Improving the Monitoring and Control of Egg Vitality of Lymantria dispar Linnaeus 1758 Using an Innovative Device and Procedure for Removing Egg Hairs
Abstract
1. Introduction
2. Materials and Methods
- The operator employs tweezers to extract the egg mass, transferring it to a Petri dish.
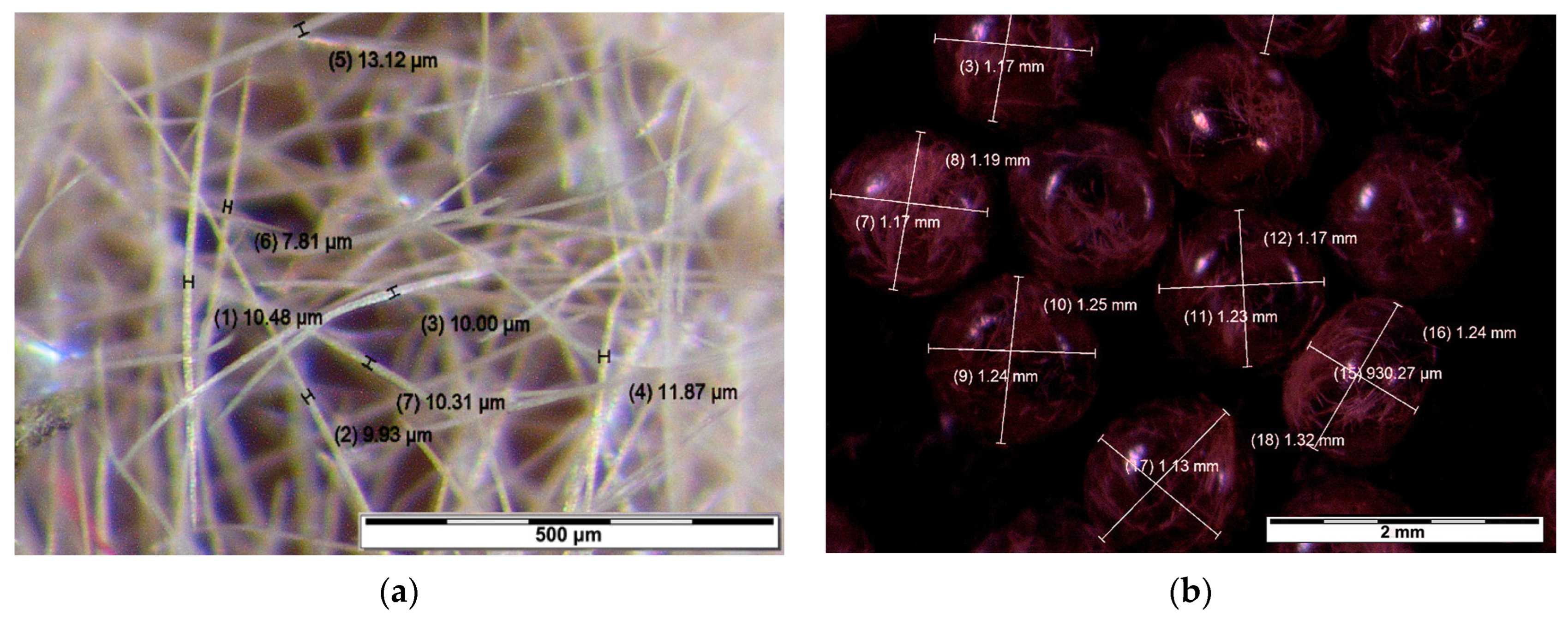
- The stopwatch is initiated, and the egg mass is delicately crushed into the smallest possible pieces using the fingertips (Figure 6a).
- Hairs separated from the eggs are eliminated through gentle blowing (Figure 6b).
- The operator notes the duration of the operation.
- Completely dehaired eggs are set aside for counting.
- The operator activates the machine with torque by plugging it into the power source.
- The operator switches on the suction unit.
- Using tweezers, the operator extracts the egg mass and transfers it to a container equipped with a sieve.
