Can CSR Strategy Classes Determined by StrateFy Explain the Species Dominance and Diversity of a Forest Community?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
2.2. Trait Measurements
2.3. Data Analysis
3. Results
4. Discussion
4.1. Functional Traits and CSR Strategy
4.2. CSR Strategy Differentiation and Community Assembly
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adler, P.B.; Salguero-Gómez, R.; Compagnoni, A.; Hsu, J.S.; Ray-Mukherjee, J.; Mbeau-Ache, C.; Franco, M. Functional Traits Explain Variation in Plant Life History Strategies. Proc. Natl. Acad. Sci. USA 2014, 111, 740–745. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 2001; ISBN 978-0-691-08836-5. [Google Scholar]
- Gadgil, M.; Solbrig, O.T. The Concept of R- and K-Selection: Evidence from Wild Flowers and Some Theoretical Considerations. Am. Nat. 1972, 106, 14–31. [Google Scholar] [CrossRef]
- Parry, G.D. The Meanings of R- and K-Selection. Oecologia 1981, 48, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Pianka, E.R. On R- and K-Selection. Am. Nat. 1970, 104, 592–597. [Google Scholar] [CrossRef]
- Stearns, S.C. The Evolution of Life History Traits: A Critique of the Theory and a Review of the Data. Annu. Rev. Ecol. Syst. 1977, 8, 145–171. [Google Scholar] [CrossRef]
- Engen, S.; Sæther, B.-E. Optimal Age of Maturity in Fluctuating Environments under R- and K-Selection. Oikos 2016, 125, 1577–1585. [Google Scholar] [CrossRef]
- Oizumi, R.; Kuniya, T.; Enatsu, Y. Reconsideration of r/K Selection Theory Using Stochastic Control Theory and Nonlinear Structured Population Models. PLoS ONE 2016, 11, e0157715. [Google Scholar] [CrossRef]
- Grime, J.P. Primary Strategies in Plants. Trans. Bot. Soc. Edinb. 1979, 43, 151–160. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the Existence of Three Primary Strategies in Plants and Its Relevance to Ecological and Evolutionary Theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Caccianiga, M.; Luzzaro, A.; Pierce, S.; Ceriani, R.M.; Cerabolini, B. The Functional Basis of a Primary Succession Resolved by CSR Classification. Oikos 2006, 112, 10–20. [Google Scholar] [CrossRef]
