Inter- and Intra-Specific Variation in Leaf Functional Traits at Different Maturity Levels in a Tropical Monsoon Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Leaf Collection
2.3. Measurement of Leaf Functional Traits
2.4. Determination of Soil Physical and Chemical Properties
2.5. Data Analysis
3. Results
3.1. Leaf Functional Traits in Three Maturity Levels
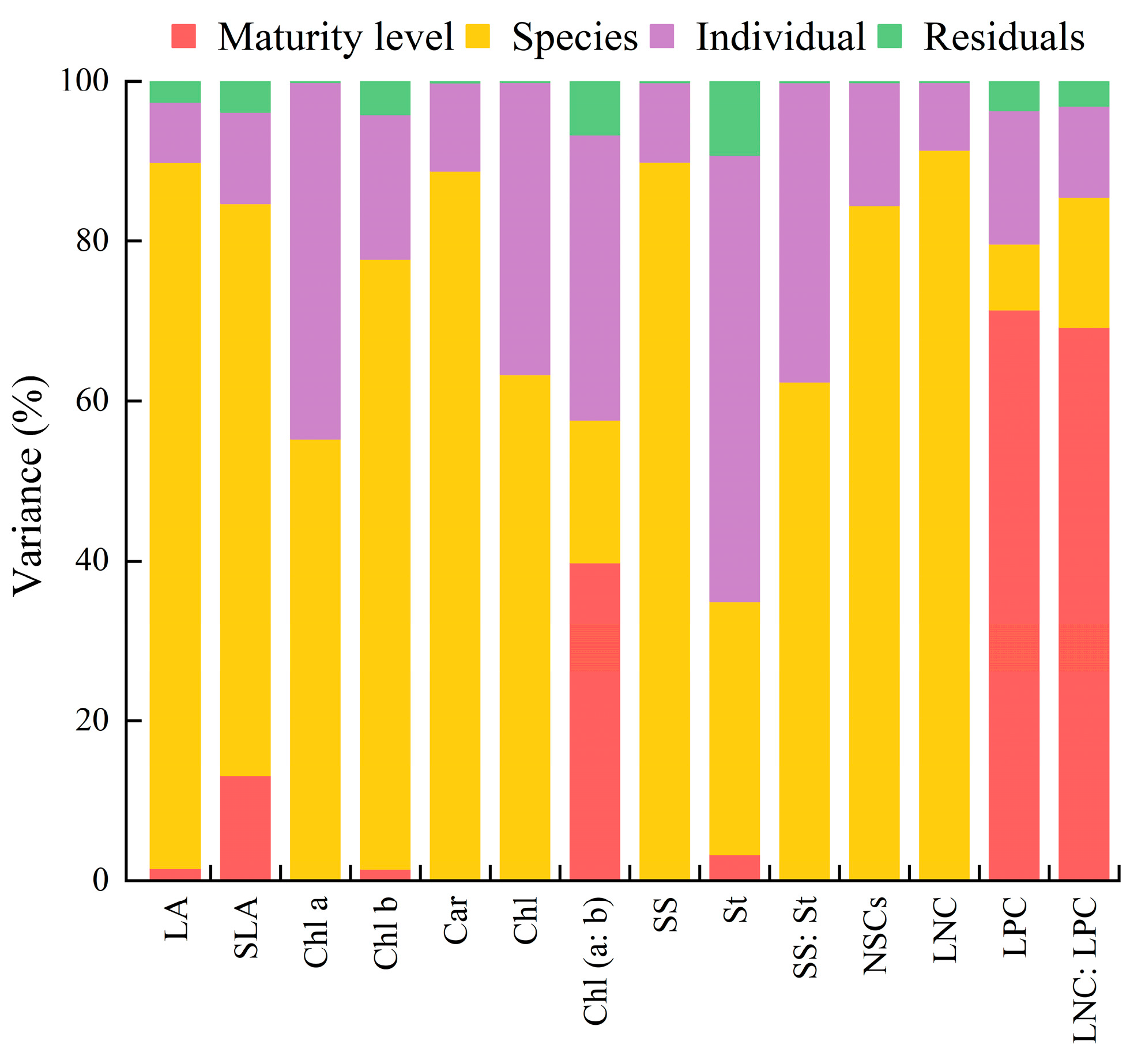
3.2. Leaf Functional Traits’ Variation Coefficient and Variance Source
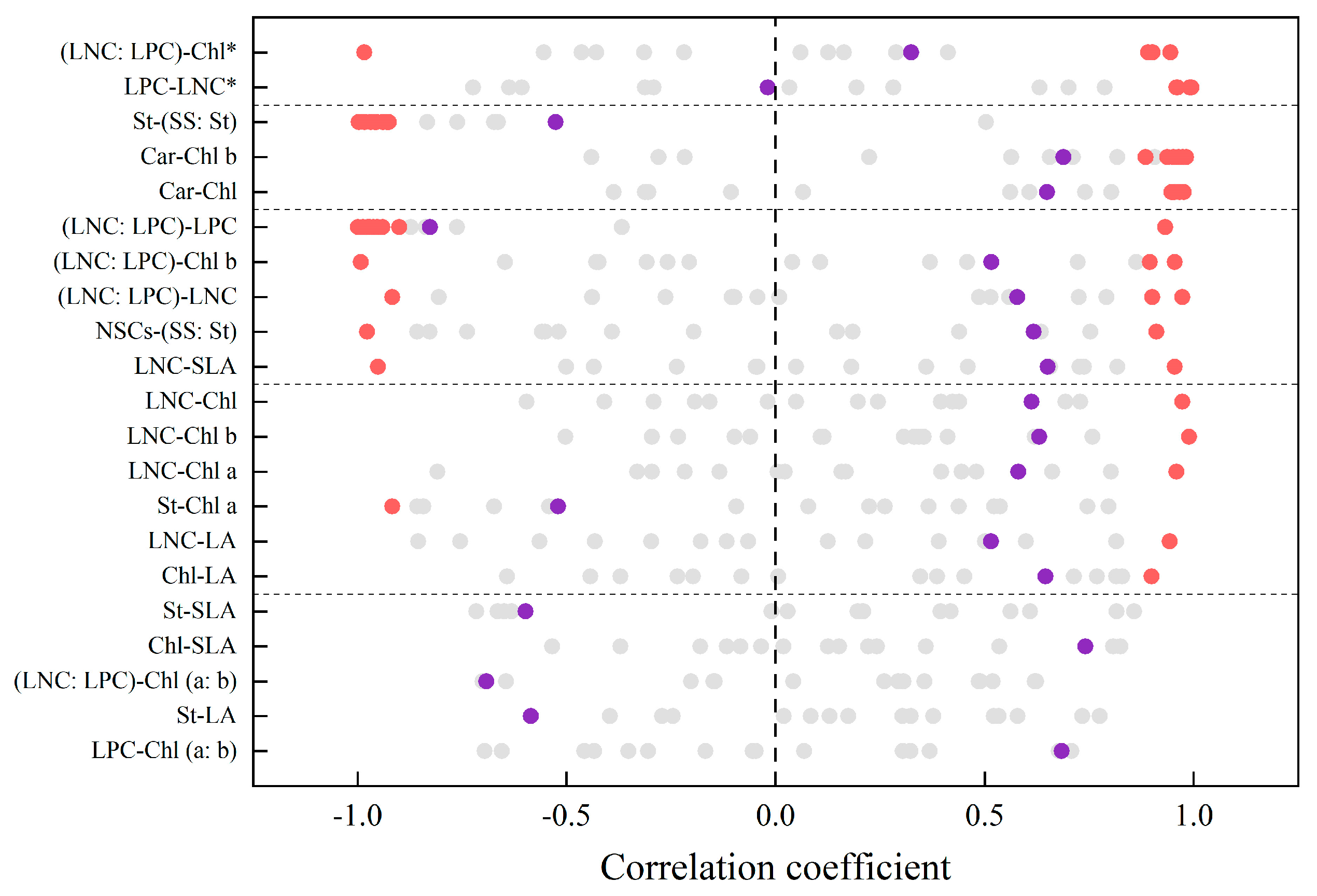
3.3. Trait–Trait Relationship and Multi-Trait Relationship
4. Discussion
4.1. Difference in Leaf Functional Traits among the Three Maturity Levels
4.2. Trait Variation and Trait–Trait Relationships at Different Scales
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Ma, K. Plant Functional Traits-Concepts, Applications and Future Directions. Sci. Sin. Vitae 2015, 45, 325–339. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, Y.; Ding, Y.; Zang, R. Interspecific and Intraspecific Variation in Functional Traits of Subtropical Evergreen and Deciduous Broad-Leaved Mixed Forests. Biodivers. Sci. 2016, 24, 262–270. [Google Scholar] [CrossRef][Green Version]
- Qin, J.; Shangguan, Z. Effects of Forest Types on Leaf Functional Traits and their Interrelationships of Pinus massoniana Coniferous And Broad-Leaved Mixed Forests in the Subtropical Mountain, Southeastern China. Ecol. Evol. 2019, 9, 6922–6932. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, Y.; An, X.; Yu, L.; Xue, Y. Leaf Functional Traits, Species Diversity and Functional Diversity of Plant Community in Tiankeng Forests. Acta Ecol. Sin. 2022, 42, 10264–10275. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Gong, J.; Zhao, L.; Xiang, W.; Cheng, X.; Wang, W.; Zhang, Y. Variation in Leaf Functional Traits and Adaptation Strategies of Dominant Tree Species in a Lithocarpus glaber-Cyclobalanopsis glauca Evergreen Broad-Leaved Forest. Acta Ecol. Sin. 2022, 42, 7256–7265. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, X.; Jiang, Y.; Liang, S.; Lu, Z.; Huang, Y.; Ni, M.; Qin, C.; Liu, R. Changes in leaf functional traits and soil environmental factors in response to slope gradient in Karst hills of Guilin. Acta Ecol. Sin. 2018, 38, 1581–1589. [Google Scholar] [CrossRef]
- Wang, Z.; Townsend, P.A.; Kruger, E.L. Leaf Spectroscopy Reveals Divergent Inter- and Intra-Species Foliar Trait Covariation and Trait-Environment Relationships across Neon Domains. New Phytol. 2022, 235, 923–938. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, L.; Lei, M.; Dang, H.; Quan, J.; Tian, T.; Chai, Y.; Yue, M. The Relationships between Leaf Economics and Hydraulic Traits of Woody Plants Depend on Water Availability. Sci. Total Environ. 2018, 621, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lohbeck, M.; Lebrija-Trejos, E.; Martinez-Ramos, M.; Meave, J.A.; Poorter, L.; Bongers, F. Functional Trait Strategies of Trees in Dry and Wet Tropical Forests are Similar but Differ in their Consequences for Succession. PLoS ONE 2014, 10, e0123741. [Google Scholar] [CrossRef]
- Shipley, B.; De Bello, F.; Cornelissen, J.H.; Laliberte, E.; Laughlin, D.C.; Reich, P.B. Reinforcing Loose Foundation Stones in Trait-Based Plant Ecology. Oecologia 2016, 180, 923–931. [Google Scholar] [CrossRef]
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.C.; Jalili, A.; Montserrat-Martí, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The Plant Traits that Drive Ecosystems: Evidence from Three Continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, C.; He, Z.; Zhu, J.; Xing, C.; Wang, X.; Liu, J.; Shen, C.; Shi, Y. Functional Trait Variation of Plant Communities in Canopy Gaps of Castanopsis kawakamii Natural Forest. Chin. J. Plant Ecol. 2022, 46, 267–279. [Google Scholar] [CrossRef]
- Maracahipes, L.; Carlucci, M.B.; Lenza, E.; Marimon, B.S.; Marimon, B.H.; Guimarães, F.A.G.; Cianciaruso, M.V. How to Live in Contrasting Habitats? Acquisitive and Conservative Strategies Emerge at Inter- and Intraspecific Levels in Savanna and Forest Woody Plants. Perspect. Plant Ecol. Evol. Syst. 2018, 34, 17–25. [Google Scholar] [CrossRef]
