
- Rodríguez-Catón, M.; Villalba, R.; Srur, A.; Williams, A.P. Radial Growth Patterns Associated with Tree Mortality in Nothofagus Pumilio Forest. Forests 2019, 10, 489. [Google Scholar] [CrossRef]
- Fasanella, M.; Suarez, M.L.; Hasbun, R.; Premoli, A.C. Individual-Based Dendrogenomic Analysis of Forest Dieback Driven by Extreme Droughts. Can. J. For. Res. 2021, 51, 420–432. [Google Scholar] [CrossRef]
- Trugman, A.T.; Anderegg, L.D.L.; Anderegg, W.R.L.; Das, A.J.; Stephenson, N.L. Why Is Tree Drought Mortality so Hard to Predict? Trends Ecol. Evol. 2021, 36, 520–532. [Google Scholar] [CrossRef] [PubMed]
- González de Andrés, E.; Suárez, M.L.; Querejeta, J.I.; Camarero, J.J. Chronically Low Nutrient Concentrations in Tree Rings Are Linked to Greater Tree Vulnerability to Drought in Nothofagus Dombeyi. Forests 2021, 12, 1180. [Google Scholar] [CrossRef]
- Mathias, J.M.; Thomas, R.B. Global Tree Intrinsic Water Use Efficiency Is Enhanced by Increased Atmospheric CO2 and Modulated by Climate and Plant Functional Types. Proc. Natl. Acad. Sci. USA 2021, 118, e2014286118. [Google Scholar] [CrossRef] [PubMed]
- Castagneri, D.; Vacchiano, G.; Hacket-Pain, A.; DeRose, R.J.; Klein, T.; Bottero, A. Meta-Analysis Reveals Different Competition Effects on Tree Growth Resistance and Resilience to Drought. Ecosystems 2022, 25, 30–43. [Google Scholar] [CrossRef]
- Cailleret, M.; Dakos, V.; Jansen, S.; Robert, E.M.; Aakala, T.; Amoroso, M.M.; Antos, J.A.; Bigler, C.; Bugmann, H.; Caccianiga, M. Early-Warning Signals of Individual Tree Mortality Based on Annual Radial Growth. Front. Plant Sci. 2019, 9, 1964. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Ogle, K. Legacies of More Frequent Drought in Ponderosa Pine across the Western United States. Glob. Change Biol. 2019, 25, 3803–3816. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Granda, E.; Camarero, J.J.; Vilà-Cabrera, A.; Jump, A.S.; Sánchez-Salguero, R.; Sangüesa-Barreda, G.; Imbert, J.B.; Gazol, A. Impacts of Recurrent Dry and Wet Years Alter Long-term Tree Growth Trajectories. J. Ecol. 2021, 109, 1561–1574. [Google Scholar] [CrossRef]
- Cabon, A.; DeRose, R.J.; Shaw, J.D.; Anderegg, W.R.L. Declining Tree Growth Resilience Mediates Subsequent Forest Mortality in the US Mountain West. Glob. Change Biol. 2023, 29, 4826–4841. [Google Scholar] [CrossRef] [PubMed]
- Serra-Maluquer, X.; Gazol, A.; Anderegg, W.R.L.; Martínez-Vilalta, J.; Mencuccini, M.; Camarero, J.J. Wood Density and Hydraulic Traits Influence Species’ Growth Response to Drought across Biomes. Glob. Change Biol. 2022, 28, 3871–3882. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Dieler, J. The Dependency of the Size-Growth Relationship of Norway Spruce (Picea abies [L.] Karst.) and European Beech (Fagus sylvatica [L.]) in Forest Stands on Long-Term Site Conditions, Drought Events, and Ozone Stress. Trees 2011, 25, 355–369. [Google Scholar] [CrossRef]
- Schmitt, A.; Trouvé, R.; Seynave, I.; Lebourgeois, F. Decreasing Stand Density Favors Resistance, Resilience, and Recovery of Quercus petraea Trees to a Severe Drought, Particularly on Dry Sites. Ann. For. Sci. 2020, 77, 52. [Google Scholar] [CrossRef]
- Bello, J.; Vallet, P.; Perot, T.; Balandier, P.; Seigner, V.; Perret, S.; Couteau, C.; Korboulewsky, N. How Do Mixing Tree Species and Stand Density Affect Seasonal Radial Growth during Drought Events? For. Ecol. Manag. 2019, 432, 436–445. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Gazol, A.; Igual, J.M.; Camarero, J.J. Silver Fir Growth Responses to Drought Depend on Interactions between Tree Characteristics, Soil and Neighbourhood Features. For. Ecol. Manag. 2021, 480, 118625. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; HilleRisLambers, J. Drought Stress Limits the Geographic Ranges of Two Tree Species via Different Physiological Mechanisms. Glob. Change Biol. 2016, 22, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Zapater, M.; Hossann, C.; Bréda, N.; Bréchet, C.; Bonal, D.; Granier, A. Evidence of Hydraulic Lift in a Young Beech and Oak Mixed Forest Using 18O Soil Water Labelling. Trees 2011, 25, 885–894. [Google Scholar] [CrossRef]