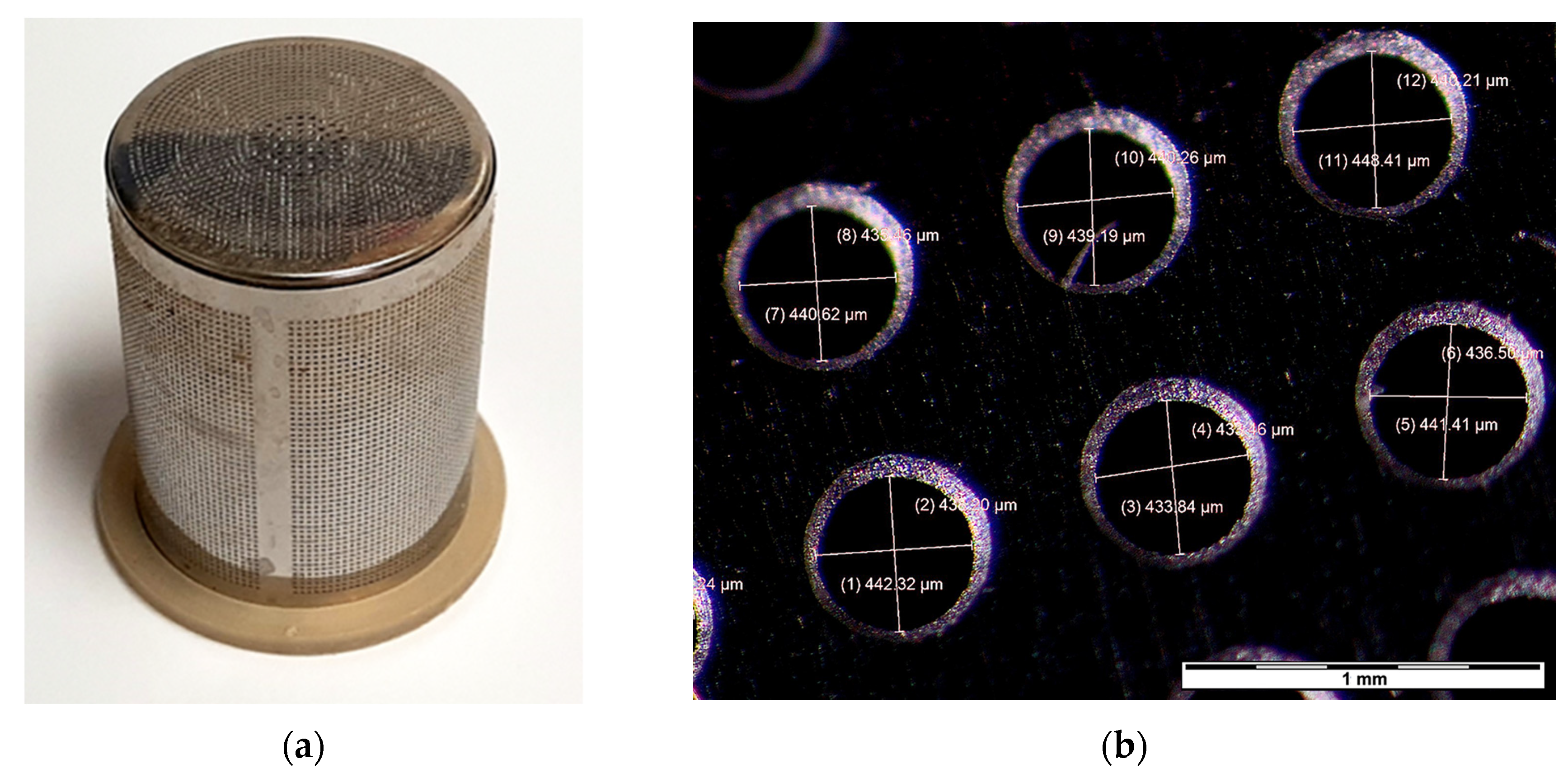
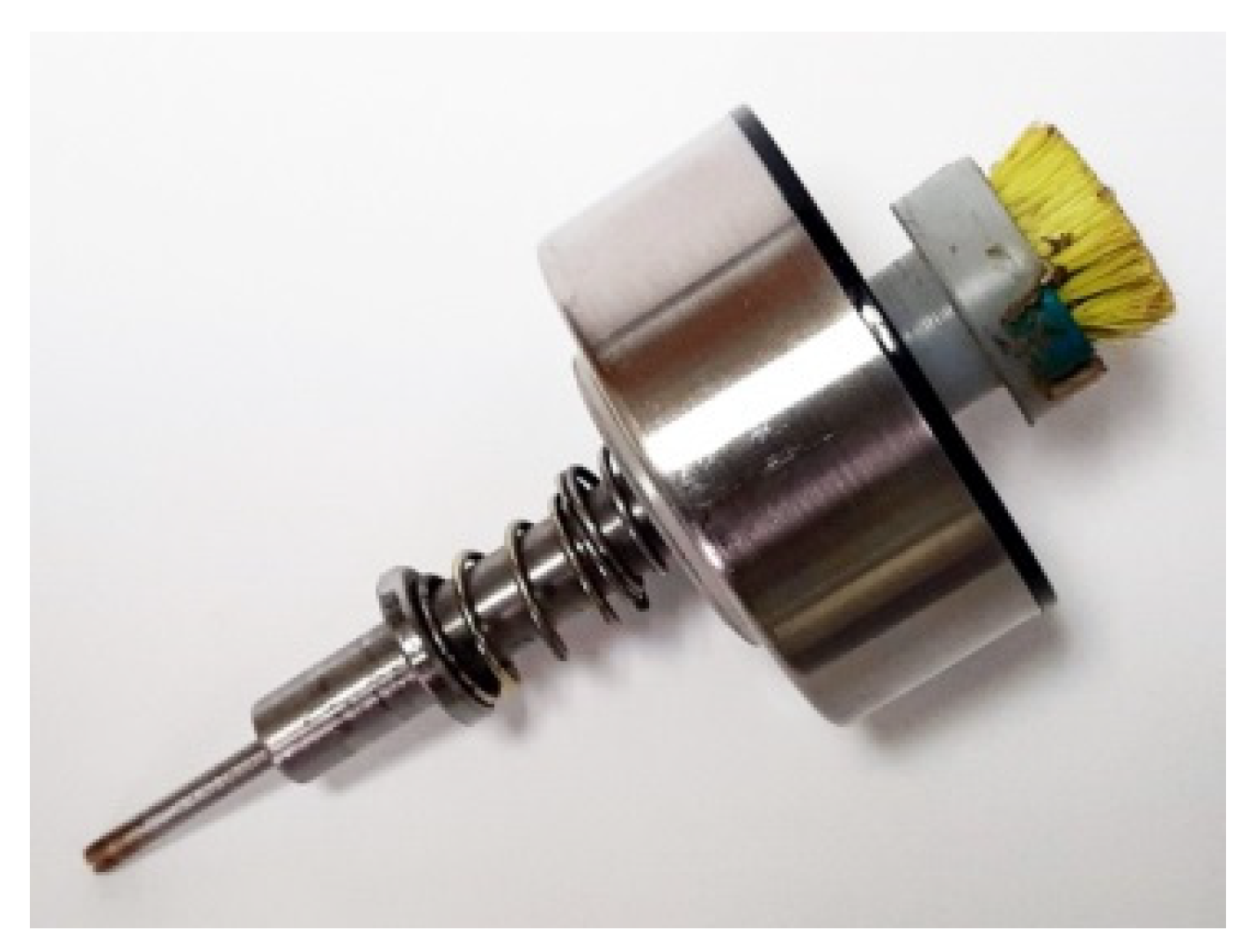
- The operator seals the container with the sieve, securing the spongy moth egg mass between the cylindrical tube of the device and the cap with the hair removal mechanism (Figure 3).
- The operator activates the torque-generating unit by selecting the corresponding rotation speed and starts the stopwatch.