- Hills, J.M.; Murphy, K.J.; Pulford, I.D.; Flowers, T.H. A Method for Classifying European Riverine Wetland Ecosystems Using Functional Vegetation Groups. Funct. Ecol. 1994, 8, 242–252. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Wilson, P.J.; Hunt, R.; Grime, J.P.; Thompson, K. Allocating C-S-R Plant Functional Types: A Soft Approach to a Hard Problem. Oikos 1999, 85, 282–294. [Google Scholar] [CrossRef]
- Li, Y.; Shipley, B. An Experimental Test of CSR Theory Using a Globally Calibrated Ordination Method. PLoS ONE 2017, 12, e0175404. [Google Scholar] [CrossRef]
- Novakovskiy, A.B.; Maslova, S.P.; Dalke, I.V.; Dubrovskiy, Y.A. Patterns of Allocation CSR Plant Functional Types in Northern Europe. Int. J. Ecol. 2016, 2016, e1323614. [Google Scholar] [CrossRef]
- Pierce, S.; Negreiros, D.; Cerabolini, B.E.L.; Kattge, J.; Díaz, S.; Kleyer, M.; Shipley, B.; Wright, S.J.; Soudzilovskaia, N.A.; Onipchenko, V.G.; et al. A Global Method for Calculating Plant CSR Ecological Strategies Applied across Biomes World-Wide. Funct. Ecol. 2017, 31, 444–457. [Google Scholar] [CrossRef]
- Schmidt, J.; Fassnacht, F.E.; Lausch, A.; Schmidtlein, S. Assessing the Functional Signature of Heathland Landscapes via Hyperspectral Remote Sensing. Ecol. Indic. 2017, 73, 505–512. [Google Scholar] [CrossRef]
- Schweiger, A.K.; Schütz, M.; Risch, A.C.; Kneubühler, M.; Haller, R.; Schaepman, M.E. How to Predict Plant Functional Types Using Imaging Spectroscopy: Linking Vegetation Community Traits, Plant Functional Types and Spectral Response. Methods Ecol. Evol. 2017, 8, 86–95. [Google Scholar] [CrossRef]
- Kattenborn, T.; Fassnacht, F.E.; Schmidtlein, S. Differentiating Plant Functional Types Using Reflectance: Which Traits Make the Difference? Remote Sens. Ecol. Conserv. 2019, 5, 5–19. [Google Scholar] [CrossRef]
- Kattenborn, T.; Fassnacht, F.E.; Pierce, S.; Lopatin, J.; Grime, J.P.; Schmidtlein, S. Linking Plant Strategies and Plant Traits Derived by Radiative Transfer Modelling. J. Veg. Sci. 2017, 28, 717–727. [Google Scholar] [CrossRef]
- Rosado, B.H.P.; de Mattos, E.A. On the Relative Importance of CSR Ecological Strategies and Integrative Traits to Explain Species Dominance at Local Scales. Funct. Ecol. 2017, 31, 1969–1974. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, D.; Bae, S. Effects of Vegetation Structure and Human Impact on Understory Honey Plant Richness: Implications for Pollinator Visitation. J. Ecol. Environ. 2017, 41, 2. [Google Scholar] [CrossRef]