- Forrester, D.I. Linking Forest Growth with Stand Structure: Tree Size Inequality, Tree Growth or Resource Partitioning and the Asymmetry of Competition. For. Ecol. Manag. 2019, 447, 139–157. [Google Scholar] [CrossRef]
- González De Andrés, E.; Seely, B.; Blanco, J.A.; Imbert, J.B.; Lo, Y.; Castillo, F.J. Increased Complementarity in Water-limited Environments in Scots Pine and European Beech Mixtures under Climate Change. Ecohydrol. Hydrobiol. 2017, 10, e1810. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-Species versus Monocultures in Plantation Forestry: Development, Benefits, Ecosystem Services and Perspectives for the Future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Gillerot, L.; Forrester, D.I.; Bottero, A.; Rigling, A.; Lévesque, M. Tree Neighbourhood Diversity Has Negligible Effects on Drought Resilience of European Beech, Silver Fir and Norway Spruce. Ecosystems 2021, 24, 20–36. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Bauhus, J. Benefits of Mixtures on Growth Performance of Silver Fir (Abies alba) and European Beech (Fagus sylvatica) Increase with Tree Size without Reducing Drought Tolerance. Front. For. Glob. Change 2019, 2, 79. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Rozas, V.; Génova, M.; Olano, J.M.; Arzac, A.; Gazol, A.; Caminero, L.; Tejedor, E.; De Luis, M.; et al. Resist, Recover or Both? Growth Plasticity in Response to Drought Is Geographically Structured and Linked to Intraspecific Variability in Pinus pinaster. J. Biogeogr. 2018, 45, 1126–1139. [Google Scholar] [CrossRef]
- Vitali, V.; Forrester, D.I.; Bauhus, J. Know Your Neighbours: Drought Response of Norway Spruce, Silver Fir and Douglas Fir in Mixed Forests Depends on Species Identity and Diversity of Tree Neighbourhoods. Ecosystems 2018, 21, 1215–1229. [Google Scholar] [CrossRef]
- Suarez, M.L.; Ghermandi, L.; Kitzberger, T. Factors Predisposing Episodic Drought-Induced Tree Mortality in Nothofagus– Site, Climatic Sensitivity and Growth Trends. J. Ecol. 2004, 92, 954–966. [Google Scholar] [CrossRef]
- Suarez, M.L.; Kitzberger, T. Differential Effects of Climate Variability on Forest Dynamics along a Precipitation Gradient in Northern Patagonia. J. Ecol. 2010, 98, 1023–1034. [Google Scholar] [CrossRef]
- Suarez, M.L.; Villalba, R.; Mundo, I.A.; Schroeder, N. Sensitivity of Nothofagus Dombeyi Tree Growth to Climate Changes along a Precipitation Gradient in Northern Patagonia, Argentina. Trees 2015, 29, 1053–1067. [Google Scholar] [CrossRef]
- Perotti, M.G.; Diéguez, M.C.; Jara, F.G. State of the Knowledge of Moist Soils of the Patagónico North (Argentina): Excellent Aspects and Importance for the Conservation of the Regional Biodiversity. Rev. Chil. Hist. Nat. 2005, 78, 723–737. [Google Scholar]
- Kitzberger, T. Fire Regime Variation along a Northern Patagonian Forest-Steppe Gradient: Stand and Landscape Response; University of Colorado, Department of Geography: Boulder, CO, USA, 1994. [Google Scholar]
- Ezcurra, C.; Brion, C. Plantas Del Nahuel Huapi Catálogo de La Flora Vascular Del Parque Nacional Nahuel Huapi, Argentina; Universidad Nacional del Comahue, Red Latinoamericana de Botánica: Bariloche, Argentina, 2005. [Google Scholar]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS Monthly High-Resolution Gridded Multivariate Climate Dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef]
- Beguería, S.; Vicente-Serrano, S.M. SPEI: Calculation of the Standardised Precipitation-Evapotranspiration Index. R Package Version 1.7. 2017. Available online: https://CRAN.R-project.org/package=SPEI (accessed on 12 October 2022).
