Abstract
Increasing temperatures, prolonged drought, the increased severity and intensity of storms, and other effects of climate change are being felt globally, and long-lived forest tree species may struggle in their current ranges. Oaks (Quercus spp.) have evolved a range of adaptations to dry and hot conditions and are believed to be a “climate change winner” by increasing their suitable habitat. However, a mixture of life history traits and increasing susceptibility to herbivores and xylovores as well as secondary pathogen infections still put oaks at risk of decline. Oak species found in the Midwestern, Great Lakes, and Northeastern United States and Canada are important keystone species with high ecological and economical importance. They are also vulnerable to existing, new, and emerging threats that have the potential to cause mortality across entire stands quickly. Current examples of insect threats include the Lymantria dispar (spongy moth), Agrilus bilineatus (twolined chestnut borer), and Nitidulidae (sap beetles) as disease vectors. Examples of emerging insects of concern include Cynipidae (oak gall wasps) and Enaphalodes rufulus (red oak borer). This study describes these insects, explains their mechanisms of action and the effects on oaks, and explores mitigation strategies for each.
Keywords:
Quercus; herbivory; spongy moth; Nitidulidae; wood borers; gall wasps; acorn weevil; invasive insect threat; oaks 1. Introduction
Anthropogenic climate change not only shifts mean temperatures higher, as it also increases the occurrence and intensity of extreme weather events, including droughts, heatwaves, and heavy rain events. Long-lived forest tree species of economic and environmental importance may be maladapted to these conditions and become increasingly susceptible to predation by insects and herbivores [1]. Opportunistic fungal and bacterial pathogenic infections have the potential to wipe out entire stands quickly, affecting the larger ecosystem [2]. As global temperatures are increasing [3], natural tree species migration is lagging and cannot keep pace to extend ranges into preferred climates to avoid temperature extremes. An ecologically and economically important hardwood genus, the long-lived oaks (Quercus, Fagaceae) move slowly at an unassisted pace of approximately 100 m per year [4,5,6,7,8] due to their heavy seeds [9].
Oaks are an integral species in many ecosystems in the Northeastern, Great Lakes, and Midwestern United States and Canada and were the most common foundation genus before widespread farming and settlement [10]. A host of mammals and birds feed on oak acorns, bark, or new growth and twigs; notable species include white-tailed deer (Odocoileus virginianus), white-footed mouse (Peromyscus leucopus), black bear (Ursus americanus), blue jay (Cyanocitta cristata), and wild turkey (Meleagris gallopavo) [11,12,13]. Ecological studies have found that abundance of black bears is directly correlated to oak populations in the United States, and when acorn yields are low due to disease or low mast years, bears venture further from their home range, leading to potential conflicts with humans in attempts to find other high-fat food sources prior to hibernation [11,14,15].
Quercus is a species-rich genus with a mostly northern hemisphere distribution [16]. The current estimate of species numbers is around 435 [16,17,18], divided into two subgenera and eight sections. Most North American species are classified as white oaks (sect. Quercus), red oaks (sect. Lobatae), and live oaks (sect. Virentes), with a few species representing two other sections: golden cups (Protobalanus) and Pontificate (one species which is found in California and Oregon) [16]. A dated phylogeny of oaks shows high diversification rates since the Pliocene in sections Quercus and Lobatae [19]. This recent diversification has been characterized by sympatric parallel adaptive radiation of Lobatae and Quercus taxa in North America [20]. Oaks have evolved a range of adaptations to dry and hot conditions [21] and, due to that, are modelled to have an increased range of suitable habitat with climate change [22]. However, increasing severe weather events (strong storms and heavy rainfall) and other factors including expanded ranges of herbivores and pathogens may still put oaks at risk [23].
Oaks have a ring-porous xylem system, the result of an evolutionary drought tolerance mechanism. This system is sensitive to freeze–thaw cycles in the spring and autumn, which provides diffuse-porous trees advantages in colder climates across the Northern United States and Canada [24]. Ring-porous trees are often more vulnerable to girdling or wood-boring insects and pathogens that move more easily through outer xylem tissues [25,26]. The ring porosity that somewhat limits the northern range of oaks allows the genus to be well equipped to handle drought stress [21,23]. However, prolonged exposure to drought and conditions at the edge of tolerance levels leave trees vulnerable to opportunistic infection. In addition, increased predation, as animals and insects struggle with their own survival, exposes vulnerable plant tissue to bacterial or fungal entry and further compounds an already dire situation. Though physical and chemical plant defenses, such as tough cell walls, cuticular waxes, and phenolic and terpenoid compounds, respectively, have traditionally been adequate to ensure survival, the migration of animals and insects north as climate temperatures increase has been outpacing that of long-lived trees [27]. As oaks are a long-lived species, the death of large trees can be a slow process, taking a decade or more [28], which can lead to entire stand declines years after environmental or predator stressors were present.
The unique forest ecosystems of the Great Lakes and Northeastern United States and Canada host a wealth of biodiversity for terrestrial and aquatic organisms and are host to several oak species and other mixed hardwood species. Several of the Northeastern states have the highest percentage of forested land in the United States (Maine at 89.46%, New Hampshire at 84.32%, Vermont at 77.81%, and New York at 62.88%), and Great Lakes states also boast heavy forest cover (56% in Michigan, 49% in Wisconsin, and 32% of Minnesota) [25,29]. The Great Lakes region is characterized by humid weather, locally heavy snowfall, and occasional heavy rains and strong storms [30]. With a changing climate, the Great Lakes region is experiencing warmer winters, with increases of more than 0.83 °C observed from 1986 to 2016 [31], as well as an average 42% increase in the time and intensity of rainfall events from 1958 to 2016 [32], and similar trends have been observed in the northeast. Such dramatic changes leave oak trees stressed from temperatures and rainfall amounts outside their preferred ranges, which can then open the door for opportunistic predation. Increasing winter temperatures increases the winter survival of insects, which can have devastating effects, as seen recently with the aggressive outbreaks of mountain pine beetles (Dendroctonus ponderosae Hopkins: Coleoptera: Scolytinae) in the Western United States [33,34]. The range of pathogens and herbivores also increases with more accommodating warmer winters, and increased cold season temperatures simultaneously provide a longer period of fecundity for potentially harmful insects. Here, we focus on several examples of current insect threats to oak stands of the Northeastern, Midwestern, and Great Lakes regions of the United States and Canada: the spongy moth (Lymantria dispar), the twolined chestnut borer (Agrilus bilineatus), the red oak borer (Enaphalodes rufulus), and the sap beetles (Nitidulidae) (which are vectors of oak wilt) (Figure 1). We discuss how these insects harm oak trees and explore what mitigation techniques have been attempted and their varying levels of success, as well as several other insects and oak diseases of current concern. We also discuss several emerging insects of concern: the oak gall wasps (Zapatella davisae, Callirhytis cornigera, and Callirhytis quercuspunctata), and oak borers (Agrilus biguttatus and Agrilus auroguttatus). Additional potential and current threats are also outlined in this paper (Table 1), as well as how future or emerging threats can be predicted, detected, and mitigated before they become devastating. Opportunistic predators are also a concern as trees stressed from drought, temperatures outside their native range, or extreme weather are vulnerable to secondary infection or infestation [35].
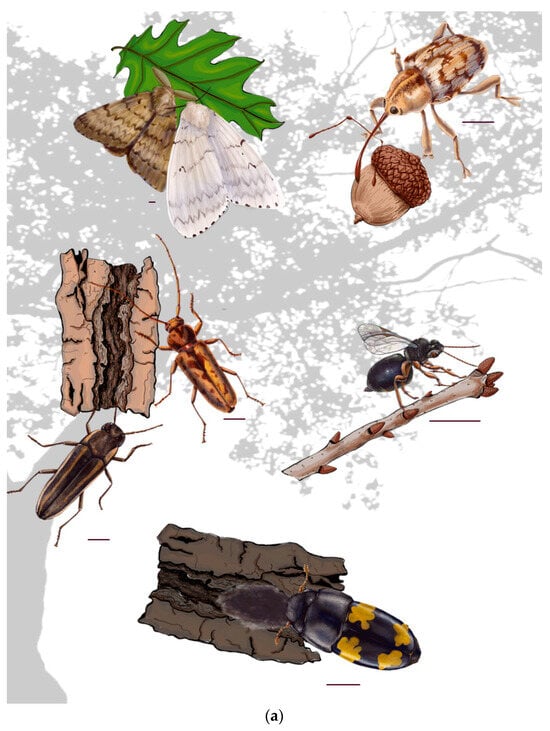
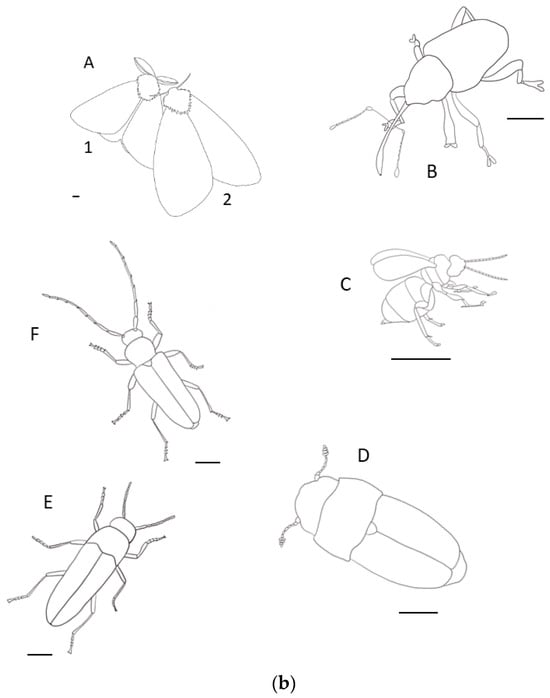
Figure 1.
Examples of existing and potential insect (a) threats to Quercus include (b: A: Lymantria dispar A1: male; A2: female (widespread defoliation), B: Curculio glandium (acorn fruit destruction), C: gall wasps (twig node disruption and crown dieback), D: Nitidulidae (spreading oak wilt via fungal hyphae), E: Agrilus bilineatus, and F: Enaphalodes rufulus (wood boring and girdling) (scale bar = 1.0 mm).

Table 1.
Examples of current and potential insect threats to oaks in the Midwestern and Northeastern United States.
2. Lymantria dispar (Spongy Moth)
The native origin of North American spongy moth (Lymantria dispar Linnaeus, Lepidoptera: Erebidae) has been disputed, with most sources pointing to Asia or Europe [100]. Lymantria dispar has been present in the United States since 1869, when captive moths escaped from silk production experiments in Massachusetts [101]. Since then, they have been present in the Northeastern United States and have migrated west to Minnesota, as far north as the Upper Peninsula of Michigan and central Ontario [95,96,97,100]. Though present in warm Southern US climates, L. dispar eggs are also tolerant of prolonged freezing temperatures and can survive temperatures down to −29 °C [102], with studies revealing that higher temperatures or radiant heat from the winter sun on southern/western exposures significantly affects survival [103]. Lymantria dispar has been long noted to be a significant threat to oak species and is still considered to be a significant threat to oak stand survival [104,105].
2.1. Mechanisms
A broad foliar generalist, L. dispar larvae willingly consume the leaves of many plant and tree species [95] and have been a known threat to oak health and growth since the early 1900s [106]. Studies of mixed hardwood stands in New England have found the greatest number of L. dispar caterpillars on Q. rubra and Q. alba [107], with Q. rubra noted to be a preferential food source [108,109]. In addition to the generalist feeding habits, spongy moths have high fecundity; even though adult female moths only live approximately one week, thousands of eggs can be laid and deposited in late summer [100,110]. The larvae hatch in late spring and feed on leaves until midsummer when they pupate. As oaks are a preferred host tree, they subsequently suffer elevated foliar damage as the larvae begin feeding immediately after hatching. Lymantria dispar caterpillars are voracious feeders and can completely denude tree foliage during heavy outbreak years [100]. Outbreaks are cyclical, occurring every five to eight years. Increased defoliation can lead to higher-than-normal concentrations of tannins in oak leaves, which can, in turn, be toxic to L. dispar [111]. While defoliated trees can recover, the resulting stress can affect leaf quality [112] and growth and can lead to tree mortality in subsequent years [108,113,114].