- Han, T.; Lu, H.; Ren, H.; Wang, J.; Song, G.; Hui, D.; Guo, Q.; Zhu, S. Are Reproductive Traits of Dominant Species Associated with Specific Resource Allocation Strategies during Forest Succession in Southern China? Ecol. Indic. 2019, 102, 538–546. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Meave, J.A.; Poorter, L.; Pérez-García, E.A.; Bongers, F. Pathways, Mechanisms and Predictability of Vegetation Change during Tropical Dry Forest Succession. Perspect. Plant. Ecol. Evol. Syst. 2010, 12, 267–275. [Google Scholar] [CrossRef]
- Becknell, J.M.; Powers, J.S. Stand Age and Soils as Drivers of Plant Functional Traits and Aboveground Biomass in Secondary Tropical Dry Forest. Can. J. For. Res. 2014, 44, 604–613. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Lebrija-Trejos, E.; Martinez-Ramos, M.; Meave, J.A.; Paz, H.; Perez-Garcia, E.A.; Romero-Perez, I.E.; Tauro, A.; Bongers, F. Successional Changes in Functional Composition Contrast for Dry and Wet Tropical Forest. Ecology 2013, 94, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Baraloto, C.; Hardy, O.J.; Paine, C.E.T.; Dexter, K.G.; Cruaud, C.; Dunning, L.T.; Gonzalez, M.-A.; Molino, J.-F.; Sabatier, D.; Savolainen, V.; et al. Using Functional Traits and Phylogenetic Trees to Examine the Assembly of Tropical Tree Communities. J. Ecol. 2012, 100, 690–701. [Google Scholar] [CrossRef]
- Jung, V.; Violle, C.; Mondy, C.; Hoffmann, L.; Muller, S. Intraspecific Variability and Trait-Based Community Assembly. J. Ecol. 2010, 98, 1134–1140. [Google Scholar] [CrossRef]
- Messier, J.; McGill, B.J.; Lechowicz, M.J. How do Traits Vary across Ecological Scales? A Case for Trait-Based Ecology. Ecol. Lett. 2010, 13, 838–848. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Douzet, R.; Aubert, S.; Lavorel, S. A Multi-Trait Approach Reveals the Structure and the Relative Importance of Intra- Vs. Interspecific Variability in Plant Traits. Funct. Ecol. 2010, 24, 1192–1201. [Google Scholar] [CrossRef]
- Plourde, B.T.; Boukili, V.K.; Chazdon, R.L.; Anten, N. Radial Changes in Wood Specific Gravity of Tropical Trees: Inter- and Intraspecific Variation during Secondary Succession. Funct. Ecol. 2014, 29, 111–120. [Google Scholar] [CrossRef]
- Su, C.; Duan, Y.; Zhenying, G.; Zheng, P.; HUang, X.; Fei, Y.; Zhou, M. Effects of Water Stress on the Physiological and Photosynthetic Characteristics of Phoebe bournei Seedlings. J. Cent. South Univ. For. Technol. 2022, 42, 85–92. [Google Scholar] [CrossRef]
- Li, X.; Pan, P.; Zang, H.; Ning, J.; Ouyang, X.; Li, X.; Gui, Y.; Wu, Z. Study on Factors Affecting Natural Regeneration of Natural Secondary Phoebe bournei Forest. For. Res. 2017, 30, 701–708. [Google Scholar] [CrossRef]
- He, Y.; Yao, Y.; Yao, Y.; Jiang, Y.; Liang, S.; Li, Y.; Liang, H.; Zhao, Q.; Huang, Y.; Ling, C. Interspecific and Intraspecific Variation of Functional Traits of Woody Species in the Dominant Cyclobalanopsis glauca Community in the Karst Area of Guilin City, Southwest China. Acta Ecol. Sin. 2021, 41, 8237–8245. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A Global Meta-Analysis of the Relative Extent of Intraspecific Trait Variation in Plant Communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The Return of the Variance: Intraspecific Variability in Community Ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Chang, S.X.; Yan, E.-R.; Wang, X.-H.; Bartha, S. Trait Variability Differs between Leaf and Wood Tissues across Ecological Scales in Subtropical Forests. J. Veg. Sci. 2014, 25, 703–714. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Diaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The Global Spectrum of Plant Form and Function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Chauvin, K.M.; Asner, G.P.; Martin, R.E.; Kress, W.J.; Wright, S.J.; Field, C.B. Decoupled Dimensions of Leaf Economic and Anti-Herbivore Defense Strategies in a Tropical Canopy Tree Community. Oecologia 2018, 186, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, L.D.L.; Berner, L.T.; Badgley, G.; Sethi, M.L.; Law, B.E.; HilleRisLambers, J. Within-Species Patterns Challenge Our Understanding of the Leaf Economics Spectrum. Ecol. Lett. 2018, 21, 734–744. [Google Scholar] [CrossRef]