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R Package for the Numerical Calibration of Proxy-Climate Relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–75. [Google Scholar]
- Biondi, F.; Qeadan, F. A Theory-Driven Approach to Tree-Ring Standardization: Defining the Biological Trend from Expected Basal Area Increment. Tree 2008, 64, 81–96. [Google Scholar] [CrossRef]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C. dplR: Dendrochronology Program Library in R. R Package Version 1.7.6. 2020. Available online: https://CRAN.R-project.org/package=dplR (accessed on 23 June 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Wood, S.; Wood, M.S. Package ‘Mgcv’. R Package Version 2015, 1, 29. [Google Scholar]
- Hui, G.; Wang, Y.; Zhang, G.; Zhao, Z.; Bai, C.; Liu, W. A Novel Approach for Assessing the Neighborhood Competition in Two Different Aged Forests. For. Ecol. Manag. 2018, 422, 49–58. [Google Scholar] [CrossRef]
- Von Gadow, K.; Hui, G.Y. Characterizing Forest Spatial Structure and Diversity. In Sustainable Forestry in Temperate Regions; Björk, L., Ed.; SUFOR, University of Lund: Lund, Sweden, 2002; pp. 20–30. [Google Scholar]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of Tree Resilience: Effects of Successive Low-Growth Episodes in Old Ponderosa Pine Forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Thurm, E.A.; Uhl, E.; Pretzsch, H. Mixture Reduces Climate Sensitivity of Douglas-Fir Stem Growth. For. Ecol. Manag. 2016, 376, 205–220. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 2013, 3, 111. [Google Scholar]
- Ramirez, K.S.; Geisen, S.; Morriën, E.; Snoek, B.L.; van der Putten, W.H. Network Analyses Can Advance Above-Belowground Ecology. Trends Plant Sci. 2018, 23, 759–768. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The Igraph Software Package for Complex Network Research. Int. J. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Nakagawa, S.; Johnson, P.C.; Schielzeth, H. The Coefficient of Determination R 2 and Intra-Class Correlation Coefficient from Generalized Linear Mixed-Effects Models Revisited and Expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef] [PubMed]
- Bucci, S.J.; Scholz, F.G.; Campanello, P.I.; Montti, L.; Jimenez-Castillo, M.; Rockwell, F.A.; Manna, L.L.; Guerra, P.; Bernal, P.L.; Troncoso, O. Hydraulic Differences along the Water Transport System of South American Nothofagus Species: Do Leaves Protect the Stem Functionality? Tree Physiol. 2012, 32, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G. Strong Hydraulic Segmentation and Leaf Senescence Due to Dehydration May Trigger Die-Back in Nothofagus Dombeyi under Severe Droughts: A Comparison with the Co-Occurring Austrocedrus Chilensis. Trees 2014, 28, 1475–1487. [Google Scholar] [CrossRef]
- Bennett, A.C.; McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.J. Larger Trees Suffer Most during Drought in Forests Worldwide. Nat. Plants 2015, 1, 15139. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Allen, C.D. Darcy’s Law Predicts Widespread Forest Mortality under Climate Warming. Nat. Clim. Change 2015, 5, 669–672. [Google Scholar] [CrossRef]