- Saenz-Pedroza, I.; Feldman, R.; Reyes-García, C.; Meave, J.A.; Calvo-Irabien, L.M.; May-Pat, F.; Dupuy, J.M. Seasonal and Successional Dynamics of Size-Dependent Plant Demographic Rates in a Tropical Dry Forest. PeerJ 2020, 8, e9636. [Google Scholar] [CrossRef]
- Cerabolini, B.E.L.; Brusa, G.; Ceriani, R.M.; De Andreis, R.; Luzzaro, A.; Pierce, S. Can CSR Classification Be Generally Applied Outside Britain? Plant Ecol. 2010, 210, 253–261. [Google Scholar] [CrossRef]
- Barba-Escoto, L.; Ponce-Mendoza, A.; García-Romero, A.; Calvillo-Medina, R.P. Plant Community Strategies Responses to Recent Eruptions of Popocatépetl Volcano, Mexico. J. Veg. Sci. 2019, 30, 375–385. [Google Scholar] [CrossRef]
- Lewis, R.J.; Pakeman, R.J.; Angus, S.; Marrs, R.H. Using Compositional and Functional Indicators for Biodiversity Conservation Monitoring of Semi-Natural Grasslands in Scotland. Biol. Conserv. 2014, 175, 82–93. [Google Scholar] [CrossRef]
- Negreiros, D.; Le Stradic, S.; Fernandes, G.W.; Rennó, H.C. CSR Analysis of Plant Functional Types in Highly Diverse Tropical Grasslands of Harsh Environments. Plant Ecol. 2014, 215, 379–388. [Google Scholar] [CrossRef]
- Smith, B.M.; Diaz, A.; Winder, L. Grassland Habitat Restoration: Lessons Learnt from Long Term Monitoring of Swanworth Quarry, UK, 1997–2014. PeerJ 2017, 5, e3942. [Google Scholar] [CrossRef]
- Stevens, C.J.; Thompson, K.; Grime, J.P.; Long, C.J.; Gowing, D.J.G. Contribution of Acidification and Eutrophication to Declines in Species Richness of Calcifuge Grasslands along a Gradient of Atmospheric Nitrogen Deposition. Funct. Ecol. 2010, 24, 478–484. [Google Scholar] [CrossRef]
- Teuber, L.M.; Hölzel, N.; Fraser, L.H. Livestock Grazing in Intermountain Depressional Wetlands—Effects on Plant Strategies, Soil Characteristics and Biomass. Agric. Ecosyst. Environ. 2013, 175, 21–28. [Google Scholar] [CrossRef]
- Lee, J.-S.; Son, D.-H.; Lee, S.-H.; Myeong, H.-H.; Cho, J.-S.; Lee, J.-C.; Lee, J.-Y.; Park, C.-S.; Kim, J.-W. Canonical Correspondence Analysis Ordinations and Competitor, Stress Tolerator, and Ruderal Strategies of Coastal Dune Plants in South Korea. J. Coast. Res. 2020, 36, 528–535. [Google Scholar] [CrossRef]
- Wollny, J.T.; Bergmann, W.; Otte, A.; Harvolk-Schöning, S. River Regulation Intensity Matters: Riverbank Vegetation Is Characterized by More Typical Riverbank Plant Species with Increasing Distance from Weirs. Ecol. Eng. 2021, 159, 106082. [Google Scholar] [CrossRef]
