Influence of Salt Concentration and Treatment Cycles on Nail-Holding Power in Dimension Lumber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nails
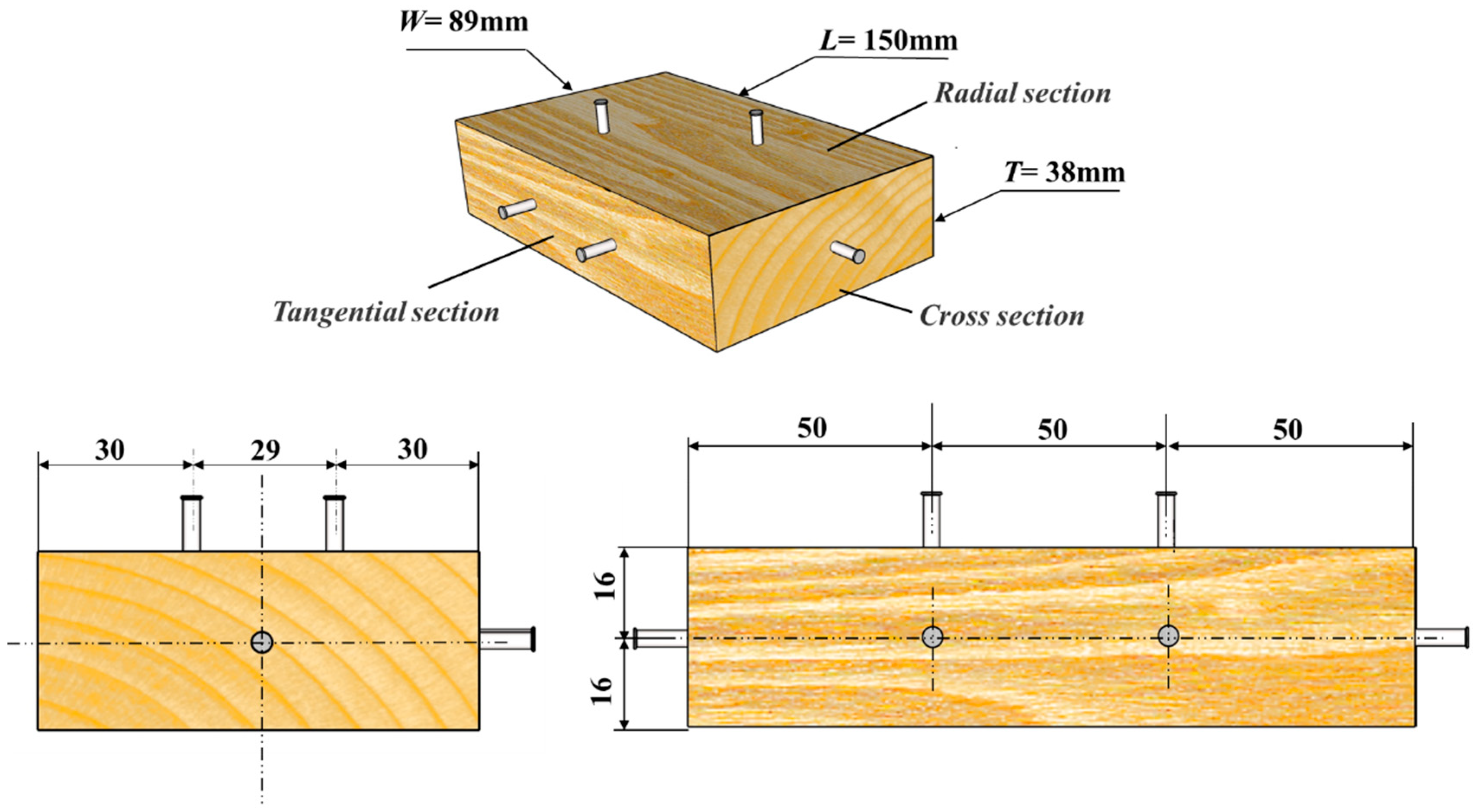
2.2. Dimension Lumber
2.3. Salt Solution Treatment
2.4. Equipment
3. Results and Discussions
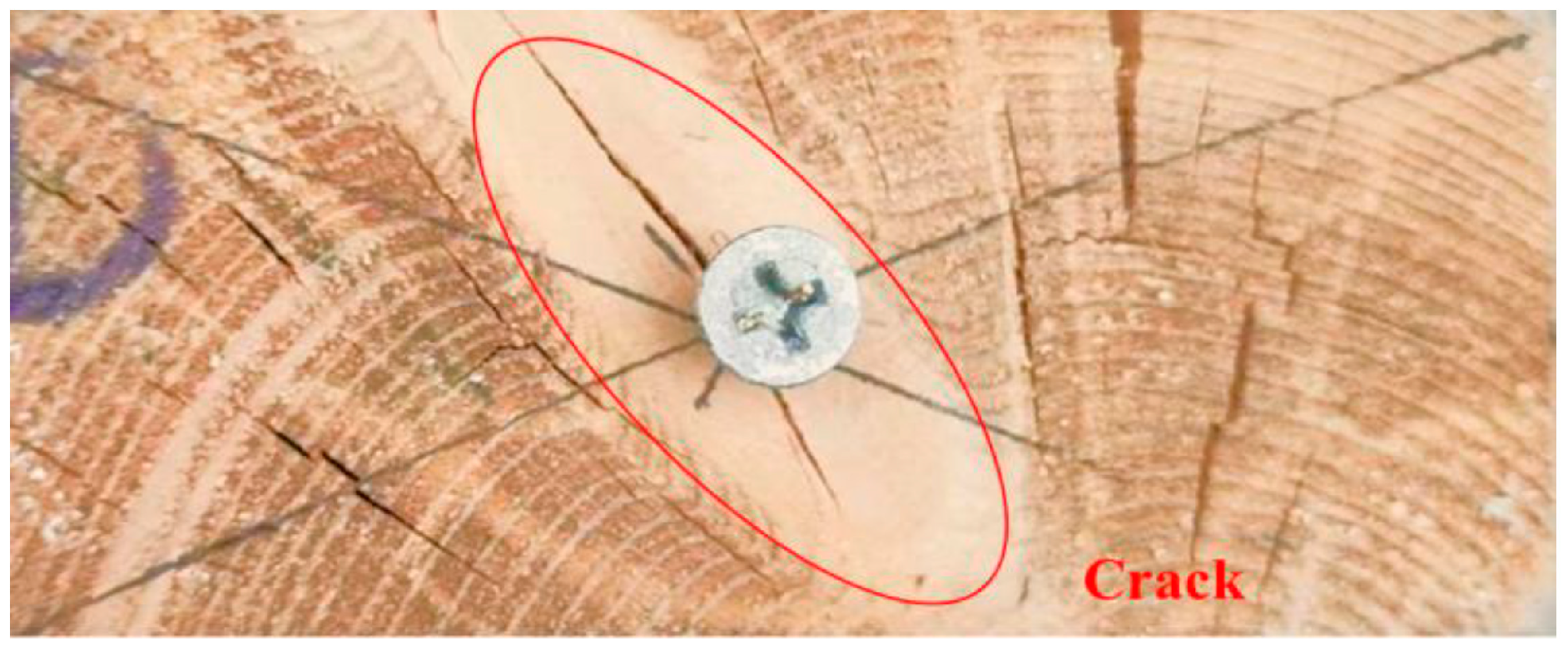
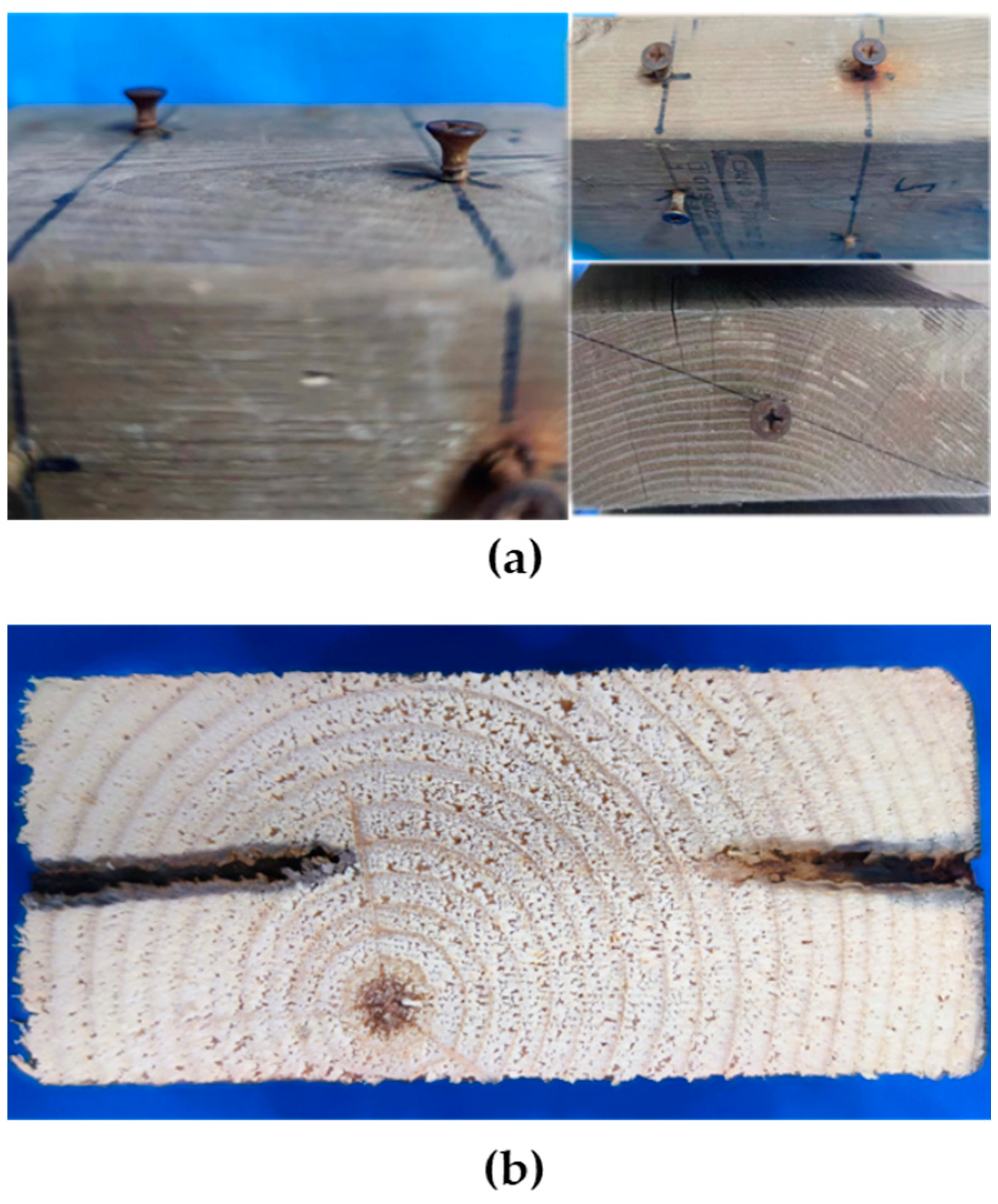
3.1. Visual Characterization of Wood Samples
3.2. Load-Displacement Characteristics
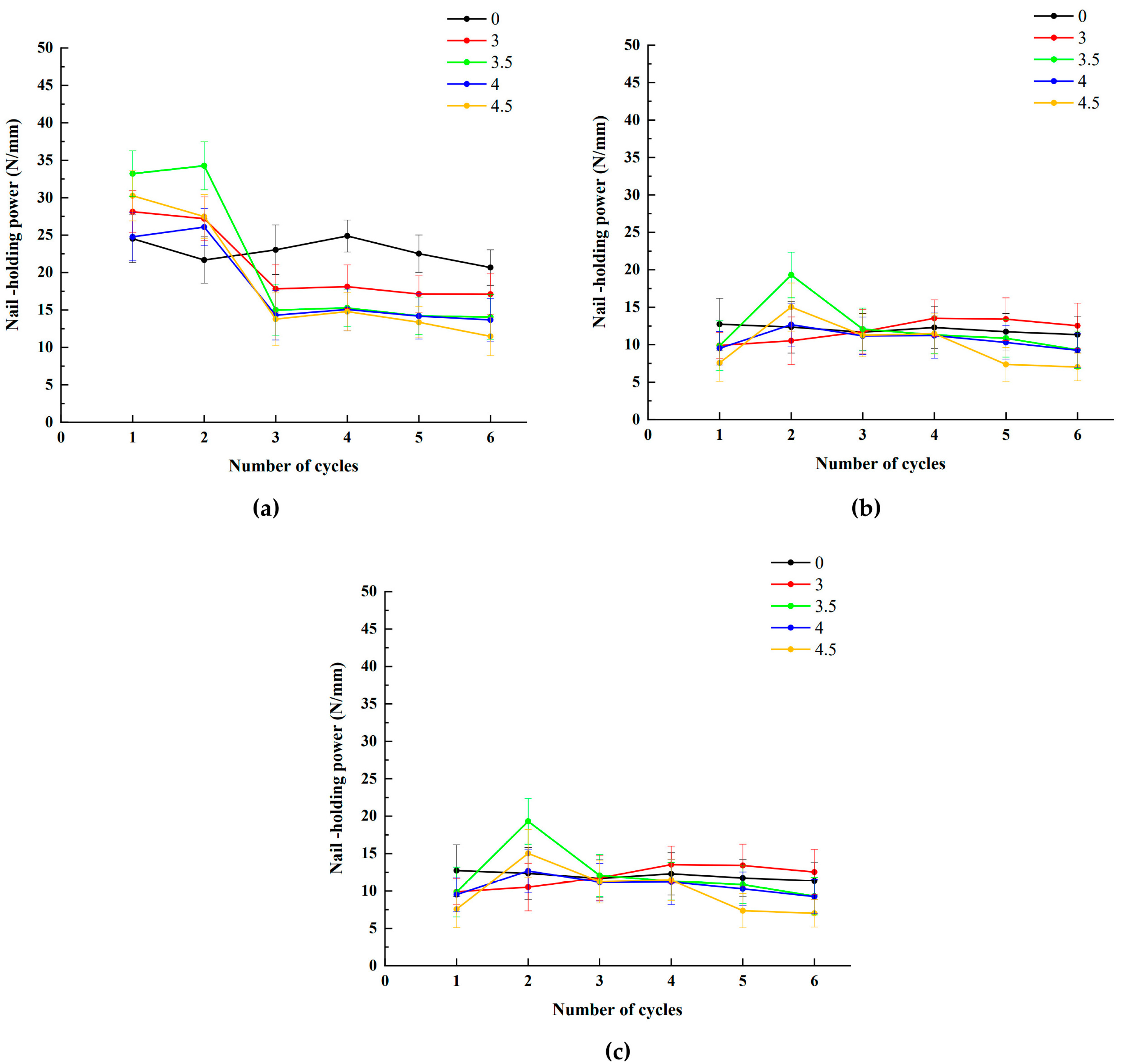
3.3. The Nail-Holding Power
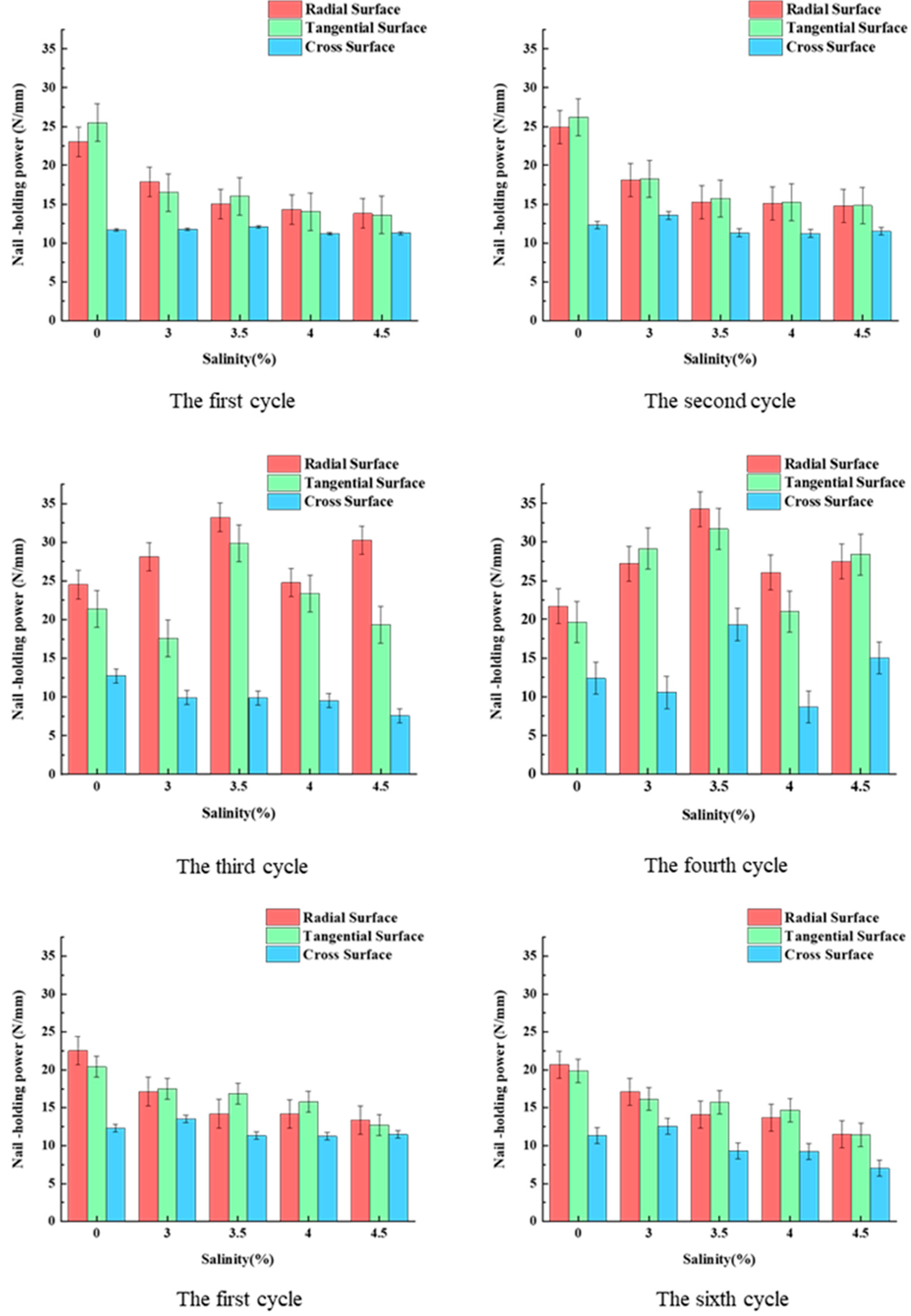