- Applying gentle hand pressure and utilizing the torque generated by the machine, the operator, while stabilizing the cylindrical tube containing the egg mass, removes hair from the eggs.
- Upon observing, through the transparent glass of the cylindrical tube, that the hairs from the egg mass have been suctioned into the unit, the operator turns off the device and stops the stopwatch.
- The operator records the duration of the operation.
- The dehaired eggs are arranged in a numbered Petri dish and are prepared for egg purity analysis.
- The container containing waste from the suction unit is transferred to a numbered container for analysis of the purity of the discarded hairs.
- Group 1, comprising 10 egg masses, was allocated for manual separation of eggs from hairs.
- Group 2, consisting of five egg masses, was allocated for mechanical separation of eggs from hairs at a speed of 10,000 rpm using a brush with stiff bristles.
- Group 3, consisting of five egg masses, was allocated for mechanical separation of eggs from hairs at a speed of 15,000 rpm using a brush with stiff bristles.
- Group 4, comprising five egg masses, was allocated for mechanical separation of eggs from hairs at a speed of 10,000 rpm using a brush with soft bristles.
- Group 5, consisting of five egg masses, was allocated for mechanical separation of eggs from hairs at a speed of 15,000 rpm using a brush with soft bristles.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patacca, M.; Lindner, M.; Lucas-Borja, M.; Cordonnier, T.; Fidej, G.; Gardiner, B.; Hauf, Y.; Jasinevičius, G.; Labonne, S.; Linkevičius, E.; et al. Significant increase in natural disturbance impacts on European forests since 1950. Glob. Change Biol. 2023, 29, 1359–1376. [Google Scholar] [CrossRef]
- Hamm, R.; Gregg, A.; Sparks, T. Intellectual property in entomology: Analysis and perspective on recent trends in global patent publications. Pest Manag. Sci. 2020, 76, 1603–1611. [Google Scholar] [CrossRef]
- Smith, H.R.; Lautenschlager, R.A. Predators of the Gypsy Moth, Combined Forest Pest Research and Development Program, 1978, Agriculture Handbook No. 534. United States Department of Agriculture. Available online: https://www.govinfo.gov/content/pkg/GOVPUB-A-PURL-gpo30570/pdf/GOVPUB-A-PURL-gpo30570.pdf (accessed on 26 July 2024).
- Gypsy Moth Management in the United States: A Cooperative Approach, Final Supplemental Environmental Impact Statement Volume I of IV Summary, Final Supplemental Environmental Impact Statement, 2012, U.S. Department of Agriculture, Forest Service. Available online: https://www.aphis.usda.gov/sites/default/files/gm-ies-vol-i-final-eis-sup.pdf (accessed on 26 July 2024).
- Gypsy Moth Program Manual, Second Edition, 2019, The U.S. Department of Agriculture (USDA). Available online: https://www.aphis.usda.gov/sites/default/files/gypsy_moth.pdf (accessed on 27 July 2024).