2.2. Threats and Solutions
To reduce the L. dispar population, multiple natural and artificial mitigation techniques have been attempted and employed, including utilizing predator species, viral, fungal, or bacterial methods, insecticides, and manual removal and disposal. In North America, multiple species consume the caterpillars, including bird species, other insects such as forest caterpillar hunter (Calasoma sycophanta) and white-footed mouse (Peromyscus leucopus) [100]. However, predation is not adequate to keep pace with the sheer number of caterpillars during outbreak years, and North American predators may not be as effective in curbing populations as those in the native spongy moth range [98]. The baculovirus Nucleopolyhedrosis virus (NPV) is always present and circulating in populations of L. dispar but is usually not fatal to caterpillars. When caterpillars are stressed during highly competitive outbreak years, or when foliage is in low supply, latent NPV begins to affect larvae and reduces populations by widespread internal cell lysis, one variable in cyclical outbreaks which assists in reducing overall outbreak intensity [115,116]. Studies have found combinations of NPV and Bacillus thuringiensis var. kurstaki (Btk) applications can be effective for killing instars in different developmental stages [117]. Additionally, a fungus native to Japan, Entomophaga maimaiga, has been employed since the early 1900s in North America to quell outbreaks and reduce L. dispar populations. Entomophaga maimaiga consumes caterpillars from the inside, then migrates outward as tissue dies. Spores disperse externally via conidiophores and can infect subsequent L. dispar generations [118]. However, E. maimaiga has limited applicability as a biocontrol as its success is very weather- and environment-dependent: spores require high humidity to reproduce [119,120]. One of the most widely employed and successful techniques in limiting L. dispar populations is the application of Bacillus thuringiensis var. kurstaki (Btk), a bacteria endemic to soil. The active insecticide contains the Cry protein produced by the bacterium and encapsulated in a spore by the bacterium. When consumed, Cry proteins bind to receptors in insect midgut cells and cause cellular rupture. Commonly applied as a foliar spray, Btk is considered very safe for mammals (including humans), birds, and most insects [100] and is effective in controlling populations. Caution must be used as the bacterium can be indiscriminately fatal to Lepidoptera species, and potential benefits should be weighed against ecosystem destruction. In heavy outbreaks, aerial spraying of Btk can be employed [121,122] but, in addition to potential harm of Lepidoptera populations [123], the overall economic cost for this method of dispersal should be considered. Recent studies have found Btk to be less harmful to total Lepidoptera populations than the insecticide tebufenozide [124].
Manual injections of insecticides into the base of a tree under the bark can reduce populations on specific trees, with azadirachtin being a common choice [119]. This method can be time-consuming and manually difficult if multiple trees are targeted. Azadirachtin is produced from oil of Neem tree (Azadiracta indica) seeds but is a broad-range insecticide (non-species-specific) and can kill beneficial indigenous insects [125]. Pheromone traps have been employed for decades in the “Slow the Spread” campaign to reduce L. dispar populations from moving south and west [126], but this method only targets male moths (though some evidence exists female spongy moths in Asia and some in Europe are flight capable and do fly [127,128], it is widely accepted that most North American females do not fly [129,130] and pheromone traps bated with female hormones will not attract them in any case). Such traps are also labor-intensive as traps need to be manually emptied and re-baited frequently. Though pheromone traps can stop the movement of spongy moths beyond the current range, it is not an effective method for reducing larval populations responsible for defoliation in an infested area. The most labor-intensive and time-consuming but safest mitigation method for curbing populations is manually scraping L. dispar egg masses and disposing of them in soapy water [131]. This effectively kills hundreds to thousands of potential L. dispar caterpillars and limits non-target impacts. Though manual scraping is unrealistic for large wooded or forested areas, it can be very effective in reducing populations in small areas. Sticky barrier bands can be applied to tree trunks in the spring [132], but care must be taken to protect birds and other small animals from injury. Burlap bands can also be applied around tree trunks in the summer to trap spongy moth caterpillars, but this also requires frequent checks and manual disposal of the caterpillars caught in soapy water [133]. “Slow the Spread” and other citizen scientist campaigns have been successful in raising awareness and curbing L. dispar infestations in urban areas, but monitoring and mitigation are still required, especially in rural and wilderness areas.
3. Agrilus bilineatus (Twolined Chestnut Borer)
Both bark and wood-boring insects, native and non-native, have been implicated in a number of major oak declines, either as primary or secondary factors [49,134,135,136]. The taxonomic families Cerambycidae and Buprestidae are generally considered the most important wood borers in oaks, along with bark beetles in Curculionidae [135,137,138]. Twolined chestnut borer (Agrilus bilineatus, Coleoptera: Buprestidae) has been commonly documented as an extensive mortality agent among oaks [25,44]. Though so named for its previous status as a pest of chestnut (Castanea dentata), A. bilineatus has a wide range in eastern North America from the Maritime provinces of Canada, west to Manitoba, south to Texas and Florida [45], and is regularly reported reaching outbreak levels in the Great Lakes states [25]. Adult A. bilineatus primarily select damaged, suppressed, or stressed oak trees to lay eggs, from which larvae bore into the bark and construct damaging galleries in the cambium layers, effectively girdling trees. Tree mortality often occurs within two to three years of attack but can occur in the first year [45]. The expansion of L. dispar throughout its range, as well as drought have been frequently linked as inciting factors to subsequent twolined chestnut borer infestation [25,48].
3.1. Mechanisms
Wood borers cause the most damage to trees by feeding on the phloem, cambium, and xylem as larvae, while adults will also consume some foliage. Adults are often attracted to stress volatile chemicals, sometimes within hours of a stress event occurring [139]. The overall time to tree mortality varies between species and events. Periodic stress impacts can also predict outbreaks associated with many wood borers. For example, tree vigor and winter starch reserves in roots are significantly correlated with twolined chestnut borer attacks [140]. Ultimately, different wood borers likely cue to suitable hosts via a range of intra- and interspecific differences in the biochemistry of leaves, bark, and wood, which is impacted by site, stress, and age of host trees. Attraction to a tree is likely within a narrow window of the stress period, not too early (as resistance compounds such as tannins and phenolics may still be present in higher levels) but not too far declined [42,46,139]. The timing in the season or host deterioration may further influence host species selection in these families. For instance, A. bilineatus prefers to develop in at least some live phloem; however, European oak borer (Agrilus sulicicollis, Coleoptera: Buprestidae) (introduced to North America in the Great Lakes region in the 1990s) prefers recently dead phloem for larval development, hence, it is likely not as aggressive of a mortality agent [25,141]. Stressed trees may be susceptible either from drought conditions or other environmental factors, or previous insect attacks, such as L. dispar outbreaks, or multiple combined factors [48,49]. The significant outbreak of the Cerambycid red oak borer (Enaphalodes rufulus, Coleoptera: Cerambycidae) tied to drought in the Ozark Mountains (Missouri, Arkansas, Oklahoma, Kansas) in the early 2000s is unique, though it had never been implicated in major tree mortality previously and is normally found in low population densities of less than one beetle emerging per tree, up to 600 beetle attacks per tree in Q. rubra were reported in 2003, causing significant decline and mortality [59]. Poor crown vigor and reduced defense mechanisms, including reduced ability to produce callus overgrowth, have been most correlated with borer attacks and outbreaks [142,143]. Callus formation has also been reported as an important defense against wood borers [44,46].
3.2. Threats and Solutions
Strong evidence supports the claim that varying stresses to trees are inciting factors leading to an increase in A. bilineatus (and other wood borer) abundance. Wood borers that are currently not strongly correlated with mortality events or considered only of minor concern could be expanding ranges throughout the region or reach outbreak level occurrences more frequently throughout the range of hosts, especially as drought is predicted to increase in the future [116,144,145,146,147]. A looming threat is the little--understood biology and ecologies of many of these insect species, which leads to surprises when optimal conditions create seemingly novel or unanticipated outbreaks, as was the case with red oak borer, leaving managers not fully prepared when such events occur. Overall, there is a continuous need for forest health specialists and taxonomic specialists to help identify novel pests and make reliable information about them accessible [148]. Preventative forest management is the most effective means of mitigation, as any silvicultural action to reduce predisposing stress factors will likely reduce or prevent an infestation. Early removal of recently dead or declining trees (sanitation) is sometimes recommended to limit pest populations from building, however, it will not prevent trees predisposed to declining vigor from eventually being attacked [42,46]. Natural biological control via native parasitoids (such as chalcid wasps, Hymenoptera: Chalcidoidea) and predators (such as woodpeckers i.e., Picidae) can act as a form of limited control [45]. Chemical control can be used on ornamental or high-value individual trees, but once wood borers are in a tree, it is not possible to save infested portions from most wood boring activity. Control of defoliating caterpillars may be a more effective management option for preventing A. bilineatus outbreaks [45]. For many wood borers, limited Integrated Pest Management (IPM) tools are developed with no effective detection tools or pheromones developed or known and limited knowledge of specific resistance or attractant volatiles. Continuous visual awareness, training for professionals, and monitoring in the field are needed.
4. Sap Beetles (Nitidulidae: Coleoptera)
Nitidulid beetles (Coleoptera: Nitidulidae), commonly known as sap or picnic beetles, are oval-shaped insects with clubbed antennae that vary in size from 0.9 to 15 mm in length, depending on species [60]. Nitidulidae feed on sap, flowers, fungi, decaying plant tissue, insects, and carrion; however, diets vary by species [60,61]. Over 4500 species of Nitidulidae can be found worldwide, with 173 species described in North America [61], and roughly 60–70 species are found within the Great Lakes and Midwestern regions [149]. Although most currently present nitidulids are native to the Great Lakes region, multiple species are confirmed vectors of the non-native, fatal fungal pathogen, oak wilt (Bretzeilla fagacearum) [64]. New records of first collections and new introductions of non-native species are seemingly continuously emerging for these often overlooked yet ubiquitous insects tied to a significant oak mortality agent [61,150,151].
4.1. Mechanisms
The majority of oak wilt spread takes place underground through root grafts of adjacent oak trees, however, overland spread from insect vectors can cause new infection centers (Figure 2). Nitidulids, especially the confirmed vectors Carpophilus sayi Parsons and Colepterus truncates Randall, have been shown to carry viable B. fagacearum spores across the landscape [61,152]; however, additional Nitidulidae, such as Glischrochilus fasciatus Olivier, G. sanguinolentus Olivier, G. quadrisignatus Say, and Epuraea corticina Erichson, are often associated with oak wilt fungal mats [153]. The fermenting scent of oak wilt fungal mats under the bark attracts beetles, where feeding and mating take place on the mat and fungal spores are accumulated [62,63]. Oak wilt disease is vectored to healthy trees when contaminated beetles leave the fungal mats and visit fresh wounds on different oak trees to feed on tree sap [152].