3.4. Differences in Nail-Holding Power across Three Sections
3.5. Effect of Salinity and Treatment Cycle on Nail-Holding Power
3.6. Statistical Analysis
3.7. Synthesize and Analyze
4. Conclusions
- (1)
- The nail-holding power of SPF lumber is significantly influenced by salt concentration and treatment duration. Radial and tangential sections exhibit a 15%–20% higher holding power than the cross-section. Initially, nail-holding power increases by up to 10% after two cycles but then declines. Each 0.5% increase in salt concentration from a 3% baseline results in about a 1% decrease in holding power, demonstrating a clear correlation. Analysis of variance shows that salinity and cycle duration significantly affect nail-holding power across all positions, with substantial differences observed between various levels and cycles. The functional relationship of each side nail-holding power changing with the high-salt treatment cycle is as follows: The radial-sectional holding power model is y = −2.204x + 23.404 (R2 = 0.91), the tangential-sectional holding power model is y = −2.628x + 23.404 (R2 = 0.86), and the cross-sectional holding power model is y = −0.148x + 12.002 (R2 = 0.63).
- (2)
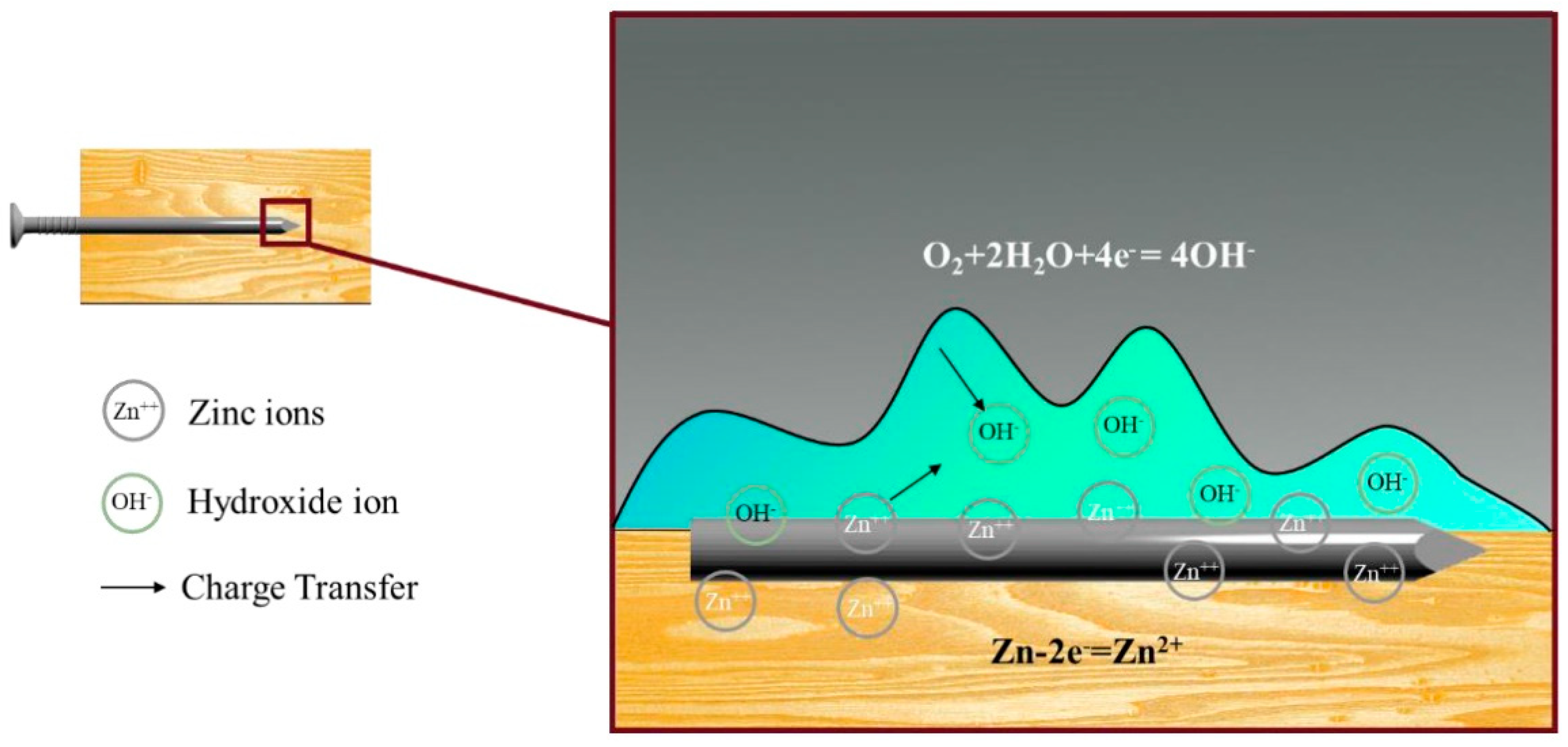
- Varying salt concentrations significantly accelerate metal corrosion, with higher concentrations leading to increased corrosion rates. For example, a 5% salt solution can increase the corrosion rate by up to 30% compared to a 3% solution. This effect is compounded by dissolved oxygen content, which further exacerbates corrosion. Corrosion rates also exhibit regional variability due to differing natural environmental conditions, underscoring the necessity for localized, quantitative analyses.