- Reich, P.B.; Cornelissen, H. The World-Wide ‘Fast-Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Wang, Y.; Zhou, Q.; Long, F.; Zhao, Q.; He, Q.; Mo, Q. Distribution of Soil Cation Exchange Capacity and Exchangeable Based Cations of E’ huangzhang Montane Rain Forest. Chin. J. Soil Sci. 2022, 53, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Wu, M.; Wang, Y.; Tao, Y.; Lin, Z.; Zhou, Q.; Mo, Q. Characteristics and maturity level analysis of four communities in E’ huangzhang tropical monsoon forests of northern edge. Guihaia 2024, 44, 815–828. [Google Scholar] [CrossRef]
- Smith, M.D.; Knapp, A.K. Dominant Species Maintain Ecosystem Function with Non-Random Species Loss. Ecol. Lett. 2003, 6, 509–517. [Google Scholar] [CrossRef]
- Hao, M.; Dai, Y.; Yue, Q.; Fan, C.; Zhang, C. Relationship between Functional Diversity of Broadleaved Korean pine Forest and Forest Carbon Sink Function. J. Beijing For. Univ. 2022, 44, 68–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Song, Y.; Su, M.; Li, X.; Li, X. C, N and P Stoichiometric Characteristics of Leaf, Litter and Soil for Subalpine Forests in Central Yunnan, China. Acta Ecol. Sin. 2020, 40, 7648–7658. [Google Scholar] [CrossRef]
- Poorter, L. Growth Responses of 15 Rain-Forest Tree Species to a Light Gradient: The Relative Importance of Morphological and Physiological Traits. Funct. Ecol. 2002, 13, 396–410. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, D.; LEE, D.K. Leaf Traits and their Interrelationships of Main Plant Species in Southeast Horqin Sandy Land. Chin. J. Ecol. 2006, 25, 921–925. [Google Scholar] [CrossRef]
- He, C.; Sun, J.; Chen, Y.; Wang, L.; Shi, S.; Qiu, F.; Wang, S.; Tagesson, T. A New Vegetation Index Combination for Leaf Carotenoid-To-Chlorophyll Ratio: Minimizing the Effect of Their Correlation. Int. J. Digit. Earth 2023, 16, 272–288. [Google Scholar] [CrossRef]
- Alvarez-Anorve, M.Y.; Quesada, M.; Sanchez-Azofeifa, G.A.; Avila-Cabadilla, L.D.; Gamon, J.A. Functional Regeneration and Spectral Reflectance of Trees During Succession in a Highly Diverse Tropical Dry Forest Ecosystem. Am. J. Bot. 2012, 99, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, A.A.d.; Castro, E.M.d.; Lima Junior, É.d.C.; Magalhães, M.M. Effects of Different Light Levels on the Initial Growth and Photosynthesis of Croton Urucurana Baill. in Southeastern Brazil. Rev. Árvore 2003, 27, 53–57. [Google Scholar] [CrossRef]
- Dias, J.; Pimenta, J.A.; Medri, M.E.; Boeger, M.R.T.; Freitas, C.T.d. Physiological Aspects of Sun and Shade Leaves of Lithraea Molleoides (Vell.) Engl. (Anacardiaceae). Braz. Arch. Biol. Technol. 2007, 50, 91–99. [Google Scholar] [CrossRef][Green Version]
- Richardson, A.D.; Carbone, M.S.; Huggett, B.A.; Furze, M.E.; Czimczik, C.I.; Walker, J.C.; Xu, X.; Schaberg, P.G.; Murakami, P. Distribution and Mixing of Old and New Nonstructural Carbon in Two Temperate Trees. New Phytol. 2015, 206, 590–597. [Google Scholar] [CrossRef]
- Li, N.; He, N.; Yu, G.; Wang, Q.; Sun, J. Leaf Non-Structural Carbohydrates Regulated by Plant Functional Groups and Climate: Evidences from A Tropical to Cold-Temperate Forest Transect. Ecol. Indic. 2016, 62, 22–31. [Google Scholar] [CrossRef]
- Signori-Muller, C.; Oliveira, R.S.; Barros, F.V.; Tavares, J.V.; Gilpin, M.; Diniz, F.C.; Zevallos, M.J.M.; Yupayccana, C.A.S.; Acosta, M.; Bacca, J.; et al. Non-Structural Carbohydrates Mediate Seasonal Water Stress Across Amazon Forests. Nat. Commun. 2021, 12, 2310. [Google Scholar] [CrossRef]
- Gusewell, S. N:P Ratios in Terrestrial Plants: Variation and Functional Significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Yu, Y.; Wen, Z.; Peng, Z.; Zhou, Q.; Mo, Q. Seasonal Changes of Leaf Non-Structural Carbohydrate of Plants in Different Layers and its Responses to Nitrogen and Phosphorus Additions in a Secondary Tropical Forest. Acta Ecol. Sin. 2022, 42, 255–265. [Google Scholar] [CrossRef]
- Liu, W.; Su, J.; Li, S.; Lang, X.; Huang, X.; Zhang, Z. Variation of Non-Structural Carbonhydrates for the Dominant Species in a Monsoon Broad-Leaved Evergreen Forest in Pu’ Er, Yunnan Province. Sci. Silvae Sin. 2017, 53, 1–9. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls A and B Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. BBA-Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Jan, B.; Roel, M. An Improved Colorimetric Method to Quantify Sugar Content of Plant Tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-162. 2023. Available online: https://CRAN.R-project.org/package=nlme (accessed on 15 May 2023).