4.2. Threats and Solutions
Oak wilt is a significant threat to oak species in the Great Lakes, Northeastern, Midwestern, and Canadian Maritimes regions. The disease was first discovered in Wisconsin in 1942 [64,154], and the current range extends through Minnesota, Wisconsin, Michigan, down through Ohio and Pennsylvania, and across the United States south to Texas. Pockets of oak wilt have also been detected in the Carolinas, New York, and recently for the first time in Ontario, Canada [155]. All oaks are susceptible to oak wilt; however, the red oak group (sect. Lobatae) is particularly susceptible with its ring-porous cells being absent [156,157,158,159]. Fungal mats are less commonly found on species in sect. Quercus and are not found in sect. Virentes [152]. Preventative measures are in place to reduce the spread of oak wilt, including protocols and regulations on harvesting and pruning during the oak growing season to reduce the spread from overland vectors entering wounds [160,161]. When oak wilt is detected, management options are available to eradicate infected trees to reduce further spread. Additionally, knowing the signs and symptoms of oak wilt can be useful so rapid treatment can be conducted to reduce the spread of oak wilt underground or overland. Control of the native nitidulid beetles is not feasible due to their ubiquitous nature and the sheer number of species that are likely visiting oak trees. However, surveying trapped nitidulidae can be a critical method for the early detection of the oak wilt fungus in new areas, increasing rapid response actions [63,162]. Refining effective methods for trapping Nitidulidae and testing for the oak wilt fungus is increasingly important as early detection tools are crucial to limit environmental and economic damage from this disease [155,163].

Figure 2.
The cycle of oak wilt infection and Nitidulidae. (A): Nitidulidae carrying the oak wilt fungal pathogen Bretzeilla fagacearum spreads the fungus to healthy oak trees, (B): oaks displaying characteristic signs of oak wilt: widespread crown dieback and leaf tissue necrosis, (C): further example of oak crown dieback and fungal hyphae mats under the outer bark layer, (C1): oak trees with oak wilt can spread the pathogen to adjacent stand trees via underground root grafts causing healthy trees (C2) to become infected (C3) and continue spreading the infection to other adjacent trees (C4), (D): Nitidulidae beetles attracted by the fermenting scent of oak wilt hyphae mats congregate to feed and mate, (E): Nitidulidae carrying the oak wilt fungal pathogen Bretzeilla fagacearum spread it to additional trees and the infection cycle continues.
5. Additional Current Threats
Though many insects do not specifically preferentially target oaks, they can still cause harm or introduce pathogens that can further weaken stressed trees. Primary threats to trees are herbivores, while secondary threats include viral, fungal, or bacterial infections. Native defoliators like the forest tent caterpillar (Malacosoma disstria, Lepidoptera: Lasiocampidae) and fall cankerworm (Alsophila pometaria, Lepidoptera: Geometridae) are generalist feeders but prefer oaks when available [74,75,76], causing significant defoliation in oak-dominated stands, which then in turn make trees more susceptible to additional pests and pathogens. Currently found in the Great Lakes/Midwest region in southern Ohio (and previously introduced and eradicated in other locations in the region), Asian long-horned beetles (Anoplophora glabripennis, Coleoptera: Cerambycidae) preferentially consume leaves and branches, and oviposit eggs in the bark of many hardwood species in North America [164,165,166]. In heavy infestations, trees will be girdled or have their heartwood destroyed, which can lead to fatality. Oviposition pits also allow entry for pathogens or other herbivores, which cause additional damage [164]. Multiple introductions in new locations of this insect pest mean that continuous monitoring and awareness programs are warranted.
5.1. Enaphalodes rufulus (Red Oak Borer)
Red oak borer (Enaphalodes rufulus, Coleoptera: Cerambycidae), historically, was only considered a minor pest in oak trees and not recognized as a secondary agent in oak decline [38,129,157]. A sequence of forest management history, drought and regional climatic patterns led to the dramatic emergence of this native species as a more aggressive, novel agent that had not caused noticeable tree mortality in the past [142]. Enaphalodes rufulus is native to the Eastern United States and likely matches the range of its preferred host species: Q. rubra, Q velutina, and Q. coccinea (sect. Lobatae) [167]. Though the seemingly rare series of events that led to the red oak borer outbreak in the Ozarks is not anticipated again soon, E. rufulus is now considered a much more significant threat to oaks in the United States and Canada. This may be exacerbated by a changing climate that may impact tree defense mechanisms [144,145,146].
5.2. Leaf Miners
Leaf miners of multiple orders are known to utilize multiple species, including Quercus, as hosts and cause tunneling, skeletonization, and other extensive leaf tissue damage [168]. Though this is rarely fatal, infestations can cause widespread defoliation and tree stress, as well as allowing entry routes for pathogens. The variable oakleaf caterpillar (Heterocampa manteo, Lepidoptera: Notodontidae) and the orange-striped oakworm (Anisota senatoria, Lepidoptera: Saturniidae) cause widespread foliar damage in late summer. An oak generalist, H. manteo prefers Q. alba, and outbreaks have been known to occur since the 1950s in Maine and appear in cycles that are rarely severe enough to require mitigation [94]. Anisota senatoria is also an oak generalist but prefers Q. rubra and has similar cyclical infestation patterns [76]. Oak leaf rollers (Archips semiferanus, Lepidoptera: Tortricidae) are oak generalists, which skeletonize leaves while larvae consume buds and leaf tissue. Insecticide mitigation is best applied during the egg or larval stages before foliar damage becomes widespread but is likely only feasible on individually, severely impacted trees or orchard plantings [85]. As cold temperatures in the winter assist with controlling populations of leaf miners, warming winters and prolonged periods without freezing temperatures observed with climate change could cause more regular and extensive infestations.
5.3. Weevils
Acorn weevils (Curculio glandium, Coleoptera: Curculionidae) oviposit in acorns by boring holes, which are subsequently healed by callus tissue (which also protects the developing egg and subsequent larvae) [57]. Though this is not fatal to the tree itself, acorn weevil larvae consume most of the acorn fruit and can damage the embryo, which affects tree reproductive rates [58]. As very few acorns avoid mammal or avian predation and find suitable environments to develop into mature trees, a heavy acorn weevil year can greatly affect oak stand populations. The invasive Asiatic oak weevil (Cyrtepistomus castaneus, Coleoptera: Curculionidae) feeds preferentially on Q. velutina and Q. rubra [56] and has been causing oak damage in the Ozark Mountains since its introduction in the 1930s. Asiatic weevils are leaf edge feeders and can cause extensive leaf damage in years of heavy infestation. Another weevil, the oak timberworm (Arrenodes minutus, Coleoptera: Brentidae) prefers Q. velutina and Q. coccinea (though they will attack other species) and finds areas of exposed sap beneath oak bark and bores single holes (“pin holes”) to oviposit [54]. The larvae enlarge the pin holes to larger wounds as they grow and age, and previously used holes are sometimes used for several years. Holes that expose sapwood cause the most damage to trees [55].
5.4. Lycorma delicatula (Spotted Lanternfly)
Spotted lanternfly (Lycorma delicatula, Hemiptera: Fulgoridae) is not considered a predominant threat to oaks but is an indiscriminate feeder [65] and will attack oaks if preferential food sources are unavailable [66]. Oaks are also capable of hosting L. delicatula through its lifecycle [67]. This invasive planthopper consumes the phloem of immature bark and leaves behind wounds from which sap flows. The fecundity of the spotted lanternfly leads to infestations and excessive wounding, which attracts opportunistic insects and opens channels for secondary infections to enter [169]. It is projected to find ample hosts as it continues to invade the Midwest and Great Lakes regions from the Northeast where it is currently located [169,170,171]. Lycorma delicatula is especially concerning in areas with Prunus and Vitis industries as infestations can decimate fruit yields and cause heavy economic losses [68].
5.5. Sudden Oak Death
In addition to oak wilt, sudden oak death has caused fatality in oaks in California and parts of Oregon. It is caused by a type of pathogenic water mold (Phytophthora ramorum) and decimated populations of Q. agrifolia (sect. Lobatae) and Notholithocarpus densiflorus (Fagaceae) in California [172,173]. Sudden oak death is a cause for concern as spores can spread by air, water, or on animals and humans and spread to new host trees through open wounds [174]. We have included mention of these two significant pathogens in with insect threats as oak wilt is vectored by Nitidulidae, and the combination of wounds from herbivore infestation and infection from P. ramorum can have widespread and devastating consequences to oak stands. Multiple states have periodically led large monitoring surveys for P. ramorum and have detected infected host plants moved in nursery stock but have not yet found sudden oak death disease in trees, yet it remains a significant concern [175,176].
6. Emerging Threats
With changing temperatures and other climatic conditions, insects which affect oaks in areas of North America or the world may be introduced to new areas and become problematic. Just as L. delicatula is currently expanding its range relatively quickly and is a large threat to commercial crops and fruits, an invasive species that targets oaks could be the next threat whose range expands rapidly and causes ecological disturbance and economic damage. Insects of known concern include oak gall wasps and multiple wood- or bark-boring beetles, while additional opportunistic secondary pests and pathogens can also threaten oak stand survival.
6.1. Oak Gall Wasps
Oak gall wasps (Hymenoptera: Cynipidae) parasitize oak tissue to deposit their eggs, thus forming a gall. In most cases, oak galls do not harm the overall health of the tree and usually only result in additional nutrients directed to the gall [177], though the exit created by emerging female wasps in twig galls can leave openings for opportunistic infections. Trees with extreme numbers of galls may undergo crown dieback [178]. The horned oak gall wasp (Callirhytis cornigera, Hymenoptera: Cynipidae) primarily targets pin oaks (Q. palustris, sect. Lobatae) [69], while the gouty oak gall wasp (Callirhytis quercuspunctata, Hymenoptera: Cynipidae) is more indiscriminate in its oak predation [179]. A newly discovered species of North American oak gall wasp, the stem gall wasp (Zapatella davisae, Hymenoptera: Cynipidae), parasitizes Q. velutina exclusively and deposits eggs in twigs [70]. Several generations of this species can be found in twig galls throughout the autumn and winter, which can cause twig node damage and widespread tissue damage due to disruption of the vascular system of the tree. In instances of widespread infestation, full tree dieback can occur up to three years after initial parasitism [70]. Similar to other species of gall wasps, exit holes from Z. davisae can leave trees susceptible to secondary infections.
Currently, Z. davisae is found in coastal New England, including Massachusetts, Rhode Island, and Connecticut [71,72], with little evidence of it having spread to other areas. However, with transfer of wood or other incidental items containing Z. davisae, there is a possibility that the species could be moved beyond New England. Currently, there is debate whether significant Q. velutina dieback coincident to Z. davisae outbreaks may also be from an opportunistic fungal pathogen Botryosphaeria spp., which may take advantage of wasp exit holes to enter tree tissue [70]. No specific mechanism of insects or other parasitism to the newly discovered oak gall wasp has yet been observed that could be used as a biologic control [71]. However, there is also no clear reason for heavy outbreak years followed by later population decreases of Z. davisae, and it has been postulated this may be from increased native insect predation [72]. For chemical mitigation, the insecticides of abamectin, imidacloprid, or bidrin applied into sapwood are effective treatments for other gall wasps, including the horned oak gall wasp and the gouty oak gall wasp, and should have similar efficacy with Z. davisae [69]. For Z. davisae in particular, treatments with imidacloprid or emamectin benzoate reduced the number of cavities in new growth, lowered leaf damage, and resulted in lowered branch mortality (which can lead to fewer instances of total dieback) [73]. For large infestations of oak gall wasps, foliar sprays of chlorpyrifos or bifenthrin have success but also indiscriminately kill other native beneficial insects and should be used with caution [69]. In most forested stands, it is unlikely that these types of treatments would be economically or ecologically feasible.