- (3)
- The load-carrying capacity of metal connectors is affected by chemical interactions between the salt solution, wood, and galvanized nails. The formation of chelates due to these interactions can reduce the load-bearing capacity by up to 25%, significantly weakening the joints in the timber frame buildings. This reduction in capacity highlights the critical risk of corrosion and structural compromise in high-salt environments.
- Examining a wider range of nail types and coatings to determine the most effective materials for use in saline environments.
- Investigating the long-term effects of saline exposure over multiple years to better understand the durability and reliability of nail connections in such conditions. Establish a model that can accurately predict corrosion failure.
- Evaluating the effectiveness of various wood preservation methods in mitigating the adverse effects of high-salt environments.
- Exploring the performance and durability of different wood species under such conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kirker, G.T.; Brischke, C.; Passarini, L.; Zelinka, S.L. Salt Damage in Wood: Controlled Laboratory Exposures and Mechanical Property Measurements. Wood Fiber Sci. 2020, 52, 44–52. [Google Scholar] [CrossRef]
- Slamová, K.; Glaser, R.; Schill, C.; Wiesmeier, S.; Köhl, M. Mapping Atmospheric Corrosion in Coastal Regions: Methods and Results. J. Phys. Conf. Ser. 2012, 2, 022003. [Google Scholar] [CrossRef]
- Crossman, M.; Simm, J.; Crossman, M. Manual on the Use of Timber in Coastal and River Engineering; Thomas Telford: London, UK, 2004. [Google Scholar]
- Prieto, A.J.; Silva, A. Service Life Prediction and Environmental Exposure Conditions of Timber Claddings in South Chile. Build. Res. Inf. 2020, 48, 191–206. [Google Scholar] [CrossRef]
- Treu, A.; Zimmer, K.; Brischke, C.; Larnøy, E.; Gobakken, L.R.; Aloui, F.; Cragg, S.M.; Flæte, P.-O.; Humar, M.; Westin, M.; et al. Durability and Protection of Timber Structures in Marine Environments in Europe: An Overview. BioResources 2019, 14, 10161–10184. [Google Scholar] [CrossRef]
- Skulteti, M.J.; Bender, D.A.; Winistorfer, S.G.; Pollock, D.G. Withdrawal Strength of Ring-Shank Nails Embedded in Southern Pine Lumber. Trans. ASAE 1997, 40, 451–456. [Google Scholar] [CrossRef]
- Fei, B.H. Nail Withdrawal Strength of Chinese Fir Plantation. Wood Process Mach. 2008, 5, 31–34. [Google Scholar]
- Wang, S.; Wang, F.; Kong, F.; Ma, P.; Chen, Z.; Que, Z. Influence of Repeated Wetting and Drying on Withdrawal Capacity of Wooden Nails and Metal Nails. Constr. Build. Mater. 2023, 409, 133991. [Google Scholar] [CrossRef]
- Teng, Q.; Que, Z.; Zeng, N. Effects of Angles on the Screw and Nail Withdrawal Strength in Dimension Lumber. Sci. Silvae Sin. 2020, 56, 154–161. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Sichel, R.J.; Stone, D.S. Exposure Testing of Fasteners in Preservative Treated Wood: Gravimetric Corrosion Rates and Corrosion Product Analyses. Corros. Sci. 2010, 52, 3943–3948. [Google Scholar] [CrossRef]
- Yerman, L.; Ottenhaus, L.-M.; Montoya, C.; Morrell, J.J. Effect of Repeated Wetting and Drying on Withdrawal Capacity and Corrosion of Nails in Treated and Untreated Timber. Constr. Build. Mater. 2021, 284, 122878. [Google Scholar] [CrossRef]
- Que, Z.; Yang, L.; Wang, F.; Zhu, X.; Wang, Y.; Mori, T. Effects of Salinity on the Nail-Holding Power of Dimension Lumber Used in Light-Frame Wood Building. J. For. Res. 2015, 26, 765–770. [Google Scholar] [CrossRef]
- GB 50005—2017; Standard for Design of Timber Structures. China Architecture and Building Press: Beijing, China, 2018.
- GB/T 1927.4-2021; Test Methods for Physical and Mechanical Properties of Small Clear Wood Specimens—Part 4: Determination of Moisture Content. China Architecture and Building Press: Beijing, China, 2021.
- GB/T 1927.5-2021; Test Methods for Physical and Mechanical Properties of Small Clear Wood Specimens—Part 5: Determination of Density. China Architecture and Building Press: Beijing, China, 2021.
- GB/T 1927–2022; Test Methods for Physical and Mechanical Properties of Small Clear Wood Specimens, Part 21: Determination of Nail and Screw Holding Power. Chinese Standards Press: Beijing, China, 2022.
- ISO 9087:1998; Wood—Determination of Nail and Screw Holding Power Under Axial Load Application. International Organization for Standardization: Geneva, Switzerland, 1998.