- Paradis, E.; Schliep, K. Ape 5.0: An Environment for Modern Phylogenetics and Evolutionary Analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Faccion, G.; Alves, A.M.; do Espírito-Santo, M.M.; Silva, J.O.; Sanchez-Azofeifa, A.; Ferreira, K.F. Intra- and Interspecific Variations on Plant Functional Traits along a Successional Gradient in a Brazilian Tropical Dry Forest. Flora 2021, 279, 151815. [Google Scholar] [CrossRef]
- Xi, X.; Zhao, Y.; Liu, Y.; Wang, X.; Gao, X. Variation and Correlation of Plant Functional Traits in Karst Area of Central Guizhou Province, China. Chinese J. Plant Ecol. 2011, 35, 1000–1008. [Google Scholar] [CrossRef]
- Bu, W.; Zang, R.G.; Ding, Y.; Zhang, J.; Ruan, Y. Relationships between Plant Functional Traits at the Community Level and Environmental Factors during Succession in a Tropical Lowland Rainforest on Hainan Island, South China. Biodivers. Sci. 2013, 21, 278–287. [Google Scholar] [CrossRef]
- Poorter, L.; Kitajima, K. Carbohydrate Storage and Light Requirements of Tropical Moist and Dry Forest Tree Species. Ecology 2007, 88, 1000–1011. [Google Scholar] [CrossRef]
- Tor-ngern, P.; Chart-asa, C.; Chanthorn, W.; Rodtassana, C.; Yampum, S.; Unawong, W.; Nathalang, A.; Brockelman, W.; Srinoppawan, K.; Chen, Y.; et al. Variation of Leaf-Level Gas Exchange Rates and Leaf Functional Traits of Dominant Trees across Three Successional Stages in A Southeast Asian Tropical Forest. Forest. Ecol. Manag. 2021, 489, 119101. [Google Scholar] [CrossRef]
- Boukili, V.K.; Chazdon, R.L. Environmental Filtering, Local Site Factors and Landscape Context Drive Changes in Functional Trait Composition during Tropical Forest Succession. Perspect. Plant Ecol. Evol. Syst. 2017, 24, 37–47. [Google Scholar] [CrossRef]
- Laughlin, D.C. Rugged Fitness Landscapes and Darwinian Demons in Trait-Based Ecology. New Phytol. 2018, 217, 501–503. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Perez-Garcia, E.A.; Meave, J.A.; Bongers, F.; Poorter, L. Functional Traits and Environmental Filtering Drive Community Assembly in a Species-Rich Tropical System. Ecology 2010, 91, 386–398. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; van Bodegom, P.M.; Witte, J.-P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A Global Study of Relationships between Leaf Traits, Climate and Soil Measures of Nutrient Fertility. Global. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Vile, D.; Shipley, B.; Garnier, E. A Structural Equation Model to Integrate Changes in Functional Strategies during Old-Field Succession. Ecology 2006, 87, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Soudant, A.; Boucher, F.; Saccone, P.; Lavorel, S. Intraspecific Functional Variability: Extent, Structure and Sources of Variation. J. Ecol. 2010, 98, 604–613. [Google Scholar] [CrossRef]