6.2. Oak Borers
The oak splendor beetle (Agrilus biguttatus, Coleoptera: Buprestidae) is an oak generalist currently found in Europe and Africa, as well as western Middle Eastern countries and Asia. A. biguttatus has been linked to the convergence of pathogens and pests responsible for acute oak decline (AOD) in Europe and is on watch lists for accidental introduction into the United States through wood importation and packing materials [40]. A. biguttatus is considered a secondary pest, selecting trees which are already stressed or have breached bark, and prefers trees with thickened bark (particularly those with south-facing surfaces), which puts old growth oak stands at particular risk [42]. Oviposition occurs in areas of broken bark and hatched larvae bore deeper into the tree, feeding off cambium. Throughout the larval stages (which can be one to two years), wood boring continues and elongates, which can girdle and kill the host tree [43]. Adult beetles exit from large D-shaped holes and consume tree foliage. As symptoms of AOD include dark sap exuding from bark cracks in mature oak trees [180], the large exit holes left by A. biguttatus can contribute to further bacterial or other pathogenic infection entering already-stressed trees [181]. As cool and warm cycles are required for larval development, it remains to be seen how climate change will affect the lifecycle of A. biguttatus and its population overall [41]. Mitigation techniques to stop the spread of A. biguttatus further into Europe, Asia, and Africa and onto other continents include preventing the movement of firewood, sanitization of exported wood and packing materials, insecticides, removal of affected twigs, limbs, or trees, and biocontrol measures including woodpeckers [42,43].
The gold-spotted oak borer (Agrilus auroguttatus, Coleoptera: Buprestidae) is a western North American species with a small native range in Arizona but has been introduced and spread in southern California (likely brought in by human transport [182]) and causes significant mortality to coast live oaks (Q. agrifolia) [36,39]. Agrilus auroguttatus has caused one hundred percent mortality of oaks in some areas and could likely establish on a number of other oak species, particularly those in sect. Lobatae, if introduced elsewhere [183]. To date, no laboratory testing has been conducted with this species to determine the suitability of eastern oaks, thus, uncertainty remains and A. auroguttatus remains on a number of invasive species watch lists for the Eastern United States [184]. Trees attacked by A. auroguttatus produce excessive sap and tend to be larger-sized mature trees [37]. In its native range, A. auroguttatus has been reported on Emory oak (Q. emoryi) and Q. hypoleucoides (sect. Lobatae) (silverleaf oak), as well as coast live oak (Q. agrifolia), canyon live oak (Q. chrysolepis, sect. Protobalanus), and California black oak (Q. kelloggii, sect. Lobatae) [182]. It has been hypothesized that this species is so successfully attacking California oaks by colonizing non-coevolved trees with low host resistance in a region with limited natural insect enemies [182]. Natural biological control shows little prospect thus far at being effective [39]. As another wood borer that is difficult to manage directly, monitoring, quarantines, and prohibiting movement of firewood are the recommended management options [37,38,39].
7. Conclusions
Predicting the next key threats to oaks of the Northeastern, Midwestern, and Great Lakes regions of the United States and Canada will be an ongoing task, compounded by rapid changes in winter temperatures, greater intensity of storms, and other fluid variables. As the adage goes, we also “don’t know what we don’t know”—in other words, we can watch for issues we know are current or emerging threats but have little knowledge about many potential unknown or unexpected threats. A question that may help oaks to better cope with new pests and changing microclimates is whether oaks change secondary metabolites across latitude gradients, and will this affect insect predation? Secondary metabolites that dominate in oaks in more southern provenances could provide advantages to oaks in northern regions with warmer overall temperatures and especially warmer winters. Knowing these advantages could aid in developing assisted migration strategies to ensure oak species survival, and knowledge of what secondary metabolites are successful in curbing predation of herbivores in warm climates can also assist in finding optimal defense strategies for oaks. This may also lead to answers regarding variation of resistance or tolerance which exists within and between populations of the same oak species, and beneficial adaptations could be used to benefit a broader range of oaks [185]. Of particular note is A. bilineatus, which has a multitude of hosts in the United States and Canada on which to feed and could explode in population with a suitable climate with a major impact on Quercus and other hardwood species [47,186]. Monitoring the magnitude and method of spread should be closely monitored to compare to Agrilus expansion elsewhere.
The largest question remains: how can we predict the next key threat to oaks? The United States Forest Service maps current and potential hazards [187], but it is still difficult to predict the next big threat. Mapping and predictive modeling may be one way to address the probability of emerging threats to a certain area or region [188,189,190]. One barrier is simply knowing the current state or locations of insect or pathogen threats, particularly in rural or wilderness areas, which are sparsely visited. Though modeling may assist with predicting threats in such areas, implementing citizen scientists to detect herbivory damage and new movement of species as “boots on the ground” could also be immensely beneficial. However, this will require more targeted education and awareness campaigns. Local agencies could provide posters of potential threats to our forests, raise awareness through publications in local newspapers and newsletters, perform outreach at public spaces like libraries, Farmer’s Markets, or Community Gardens, and provide paths to document insect species movements (similar to what we have seen with L. dispar and L. delicatula in multiple citizen scientist web platforms and mapping databases). Data from such efforts could also lead, in turn, to better predictive modeling as herbivore movement is better documented and understood. Funding for non-targeted trapping and identification of insects in longitudinal and latitudinal sections could provide a broader picture of current insect species and population densities. For example, the emerald ash borer (Agrilus planipennis, Coleoptera: Buprestidae) is native to China and East Asia but first received widespread recognition as an invasive insect in Michigan in the early 2000s after it caused dieback and huge economic loss in ash trees (Fraxinus spp.) [191,192]. It has been postulated by dendrochronological studies that A. planipennis was actually present in the United States for a number of years before 2002 (even as far back as 1997) [193] and evaded detection, only becoming noticeable when it caused a significant amount of mortality in rows and rows of street trees. There could easily be enough oak stand density to see similar devastation in areas of the United States and Canada. Remaining vigilant of new and emerging threats with a combination of predictive modeling, citizen scientist observations, and continuing mitigation and remediation of current threats will help assist oak survival as we face fluid and vast ecological changes globally.
Author Contributions
Conceptualization, A.J.S. and C.K.; investigation, A.J.S., K.B., T.L.B. and C.K.; writing—A.J.S., K.B., T.L.B. and C.K.; writing—review and editing, A.J.S., K.B., T.L.B. and C.K.; visualization and illustrations, Figure 1a: A.J.S.; Figure 1b: A.J.S.; Figure 2: K.B. All authors have read and agreed to the published version of the manuscript.
Funding
Partial funding support was provided for by the USDA Forest Service, Forest Health Protection, Forest Health Monitoring Program, Evaluation Monitoring (EM 21-DG-11094200-063). This study was supported in part by a US Department of Agriculture National Institute of Food and Agriculture, McIntire Stennis (grant Accession number 1017893).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Champagne, E.; Royo, A.A.; Tremblay, J.P.; Raymond, P. Tree Assisted Migration in a Browsed Landscape: Can We Predict Susceptibility to Herbivores? For. Ecol. Manag. 2021, 498, 119576. [Google Scholar] [CrossRef]
- Tubby, K.V.; Webber, J.F. Pests and Diseases Threatening Urban Trees under a Changing Climate. Forestry 2010, 83, 451–459. [Google Scholar] [CrossRef]
- NOAA National Centers for Environmental Information. Monthly Global Climate Report for Annual 2021; NCEI: Silver Spring, MD, USA, 2022.
- Lazarus, E.D.; McGill, B.J. Pushing the Pace of Tree Species Migration. PLoS ONE 2014, 9, e105380. [Google Scholar] [CrossRef] [PubMed]
- Huntley, B.; Birks, H.J.B. An Atlas of Past and Present Pollen Maps for Europe: 0–13000 B.P.; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Clark, J.; Fastie, C.; Hurtt, G.; Jackson, S.T.; Johnson, C.; King, G.A.; Lewis, M.; Lynch, J.; Pacala, S.; Prentice, C.; et al. Reid’s Paradox of Rapid Plant Migration Dispersal Theory and Interpretation of Paleoecological Records. BioScience 1998, 48, 13–24. [Google Scholar] [CrossRef]
- Nathan, R.; Horvitz, N.; He, Y.; Kuparinen, A.; Schurr, F.M.; Katul, G.G. Spread of North American Wind-Dispersed Trees in Future Environments. Ecol. Lett. 2011, 14, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.I.; Richardson, D.M. Predicting Plant Migration Rates in a Changing World: The Role of Long-Distance Dispersal. Am. Nat. 1999, 153, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.V.; Clark, J.S. Between-Site Differences in the Scale of Dispersal and Gene Flow in Red Oak. PLoS ONE 2012, 7, e36492. [Google Scholar] [CrossRef] [PubMed]
- Hanberry, B.B.; Nowacki, G.J. Oaks Were the Historical Foundation Genus of the East-Central United States. Quat. Sci. Rev. 2016, 145, 94–103. [Google Scholar] [CrossRef]
- Clark, J.D. Oak-Black Bear Relationships in Southeastern Uplands; General Technical Report SRS-73; U.S. Department of Agriculture, Forest Service: Asheville, NC, USA, 2004. Available online: https://pubs.usgs.gov/publication/70170380 (accessed on 10 June 2024).
- Dickson, J.G. Wildlife and Upland Oak Forests; General Technical Report SRS-73; U.S. Department of Agriculture, Forest Service: Asheville, NC, USA, 2004. Available online: https://www.fs.usda.gov/research/treesearch/6506 (accessed on 10 June 2024).
- Norman, G.W.; Steffen, D.E. Effects of Recruitment, Oak Mast, and Fall-Season Format on Wild Turkey Harvest Rates in Virginia. Wildlife Soc. Bull. 2003, 31, 553–559. [Google Scholar]
- Rogers, L.L.; Lindquist, E.L. Black Bears and the Oak Resource in Northeastern Minnesota. In Proceedings of the Oak Resource in the Upper Midwest Conference, Winona, MN, USA, 3–6 June 1991; pp. 107–114. [Google Scholar]
- Cottam, C.; Nelson, A.L.; Clarke, T.E. Notes on Early Winter Food Habits of the Black Bear in George Washington National Forest. J. Mammal. 1939, 20, 310–314. [Google Scholar] [CrossRef]
- Hipp, A.L.; Manos, P.S.; González-Rodríguez, A.; Hahn, M.; Kaproth, M.; McVay, J.D.; Avalos, S.V.; Cavender-Bares, J. Sympatric Parallel Diversification of Major Oak Clades in the Americas and the Origins of Mexican Species Diversity. New Phytol. 2018, 217, 439–452. [Google Scholar] [CrossRef]
- Le Hardy de Beaulieu, A.; Lamant, T. Guide Illustré des Chênes; Edilens: Geer, Belgium, 2010; ISBN 2960097408. [Google Scholar]
- Denk, T.; Grimm, G.W.; Manos, P.S.; Deng, M.; Hipp, A.L. An Updated Infrageneric Classification of the Oaks: Review of Previous Taxonomic Schemes and Synthesis of Evolutionary Patterns. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus quercus L.; Eustaquio Gil-Pelegrín, J., Javier Peguero-Pina, D.S.-K., Eds.; Springer: New York, NY, USA, 2017; pp. 13–38. ISBN 3319690981. [Google Scholar]
- Hipp, A.L.; Manos, P.S.; Hahn, M.; Avishai, M.; Bodénès, C.; Cavender-Bares, J.; Crowl, A.A.; Deng, M.; Denk, T.; Fitz-Gibbon, S.; et al. Genomic Landscape of the Global Oak Phylogeny. New Phytol. 2020, 226, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J. Diversification, Adaptation, and Community Assembly of the American Oaks (Quercus), a Model Clade for Integrating Ecology and Evolution. New Phytol. 2019, 221, 669–692. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.D. Adaptations and Responses to Drought in Quercus Species of North America. Tree Physiol. 1990, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Iverson, L.; Matthews, S.; Peters, M. Climate Change Tree Atlas, Version 4 (A Spatial Database of 134 Tree Species of the Eastern USA). Available online: https://www.fs.usda.gov/nrs/atlas/tree/ (accessed on 11 May 2024).