- Leifsson, C.; Buras, A.; Klesse, S.; Baittinger, C.; Bat-Enerel, B.; Battipaglia, G.; Biondi, F.; Stajić, B.; Budeanu, M.; Čada, V.; et al. Identifying Drivers of Non-Stationary Climate-Growth Relationships of European Beech. Sci. Total Environ. 2024, 937, 173321. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.G.; Ignazi, G.; Mathiasen, P.; Premoli, A.C. Climate-Driven Adaptive Responses to Drought of Dominant Tree Species from Patagonia. New For. 2022, 53, 57–80. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gazol, A.; Gentilesca, T.; Ripullone, F. Size Matters a Lot: Drought-Affected Italian Oaks Are Smaller and Show Lower Growth Prior to Tree Death. Front. Plant Sci. 2017, 8, 135. [Google Scholar] [CrossRef]
- Galiano, L.; Martínez-Vilalta, J.; Sabaté, S.; Lloret, F. Determinants of Drought Effects on Crown Condition and Their Relationship with Depletion of Carbon Reserves in a Mediterranean Holm Oak Forest. Tree Physiol. 2012, 32, 478–489. [Google Scholar] [CrossRef]
- Cranston, B.M.; Powers, B.F.; Macinnis-Ng, C. Inexpensive Throughfall Exclusion Experiment for Single Large Trees. Appl. Plant Sci. 2020, 8, e11325. [Google Scholar] [CrossRef]
- Molowny-Horas, R.; Suarez, M.L.; Lloret, F. Changes in the Natural Dynamics of Nothofagus Dombeyi Forests: Population Modeling with Increasing Drought Frequencies. Ecosphere 2017, 8, e01708. [Google Scholar] [CrossRef]
- Neycken, A.; Scheggia, M.; Bigler, C.; Lévesque, M. Long-Term Growth Decline Precedes Sudden Crown Dieback of European Beech. Agric. For. Meteorol. 2022, 324, 109103. [Google Scholar] [CrossRef]
- Cailleret, M.; Jansen, S.; Robert, E.M.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M. A Synthesis of Radial Growth Patterns Preceding Tree Mortality. Glob. Change Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Novick, K.A.; Alexander, M.R.; Maxwell, J.T.; Moore, D.J.; Phillips, R.P.; Anderegg, W.R. Linking Drought Legacy Effects across Scales: From Leaves to Tree Rings to Ecosystems. Glob. Change Biol. 2019, 25, 2978–2992. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Sánchez-Salguero, R.; Vicente-Serrano, S.M.; Serra-Maluquer, X.; Gutiérrez, E.; de Luis, M.; Sangüesa-Barreda, G.; Novak, K.; Rozas, V. Drought Legacies Are Short, Prevail in Dry Conifer Forests and Depend on Growth Variability. J. Ecol. 2020, 108, 2473–2484. [Google Scholar] [CrossRef]
- Vilonen, L.; Ross, M.; Smith, M.D. What Happens after Drought Ends: Synthesizing Terms and Definitions. New Phytol. 2022, 235, 420–431. [Google Scholar] [CrossRef]
- Chen, Y.; Wright, S.J.; Muller-Landau, H.C.; Hubbell, S.P.; Wang, Y.; Yu, S. Positive Effects of Neighborhood Complementarity on Tree Growth in a Neotropical Forest. Ecology 2016, 97, 776–785. [Google Scholar] [CrossRef]