- Fajardo, A.; Siefert, A. Intraspecific Trait Variation and the Leaf Economics Spectrum across Resource Gradients and Levels of Organization. Ecology 2018, 99, 1024–1030. [Google Scholar] [CrossRef]
- Fang, S.; Cadotte, M.W.; Yuan, Z.; Lin, F.; Ye, J.; Hao, Z.; Wang, X. Intraspecific Trait Variation Improves the Detection of Deterministic Community Assembly Processes in Early Successional Forests, but not in Late Successional Forests. J. Plant Ecol. 2019, 12, 593–602. [Google Scholar] [CrossRef]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Diaz, S.; et al. Functional Traits and the Growth-Mortality Trade-Off in Tropical Trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Chen, Y.; Yu, S.; Fan, Y.; Peng, Z.; Wang, W.; Li, Z.; Wang, F. Leaf Nonstructural Carbohydrate Concentrations of Understory Woody Species Regulated by Soil Phosphorus Availability in a Tropical Forest. Ecol. Evol. 2020, 10, 8429–8438. [Google Scholar] [CrossRef] [PubMed]
- Favaretto, V.F.; Martinez, C.A.; Soriani, H.H.; Furriel, R.P.M. Differential Responses of Antioxidant Enzymes in Pioneer and Late-Successional Tropical Tree Species Grown Under Sun and Shade Conditions. Environ. Exp. Bot. 2011, 70, 20–28. [Google Scholar] [CrossRef]
- Brancalion, P.H.S.; Campoe, O.; Mendes, J.C.T.; Noel, C.; Moreira, G.G.; van Melis, J.; Stape, J.L.; Guillemot, J. Intensive Silviculture Enhances Biomass Accumulation and Tree Diversity Recovery in Tropical Forest Restoration. Ecol. Appl. 2019, 29, e01847. [Google Scholar] [CrossRef]
- Kollmann, J.; Meyer, S.T.; Bateman, R.; Conradi, T.; Gossner, M.M.; de Souza Mendonça, M.; Fernandes, G.W.; Hermann, J.-M.; Koch, C.; Müller, S.C.; et al. Integrating Ecosystem Functions into Restoration Ecology-Recent Advances and Future Directions. Restor. Ecol. 2016, 24, 722–730. [Google Scholar] [CrossRef]
- Laughlin, D.C. Applying Trait-Based Models to Achieve Functional Targets for Theory-Driven Ecological Restoration. Ecol. Lett. 2014, 17, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, M.B.; Brancalion, P.H.S.; Rodrigues, R.R.; Loyola, R.; Cianciaruso, M.V. Functional Traits and Ecosystem Services in Ecological Restoration. Restor. Ecol. 2020, 28, 1372–1383. [Google Scholar] [CrossRef]