- Rauschendorfer, J.; Rooney, R.; Külheim, C. Strategies to Mitigate Shifts in Red Oak (Quercus Sect. Lobatae) Distribution under a Changing Climate. Tree Physiol. 2022, 42, 2383–2400. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.V.; Wagner, W.H., Jr. Michigan Trees: A Guide to the Trees of the Great Lakes Region, 2nd ed.; The University of Michigan Press: Ann Arbor, MI, USA, 2004; pp. 230–231. ISBN 0472089218. [Google Scholar]
- Haack; Robert, A.; Toby, P. Historical Population Increases and Related Inciting Factors of Agrilus anxius, Agrilus bilineatus, and Agrilus granulatus liragus (Coleoptera: Buprestidae) in the Lake States (Michigan, Minnesota, and Wisconsin). Great Lakes Entomol. 2019, 52, 21–33. [Google Scholar] [CrossRef]
- Cowling, E.B.; Horsfall, J.G. Prologue: How Plants Suffer from Disease. In How Plants Suffer from Disease; Elsevier: Amsterdam, The Netherlands, 1978; pp. 1–18. [Google Scholar]
- Sittaro, F.; Paquette, A.; Messier, C.; Nock, C.A. Tree Range Expansion in Eastern North America Fails to Keep Pace with Climate Warming at Northern Range Limits. Glob. Change Biol. 2017, 23, 3292–3301. [Google Scholar] [CrossRef] [PubMed]
- Pederson, B.S. The Role of Stress in the Mortality of Midwestern Oaks Indicated by Growth Prior to Death. Ecology 1998, 79, 79–93. [Google Scholar] [CrossRef]
- Vogt, J.T.; Smith, W.B. Forest Inventory and Analysis Fiscal Year 2015 Business Report; United States Department of Agriculture, Forest Service: Knoxville, TN, USA, 2016.
- Botts, L.; Krushelnicki, B. The Great Lakes: An Environmental Atlas and Resource Book, 3rd ed.; United States Environmental Protection Agency, Government of Canada: Washington, DC, USA, 1995.
- Vose, R.S.; Easterling, D.R.; Kunkel, K.E.; LeGrande, A.N.; Wehner, M.F. Temperature Changes in the United States. In Climate Science Special Report: Fourth National Climate Assessment; U.S. Global Change Research Program: Washington, DC, USA, 2017; Volume 1. [Google Scholar]
- Easterling, D.R.; Arnold, J.R.; Knutson, T.; Kunkel, K.E.; LeGrande, A.N.; Leung, L.R.; Vose, R.S.; Waliser, D.E.; Wehner, M.F. Precipitation Change in the United States. In Climate Science Special Report: Fourth National Climate Assessment; U.S. Global Change Research Program: Washington, DC, USA, 2017; Volume 1. [Google Scholar]
- Sambaraju, K.R.; Carroll, A.L.; Zhu, J.; Stahl, K.; Moore, R.D.; Aukema, B.H. Climate Change Could Alter the Distribution of Mountain Pine Beetle Outbreaks in Western Canada. Ecography 2012, 35, 211–223. [Google Scholar] [CrossRef]
- Audley, J.P.; Fettig, C.J.; Steven Munson, A.; Runyon, J.B.; Mortenson, L.A.; Steed, B.E.; Gibson, K.E.; Jørgensen, C.L.; McKelvey, S.R.; McMillin, J.D.; et al. Impacts of Mountain Pine Beetle Outbreaks on Lodgepole Pine Forests in the Intermountain West, U.S., 2004–2019. For. Ecol. Manag. 2020, 475, 118403. [Google Scholar] [CrossRef]
- Mattson, W.J.; Haack, R.A. The Role of Drought in Outbreaks of Plant-Eating Insects. Bioscience 1987, 37, 110–118. [Google Scholar] [CrossRef]
- Coleman, T.W.; Seybold, S.J. Previously Unrecorded Damage to Oak, Quercus Spp., in Southern California by the Goldspotted Oak Borer, Agrilus coxalis Waterhouse (Coleoptera: Buprestidae). Pan-Pac. Entomol. 2008, 84, 288–300. [Google Scholar] [CrossRef]
- Smith, S. Goldspotted Oak Borer Strategic Plan; United States Department of Agriculture, Forest Service: Washington, DC, USA, 2009.
- Coleman, T.W.; Jones, M.I.; Smith, S.L.; Venette, R.C.; Flint, M.L.; Seybold, S.J. Gold-Spotted Oak Borer; United States Department of Agriculture, Forest Service: Portland, OR, USA, 2017.
- Coleman, T.W.; Lopez, V.; Rugman-Jones, P.; Stouthamer, R.; Seybold, S.J.; Reardon, R.; Hoddle, M.S. Can the Destruction of California’s Oak Woodlands Be Prevented? Potential for Biological Control of the Goldspotted Oak Borer, Agrilus auroguttatus. BioControl 2012, 57, 211–225. [Google Scholar] [CrossRef]
- Domingue, M.J.; Baker, T.C. Invasive Species: Threats, Ecological Impact and Control Methods. In Invasive Species; Blanco, J.J., Fernandes, A.T., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 77–100. ISBN 978-1-61942-761-7. [Google Scholar]
- Reed, K.; Denman, S.; Leather, S.R.; Forster, J.; Inward, D.J.G. The Lifecycle of Agrilus biguttatus: The Role of Temperature in Its Development and Distribution, and Implications for Acute Oak Decline. Agric. For. Entomol. 2018, 20, 334–346. [Google Scholar] [CrossRef]
- Vansteenkiste, D.; Tirry, L.; Van Acker, J.; Stevens, M. Predispositions and Symptoms of Agrilus Borer Attack in Declining Oak Trees. Ann. For. Sci. 2004, 61, 815–823. [Google Scholar] [CrossRef]
- Dueñas-López, M.A. Agrilus biguttatus (Oak Splendour Beetle). CABI Compend. 2022, 631, 10.1079. [Google Scholar] [CrossRef]
- Haack, R.A.; Benjamin, D.M. The Biology and Ecology of the Twolined Chestnut Borer, Agrilus bilineatus (Coleoptera: Buprestidae), On Oaks, Quercus spp., in Wisconsin. Can. Entomol. 1982, 114, 385–396. [Google Scholar] [CrossRef]
- Haack, R.A.; Acciavatti, R.E. Twolined Chestnut Borer. In Forest Insect and Disease Leaflet 168; United States Department of Agriculture, Forest Service, Rocky Mountain Region (R2): Lakewood, CO, USA, 1992. [Google Scholar]
- Muzika, R.M.; Liebhold, A.M.; Twery, M.J. Dynamics of Twolined Chestnut Borer Agrilus bilineatus as Influenced by Defoliation and Selection Thinning. Agric. For. Entomol. 2000, 2, 283–289. [Google Scholar] [CrossRef]
- Petrice, T.R.; Haack, R.A. Biology of the European Oak Borer in Michigan, United States of America, with Comparisons to the Native Twolined Chestnut Borer. Can. Entomol. 2014, 146, 36–51. [Google Scholar] [CrossRef]
- Dunn, J.P.; Potter, D.A.; Kimmerer, T.W. Carbohydrate Reserves, Radial Growth, and Mechanisms of Resistance of Oak Trees to Phloem-Boring Insects. Oecologia 1990, 83, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent Insects, Pathogens and Drought Shape Changing Patterns in Oak Decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Peterson, A.T.; Scachetti-Pereira, R.; Hargrove, W.W. Potential Geographic Distribution of Anoplophora glabripennis (Coleoptera: Cerambycidae) in North America. Am. Midl. Nat. 2004, 151, 170–178. [Google Scholar] [CrossRef]
- Haack, R.A.; Bauer, L.S.; Gao, R.; McCarthy, J.J.; Miller, D.L.; Petrice, T.R.; Poland, T.M. Anoplophora glabripennis Within-Tree Distribution, Seasonal Development, and Host Suitability in China and Chicago. Great Lakes Entomol. 2006, 39, 169–183. [Google Scholar] [CrossRef]
- Smith, M.; Turgeon, J.; De Groot, P.; Gasman, B. Asian Longhorned Beetle Anoplophora glabripennis (Motschulsky): Lessons Learned and Opportunities to Improve the Process of Eradication and Management. Am. Entomol. 2009, 55, 21–22. [Google Scholar] [CrossRef]
- Hu, J.; Angeli, S.; Schuetz, S.; Luo, Y.; Hajek, A.E. Ecology and Management of Exotic and Endemic Asian Longhorned Beetle Anoplophora glabripennis. Agric. For. Entomol. 2009, 11, 359–375. [Google Scholar] [CrossRef]
- Buchanan, W.D. Biology of the Oak Timberworm, Arrhenodes minutus. J. Econ. Entomol. 1960, 53, 510–513. [Google Scholar] [CrossRef]
- Solomon, J.D. Order Coleoptera; Family Brentidae—Timber Worms or Primitive Weevils; Arrhenodes minutus. In Guide to Insect Borers in North American Broadleaf Trees and Shrubs; Forest Service Agricultural Handbook AH-706; United States Department of Agriculture, Forest Service: Washington, DC, USA, 1995; pp. 476–479. [Google Scholar]
- Linit, M.J.; Johnson, P.S.; McKinney, R.A.; Kearby, W.H. Insects and Leaf Area Losses of Planted Northern Red Oak Seedlings in an Ozark Forest. For. Sci. 1986, 32, 11–20. [Google Scholar] [CrossRef]
- Reut, M.; Jakubczyk, E.; Chrabąszcz, M.; Moniuszko, H. Hard Nut to Crack. Acorn Hardness Implications on Oviposition of the Acorn Weevil Curculio glandium Marsham, 1802 (Coleoptera: Curculionidae). Diversity 2022, 14, 922. [Google Scholar] [CrossRef]
- Udaka, H.; Sinclair, B.J. The Overwintering Biology of the Acorn Weevil, Curculio glandium in Southwestern Ontario. J. Therm. Biol. 2014, 44, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Fierke, M.K.; Kinney, D.L.; Salisbury, V.B.; Crook, D.J.; Stephen, F.M. A Rapid Estimation Procedure for Within-Tree Populations of Red Oak Borer (Coleoptera: Cerambycidae). For. Ecol. Manag. 2005, 215, 163–168. [Google Scholar] [CrossRef]
- Parsons, C.T. A Revision of Nearctic Nitidulidae (Coleoptera). Bull. Comp. Zool. 1943, 92, 121–248. [Google Scholar]
- Reed, S.E.; Dutkiewicz, D.; Ross, F.; Llewellyn, J.; Fraser, H. New Records of Nitidulidae (Nitidulidae, Coleoptera) Species in Canada, Ontario, and Manitoba. Zookeys 2023, 1156, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Cease, K.R.; Juzwik, J. Predominant Nitidulid Species (Coleoptera: Nitidulidae) Associated with Spring Oak Wilt Mats in Minnesota. Can. J. For. Res. 2001, 31, 635–643. [Google Scholar] [CrossRef]
- DiGirolomo, M.F.; Munck, I.A.; Dodds, K.J.; Cancelliere, J. Sap Beetles (Coleoptera: Nitidulidae) in Oak Forests of Two Northeastern States: A Comparison of Trapping Methods and Monitoring for Phoretic Fungi. J. Econ. Entomol. 2020, 113, 2758–2771. [Google Scholar] [CrossRef] [PubMed]
- Juzwik, J. Epidemiology and Occurrence of Oak Wilt in Midwestern, Middle, and South Atlantic States. In Proceedings of the National Oak Wilt Symposium, Austin, TX, USA, 4–7 June 2007; Billings, R.F., Appel, D.N., Eds.; 2007. [Google Scholar]
- Kim, J.G.; Lee, E.H.; Seo, Y.M.; Kim, N.Y. Cyclic Behavior of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae) on Host Plants. J. Insect Behav. 2011, 24, 423–435. [Google Scholar] [CrossRef]
- Uyi, O.; Keller, J.A.; Johnson, A.; Long, D.; Walsh, B.; Hoover, K. Spotted Lanternfly (Hemiptera: Fulgoridae) Can Complete Development and Reproduce without Access to the Preferred Host, Ailanthus Altissima. Environ. Entomol. 2020, 49, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.M.; Leach, H. Biology and Management of the Spotted Lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae), in the United States. Annu. Rev. Entomol. 2023, 68, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Murman, K.; Setliff, G.P.; Pugh, C.V.; Toolan, M.J.; Canlas, I.; Cannon, S.; Abreu, L.; Fetchen, M.; Zhang, L.; Warden, M.L.; et al. Distribution, Survival, and Development of Spotted Lanternfly on Host Plants Found in North America. Environ. Entomol. 2020, 49, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Eliason, E.A.; Potter, D.A. Biology and Management of the Horned Oak Gall Wasp on Pin Oak. J. Arboric. 2001, 27, 92–101. [Google Scholar] [CrossRef]
- Buffington, M.L.; Melika, G.; Davis, M.; Elkinton, J.S. The Description of Zapatella davisae, New Species, (Hymenoptera: Cynipidae) a Pest Gallwasp of Black Oak (Quercus velutina) in New England, USA. Proc. Entomol. Soc. Wash. 2016, 118, 14–26. [Google Scholar] [CrossRef]
- Davis, M.J.; Andersen, J.C.; Elkinton, J. Identification of the Parasitoid Community Associated with an Outbreaking Gall Wasp, Zapatella davisae, and Their Relative Abundances in New England and Long Island, New York. Ecol. Evol. 2019, 9, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Smith-Freedman, C.J.; Andersen, J.C.; Griffin, B.P.; Schick, K.; Elkinton, J.S.; Schmidt-Jeffris, R. Rise and Fall of an Oak Gall Wasp (Hymenoptera: Cynipidae) Outbreak in Massachusetts. Environ. Entomol. 2019, 48, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Elkinton, J.S. Efficacy of Two Systemic Insecticides with Stem Gall Wasp, Zapatella davisae (Hymenoptera: Cynipidae) on Black Oak. J. Econ. Entomol. 2018, 111, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Schowalter, T.D. Biology and Management of the Forest Tent Caterpillar (Lepidoptera: Lasiocampidae). J. Integr. Pest Manag. 2017, 8, 24. [Google Scholar] [CrossRef]
- Ciesla, W.M.; Asaro, C. Fall Cankerworm. Forest Insect & Disease Leaflet 182; United States Department of Agriculture, Forest Service, Pacific Northwest Region (R6): Portland, OR, USA, 2013. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5426975.pdf (accessed on 13 May 2024).