- Tang, Y.J.; Meng, Q.; Ren, P. Spatial Distribution and Concentrations of Salt Fogs in a Coastal Urban Environment: A Case Study in Zhuhai City. Build. Environ. 2023, 234, 110156. [Google Scholar] [CrossRef]
- Celebi, G.; Kilic, M. Nail and Screw Withdrawal Strength of Laminated Veneer Lumber Made Up Hardwood and Softwood Layers. Constr. Build. Mater. 2007, 21, 894–900. [Google Scholar] [CrossRef]
- Wu, G.F.; Lang, Q.; Song, S.; Pu, J. Impregnation and Drying Schedule of Eucalyptus Wood. Adv. Mater. Sci. Eng. 2011, 71–78, 860–863. [Google Scholar] [CrossRef]
- Permeh, S.; Lau, K. Corrosion of Galvanized Steel in Alkaline Solution Associated with Sulfate and Chloride Ions. Constr. Build. Mater. 2023, 392, 131889. [Google Scholar] [CrossRef]
- Francis, R. Galvanic Corrosion. In Corrosion in Seawater Systems; Francis, R., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 109–126. [Google Scholar]
- Morcillo, M.; Diaz, I.; Cano, H.; Chico, B.; de la Fuente, D. Atmospheric Corrosion of Weathering Steels. Overview for Engineers. Part II: Testing, Inspection, Maintenance. Constr. Build. Mater. 2019, 222, 750–765. [Google Scholar] [CrossRef]
- Soufeiani, L.; Foliente, G.; Nguyen, K.T.Q.; San Nicolas, R. Corrosion Protection of Steel Elements in Facade Systems—A Review. J. Build. Eng. 2020, 32, 101759. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Glass, S.V.; Boardman, C.R.; Derome, D. Comparison of the Corrosion of Fasteners Embedded in Wood Measured in Outdoor Exposure with the Predictions from a Combined Hygrothermal-Corrosion Model. Corros. Sci. 2016, 102, 178–185. [Google Scholar] [CrossRef]
- Yu, S.; Liu, Y.; Chen, Q.; Yu, X.; Hyun, G.; Wang, S.; Ye, Y.; Feng, J.; Chen, Z.; Jiang, F.; et al. Damage-Tolerant Wood Layers for Corrosion Protection of Metal Structures. Nano Lett. 2023, 24, 245–253. [Google Scholar] [CrossRef]
- Luszczki, G.E.; Clapp, J.D.; Davids, W.G.; Lopez-Anido, R. Withdrawal Capacity of Plain, Annular Shank, and Helical Shank Nail Fasteners in Spruce-Pine-Fir Lumber. For. Prod. J. 2013, 63, 213–220. [Google Scholar] [CrossRef]
- Ceylan, A.; Girgin, Z.C. Comparisons on Withdrawal Resistance of Resin and Phosphate Coated Annular Ring Nails in CLT Specimens. Constr. Build. Mater. 2020, 63, 213–220. [Google Scholar] [CrossRef]
- Brandner, R.; Ringhofer, A.; Grabner, M. Probabilistic Models for the Withdrawal Behavior of Single Self-Tapping Screws in the Narrow Face of Cross Laminated Timber (CLT). Eur. J. Wood Wood Prod. 2018, 76, 13–30. [Google Scholar] [CrossRef]
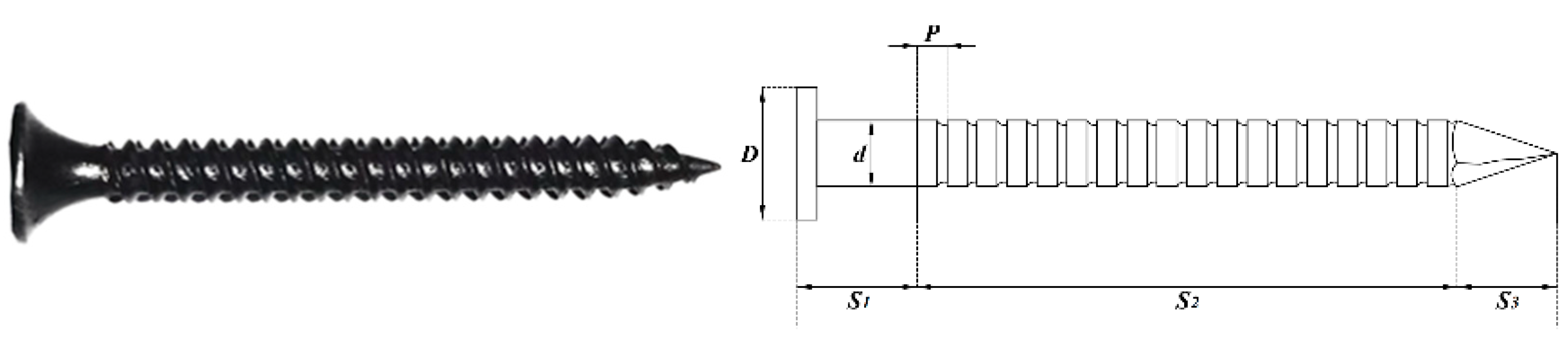




| Type | L | S1 | S2 | S3 | D | d | P |
|---|---|---|---|---|---|---|---|
| Spiral nails | 38 | 6 | 27 | 5 | 3.5 | 3.3 | 1.5 |
| Step | Comments | |
|---|---|---|
| 1 | Cut timber specimens | See Figure 2 for size |
| 2 | Determine the moisture content of each specimen | Using small samples. Oven-dry method |
| 3 | Manually drive nails in the wood | Refer to Table 1 for nail sizes and Figure 1 for nail geometry |
| 4 | Perform the nail-holding power tests | Values corresponding to Cycle 0 |
| 5 | Wet-dry cycle: soak samples in salt solution at ambient temperature for three days | Soak until the moisture content reaches 50%–60%, determined by gravimetry |
| 6 | Wet-dry cycle: the sample is dried outdoors for two days | Dry until the moisture content drops to 10%–20%, determined by gravimetry |
| 7 | Perform the nail-holding power tests | Values corresponding to Cycle 1 |
| 8 | Assess fastener condition | Check the condition of the wood around the nails |
| Salinity/% | Crude Salt/Kg | Distilled Water/L |