- Emerson, B.C.; Gillespie, R.G. Phylogenetic Analysis of Community Assembly and Structure Over Space and Time. Trends Ecol. Evol. 2008, 23, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, M.; Swenson, N.G. Why Functional Traits Do Not Predict Tree Demographic Rates. Trends Ecol. Evol. 2018, 33, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Shen, J.; Tian, J.; Jin, K.; Zhang, F. Soil Health and Agriculture Green Development: Opportunities and Challenges. Acta Pedol. Sin. 2020, 57, 783–796. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Swenson, N.G. Variation in Leaf Functional Trait Values within and Across Individuals and Species: An Example from a Costa Rican Dry Forest. Funct. Ecol. 2010, 24, 217–223. [Google Scholar] [CrossRef]
- Messier, J.; McGill, B.J.; Enquist, B.J.; Lechowicz, M.J. Trait Variation and Integration across Scales: Is the Leaf Economic Spectrum Present at Local Scales? Ecography 2017, 40, 685–697. [Google Scholar] [CrossRef]
- Silva, M.C.; Teodoro, G.S.; Bragion, E.F.A.; van den Berg, E. The Role of Intraspecific Trait Variation in the Occupation of Sharp Forest-Savanna Ecotones. Flora 2019, 253, 35–42. [Google Scholar] [CrossRef]
- Benito Garzón, M.; Alía, R.; Robson, T.M.; Zavala, M.A. Intra-Specific Variability and Plasticity Influence Potential Tree Species Distributions under Climate Change. Global. Ecol. Biogeogr. 2011, 20, 766–778. [Google Scholar] [CrossRef]
- Funk, J.L.; Cornwell, W.K. Leaf Traits within Communities: Context May Affect the Mapping of Traits to Function. Ecology 2013, 94, 1893–1897. [Google Scholar] [CrossRef]
- Mason, C.M.; Donovan, L.A. Evolution of the Leaf Economics Spectrum in Herbs: Evidence from Environmental Divergences in Leaf Physiology across Helianthus (Asteraceae). Evolution 2015, 69, 2705–2720. [Google Scholar] [CrossRef] [PubMed]

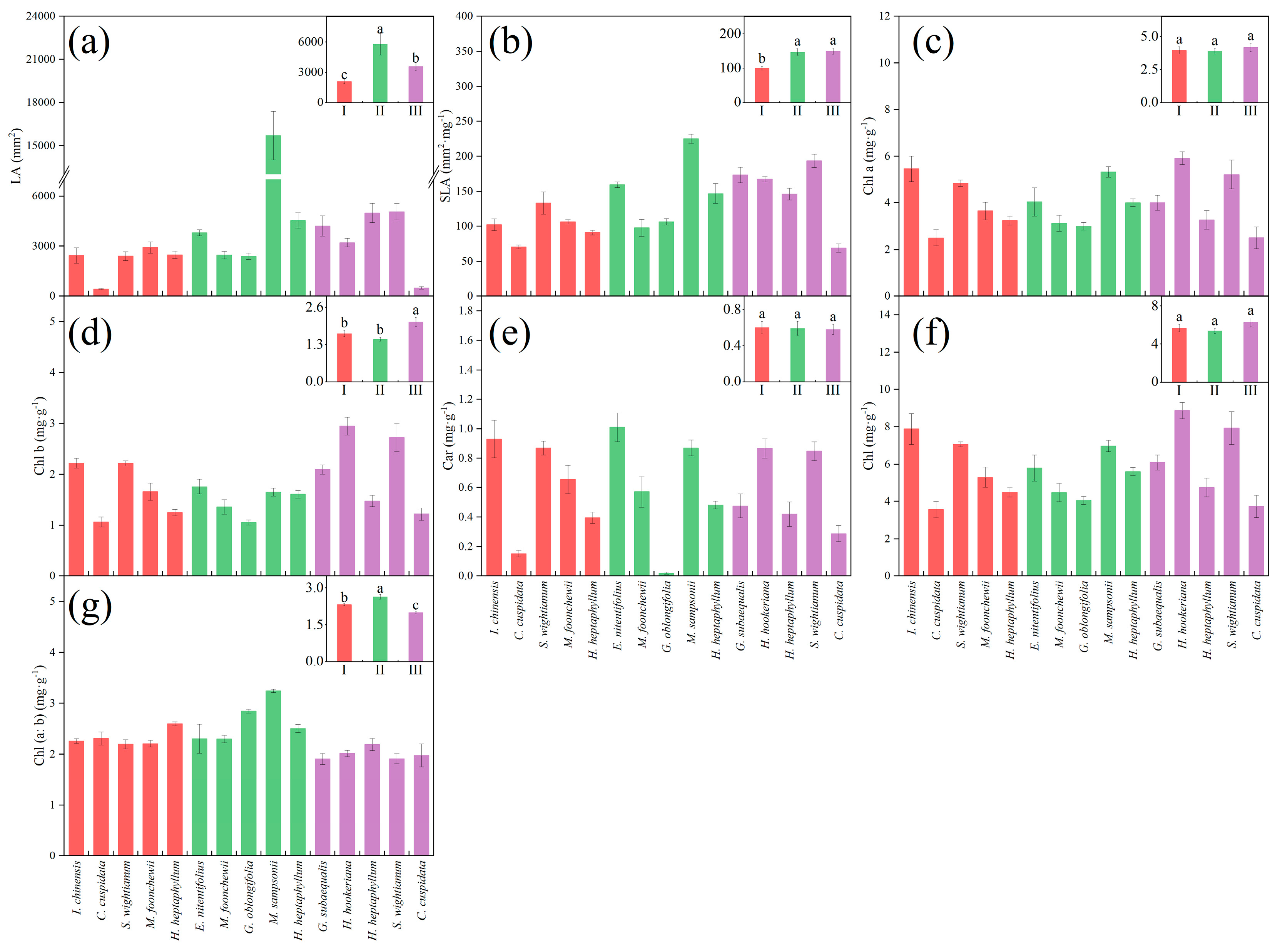
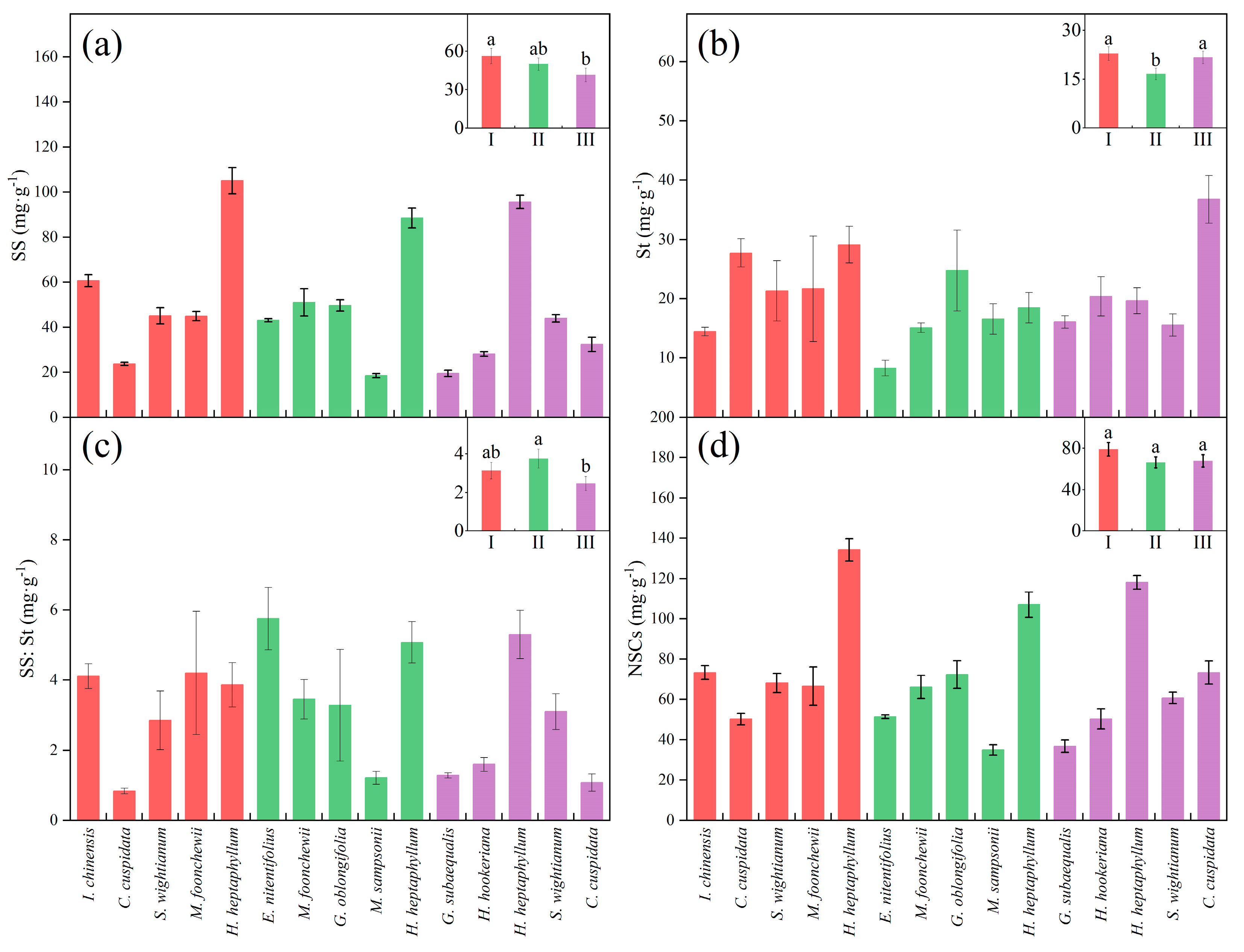
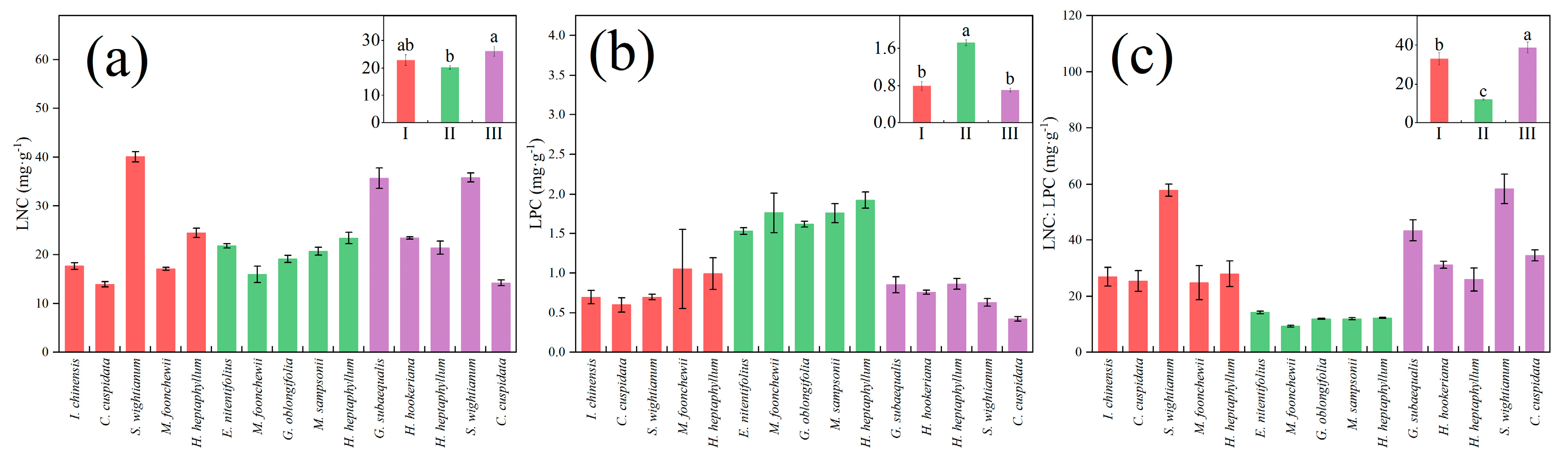
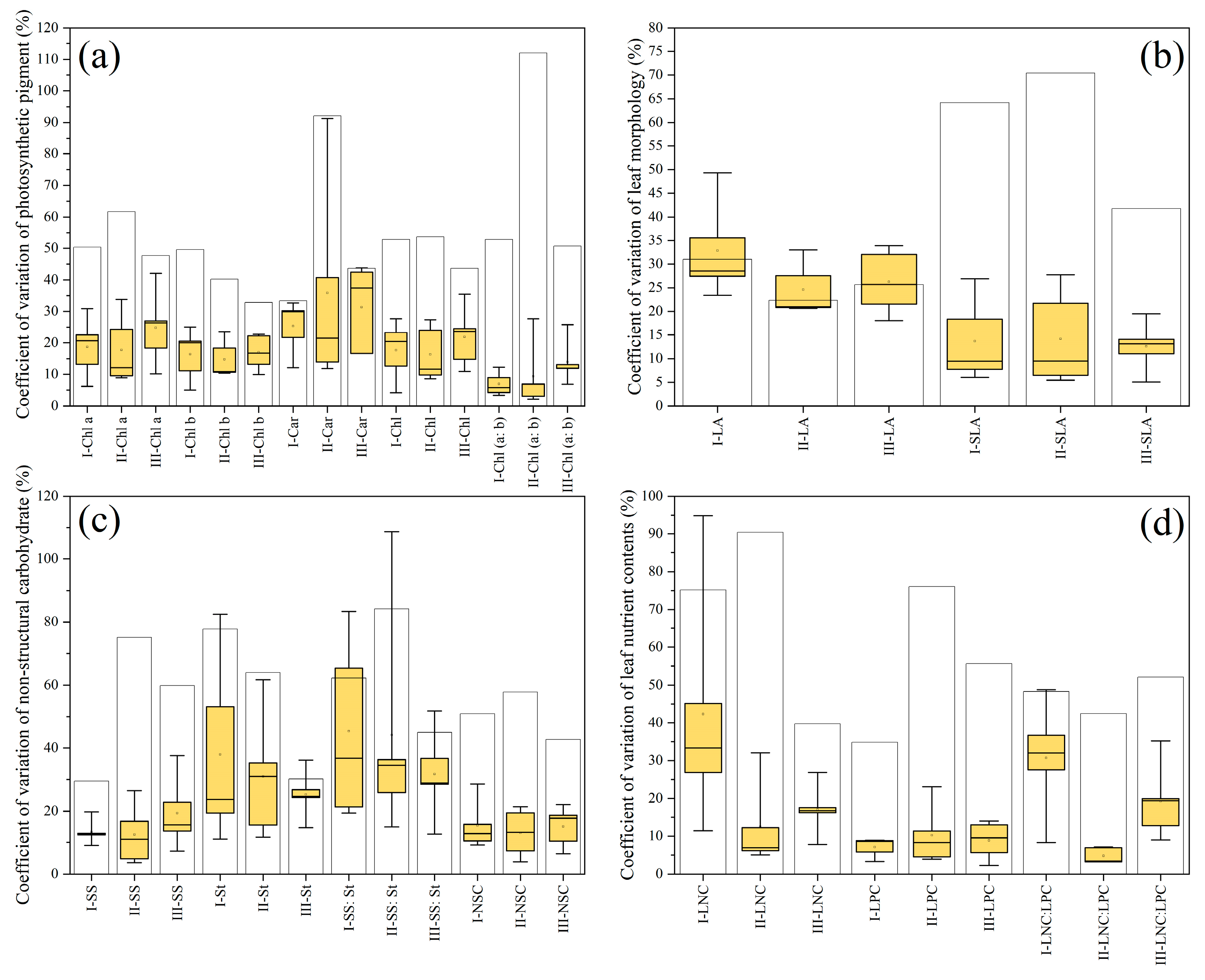

| Functional Trait | Unit | Biological and Ecological Significance |
|---|---|---|
| LA | mm2 | Leaf area (LA) reflects the ability of plants to capture light [37]. When suffering drought resistance, it first adapts to the environment by reducing leaf area and reducing leaf width. In wet years, leaf area will increase and leaf length will increase [38] |
| SLA | mg·g−1 | Specific leaf area (SLA) is one of the leaf economic spectrum traits, reflecting the investment made by plants to capture light per unit area and the plants’ shade tolerance [37]. It reflects the ability of plants to capture resources, and is related to plant growth rate and photosynthetic rate [39]. Plants with larger SLA have a larger resource capture area in their leaves, and thus have a higher net photosynthetic rate. Plants with lower SLA can better adapt to resource-poor and drought environments [40]. |