- Solomon, J.D.; McCracken, F.I.; Anderson, R.L.; Lewis, R., Jr.; Oliveria, F.L.; Filer, T.H.; Barry, P.J. Oak Pests: A Guide to Major Insects, Diseases, Air Pollution and Chemical Injury; General Report SA-GR11; United States Department of Agriculture, Forest Service State & Private Forestry Southeastern Area: Asheville, NC, USA, 1980.
- Noukoun, C.; Bryant, G.; Frank, S.D. The Effect of Sticky Bands on Cankerworm Abundance and Defoliation in Urban Trees. Arboric. Urban For. 2014, 40, 135–142. [Google Scholar] [CrossRef]
- Darr, M.N.; Coyle, D.R. Fall Cankerworm (Lepidoptera: Geometridae), a Native Defoliator of Broadleaved Trees and Shrubs in North America. J. Integr. Pest Manag. 2021, 12, 23. [Google Scholar] [CrossRef]
- Walter, J.A.; Finch, F.T.; Johnson, D.M. Re-evaluating Fall Cankerworm Management Thresholds for Urban and Suburban Forests. Agric. For. Entomol. 2016, 18, 145–150. [Google Scholar] [CrossRef]
- Coffelt, M.A.; Schultz, P.B.; Wolf, D.D. Impact of Late-Season Orangestriped Oakworm (Lepidoptera: Satumiidae) Defoliation on Oak Growth and Vigor. Environ. Entomol. 1993, 22, 1318–1324. [Google Scholar] [CrossRef]
- Coffelt, M.A.; Schultz, P.B. Population Biology of Orangestriped Oakworm (Lepidoptera: Saturniidae) in Southeastern Virginia. J. Entomol. Sci. 1993, 28, 218–229. [Google Scholar] [CrossRef]
- Coffelt, M.A.; Schultz, P.B. Host Plant Suitability of the Orangestriped Oakworm (Lepidoptera: Saturniidae). J. Environ. Hortic. 1993, 11, 182–186. [Google Scholar] [CrossRef]
- Balduf, W.V. Observations on Archips cerasivoranus (Fitch) (Tortricidae: Lepidoptera) and Certain Parasites (Diptera: Hymenoptera). Ohio J. Sci. 1965, 65, 60–70. [Google Scholar]
- Schultz, P.B.; Shetlar, D.J. Major Insect Pests of Ornamental Trees and Shrubs. In Handbook of Integrated Pest Management; CRC Press: Boca Raton, FL, USA, 2020; pp. 237–247. [Google Scholar]
- United States Department of Agriculture, Forest Service. Pest Alert Oak Leaftier and Oak Leaf Roller; NA-PR-03-98; Northeastern Area Southern Region: Asheville, NC, USA, 1998.
- Carroll, M.R.; Wooster, M.T.; Kearby, W.H.; Allen, D.C. Biological Observations on Three Oak Leaf Tiers: Psilocorsis quercicella, P. reflexella, and P. cryptolechiella in Massachusetts and Missouri. Ann. Entomol. Soc. Am. 1979, 72, 441–447. [Google Scholar] [CrossRef]
- Mumma, R.O.; Zettle, A.; Hendry, L.B.; Cameron, E.A. Adult Emergence Profiles of the Oak Leaf Roller Archips semiferanus and Its Pupal Parasites. Environ. Entomol. 1974, 3, 329–332. [Google Scholar] [CrossRef]
- Gelok, E.; McGregor, R.; Henderson, D.; Poirier, L. Seasonal Occurrence and Parasitism of Bucculatrix ainsliella (Lepidoptera: Lyonetiidae) on Quercus rubra in Burnaby, British Columbia. J. Entomol. Soc. Br. Columbia. 1998, 95, 111–116. [Google Scholar]
- Murtfeldt, M.E. A New Species of Bucculatrix. Can. Entomol. 1905, 37, 218–219. [Google Scholar] [CrossRef]
- Gibbons, C.F.; Butcher, J.W. The Oak Skeletonizer, Bucculatrix ainsliella, in a Michigan Woodlot. J. Econ. Entomol. 1961, 54, 681–684. [Google Scholar] [CrossRef]
- Karban, R.; Ricklefs, R.E. Leaf traits and the species richness and abundance of Lepidopteran larvae on deciduous trees in southern Ontario. Oikos 1984, 43, 165–170. [Google Scholar] [CrossRef]
- Asaro, C.; Chamberlin, L.A. Impacts of Oak Decline, Gypsy Moth, and Native Spring Defoliators on The Oak Resource in Virginia. In Oak Symposium: Sustaining Oak Forests in the 21st Century Through Science-Based Management; e-General Technical Report SRS-237; United States Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2019. [Google Scholar]
- Evans, D. The Stages of Erannis Vancouverensis (Lepidoptera: Geometridae) and Life History Notes. Can. Entomol. 1973, 105, 605–612. [Google Scholar] [CrossRef]
- Oten, K.L.; Jetton, R.M.; Coyle, D.R. Ecology, Impacts, and Management of Common Late-season Defoliators of Southern Hardwoods. J. Integr. Pest Manag. 2023, 14, 4. [Google Scholar] [CrossRef]
- Eaton, E.R.; Kaufman, K. Field Guide to Insects of North America, 1st ed.; Mariner Books: Boston, MA, USA, 2007. [Google Scholar]
- Evans, H.J.; Hopkin, A.A.; Scarr, T.A. Status of Important Forest Pests in Ontario in 2005; Natural Resource Canada and Ontario Ministry of Natural Resources: Sault Ste Marie, ON, Canada, 2006. [Google Scholar]
- Juzwik, J.; Haugen, L.; Kyhl, J.; Schneeberger, N.F.; Rothlisberger, J.D.; Poland, T.M. Appendix: Regional Summaries: Midwest Region. In Invasive Species in Forests and Rangelands of the United States: A Comprehensive Science Synthesis for the United States Forest Sector; Poland, T.M., Patel-Weynand, T., Finch, D.M., Miniat, C.F., Hayes, D.C., Lopez, V.M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 414–419. [Google Scholar]
- Alalouni, U.; Schädler, M.; Brandl, R. Natural Enemies and Environmental Factors Affecting the Population Dynamics of the Gypsy Moth. J. Appl. Entomol. 2013, 137, 721–738. [Google Scholar] [CrossRef]
- Murray, K.D.; Shields, K.S.; Burand, J.P.; Elkinton, J.S. The effect of gypsy moth metamorphosis on the development of nuclear polyhedrosis virus infection. J. Invertebr. Pathol. 1991, 57, 352–361. [Google Scholar] [CrossRef]
- Rindos, M.; Liebhold, A.W. The Spongy Moth, Lymantria dispar. Curr. Biol. 2023, 33, R665–R668. [Google Scholar] [CrossRef] [PubMed]
- Forbush, E.H.; Fernald, C.H. The Gypsy Moth. Porthetria Dispar (Linn.). A Report of the Work of Destroying the Insect in the Commonwealth of Massachusetts, Together with an Account of Its History and Habits Both in Massachusetts and Europe; Wright & Potter Printing Co.: Boston, MA, USA, 1896. [Google Scholar]
- Ananko, G.G.; Kolosov, A.V. Asian Gypsy Moth (Lymantria dispar L.) Populations: Tolerance of Eggs to Extreme Winter Temperatures. J. Therm. Biol. 2021, 102, 103123. [Google Scholar] [CrossRef] [PubMed]
- Andresen, J.A.; Mccullough, D.G.; Potter, B.E.; Koller, C.N.; Bauer, L.S.; Lusch, D.P.; Ramm, C.W. Effects of Winter Temperatures on Gypsy Moth Egg Masses in the Great Lakes Region of the United States. Agric. For. Meteorol. 2001, 110, 85–110. [Google Scholar] [CrossRef]
- Conrad, A.O.; Crocker, E.V.; Li, X.; Thomas, W.R.; Ochuodho, T.O.; Holmes, T.P.; Nelson, C.D. Threats to Oaks in the Eastern United States: Perceptions and Expectations of Experts. J. For. 2020, 118, 14–27. [Google Scholar] [CrossRef]
- Rexrode, C.O. Insect Damage to Oaks. In Proceedings of the Upland Oak Ecology Symposium, Morgantown, WV, USA, 16–20 August 1971; United States Department of Agriculture, Forest Service, Northeastern Forest Experimental Station: Durham, NH, USA, 1971; pp. 129–134. [Google Scholar]
- Baker, W.L. Effect of Gypsy Moth Defoliation on Certain Forest Trees. J. For. 1941, 39, 1017–1022. [Google Scholar] [CrossRef]
- Barbosa, P. Host Plant Exploitation by the Gypsy Moth, Lymantria dispar. Entomol. Exp. Appl. 1978, 24, 228–237. [Google Scholar] [CrossRef]
- Lechowicz, M.J.; Jobin, L. Estimating the Susceptibility of Tree Species to Attack by the Gypsy Moth, Lymantria dispar. Ecol. Entomol. 1983, 8, 171–183. [Google Scholar] [CrossRef]
- Valentine, H.T.; Talerico, R.L. Gypsy Moth Larval Growth and Consumption on Red Oak. For. Sci. 1980, 26, 599–605. [Google Scholar] [CrossRef]
- Coleman, T.W. Lymantria dispar and Progression of Management Strategies in the United States. In Slow the Spread: A 20-Year Reflection on the National Lymantria dispar Integrated Pest Management Program; Coleman, T.W., Liebhold, A.M., Eds.; General Technical Report NRS-212; United States Department of Agriculture, Forest Service, Northern Research Station: Madison, WI, USA, 2023; pp. 1–27. [Google Scholar]
- Rossiter, M.; Schultz, J.C.; Baldwin, I.T. Relationships among Defoliation, Red Oak Phenolics, and Gypsy Moth Growth and Reproduction. Ecology 1988, 69, 267–277. [Google Scholar] [CrossRef]
- Schultz, J.C.; Baldwin, I.T. Oak Leaf Quality Declines in Response to Defoliation by Gypsy Moth Larvae. Science 1982, 217, 149–151. Available online: https://www.jstor.org/stable/1689717 (accessed on 13 May 2024). [CrossRef] [PubMed]
- Davidson, C.B.; Gottschalk, K.W.; Johnson, J.E. Tree Mortality Following Defoliation by the European Gypsy Moth (Lymantria dispar L.) in the United States: A Review. For. Sci. 1999, 45, 74–84. [Google Scholar] [CrossRef]
- Hilmers, T.; Leroy, B.M.L.; Bae, S.; Hahn, W.A.; Hochrein, S.; Jacobs, M.; Lemme, H.; Müller, J.; Schmied, G.; Weisser, W.W.; et al. Growth Response of Oaks to Insect Defoliation: Immediate and Intermediate Perspectives. For. Ecol. Manag. 2023, 549, 121465. [Google Scholar] [CrossRef]