|---|---|---|
| 0.0% | 0.0 | 30.00 |
| 3.0% | 1.0 | 32.33 |
| 3.5% | 1.0 | 27.57 |
| 4.0% | 1.5 | 36.00 |
| 4.5% | 1.5 | 31.83 |
| Salinity (%) | The First Cycle | The Second Cycle | The Third Cycle | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nail-Holding Power (N∙mm−1) | Nail-Holding Power (N∙mm−1) | Nail-Holding Power (N∙mm−1) | |||||||
| PS1 | PS2 | PS3 | PS1 | PS2 | PS3 | PS1 | PS2 | PS3 | |
| 0 | 24.52 | 21.39 | 12.73 | 21.67 | 19.07 | 12.35 | 23.04 | 25.50 | 11.67 |
| (3.19) | (2.95) | (3.46) | (3.10) | (2.61) | (3.46) | (3.32) | (2.47) | (2.49) | |
| 3 | 28.13 | 17.61 | 9.91 | 27.20 | 29.15 | 10.53 | 17.83 | 16.50 | 11.74 |
| (2.81) | (3.03) | (1.73) | (2.92) | (2.55) | (3.18) | (3.21) | (2.85) | (2.97) | |
| 3.5 | 33.20 | 29.87 | 9.86 | 34.27 | 31.71 | 19.31 | 15.00 | 16.00 | 12.08 |
| (3.07) | (2.47) | (3.32) | (3.21) | (2.43) | (3.05) | (3.45) | (2.92) | (2.81) | |
| 4 | 24.78 | 23.34 | 9.52 | 26.07 | 21.01 | 12.67 | 14.31 | 14.02 | 11.18 |
| (3.21) | (3.18) | (2.24) | (2.47) | (3.15) | (2.86) | (3.32) | (3.07) | (2.51) | |
| 4.5 | 30.26 | 19.30 | 7.57 | 27.48 | 28.40 | 15.02 | 13.78 | 13.60 | 11.26 |
| (3.36) | (3.54) | (2.45) | (2.92) | (2.43) | (3.22) | (3.51) | (3.22) | (2.86) | |
| Salinity (%) | The fourth cycle | The fifth cycle | The sixth cycle | ||||||
| Nail-holding power (N∙mm−1) | Nail-holding power (N∙mm−1) | Nail-holding power (N∙mm−1) | |||||||
| PS1 | PS2 | PS3 | PS1 | PS2 | PS3 | PS1 | PS2 | PS3 | |
| 0 | 24.89 | 26.17 | 12.30 | 22.52 | 20.39 | 11.73 | 20.67 | 19.87 | 11.35 |
| (2.14) | (2.41) | (2.83) | (2.49) | (1.92) | (2.45) | (2.37) | (1.95) | (2.45) | |
| 3 | 18.11 | 18.25 | 13.53 | 17.13 | 17.51 | 13.41 | 17.10 | 16.15 | 12.53 |
| (2.92) | (1.87) | (2.47) | (2.43) | (2.68) | (2.85) | (2.74) | (2.12) | (3.02) | |
| 3.5 | 15.27 | 15.71 | 11.31 | 14.20 | 16.87 | 10.86 | 14.07 | 15.71 | 9.31 |
| (2.51) | (2.21) | (2.51) | (2.51) | (2.98) | (2.51) | (2.98) | (2.51) | (2.51) | |
| 4 | 15.07 | 15.23 | 11.22 | 14.17 | 15.79 | 10.30 | 13.67 | 14.67 | 9.25 |
| (2.85) | (3.15) | (3.03) | (3.05) | (2.66) | (2.24) | (2.85) | (3.30) | (2.28) | |
| 4.5 | 14.78 | 14.80 | 11.48 | 13.36 | 12.73 | 7.37 | 11.48 | 11.40 | 7.02 |
| (2.55) | (2.21) | (2.72) | (2.07) | (3.39) | (2.28) | (2.55) | (2.21) | (1.84) | |
| Properties | Factor | Sum of Squares | DF | F Value | P | Partial η2 |
|---|---|---|---|---|---|---|
| PS1 | Salinity | 264.235 | 4 | 8.695 | <0.001 | 0.225 |
| Cycle | 4975.578 | 5 | 130.981 | <0.001 | 0.845 | |
| Salinity × Cycle | 1709.941 | 20 | 11.253 | <0.001 | 0.652 | |
| Error | 911.691 | 120 | ||||
| PS2 | Salinity | 689.326 | 4 | 24.787 | <0.001 | 0.452 |
| Cycle | 1863.134 | 5 | 53.596 | <0.001 | 0.691 | |
| Salinity × Cycle | 1600.099 | 20 | 11.507 | <0.001 | 0.657 | |
| Error | 834.300 | 120 | ||||
| PS3 | Salinity | 161.559 | 4 | 6.774 | <0.001 | 0.184 |
| Cycle | 314.267 | 5 | 10.542 | <0.001 | 0.305 | |
| Salinity × Cycle | 463.273 | 20 | 3.885 | <0.001 | 0.393 | |
| Error | 715.496 | 120 |
| Properties | Salinity | (I) Cycle | (J) Cycle | Mean Difference | P | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| PS1 | 0.0 | 2.0 | 4.0 | −6.104 * | 0.010 | −11.325 | −0.883 |
| 3.0 | 4.0 | −6.150 * | 0.009 | −11.371 | −0.929 | ||
| 6.0 | 11.944 * | 0.000 | 6.723 | 17.165 | |||
| 4.0 | 6.0 | 11.238 * | 0.000 | 6.017 | 16.459 | ||
| 5.0 | 6.0 | 10.974 * | 0.000 | 5.753 | 16.195 | ||
| 3.0 | 1.0 | 3.0 | −11.944 * | 0.000 | −17.165 | −6.723 | |
| 4.0 | −11.238 * | 0.000 | −16.459 | −6.017 | |||
| 5.0 | −10.974 * | 0.000 | −16.195 | −5.753 | |||