| Chl | mg·g−1 | Chlorophyll (Chl), the main pigment in plant photosynthesis, converts absorbed light energy into stored chemical energy [41]. |
| Chl (a:b) | / | Chlorophyll (a:b) is an acquisition strategy related to light capture [42]. The smaller the value, the stronger the shade tolerance of plants [43,44]. |
| Car | mg·g−1 | Carotenoids (Car) are photoprotective to chlorophyll and transfer absorbed light energy to chlorophyll, playing an auxiliary role in plant photosynthesis [41]. |
| NSCs | mg·g−1 | Non-structural carbohydrates (NSCs), which include soluble sugars and starch, reflects the adaptability of plants to environmental stresses [45] and may be a promising criterion for successional classification [46]. |
| SS | mg·g−1 | Soluble sugars (SS) provides immediate energy substrates for respiration, defense, plant stress signaling, phloem transport and osmoregulation [47]. It is also associated with cold tolerance, and the accumulation of soluble sugars helps to stop intracellular ice from harming the plant [48]. |
| St | mg·g−1 | Starch (St) represents a transient or long-term energy store that plants can convert to SS for use when carbon demand exceeds supply [47]. |
| SS:St | / | SS:St reflects the plant’s carbon utilization strategy, with higher values indicating that the plant converts more photosynthetic products into soluble sugars to supply vigorous life activities and growth, and lower values indicating that the plant converts more carbon into starch for long term storage and adaptation to a shaded environment [49,50]. |
| LNC | mg·g−1 | Leaf nitrogen content (LNC) is one of the leaf economic spectrum traits, reflecting the ability of plants to acquire nitrogen as well as the maximum photosynthetic rate [37], which is mainly fixed by plants from the atmosphere [29]. |
| LPC | mg·g−1 | Leaf phosphorus content (LPC) is one of the leaf economic spectrum traits and is mainly absorbed from weathering of soil minerals [29]. |
| LNC:LPC | / | LNC:LPC reflects plant nutrient limitation, when LNC:LPC < 14 for N limitation, LNC:LPC > 16 for P limitation, and 14 < LNC:LPC < 16a for N and P co-limitation [48]. |
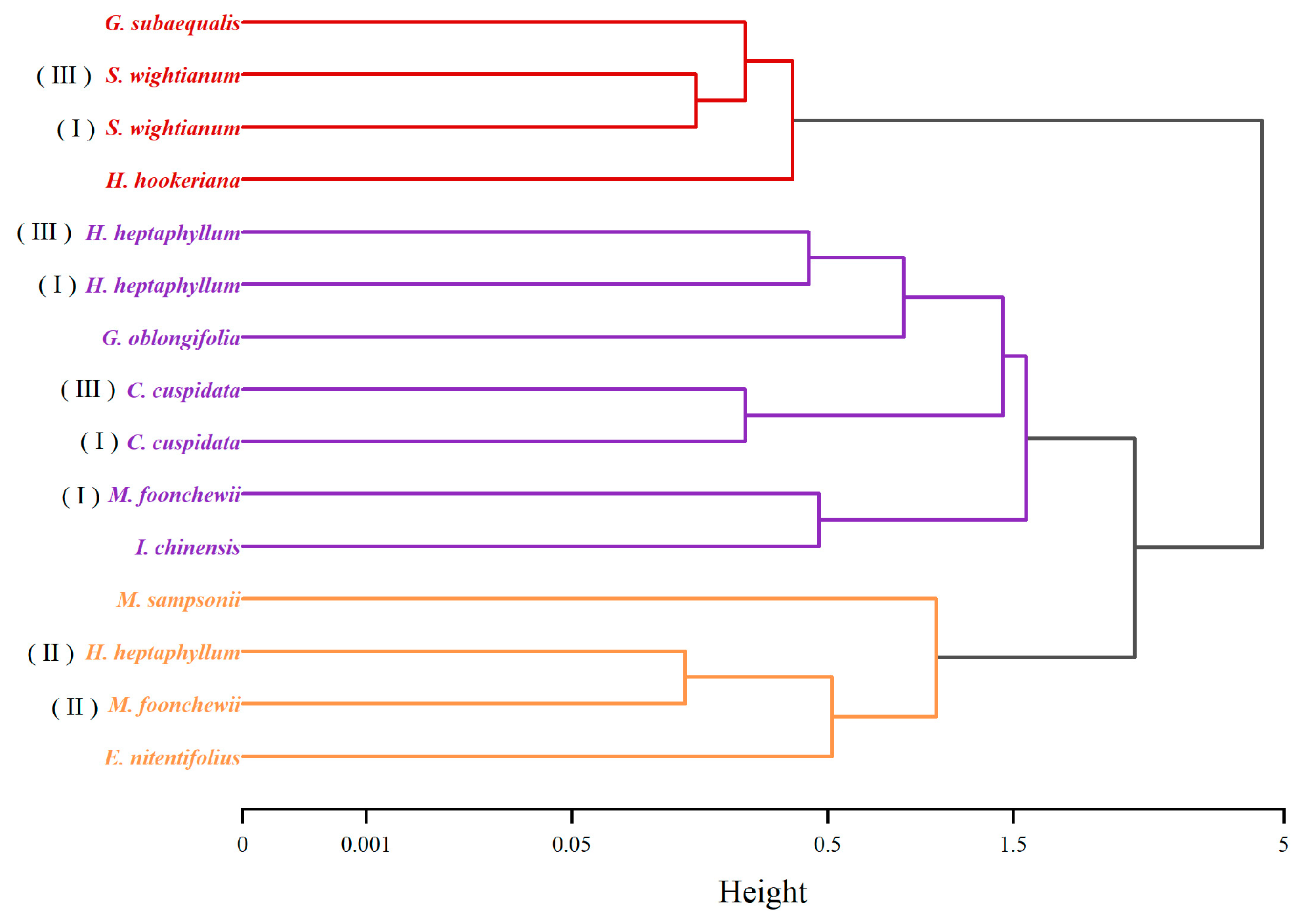
| Community | Latitude and Longitude | Altitude | Stand Density/(Stems hm−2) | Average DBH/(cm) | Average Height/(m) | Simpson Index | Pielou Evenness Index | Maturity Level |
|---|---|---|---|---|---|---|---|---|
| A | 111°31′25″ E, 21°55′11″ N | 100 | 6391.67 | 4.66 ± 0.15 a | 5.675 ± 0.10 a | 0.94 ± 0.02 a | 0.87 ± 0.03 a | III |