- McClintock, J.T.; Dougherty, E.M.; Weiner, R.M. Protein Synthesis in Gypsy Moth Cells Infected with a Nuclear Polyhedrosis Virus of Lymantria dispar. Virus Res. 1986, 5, 307–322. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; (Ted) Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Hwang, H.; Acharya, R.; de Lucas, M.C.; Sharma, S.R.; Lee, Y.; Lee, K. Effects of Lymantria dispar Multiple Nucleopolyhedrovirus and Bacillus thuringiensis Var. Kurstaki on Different Larval Instars of Lymantria dispar Asiatica. Arch. Insect Biochem. Physiol. 2023, 113, e22002. [Google Scholar] [CrossRef]
- Hajek, A.E. Pathology and Epizootiology of Entomophaga maimaiga Infections in Forest Lepidoptera. Microbiol. Mol. Biol. Rev. 1999, 63, 814–835. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.R.; Hajek, A.E.; Liebhold, A.M.; Plymale, R. Impact of Entomophaga maimaiga (Entomophthorales: Entomophthoraceae) on Outbreak Gypsy Moth Populations (Lepidoptera: Erebidae): The Role of Weather. Environ. Entomol. 2014, 43, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, T.G.; Weseloh, R.M. Discovery of Entomophaga maimaiga in North American Gypsy Moth, Lymantria dispar. Proc. Natl. Acad. Sci. 1990, 87, 2461–2465. [Google Scholar] [CrossRef] [PubMed]
- Teschke, K.; Chow, Y.; Bartlett, K.; Ross, A.; van Netten, C. Spatial and Temporal Distribution of Airborne Bacillus thuringiensis Var. Kurstaki during an Aerial Spray Program for Gypsy Moth Eradication. Environ. Heal. Perspect. 2001, 109, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, C. Aerial Spraying of Bacillus thuringiensis Kurstaki (Btk). J. Pestic. Reform 2006, 26, 13–16. [Google Scholar]
- Boulton, T.J.; Otvos, I.S.; Halwas, K.L.; Rohlfs, D.A. Recovery of Nontarget Lepidoptera on Vancouver Island, Canada: One and Four Years after a Gypsy Moth Eradication Program. Environ. Toxicol. Chem. 2007, 26, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Leroy, B.M.L.; Rabl, D.; Püls, M.; Hochrein, S.; Bae, S.; Müller, J.; Hebert, P.D.N.; Kuzmina, M.L.; Zakharov, E.V.; Lemme, H.; et al. Trait-Mediated Responses of Caterpillar Communities to Spongy Moth Outbreaks and Subsequent Tebufenozide Treatments. Ecol. Appl. 2023, 33, e2890. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.P.; Webb, R.E.; Thorpe, K.W. Potential Enhancement of the Gypsy Moth (Lepidoptera: Lymantriidae) Nuclear Polyhedrosis Virus with the Triterpene Azadirachtin. Environ. Entomol. 1996, 25, 1209–1214. [Google Scholar] [CrossRef]
- Sharov, A.A.; Leonard, D.; Libehold, A.M.; Roberts, E.A.; Dickerson, W. “Slow the Spread” A National Program to Contain the Gypsy Moth. J. For. 2002, 100, 30–36. [Google Scholar] [CrossRef]
- Keena, M.A.; Wallner, W.E.; Grinberg, P.S.; Cardé, A.R.T. Female Flight Propensity and Capability in Lymantria dispar (Lepidoptera: Lymantriidae) from Russia, North America, and Their Reciprocal F 1 Hybrids. Environ. Entomol. 2001, 30, 380–387. [Google Scholar] [CrossRef]
- Reineke, A.; Zebitz, C.P.W. Flight Ability of Gypsy Moth Females (Lymantria dispar L.) (Lep., Lymantriidae): A Behavioural Feature Characterizing Moths from Asia? J. Appl. Entomol. 1998, 122, 307–310. [Google Scholar] [CrossRef]
- Elkinton, J.S.; Liebhold, A.M. Population Dynamics of Gypsy Moth in North America. Annu. Rev. Entmol. 1990, 35, 571–596. [Google Scholar] [CrossRef]
- Sandquist, R.E.; Richerson, J.V.; Cameron, E.C. Flight of North American Female Gypsy Moths. Environ. Entmol. 1973, 2, 957–958. [Google Scholar] [CrossRef]
- Webb, R.E.; McLane, W.H.; Finney, J.A.; Venables, L.; White, G.B.; Wieber, A.M.; Cohen, D.L. Destruction of Gypsy Moth Egg Masses (Using Surfactants, Detergents, Oils or Conventional Insecticides) for Quarantine and Community Action Programs. J. Entomol. Sci. 1994, 29, 305–317. [Google Scholar] [CrossRef]
- Thorpe, K.W.; Webb, R.E.; Ridgway, R.L.; Venables, L.; Tatman, K.M. Sticky Barrier Bands Affect Density of Gypsy Moth (Lepidoptera: Lymantriidae) and Damage in Oak Canopies. J. Econ. Entomol. 1993, 86, 1497–1501. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Elkinton, J.S.; Wallner, W.E. Effect of Burlap Bands on Between-Tree Movement of Late-Instar Gypsy Moth, Lymantria dispar (Lepidoptera: Lymantriidae). Environ. Entomol. 1986, 15, 373–379. [Google Scholar] [CrossRef]
- Wargo, P.M. Armillariella mellea and Agrilus bilineatus and Mortality of Defoliated Oak Trees. For. Sci. 1977, 23, 485–492. [Google Scholar]
- Sallé, A.; Nageleisen, L.-M.; Lieutier, F. Bark and Wood Boring Insects Involved in Oak Declines in Europe: Current Knowledge and Future Prospects in a Context of Climate Change. For. Ecol. Manag. 2014, 328, 79–93. [Google Scholar] [CrossRef]
- Millers, I.; Shriner, D.S.; Rizzo, D. History of Hardwood Decline in the Eastern United States; US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Durham, NH, USA, 1989; Volume 126.
- Dodds, K.J.; Sweeney, J.; Allison, J.D. Woodborers in Forest Stands. In Forest Entomology and Pathology; Springer International Publishing: Cham, Switzerland, 2023; pp. 361–415. [Google Scholar]
- Haack, R.A.; Petrice, T.R.; Zablotny, J.E. First Report of the European Oak Borer, Agrilus sulcicollis; (Coleoptera: Buprestidae), in the United States. Great Lakes Entomol. 2018, 42, 1. [Google Scholar] [CrossRef]
- Dunn, J.P.; Kimmerer, T.W.; Nordin, G.L. The Role of Host Tree Condition in Attack of White Oaks by the Twolined Chestnut Borer, Agrilus bilineatus (Weber) (Coleoptera: Buprestidae). Oecologia 1986, 70, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.P.; Kimmerer, T.W.; Potter, D.A. Winter Starch Reserves of White Oak as a Predictor of Attack by the Twolined Chestnut Borer, Agrilus bilineatus (Weber) (Coleoptera: Buprestidae). Oecologia 1987, 74, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Jendek, E.; Grebennikov, V.V. Agrilus sulcicollis (Coleoptera: Buprestidae), a New Alien Species in North America. Can. Entomol. 2009, 141, 236–245. [Google Scholar] [CrossRef]
- Haavik, L.J.; Fierke, M.K.; Stephen, F.M. Factors Affecting Suitability of Quercus Rubra as Hosts for Enaphalodes rufulus (Coleoptera: Cerambycidae). Environ. Entomol. 2010, 39, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Fierke, M.K.; Stephen, F.M. Callus Formation and Bark Moisture as Potential Physical Defenses of Northern Red Oak, Quercus Rubra, against Red Oak Borer, Enaphalodes rufulus (Coleoptera: Cerambycidae). Can. Entomol. 2008, 140, 149–157. [Google Scholar] [CrossRef]
- Duan, S.; He, H.S.; Spetich, M.A.; Wang, W.J.; Fraser, J.S.; Xu, W. Long-Term Effects of Succession, Climate Change and Insect Disturbance on Oak-Pine Forest Composition in the U.S. Central Hardwood Region. Eur. J. For. Res. 2022, 141, 153–164. [Google Scholar] [CrossRef]
- Duan, S.; He, H.S.; Spetich, M.A.; Wang, W.J.; Fraser, J.S.; Thompson, F.R. Indirect Effects Mediate Direct Effects of Climate Warming on Insect Disturbance Regimes of Temperate Broadleaf Forests in the Central U.S. J. Appl. Ecol. 2021, 58, 2626–2636. [Google Scholar] [CrossRef]
- Haavik, L.J.; Crook, D.J.; Fierke, M.K.; Galligan, L.D.; Stephen, F.M. Partial Life Tables from Three Generations of Enaphalodes rufulus; (Coleoptera: Cerambycidae). Environ. Entomol. 2012, 41, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Kolb, T.E.; Fettig, C.J.; Ayres, M.P.; Bentz, B.J.; Hicke, J.A.; Mathiasen, R.; Stewart, J.E.; Weed, A.S. Observed and Anticipated Impacts of Drought on Forest Insects and Diseases in the United States. For. Ecol. Manag. 2016, 380, 321–334. [Google Scholar] [CrossRef]
- Wheeler, Q.D.; Miller, K.B. The Science of Insect Taxonomy: Prospects and Needs. In Insect Biodiversity: Science and Society, 2nd ed.; Footit, R.G., Adler, P.H., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 499–526. ISBN 9781118945568. [Google Scholar]
- Marske, K.A.; Ivie, M.A. Beetle Fauna of the United States and Canada. Coleopt. Bull. 2003, 57, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, L.; Bloin, P.; Pelletier, F.; Hébert, C.; Sambaraju, K. First Records of Three Carpophilinae (Coleoptera: Nitidulidae) in Quebec. Great Lakes Entomol. 2023, 56, 17. [Google Scholar] [CrossRef]
- Cline, A.R.; Skelley, P.E. Discovery of New Species and Country Records for the North American Sap Beetle Fauna (Coleoptera: Nitidulidae). Zootaxa 2013, 3683, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Juzwik, J.; Skalbeck, T.C.; Neuman, M.F. Sap Beetle Species (Coleoptera: Nitidulidae) Visiting Fresh Wounds on Healthy Oaks During Spring in Minnesota. For. Sci. 2004, 50, 757–764. [Google Scholar] [CrossRef]
- Cervenka, V.J.; Skalbeck, T.C.; Kyhl, J.F.; Blackford, D.C.; Juzwik, J.J.; Seybold, S.J. How to Identify Common Nitidulid Beetles Associated with Oak Wilt Mats in Minnesota; HT-71; United States Department of Agriculture, Forest Service, North Central Research Station: Saint Paul, MN, USA, 2001.