- Kim, M.-J.; Kim, K.E.; Lee, C.Y.; Park, Y.; Jung, J.-K.; Nam, Y. Effect of Chilling Temperature on Survival and Post-Diapause Development of Korean Population of Lymantria dispar asiatica (Lepidoptera: Erebidae) Eggs. Forests 2022, 13, 2117. [Google Scholar] [CrossRef]
- Sparks, M.E.; Wang, Y.-M.; Shi, J.; Harrison, R.L. Lymantria dispar Iflavirus 1 RNA Comprises a Large Proportion of RNA in Adult L. dispar Moths. Insects 2023, 14, 466. [Google Scholar] [CrossRef] [PubMed]
- Streifel, M.A.; Tobin, P.C.; Kees, A.M.; Aukema, B.H. Range expansion of Lymantria dispar dispar (L.) (Lepidoptera: Erebidae) along its north-western margin in North America despite low predicted climatic suitability. J. Biogeogr. 2018, 46, 58–69. [Google Scholar] [CrossRef]
- AbdelMonem, A.E.; Mumma, R.O. Fate of an Insect Growth Regulator EL-494 in Soybean Callus Tissue, Soybean Plants, and Gypsy Moth Larvae. J. Agric. Food Chem. 1982, 30, 536–542. [Google Scholar] [CrossRef]
- Villemant, C.; Andreï-Ruiz, M.C. Life-cycles and biological features of eggs predators of Lymantria dispar (Lepidoptera: Lymantriidae) in the Mamora cork oak forest, Morocco. Eur. J. Entomol. 1999, 96, 29–36. [Google Scholar]
- Barbosa, P.; Waldvogel, M.; Martinat, P.; Douglass, W. Developmental and Reproductive Performance of the Gypsy Moth, Lymantria dispar (L.) (Lepidoptera: Lymantriidae), on Selected Hosts Common to Mid-Atlantic and Southern Forests. Environ. Entomol. 1983, 12, 1858–1862. [Google Scholar] [CrossRef]
- Ananko, G.; Kolosov, A. Asian gypsy moth (Lymantria dispar L.) populations: Tolerance of eggs to extreme winter temperatures. J. Therm. Biol. 2021, 103, 103123. [Google Scholar] [CrossRef]
- Boukouvala, M.C.; Kavallieratos, N.G.; Skourti, A.; Pons, X.; Alonso, C.L.; Eizaguirre, M.; Fernandez, E.B.; Solera, E.D.; Fita, S.; Bohinc, T.; et al. Lymantria dispar (L.) (Lepidoptera: Erebidae): Current Status of Biology, Ecology, and Management in Europe with Notes from North America. Insects 2022, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Mihajlović, L.J.; Tabaković-Tošić, M.; Jančić, G.; Jovanović, V. Spongy moth (Lymantria dispar L.) The most dangerous pest of our forests and orchards [Gubar (Lymantria dispar L.) Najopasnija Štetočina Naših Šuma i Voćnjaka]; The Ministry of Agriculture, Forestry and Water Industry and PE “Srbijašume”: Belgrade, Serbia, 2004; p. 32. [Google Scholar]
- Mihajlović, L.J. Spongy moth (Lymantria dispar L.) (Lepidoptera, Lymantridae) in Serbia, Forestry [Gubar (Lymantria dispar L.) (Lepidoptera, Lymantridae) u Srbiji, Šumarstvo]. Serbian Engl. Summ. 2008, 60, 1–26. [Google Scholar]
- Tabaković-Tošić, M. Gypsy moth (Lymantria dispar L.): Outbreak in the central part of republic of Serbia in the period 2010–2013. Sustain. For. Collect. 2013, 67–68, 141–150. [Google Scholar]
- Hlásny, T.; Trombik, J.; Holuša, J.; Lukášová, K.; Grendár, M.; Turčáni, M.; Zúbrik, M.; Tabaković-Tošić, M.; Hirka, A.; Buksha, I.; et al. Multi-decade patterns of gypsy moth fluctuations in the Carpathian Mountains and options for outbreak forecasting. J. Pest Sci. 2016, 89, 413–425. [Google Scholar] [CrossRef]
- Haynes, K.J.; Liebhold, A.M.; Johnson, D.M. Spatial analysis of harmonic oscillation of gypsy moth outbreak intensity. Oecologia 2009, 159, 249–256. [Google Scholar] [CrossRef]