- De Keersmaeker, L.; Vandekerkhove, K.; Verstraeten, A.; Baeten, L.; Verschelde, P.; Thomaes, A.; Hermy, M.; Verheyen, K. Clear-Felling Effects on Colonization Rates of Shade-Tolerant Forest Herbs into a Post-Agricultural Forest Adjacent to Ancient Forest. Appl. Veg. Sci. 2011, 14, 75–83. [Google Scholar] [CrossRef]
- Verstraeten, G.; Baeten, L.; Van den Broeck, T.; De Frenne, P.; Demey, A.; Tack, W.; Muys, B.; Verheyen, K. Temporal Changes in Forest Plant Communities at Different Site Types. Appl. Veg. Sci. 2013, 16, 237–247. [Google Scholar] [CrossRef]
- Ciccarelli, D. Mediterranean Coastal Dune Vegetation: Are Disturbance and Stress the Key Selective Forces That Drive the Psammophilous Succession? Estuar. Coast. Shelf Sci. 2015, 165, 247–253. [Google Scholar] [CrossRef]
- da Costa, H.d.J.A.; Gurgel, E.S.C.; do Amaral, D.D.; Vasconcelos, L.V.; Rebelo, L.G.B.; Teodoro, G.S. CSR Ecological Strategies, Functional Traits and Trade-Offs of Woody Species in Amazon Sandplain Forest. Flora 2020, 273, 151710. [Google Scholar] [CrossRef]
- Rosenfield, M.F.; Müller, S.C.; Overbeck, G.E. Short Gradient, but Distinct Plant Strategies: The CSR Scheme Applied to Subtropical Forests. J. Veg. Sci. 2019, 30, 984–993. [Google Scholar] [CrossRef]
- Munoz, F.; Violle, C.; Cheptou, P.-O. CSR Ecological Strategies and Plant Mating Systems: Outcrossing Increases with Competitiveness but Stress-Tolerance Is Related to Mixed Mating. Oikos 2016, 125, 1296–1303. [Google Scholar] [CrossRef]
- Good, M.; Morgan, J.W.; Venn, S.; Green, P. Timing of Snowmelt Affects Species Composition via Plant Strategy Filtering. Basic Appl. Ecol. 2019, 35, 54–62. [Google Scholar] [CrossRef]
- Eler, K.; Kermavnar, J.; Marinšek, A.; Kutnar, L. Short-Term Changes in Plant Functional Traits and Understory Functional Diversity after Logging of Different Intensities: A Temperate Fir-Beech Forest Experiment. Ann. For. Res. 2018, 50, 223–241. [Google Scholar] [CrossRef]
- Darling, E.S.; Alvarez-Filip, L.; Oliver, T.A.; McClanahan, T.R.; Côté, I.M. Evaluating Life-History Strategies of Reef Corals from Species Traits. Ecol. Lett. 2012, 15, 1378–1386. [Google Scholar] [CrossRef]