- Fichtner, A.; Härdtle, W.; Bruelheide, H.; Kunz, M.; Li, Y.; von Oheimb, G. Neighbourhood Interactions Drive Overyielding in Mixed-Species Tree Communities. Nat. Commun. 2018, 9, 1144. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European Tree Species to Drought Stress in Mixed versus Pure Forests: Evidence of Stress Release by Inter-specific Facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef]
- Gyenge, J.E.; Fernández, M.E.; Dalla Salda, G.; Schlichter, T. Leaf and Whole-Plant Water Relations of the Patagonian Conifer Austrocedrus Chilensis (D. Don) Pic. Ser. et Bizzarri: Implications on Its Drought Resistance Capacity. Ann. For. Sci. 2005, 62, 297–302. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Camarero, J.J.; Oliva, J.; Montes, F.; Gazol, A. Past Logging, Drought and Pathogens Interact and Contribute to Forest Dieback. Agric. For. Meteorol. 2015, 208, 85–94. [Google Scholar] [CrossRef]
- Grossiord, C. Having the Right Neighbors: How Tree Species Diversity Modulates Drought Impacts on Forests. New Phytol. 2020, 228, 42–49. [Google Scholar] [CrossRef]
- Ford, K.R.; Breckheimer, I.K.; Franklin, J.F.; Freund, J.A.; Kroiss, S.J.; Larson, A.J.; Theobald, E.J.; HilleRisLambers, J. Competition Alters Tree Growth Responses to Climate at Individual and Stand Scales. Can. J. For. Res. 2017, 47, 53–62. [Google Scholar] [CrossRef]
- Versace, S.; Gianelle, D.; Garfì, V.; Battipaglia, G.; Lombardi, F.; Marchetti, M.; Tognetti, R. Interannual Radial Growth Sensitivity to Climatic Variations and Extreme Events in Mixed-Species and Pure Forest Stands of Silver Fir and European Beech in the Italian Peninsula. Eur. J. For. Res. 2020, 139, 627–645. [Google Scholar] [CrossRef]
- González De Andrés, E.; Camarero, J.J.; Blanco, J.A.; Imbert, J.B.; Lo, Y.; Sangüesa-Barreda, G.; Castillo, F.J. Tree-to-tree Competition in Mixed European Beech–Scots Pine Forests Has Different Impacts on Growth and Water-use Efficiency Depending on Site Conditions. J. Ecol. 2018, 106, 59–75. [Google Scholar] [CrossRef]

| Predictor Variables | Estimate/Edf | Std. Error/ F-Value | p |
|---|---|---|---|
| Linear Terms | |||
| Height | 0.037 | 0.027 | 0.164 |
| DBH | 0.377 | 0.025 | <0.001 |
| Vitality (dead) | 0.069 | 0.049 | 0.159 |
| Smooth terms | |||
| SPEI3Jan | 1.494 | 9.286 | 0.007 |
| Year | 3.910 | 70.40 | <0.001 |
| Year × Vitality (dead) | 1.000 | 0.305 | 0.581 |
| SPEI3Jan × Vitality (dead) | 1.000 | 0.140 | 0.708 |
| Year × SPEI3Jan | 10.985 | 13.759 | <0.001 |
| Year × SPEI3Jan × Vitality (dead) | 1.028 | 0.178 | 0.072 |
| Observations | 4533 | ||
| Adjusted R2 | 0.554 | ||
| Variables | Estimates | Std. Error | p |
|---|---|---|---|
| Fixed effects | |||
| Intercept | −3.39 | 4.03 | 0.360 |
| Vitality (vital) | 0.71 | 0.37 | 0.060 |
| Year | 0.002 | 0.002 | 0.181 |
| SPEI3Jan | 0.03 | 0.01 | <0.001 |
| CI | −0.03 | 0.05 | 0.466 |
| M | 1.16 | 0.40 | 0.005 |
| Vitality (vital) × CI | 0.03 | 0.08 | 0.719 |
| Vitality (vital) × M | −1.35 | 0.60 | 0.027 |
| Random Effects | |||
| σi2 * | 0.14 | ||
| N | 59ID | ||
| Observations | 1457 | ||
| Marginal R2/Conditional R2 | 0.176/0.644 | ||