- Henry, B.W.; Moses, C.S.; Richard, C.A.; Riker, A.J. Oak Wilt: Its Significance, Symptoms, and Cause. Phytopathology 1944, 34, 636–647. [Google Scholar]
- Gauthier, M.-K.; Bourgault, É.; Potvin, A.; Bilodeau, G.J.; Gustavsson, S.; Reed, S.; Therrien, P.; Barrette, É.; Tanguay, P. Biosurveillance of Oak Wilt Disease in Canadian Areas at Risk. Can. J. Plant Pathol. 2024, 46, 27–38. [Google Scholar] [CrossRef]
- Nair, V.M.G.; Kuntz, J.E. Mat Formation in Bur Oaks Infected with Ceratocystis Fagacearum; No. 95; Forestry Research Notes, Wisconsin University College of Agriculture and Conservation Department: Madison, WI, USA, 1963. [Google Scholar]
- Cones, W.L. Oak Wilt Mats on White Oak in West Virginia. Plant Dis. 1967, 51, 430. [Google Scholar]
- Gibbs, J.N.; French, D.W. The Transmission of Oak Wilt; United States Department of Agriculture, Forest Service, North Central Forest Experiment Station: Saint Paul, MN, USA, 1980; Volume 185.
- Appel, D.N. The Oak Wilt Enigma: Perspectives from the Texas Epidemic. Annu. Rev. Phytopathol. 1995, 33, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Sakalidia, M.; Chahal, K.; Morris, O.; Cregg, B.; McCullough, D. Worried About Oak Wilt? Michigan State University Extension: East Lansing, MI, USA, 2020; Available online: https://www.canr.msu.edu/news/worried_about_oak_wilt? (accessed on 11 May 2024).
- Minnesota Department of Natural Resources. Oak Wilt Management. Available online: https://www.dnr.state.mn.us/treecare/forest_health/oakwilt/management.html (accessed on 16 June 2024).
- Juzwik, J.; Appel, D.N.; MacDonald, W.L.; Burks, S. Challenges and Successes in Managing Oak Wilt in the United States. Plant Dis. 2011, 95, 888–900. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.; Snover-Clift, K.; Somers, L.; Cancelliere, J.; Cole, R. Early Detection of the Oak Wilt Fungus (Bretziella fagacearum) Using Trapped Nitidulid Beetle Vectors. For. Pathol. 2022, 52, e12767. [Google Scholar] [CrossRef]
- van der Gaag, D.J.; Loomans, A.J.M. Host Plants of Anoplophora glabripennis, a Review. EPPO Bull. 2014, 44, 518–528. [Google Scholar] [CrossRef]
- Coyle, D.R.; Trotter, R.T.; Bean, M.S.; Pfister, S.E. First Recorded Asian Longhorned Beetle (Coleoptera: Cerambycidae) Infestation in the Southern United States. J. Integ. Pest Manag. 2021, 12, 1–6. [Google Scholar] [CrossRef]
- Cavey, J.F.; Hoebeke, E.R.; Passoa, S.; Lingafelter, S.W. A New Exotic Threat To North American Hardwood Forests: An Asian Longhorned Beetle, Anoplophora Glabripennis (Motschulsky) (Coleoptera: Cerambycidae). I. Larval Description And Diagnosis. Proc. Entmol. Soc. Wash. 1998, 100, 373–381. [Google Scholar]
- Stephen, F.M. Red Oak Borer: Forest Insect and Disease Leaflet 163; United States Department of Agriculture, Forest Service: Fayetteville, AK, USA, 2022. Available online: https://www.fs.usda.gov/foresthealth/docs/fidls/FIDL-163-RedOakBorer.pdf (accessed on 22 June 2024).
- Priest, R.J.; Kula, R.R.; Gates, M.W. Leaf Mining Insects and Their Parasitoids in the Old-Growth Forest of the Huron Mountains. Great Lakes Entomol. 2019, 52, 117–160. [Google Scholar] [CrossRef]
- Dara, S.K.; Barringer, L.; Arthurs, S.P. Lycorma delicatula (Hemiptera: Fulgoridae): A New Invasive Pest in the United States. J Integr. Pest Manag. 2015, 6, 20. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Forest Service. Spotted Lanternfly Map: Confirmed Spotted Lanternfly Locations. Available online: https://www.stopslf.org/where-is-slf/slf-map/ (accessed on 11 May 2024).
- Barringer, L.; Ciafré, C.M. Worldwide Feeding Host Plants of Spotted Lanternfly, With Significant Additions from North America. Environ. Entomol. 2020, 49, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- McPherson, B.A.; Wood, D.L.; Storer, A.J.; Maggi Kelly, N.; Standiford, R.B. Sudden Oak Death, A New Forest Disease in California. Integr. Pest Manag. Rev. 2001, 6, 243–246. [Google Scholar] [CrossRef]
- Garbelotto, M.; Svihra, P.; Rizzo, D.M. New Pests and Diseases: Sudden Oak Death Syndrome Fells 3 Oak Species. Calif. Agric. 2001, 55, 9–19. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Goss, E.M.; Press, C.M. Phytophthora Ramorum: A Pathogen with a Remarkably Wide Host Range Causing Sudden Oak Death on Oaks and Ramorum Blight on Woody Ornamentals. Mol. Plant Pathol. 2008, 9, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Venette, R.C.; Cohen, S.D. Potential Climatic Suitability for Establishment of Phytophthora Ramorum within the Contiguous United States. For. Ecol. Manag. 2006, 231, 18–26. [Google Scholar] [CrossRef]
- Ockels, F.S. Sudden Oak Death: Monitoring Phytophthora Ramorum in the North Central United States. Ornam. Plants 2005, 20, 127–130. [Google Scholar]
- Stone, G.N.; Schönrogge, K.; Atkinson, R.J.; Bellido, D.; Pujade-Villar, J. The Population Biology of Oak Gall Wasps (Hymenoptera: Cynipidae). Annu. Rev. Entomol. 2001, 47, 633–668. [Google Scholar] [CrossRef]
- Johnson, W.T.; Lyon, H.H. Insects That Feed on Trees and Shrubs, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1988. [Google Scholar]
- Csóka, G.; Stone, G.N.; Melika, G. Biology, Ecology, and Evolution of Gall-inducing Cynipidae. In Biology, Ecology, and Evolution of Gall-inducing Arthropods; Raman, A., Schaefer, C.W., Withers, T.M., Eds.; Science Publishers, Inc.: Enfield, NH, USA, 2005; Volume 2, pp. 573–642. ISBN 1-57808-346-X. [Google Scholar]
- Denman, S. Tools and Resources: Acute Oak Decline. Available online: https://www.forestresearch.gov.uk/tools-and-resources/fthr/pest-and-disease-resources/acute-oak-decline/ (accessed on 18 May 2024).
- Brown, N.; Inward, D.J.G.; Jeger, M.; Denman, S. A Review of Agrilus biguttatus in UK Forests and Its Relationship with Acute Oak Decline. Forestry 2015, 88, 53–63. [Google Scholar] [CrossRef]
- Coleman, T.W.; Seybold, S.J. Collection History and Comparison of the Interactions of the Goldspotted Oak Borer, Agrilus auroguttatus Schaeffer (Coleoptera: Buprestidae), with Host Oaks in Southern California and Southeastern Arizona, U.S.A. Coleopt. Bull. 2011, 65, 93–108. [Google Scholar] [CrossRef]
- Venette, R.C.; Coleman, T.W.; Seybold, S.J. Assessing the Risks Posed by Goldspotted Oak Borer to California and Beyond. In Proceedings of the Seventh California Oak Symposium: Managing Oak Woodlands in a Dynamic World, Berkeley, CA, USA, 3–6 November 2014; General Technical Report PSW-GTR-251. US Department of Agriculture, Forest Service, Pacific Southwest Research Station: Berkeley, CA, USA. [Google Scholar]
- Loehle, C.; Hulcr, J.; Smith, J.A.; Munro, H.L.; Fox, T. Preventing the Perfect Storm of Forest Mortality in the United States Caused by Invasive Species. J. For. 2023, 121, 104–117. [Google Scholar] [CrossRef]
- Etterson, J.R.; Cornett, M.W.; White, M.A.; Kavajecz, L.C. Assisted Migration across Fixed Seed Zones Detects Adaptation Lags in Two Major North American Tree Species. Ecol. Appl. 2020, 30, e02092. [Google Scholar] [CrossRef] [PubMed]
- Hızal, E.; Arslangündoğdu, Z. The First Record of Two-Lined Chestnut Borer Agrilus bilineatus (Weber, 1801) (Coleoptera: Buprestidae) from Europe. Entomol. News 2018, 127, 333–335. [Google Scholar] [CrossRef]
- Krist, F.J.; Ellenwood, J.R.; Woods, M.E.; McMahan, A.J.; Cowardin, J.P.; Ryerson, D.E.; Sapio, F.J.; Zweifler, M.O.; Romero, S.A. National Insect & Disease Risk and Hazard Mapping; United States Department of Agriculture, Forest Service: Washington, DC, USA, 2024. Available online: https://www.fs.usda.gov/foresthealth/applied-sciences/mapping-reporting/national-risk-maps.shtml (accessed on 22 June 2024).
- Régnière, J.; Logan, J.A.; Carroll, A.; Safranyik, L. Phenological Modelling of Climate Change Impacts on North American Forest Insects. In Proceedings of the XII World Forestry Congress Proceedings Synthesis, Quebec, QC, Canada, 21–28 September 2013; Food and Agriculture Organization of the United Nations: Quebec, QC, Canada, 2003. [Google Scholar]
- Pedlar, J.H.; McKenney, D.W.; Hope, E.; Reed, S.; Sweeney, J. Assessing the Climate Suitability and Potential Economic Impacts of Oak Wilt in Canada. Sci. Rep. 2020, 10, 19391. [Google Scholar] [CrossRef] [PubMed]
- Potter, K.M.; Escanferla, M.E.; Jetton, R.M.; Man, G. Important Insect and Disease Threats to United States Tree Species and Geographic Patterns of Their Potential Impacts. Forests 2019, 10, 304. [Google Scholar] [CrossRef]
- Van Driesche, R.G.; Reardon, R.C. Technology Transfer Non-Native Pest Biology and Control of Emerald Ash Borer, FHTET-2014-09; United States Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2015. Available online: https://bugwoodcloud.org/resource/pdf/Biology_&_Control_EAB_FHTET-2014-09.pdf (accessed on 14 June 2024).
- Sun, J.; Koski, T.-M.; Wickham, J.D.; Baranchikov, Y.N.; Bushley, K.E. Emerald Ash Borer Management and Research: Decades of Damage and Still Expanding. Annu. Rev. Entomol. 2023, 69, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Siegert, N.W.; Mccullough, D.G.; Liebhold, A.M.; Telewski, F.W. Dendrochronological Reconstruction of the Epicentre and Early Spread of Emerald Ash Borer in North America. Divers. Distrib. 2014, 20, 847–858. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).