- Takou, M.; Wieters, B.; Kopriva, S.; Coupland, G.; Linstädter, A.; De Meaux, J. Linking Genes with Ecological Strategies in Arabidopsis Thaliana. J. Exp. Bot. 2019, 70, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- May, R.-L.; Warner, S.; Wingler, A. Classification of Intra-Specific Variation in Plant Functional Strategies Reveals Adaptation to Climate. Ann. Bot. 2017, 119, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, R.; Panseri, S.; Giupponi, L.; Leoni, V.; Citti, C.; Cattaneo, C.; Cavaletto, M.; Giorgi, A. Phytochemical and Ecological Analysis of Two Varieties of Hemp (Cannabis Sativa L.) Grown in a Mountain Environment of Italian Alps. Front. Plant Sci. 2019, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, D.; Zhou, T.; Huang, B.; Zhang, H.; Chen, B.; Peng, S. The Role of Functional Strategies in Global Plant Distribution. Ecography 2021, 44, 493–503. [Google Scholar] [CrossRef]
- Davison, J.; García de León, D.; Zobel, M.; Moora, M.; Bueno, C.G.; Barceló, M.; Gerz, M.; León, D.; Meng, Y.; Pillar, V.D.; et al. Plant Functional Groups Associate with Distinct Arbuscular Mycorrhizal Fungal Communities. New Phytol. 2020, 226, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-Y.; van Kleunen, M.; Winter, M.; Weigelt, P.; Stein, A.; Pierce, S.; Pergl, J.; Moser, D.; Maurel, N.; Lenzner, B.; et al. The Role of Adaptive Strategies in Plant Naturalization. Ecol. Lett. 2018, 21, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Dalle Fratte, M.; Bolpagni, R.; Brusa, G.; Caccianiga, M.; Pierce, S.; Zanzottera, M.; Cerabolini, B.E.L. Alien Plant Species Invade by Occupying Similar Functional Spaces to Native Species. Flora 2019, 257, 151419. [Google Scholar] [CrossRef]
- Chagnon, P.-L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A Trait-Based Framework to Understand Life History of Mycorrhizal Fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef]
- Blumenthal, D.; Mitchell, C.E.; Pyšek, P.; Jarošík, V. Synergy between Pathogen Release and Resource Availability in Plant Invasion. Proc. Natl. Acad. Sci. USA 2009, 106, 7899–7904. [Google Scholar] [CrossRef]
- Craine, J.M. Reconciling Plant Strategy Theories of Grime and Tilman. J. Ecol. 2005, 93, 1041–1052. [Google Scholar] [CrossRef]
- Herben, T.; Klimešová, J.; Chytrý, M. Philip Grime’s Fourth Corner: Are There Plant Species Adapted to High Disturbance and Low Productivity? Oikos 2018, 127, 1125–1131. [Google Scholar] [CrossRef]