- Tardif, R.; Secrest, J.P. Devices for cleaning and counting eggs of the gypsy moth. J. Econ. Entomol. 1970, 63, 678–679. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Simons, E.E.; Sior, A.; Unger, J.D. Forecasting Defoliation Caused by the Gypsy Moth from Field Measurements. Environ. Entomol. 1993, 22, 26–32. [Google Scholar] [CrossRef]
- Čakar, L.J. Some data on the parasite of sponge moth eggs Anastatus disparis Ruschka [Neki podaci o parazitu gubarevih jaja Anastatus disparis Ruschka]. Plant Prot. 1952, 9, 13–27. [Google Scholar]
- Maksimović, M. Egg abundance in spongy moth egg masses. [Brojnost jaja u leglima gubara]. Plant Prot. 1954, 26, 57–61. [Google Scholar]
- Vasić, K. Parasitic Hymenoptera of the spongy moth [Parazitske Hymenoptere gubara]. Plant Prot. 1958, 41–42, 17–21. [Google Scholar]
- Vasić, K.; Salatić, S. A new contribution to the knowledge of parasitic Hymenoptera of the sponge moth [Novi prilog poznavanju parazitskih Hymenoptera gubara]. Plant Prot. 1959, 52, 47–50. [Google Scholar]
- Nonveiller, G. Predators of spongy moth egg masses identified in Yugoslavia during the outbreak of 1945–1950 [Predatori gubarevih jajnih legala utvrđeni u Jugoslaviji tokom gradacije 1945–1950. godine]. Plant Prot. 1959, 52/53, 15–35. [Google Scholar]
- Salatić, S. Results of investigating certain factors affecting the efficiency of spongy moth egg parasites [Rezultati ispitivanja nekih faktora efikasnosti jajnih parazita gubara]. Plant Prot. 1963, 76, 693–699. [Google Scholar]
- Bjegović, P. Competitive relations between Ooencyrtus kuwanae How. and Anastatus disparis R [Kompetitorni odnosi između Ooencyrtus kuwanae How. i Anastatus disparis R.]. Plant Prot. 1963, 75, 543–552. [Google Scholar]
- Bjegović, P. Distribution and reduction role of egg parasites of spongymoth in Yugoslavia [Rasprostranjenje i redukciona uloga jajnih parazita gubara u Jugoslaviji]. Plant Prot. 1974, 128/129, 173–182. [Google Scholar]
- Krnjajić, S. Overview of species and abundance of egg parasitoids in some localities in Yugoslavia [Pregled vrsta i brojnosti jajnih parazitoida u nekim lokalitetima Jugoslavije]. Plant Prot. 1976, 93–95, 247–255. [Google Scholar]
- Sisojević, P.; Vasić, K. Parasitoids and hyperparasitoids of the spongy moth and their role in reducing the host population in Yugoslavia [Parazitoidi i hiperparazitoidi gubara i njihova uloga u smanjenju populacije domaćina u Jugoslaviji]. In Book of Abstracts of the 2nd Annual Scientific Conference of the Entomological Science of Serbia; Entomological Society of Serbia: Belgrade, Serbia, 1980; pp. 10–11. [Google Scholar]
- Ristić, M.; Sisojević, P.; Brajković, M. Parasitoids, hyperparasitoids and predators of Lymantria dispar L. (Lepidoptera, Lymantridae) in Yugoslavia [Parazitoidi, hiperparazitoidi i predatori gubara Lymantria dispar L. (Lepidoptera, Lymantridae) u jugoslovenskim zemljama]. Acta Entomol. Serb. Specc. Iss. 1998, 39–59. [Google Scholar]
- Drekić, M.; Poljaković-Pajnik, L.; Vasić, V.; Kovačević, B.; Marković, M.; Milović, M.; Pilipović, A. Short-term forecast of damage caused by the ash weevil [Kratkoročna prognoza šteta od jasenovog surlaša]. Poplar 2019, 204, 51–57. [Google Scholar]
- Georgiev, G.; Tabaković-Tošić, M.; Georgieva, M.; Mirchev, P. Lymantria dispar mortality in pupal stage caused by Entomophaga maimaiga in Bulgaria and Serbia. Poplar 2019, 203, 71–78. [Google Scholar]