| B | 111°33′51″ E, 21°54′55″ N | 150 | 11,558.33 | 3.68 ± 0.09 b | 4.934 ± 0.07 c | 0.90 ± 0.02 b | 0.74 ± 0.03 c | I |
| C | 111°32′8″ E, 21°55′4″ N | 120 | 6500.00 | 4.48 ± 0.13 a | 5.063 ± 0.08 b | 0.94 ± 0.01 a | 0.84 ± 0.03 b | II |
| Community | Species | Abbreviation | IV | IV in Total |
|---|---|---|---|---|
| A | Sinosideroxylon wightianum | S. wightianum | 11.07 | 35.74 |
| A | Heptapleurum heptaphyllum | H. heptaphyllum | 8.27 | |
| A | Camellia cuspidata | C. cuspidata | 7.39 | |
| A | Hancea hookeriana | H. hookeriana | 5.40 | |
| A | Gironniera subaequalis | G. subaequalis | 3.62 | |
| B | Sinosideroxylon wightianum | S. wightianum | 13.29 | 49.88 |
| B | Itea chinensis | I. chinensis | 11.51 | |
| B | Camellia cuspidata | C. cuspidata | 10.02 | |
| B | Heptapleurum heptaphyllum | H. heptaphyllum | 9.12 | |
| B | Machilus foonchewii | M. foonchewii | 5.94 | |
| C | Heptapleurum heptaphyllum | H. heptaphyllum | 11.96 | 33.19 |
| C | Machilus foonchewii | M. foonchewii | 6.04 | |
| C | Garcinia oblongifolia | G. oblongifolia | 6.01 | |
| C | Macaranga sampsonii | M. sampsonii | 5.52 | |
| C | Elaeocarpus nitentifolius | E. nitentifolius | 3.65 |
| Community | Maturity Level | AP | TP | TN | pH |
|---|---|---|---|---|---|
| A | III | 4.53 ± 0.45 b | 0.1 ± 0.00 b | 2.77 ± 0.04 b | 5.06 ± 0.04 a |
| B | I | 2.76 ± 0.59 c | 0.08 ± 0.01 b | 1.91 ± 0.01 c | 4.78 ± 0.02 b |
| C | II | 7.49 ± 0.55 a | 0.16 ± 0.04 a | 3.42 ± 0.26 a | 4.83 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Liu, Y.; He, Z.; Gu, X.; Yu, Y.; Tao, Y.; Zhou, Q.; Mo, Q. Inter- and Intra-Specific Variation in Leaf Functional Traits at Different Maturity Levels in a Tropical Monsoon Forest. Forests 2024, 15, 1383. https://doi.org/10.3390/f15081383
Wu M, Liu Y, He Z, Gu X, Yu Y, Tao Y, Zhou Q, Mo Q. Inter- and Intra-Specific Variation in Leaf Functional Traits at Different Maturity Levels in a Tropical Monsoon Forest. Forests. 2024; 15(8):1383. https://doi.org/10.3390/f15081383
Chicago/Turabian StyleWu, Miaolan, Yue Liu, Zhihang He, Xiaojuan Gu, Yaohong Yu, Yuzhu Tao, Qing Zhou, and Qifeng Mo. 2024. "Inter- and Intra-Specific Variation in Leaf Functional Traits at Different Maturity Levels in a Tropical Monsoon Forest" Forests 15, no. 8: 1383. https://doi.org/10.3390/f15081383
APA StyleWu, M., Liu, Y., He, Z., Gu, X., Yu, Y., Tao, Y., Zhou, Q., & Mo, Q. (2024). Inter- and Intra-Specific Variation in Leaf Functional Traits at Different Maturity Levels in a Tropical Monsoon Forest. Forests, 15(8), 1383. https://doi.org/10.3390/f15081383