- Wilson, J.B.; Lee, W.G. C-S-R Triangle Theory: Community-Level Predictions, Tests, Evaluation of Criticisms, and Relation to Other Theories. Oikos 2000, 91, 77–96. [Google Scholar] [CrossRef]
- Xie, L.; Feng, Y.; Zhao, R.; Lv, T.; Wang, N.; Li, Y.; Zheng, X.; Chen, S.; Ding, H.; Fang, Y. Positive Relationships between Species Diversity and Genetic Diversity on a Local Scale at Mt. Wu Yi, China. Biodivers. Conserv. 2023, 32, 4295–4311. [Google Scholar] [CrossRef]
- Ding, H.; Fang, Y.; Yang, Q.; Chen, X.; Yuan, F.; Xu, H.; He, L.; Yan, J.; Chen, T.; Yu, C.; et al. Community Characteristics of a Mid-Subtropical Evergreen Broad-Leaved Forest Plot in the Wuyi Mountains, Fujian Province, Southeastern China. Biodivers. Sci. 2016, 23, 479–492. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Harrison, S.P.; Prentice, I.C.; Yang, Y.; Bai, F.; Togashi, H.F.; Wang, M.; Zhou, S.; Ni, J. The China Plant Trait Database: Toward a Comprehensive Regional Compilation of Functional Traits for Land Plants. Ecology 2018, 99, 500. [Google Scholar] [CrossRef] [PubMed]
- Kilinç, M.; Karavin, N.; Kutbay, H. Classification of Some Plant Species According to Grime’s Strategies in a Quercus Cerris L. Var. Cerris Woodland in Samsun, Northern Turkey. Turk. J. Bot. 2010, 34, 521–529. [Google Scholar] [CrossRef]
- Pierce, S.; Brusa, G.; Vagge, I.; Cerabolini, B.E.L. Allocating CSR Plant Functional Types: The Use of Leaf Economics and Size Traits to Classify Woody and Herbaceous Vascular Plants. Funct. Ecol. 2013, 27, 1002–1010. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Montserrat Marti, G.; Šerá, B.; Jones, G.; Bogaard, A.; Charles, M.; Font, X.; Ater, M.; Taleb, A.; Santini, B.A.; et al. Seed Size, Number and Strategies in Annual Plants: A Comparative Functional Analysis and Synthesis. Ann. Bot. 2020, 126, 1109–1128. [Google Scholar] [CrossRef]
- de Paula Oliveira, R.; Zotz, G.; Wanek, W.; Franco, A.C. Leaf Trait Co-Variation and Trade-Offs in Gallery Forest C3 and CAM Epiphytes. Biotropica 2021, 53, 520–535. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Harrison, S.P.; Prentice, I.C.; Wright, I.J.; Peng, C.; Lin, G. Quantifying Leaf-Trait Covariation and Its Controls across Climates and Biomes. New Phytol. 2019, 221, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Candeias, M.; Fraterrigo, J. Trait Coordination and Environmental Filters Shape Functional Trait Distributions of Forest Understory Herbs. Ecol. Evol. 2020, 10, 14098–14112. [Google Scholar] [CrossRef] [PubMed]