| Variables | Log (Resistance) | Log (Recovery) | Log (Resilience) | |||
|---|---|---|---|---|---|---|
| Est. (Std. Error) | p | Est. (Std. Error) | p | Est. (Std. Error) | p | |
| Fixed effects | ||||||
| Intercept | −0.33 (0.38) | 0.39 | 0.39 (0.45) | 0.39 | 0.21 (0.29) | 0.47 |
| SPEI3Jan | 0.08 (0.13) | 0.55 | 0.04 (0.13) | 0.75 | 0.10 (0.12) | 0.43 |
| DBH | −0.001 (0.01) | 0.84 | 0.003 (0.01) | 0.76 | −0.002(0.01) | 0.72 |
| Vitality (dead) | 0.87 (0.50) | 0.08 | −0.76 (0.59) | 0.21 | −0.16 (0.45) | 0.73 |
| CI | 0.03 (0.04) | 0.50 | 0.03 (0.06) | 0.59 | 0.02 (0.03) | 0.50 |
| M | −0.24 (0.28) | 0.40 | 0.29 (0.36) | 0.42 | 0.15 (0.24) | 0.53 |
| DBH × Vitality (dead) | −0.03 (0.01) | 0.006 | 0.03 (0.01) | 0.05 | 0.004 (0.01) | 0.68 |
| CI × Vitality (dead) | −0.05 (0.05) | 0.37 | 0.01 (0.07) | 0.91 | 0.04 (0.05) | 0.38 |
| M × Vitality (dead) | 0.24 (0.39) | 0.55 | −0.13 (0.50) | 0.79 | −0.10 (0.35) | 0.76 |
| SPEI3Jan × Vitality (dead) | 0.07 (0.19) | 0.72 | −0.10 (0.18) | 0.58 | 0.02 (0.17) | 0.88 |
| Random Effects | ||||||
| σi2 * | 0.16 | 0.15 | 0.12 | |||
| N | 61 | 61 | 61 | |||
| Observations | 122 | 120 | 121 | |||
| Marginal R2/Conditional R2 | 0.150/0.154 | 0.108/0.333 | 0.044/0.044 | |||
| Variables | Group | Centrality | |
|---|---|---|---|
| Tree Vitality | Vital | Dead | |
| Diameter at breast height (DBH) | Tree characteristic | 2 | 4 |
| 25-year mean BAI (BAI25) | Tree characteristic | 1 | 5 |
| Tree height (ht) | Tree characteristic | 2 | - |
| Crown spread (W) | Tree characteristic | 1 | 1 |
| Temperature relationship (T) | Climate–growth | 1 | 2 |
| Precipitation relationship (P | Climate–growth | 2 | 1 |
| Resistance (Rt) | Drought response indices | 2 | 3 |
| Recovery (Rc) | Drought response indices | 4 | 4 |
| Resilience (Rs) | Drought response indices | 2 | 1 |
| 10-year post-drought response (leg98) | Drought response indices | 2 | 1 |
| Competition index (CI) | Neighbour pressure | 1 | 1 |
| Percentage of N. dombeyi (%Nd) | Neighbour pressure | 3 | 5 |
| Percentage of co-dominant broadleaf (%Bl) | Neighbour pressure | 1 | 1 |
| Percentage of A. chilensis (%Au) | Neighbour pressure | 3 | 1 |
| Percentage of subcanopy species (%sub) * | Neighbour pressure | 2 | 2 |
| Percentage of dead N. dombeyi (%NDT) | Neighbour pressure | 4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suarez, M.L.; Sasal, Y.; Facciano, L. Factors Driving Unexpected Drought-Induced Nothofagus dombeyi Mortality in a Valdivian Temperate Rainforest, Argentina. Forests 2024, 15, 1355. https://doi.org/10.3390/f15081355
Suarez ML, Sasal Y, Facciano L. Factors Driving Unexpected Drought-Induced Nothofagus dombeyi Mortality in a Valdivian Temperate Rainforest, Argentina. Forests. 2024; 15(8):1355. https://doi.org/10.3390/f15081355
Chicago/Turabian StyleSuarez, María Laura, Yamila Sasal, and Loreta Facciano. 2024. "Factors Driving Unexpected Drought-Induced Nothofagus dombeyi Mortality in a Valdivian Temperate Rainforest, Argentina" Forests 15, no. 8: 1355. https://doi.org/10.3390/f15081355