- Stojanović, D.V.; Jerinić-Prodanović, D.; Kereši, T.; Graora, D.; Marković, M. Choreutis nemorana (Hübner, 1799) (Lepidoptera: Choreutidae) in Serbia. Poplar 2020, 206, 29–34. [Google Scholar] [CrossRef]
- Hossler, E.W. Caterpillars and moths: Part I. Dermatologic manifestations of encounters with Lepidoptera. J. Am. Acad. Dermatol. 2010, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]




| Standardized Operational Time (s per 100 Eggs) | Old (Manual) Procedure | New (Mechanical) Procedure |
|---|---|---|
| Number of egg masses | 10 | 16 |
| Mean | 61.88 | 5.89 |
| 95% CI for mean | (47.42, 76.34) | (3.59, 8.18) |
| Median | 58.02 | 4.83 |
| Minimum | 39.47 | 1.35 |
| Maximum | 93.67 | 17.14 |
| Standard Deviation | 20.21 | 4.31 |
| Coefficient of Variation | 32.66% | 73.23% |
| Standardized Operational Time (s per 100 Eggs) | Speed 1 (10,000 rpm) | Speed 2 (15,000 rpm) |
|---|---|---|
| Number of egg masses | 8 | 8 |
| Mean | 8.59 | 3.18 |
| 95% CI for mean | (4.73, 12.44) | (2.04, 4.33) |
| Median | 6.90 | 3.05 |
| Minimum | 2.71 | 1.35 |
| Maximum | 17.14 | 5.77 |
| Standard Deviation | 4.61 | 1.37 |
| Coefficient of Variation | 53.71% | 42.95% |
| Standardized Operational Time (s per 100 Eggs) | Brush with Stiff Bristles | Brush with Soft Bristles |
|---|---|---|
| Number of egg masses | 8 | 8 |
| Mean | 7.48 | 4.29 |
| 95% CI for mean | (3.03, 11.93) | (2.31, 6.27) |
| Median | 4.92 | 4.15 |
| Minimum | 2.80 | 1.35 |
| Maximum | 17.14 | 7.06 |
| Standard Deviation | 5.33 | 2.37 |
| Coefficient of Variation | 71.21% | 55.18% |
| Method | % Cracked Eggs (Mean ± SD) | % Crushed Eggs (Mean ± SD) |
|---|---|---|
| Mechanical Pr., Speed 1 | 0.10 ± 0.18 | 0.58 ± 0.89 |
| Mechanical Pr., Speed 2 | 0.79 ± 1.07 | 0.10 ± 0.19 |
| Mechanical Pr., Stiff Bristles | 0.71 ± 1.08 | 0.60 ± 0.88 |
| Mechanical Pr., Soft Bristles | 0.12 ± 0.18 | 0.08 ± 0.17 |
| Mechanical Pr., all groups | 0.44 ± 0.82 | 0.34 ± 0.67 |
| Manual Procedure | 0.82 ± 0.74 | 0.74 ± 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, D.V.; Ranđelović, D.; Ivetić, J.; Pap, P.; Zlatković, M.; Đilas, M.; Orlović, S. Improving the Monitoring and Control of Egg Vitality of Lymantria dispar Linnaeus 1758 Using an Innovative Device and Procedure for Removing Egg Hairs. Forests 2024, 15, 1426. https://doi.org/10.3390/f15081426
Stojanović DV, Ranđelović D, Ivetić J, Pap P, Zlatković M, Đilas M, Orlović S. Improving the Monitoring and Control of Egg Vitality of Lymantria dispar Linnaeus 1758 Using an Innovative Device and Procedure for Removing Egg Hairs. Forests. 2024; 15(8):1426. https://doi.org/10.3390/f15081426
Chicago/Turabian StyleStojanović, Dejan V., Dragana Ranđelović, Jelena Ivetić, Predrag Pap, Milica Zlatković, Milutin Đilas, and Saša Orlović. 2024. "Improving the Monitoring and Control of Egg Vitality of Lymantria dispar Linnaeus 1758 Using an Innovative Device and Procedure for Removing Egg Hairs" Forests 15, no. 8: 1426. https://doi.org/10.3390/f15081426
APA StyleStojanović, D. V., Ranđelović, D., Ivetić, J., Pap, P., Zlatković, M., Đilas, M., & Orlović, S. (2024). Improving the Monitoring and Control of Egg Vitality of Lymantria dispar Linnaeus 1758 Using an Innovative Device and Procedure for Removing Egg Hairs. Forests, 15(8), 1426. https://doi.org/10.3390/f15081426