- Rosas, T.; Mencuccini, M.; Barba, J.; Cochard, H.; Saura-Mas, S.; Martínez-Vilalta, J. Adjustments and Coordination of Hydraulic, Leaf and Stem Traits along a Water Availability Gradient. New Phytol. 2019, 223, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Umaña, M.N.; Li, W.; Fang, M.; Chen, Y.; Lu, H.; Yu, S. Coordination of Leaf, Stem and Root Traits in Determining Seedling Mortality in a Subtropical Forest. For. Ecol. Manag. 2019, 446, 285–292. [Google Scholar] [CrossRef]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and Structural Tradeoffs Underlying the Leaf Economics Spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Marquet, P.A.; Allen, A.P.; Brown, J.H.; Dunne, J.A.; Enquist, B.J.; Gillooly, J.F.; Gowaty, P.A.; Green, J.L.; Harte, J.; Hubbell, S.P.; et al. On Theory in Ecology. BioScience 2014, 64, 701–710. [Google Scholar] [CrossRef]
- Allesina, S.; Levine, J.M. A Competitive Network Theory of Species Diversity. Proc. Natl. Acad. Sci. USA 2011, 108, 5638–5642. [Google Scholar] [CrossRef] [PubMed]
- Karger, D.N.; Tuomisto, H.; Amoroso, V.B.; Darnaedi, D.; Hidayat, A.; Abrahamczyk, S.; Kluge, J.; Lehnert, M.; Kessler, M. The Importance of Species Pool Size for Community Composition. Ecography 2015, 38, 1243–1253. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Rodriguez-Velázquez, J.; van Breugel, M.; Bongers, F. Changing Drivers of Species Dominance during Tropical Forest Succession. Funct. Ecol. 2014, 28, 1052–1058. [Google Scholar] [CrossRef]
- He, X.; Lin, Y.; Han, G.; Tian, X. What Determines the Number of Dominant Species in Forests? J. For. Res. 2010, 21, 287–292. [Google Scholar] [CrossRef]
- Chen, T.; Xu, F.; Yang, Q.; Chen, S.; Ge, X.; Wu, J.; Cui, P.; Fang, Y.; Ding, H. Spatial Distribution Characteristics of an Evergreen Broad-Leaved Forest in the Wuyi Mountains, Fujian Province, Southeastern China. J. Ecol. 2018, 38, 1817–1825. [Google Scholar]
- Grime, J.P. Vegetation Classification by Reference to Strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
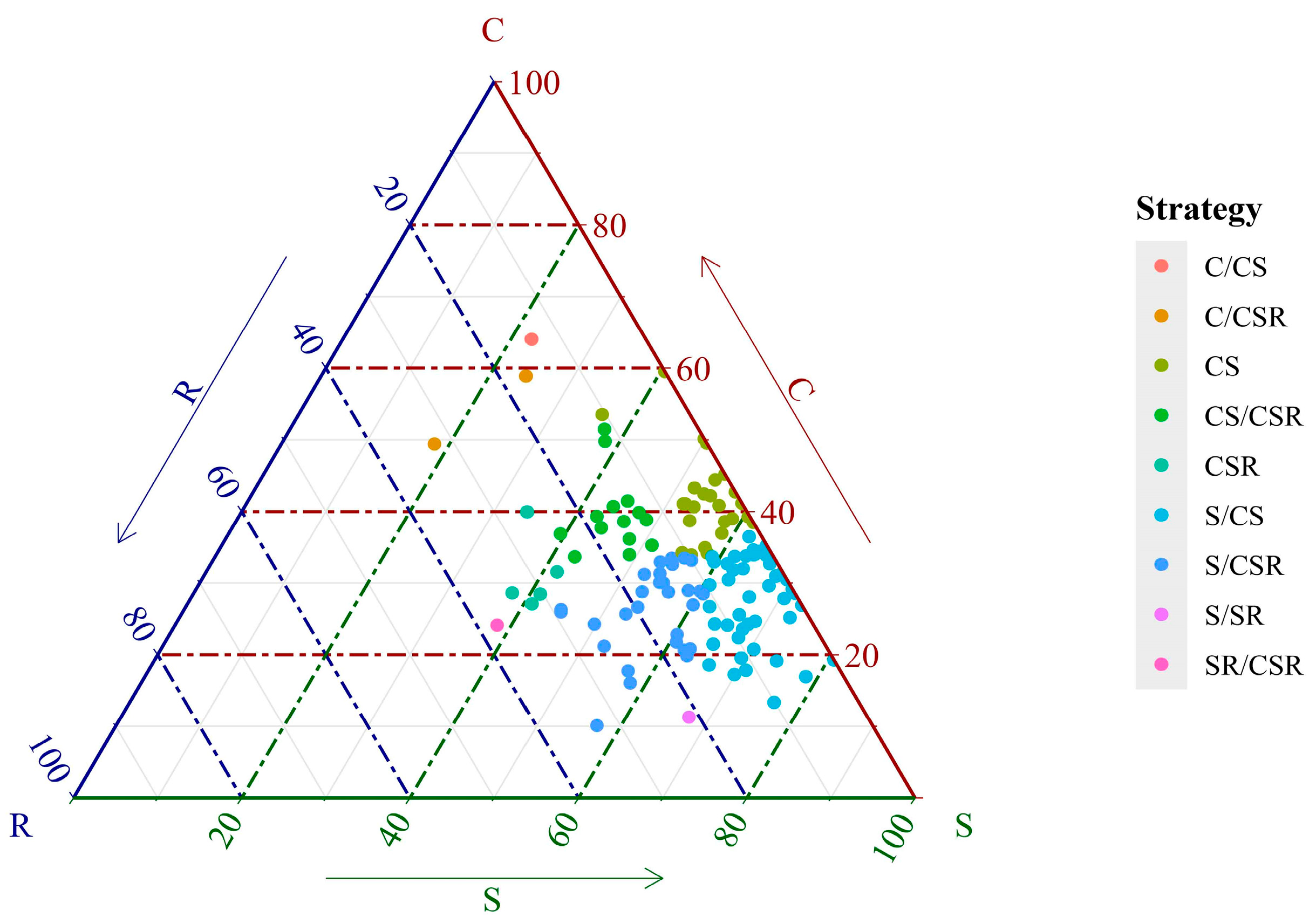
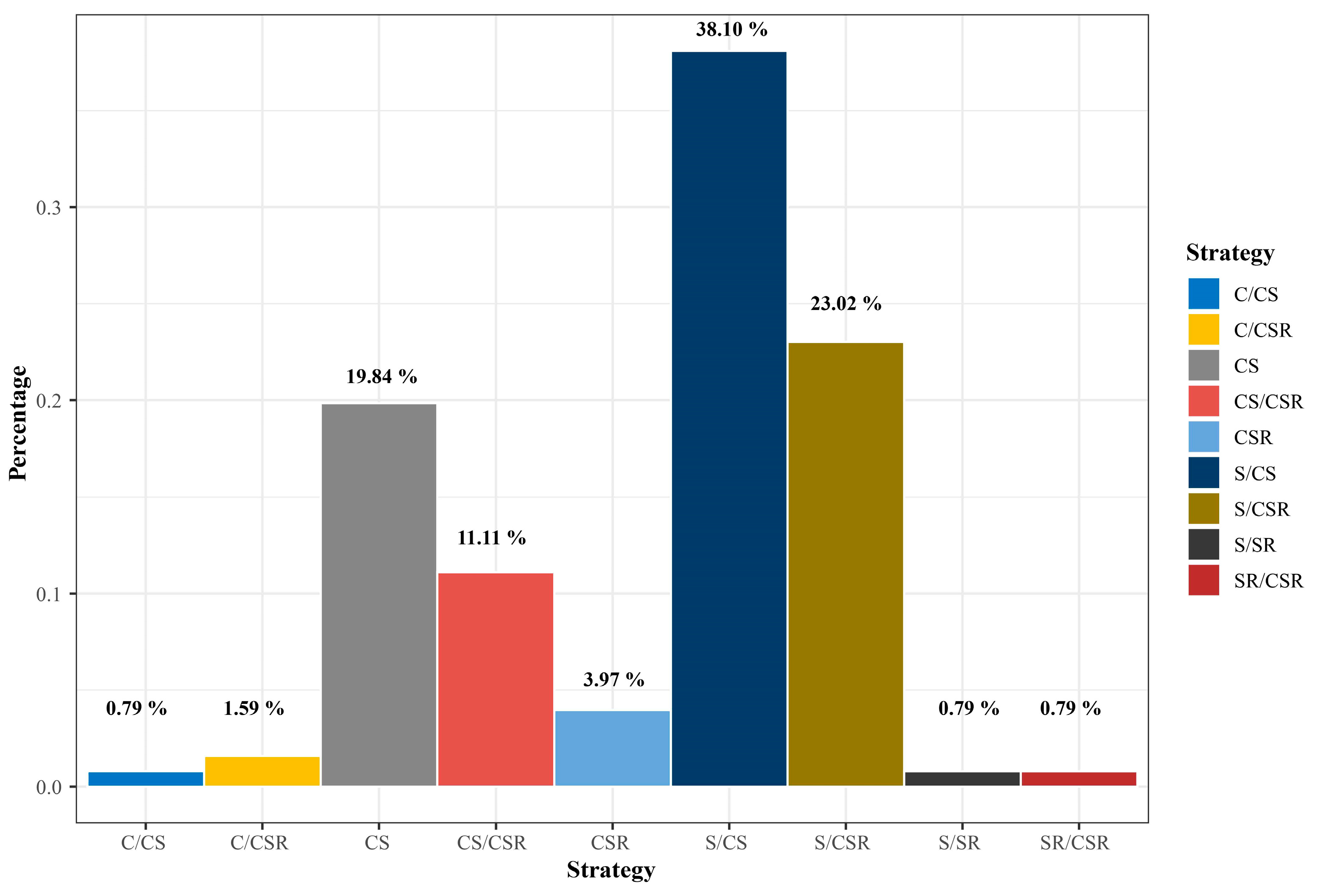

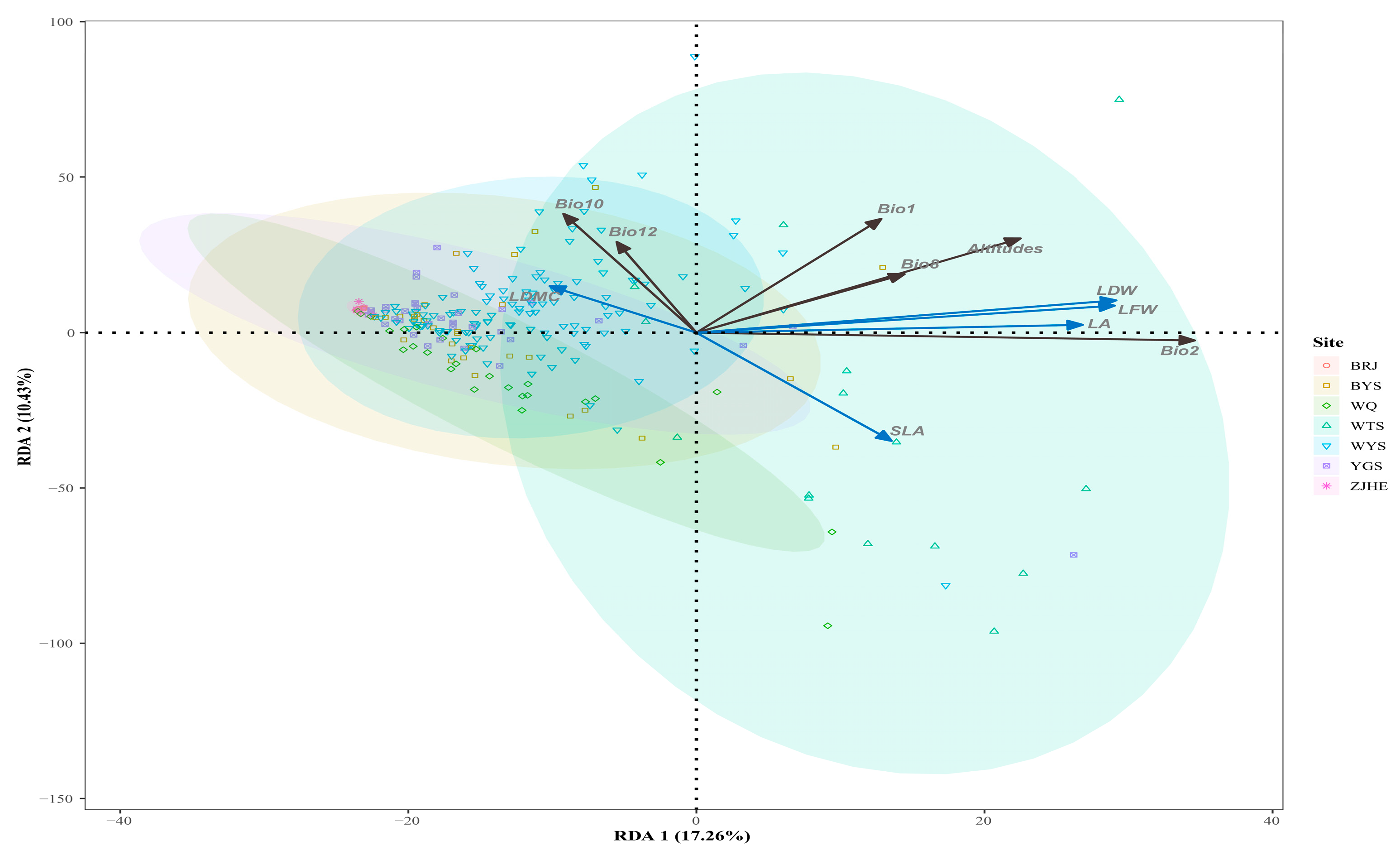
| Species | IV% | C% | S% | R% | CSR Strategy Class |