| 6.0 | 12.416 * | 0.000 | 7.195 | 17.637 | |||
| 2.0 | 3.0 | 12.328 * | 0.000 | 7.107 | 17.549 | ||
| 4.0 | 11.622 * | 0.000 | 6.401 | 16.843 | |||
| 5.0 | 11.358 * | 0.000 | 6.137 | 16.579 | |||
| 6.0 | 12.800 * | 0.000 | 7.579 | 18.021 | |||
| 3.5 | 1.0 | 3.0 | 19.494 * | 0.000 | 14.273 | 24.715 | |
| 4.0 | 18.808 * | 0.000 | 13.587 | 24.029 | |||
| 5.0 | 20.006 * | 0.000 | 14.785 | 25.227 | |||
| 6.0 | 21.808 * | 0.000 | 16.587 | 27.029 | |||
| 2.0 | 3.0 | 17.392 * | 0.000 | 12.171 | 22.613 | ||
| 4.0 | 16.706 * | 0.000 | 11.485 | 21.927 | |||
| 5.0 | 17.904 * | 0.000 | 12.683 | 23.125 | |||
| 6.0 | 19.706 * | 0.000 | 14.485 | 24.927 | |||
| 4.0 | 1.0 | 2.0 | 9.156 * | 0.000 | 3.935 | 14.377 | |
| 3.0 | 14.756 * | 0.000 | 9.535 | 19.977 | |||
| 4.0 | 18.594 * | 0.000 | 13.373 | 23.815 | |||
| 5.0 | 18.914 * | 0.000 | 13.693 | 24.135 | |||
| 6.0 | 19.286 * | 0.000 | 14.065 | 24.507 | |||
| 2.0 | 3.0 | 5.600 * | 0.025 | 0.379 | 10.821 | ||
| 4.0 | 9.438 * | 0.000 | 4.217 | 14.659 | |||
| 5.0 | 9.758 * | 0.000 | 4.537 | 14.979 | |||
| 6.0 | 10.130 * | 0.000 | 4.909 | 15.351 | |||
| 4.5 | 1.0 | 3.0 | 15.930 * | 0.000 | 10.709 | 21.151 | |
| 4.0 | 16.986 * | 0.000 | 11.765 | 22.207 | |||
| 5.0 | 17.690 * | 0.000 | 12.469 | 22.911 | |||
| 6.0 | 17.692 * | 0.000 | 12.471 | 22.913 | |||
| 2.0 | 3.0 | 13.508 * | 0.000 | 8.287 | 18.729 | ||
| 4.0 | 14.564 * | 0.000 | 9.343 | 19.785 | |||
| 5.0 | 15.268 * | 0.000 | 10.047 | 20.489 | |||
| 6.0 | 15.270 * | 0.000 | 10.049 | 20.491 | |||
| Properties | Cycle | (I) Salinity | (J) Salinity | Mean Difference | P | 95% Confidence Interval for Difference | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| PS1 | 1.0 | 0.0 | 3.5 | −9.396 * | 0.000 | −14.381 | −4.411 |
| 4.0 | −9.396 * | 0.000 | −14.381 | −4.411 | |||
| 4.5 | −5.790 * | 0.012 | −10.775 | −0.805 | |||
| 3.0 | 3.5 | −5.492 * | 0.021 | −10.477 | −0.507 | ||
| 4.0 | −5.492 * | 0.021 | −10.477 | −0.507 | |||
| 2.0 | 0.0 | 3.0 | −8.240 * | 0.000 | −13.225 | −3.255 | |
| 3.5 | −11.246 * | 0.000 | −16.231 | −6.261 | |||
| 4.5 | −7.320 * | 0.001 | −12.305 | −2.335 | |||
| 3.0 | 0.0 | 8.240 * | 0.000 | 3.255 | 13.225 | ||
| 3.5 | 4.0 | 7.054 * | 0.001 | 2.069 | 12.039 | ||
| 3.0 | 0.0 | 3.5 | 6.100 * | 0.007 | 1.115 | 11.085 | |
| 4.5 | 6.142 * | 0.006 | 1.157 | 11.127 | |||
| 4.0 | 0.0 | 3.0 | 9.486 * | 0.000 | 4.501 | 14.471 | |
| 3.5 | 11.564 * | 0.000 | 6.579 | 16.549 | |||
| 4.0 | 11.350 * | 0.000 | 6.365 | 16.335 | |||
| 4.5 | 13.348 * | 0.000 | 8.363 | 18.333 | |||
| 5.0 | 0.0 | 3.5 | 8.076 * | 0.000 | 3.091 | 13.061 | |
| 4.0 | 6.984 * | 0.001 | 1.999 | 11.969 | |||
| 4.5 | 9.366 * | 0.000 | 4.381 | 14.351 | |||
| 6.0 | 0.0 | 3.0 | 5.826 * | 0.011 | 0.841 | 10.811 | |
| 3.5 | 9.726 * | 0.000 | 4.741 | 14.711 | |||
| 4.0 | 7.204 * | 0.001 | 2.219 | 12.189 | |||
| 4.5 | 9.216 * | 0.000 | 4.231 | 14.201 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, J.; Lin, J.; Chen, Z.; Jia, S.; Zi, Y.; Que, Z. Influence of Salt Concentration and Treatment Cycles on Nail-Holding Power in Dimension Lumber. Forests 2024, 15, 1387. https://doi.org/10.3390/f15081387
Lei J, Lin J, Chen Z, Jia S, Zi Y, Que Z. Influence of Salt Concentration and Treatment Cycles on Nail-Holding Power in Dimension Lumber. Forests. 2024; 15(8):1387. https://doi.org/10.3390/f15081387
Chicago/Turabian StyleLei, Jia, Jingkang Lin, Zhiyuan Chen, Shuke Jia, Youying Zi, and Zeli Que. 2024. "Influence of Salt Concentration and Treatment Cycles on Nail-Holding Power in Dimension Lumber" Forests 15, no. 8: 1387. https://doi.org/10.3390/f15081387