|---|---|---|---|---|---|
| Castanopsis carlesii | 7.21 | 18 | 71 | 11 | S/CS |
| Castanopsis fordii | 5.28 | 42 | 55 | 3 | CS |
| Castanopsis eyrei | 4.58 | 19 | 81 | 0 | S/CS |
| Engelhardia roxburghiana | 4.43 | 30 | 63 | 7 | S/CS |
| Syzygium buxifolium | 3.69 | 20 | 70 | 11 | S/CS |
| Schima superba | 3.20 | 37 | 59 | 5 | CS |
| Rhododendron henryi | 2.96 | 34 | 64 | 2 | S/CS |
| Itea oblonga | 2.90 | 41 | 53 | 6 | CS |
| Eurya muricata | 2.79 | 22 | 61 | 17 | S/CSR |
| Castanopsis faberi | 2.79 | 34 | 64 | 2 | S/CS |
| Families | C% | S% | R% | CSR-Score Width | CSR Strategy Classes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CS | S/CS | S/CSR | C/CS | CSR | S/SR | |||||
| Fagaceae | 18–50 | 50–81 | 0–11 | broad | √ | √ | √ | - | - | - |
| Lauraceae | 19–43 | 41–74 | 0–32 | broad | √ | √ | - | - | √ | - |
| Aquifoliaceae | 10–60 | 40–70 | 0–33 | broad | √ | √ | √ | - | - | √ |
| Magnoliaceae | 31–64 | 22–59 | 0–26 | broad | √ | √ | - | √ | √ | - |
| Ericaceae | 17–35 | 50–70 | 8–26 | median | √ | √ | √ | - | - | - |
| Symplocaceae | 21–50 | 50–63 | 0–17 | median | √ | √ | - | - | - | - |
| Theaceae | 19–38 | 56–69 | 0–17 | narrow | √ | √ | √ | - | - | - |
| Elaeocarpaceae | 24–34 | 56–66 | 4–12 | narrow | √ | √ | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Cui, G.; Li, H.; Wang, N.; Zheng, X.; Ding, H.; Lv, T.; Fang, Y. Can CSR Strategy Classes Determined by StrateFy Explain the Species Dominance and Diversity of a Forest Community? Forests 2024, 15, 1412. https://doi.org/10.3390/f15081412
Peng Y, Cui G, Li H, Wang N, Zheng X, Ding H, Lv T, Fang Y. Can CSR Strategy Classes Determined by StrateFy Explain the Species Dominance and Diversity of a Forest Community? Forests. 2024; 15(8):1412. https://doi.org/10.3390/f15081412
Chicago/Turabian StylePeng, Ye, Gansha Cui, Hengyi Li, Ningjie Wang, Xiao Zheng, Hui Ding, Ting Lv, and Yanming Fang. 2024. "Can CSR Strategy Classes Determined by StrateFy Explain the Species Dominance and Diversity of a Forest Community?" Forests 15, no. 8: 1412. https://doi.org/10.3390/f15081412
APA StylePeng, Y., Cui, G., Li, H., Wang, N., Zheng, X., Ding, H., Lv, T., & Fang, Y. (2024). Can CSR Strategy Classes Determined by StrateFy Explain the Species Dominance and Diversity of a Forest Community? Forests, 15(8), 1412. https://doi.org/10.3390/f15081412






