Abstract
We investigated the utility of nuclear and cytoplasmic single nucleotide polymorphism (SNP) markers for timber tracking of the intensively logged and commercialized Amazonian tree Jacaranda copaia. Eight hundred and thirty-two trees were sampled (cambium or leaves) from 38 sampling sites in Bolivia, Brazil, French Guiana, and Peru. A total of 128 SNP markers (113 nuclear, 11 chloroplastic, and 4 mitochondrial) were used for genotyping the samples. Bayesian cluster analyses were carried out to group individuals into homogeneous genetic groups for tests to self-assign groups of individuals or individuals to their population of origin. Cluster analysis based on all the SNP markers detected seven main genetic groups. Genetic differentiation was high among populations (0.484) and among genetic groups (0.415), and populations showed a strong isolation-by-distance pattern. Self-assignment testing of the groups of individuals for all loci was able to determine the population origin of all the samples (accuracy = 100%). Self-assignment tests of individuals were able to assign the origin of 94.5%–100% of individuals (accuracy: 91.7%–100%). Our results show that the use of the 128 SNP markers is suitable to correctly determine the origin of J. copaia timber, and they should be considered a useful tool for customs and local and international police.
Keywords:
illegal logging; forensics; SNP markers; timber tracking; tropical trees; Jacaranda copaia 1. Introduction
Timber logging, whether legal or illegal, contributes to biodiversity loss. Illegal logging is also an economic problem for the legal market, as extremely low-priced wood competes with legal logging, which have higher costs [1,2]. International laws have been established in countries around the world to avoid the importation of illegal timber; for example, timber regulations of the United States (Lacey Act), the European Union (The EU Timber Regulation No. 995/2010), and Australia (Illegal Logging Prohibition Act), which declare it illegal to import timber and its derived products (furniture, musical instruments, etc.) sourced from illegal timber extraction [1]. These regulations require that importing companies declare (1) the country and specific place of origin and (2) the names of the exact species of all plants contained in their wood products and (3) guarantee that these wood products have a legal origin and have been extracted in a sustainable way in accordance with the laws of the country of origin. In addition to the legislation targeting illegal timber trading, the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) [3,4] allows the international trade of species listed in CITIES Appendix II only when sourced from plantations.
The main problems in the illegal timber trade are in the documentation of the origin of the wood, with falsified certificates and documentation and missing or false information [5]. According to the Brazilian Federal Police, in 2021, 90% of the wood extracted from the Amazon Forest had an illegal origin [5,6]. Timber tracking methods are required to fight against illegal timber trading, but methods based only on documentation are sensitive to manipulation and forgery. Therefore, timber export companies and institutions responsible for controlling the origin of imported timber, such as customs, federal police, and Interpol need reliable tools to prove and confirm the declared origin of wood and its derivative products when traded internationally.
To be able to track the species, country, and specific site of origin of the imported timber, several methods exist, such as chemical analysis or mass spectrometry [7,8], the use of stable isotopes [9], near-infrared spectroscopy [10], wood anatomy [11,12], and DNA fingerprinting [13,14,15,16,17,18], which all exploit differences in the profiles among species and specific origins. These methods have shown strong potential for species, country, and site origin determination, especially DNA fingerprinting. Some methods, such as wood anatomy, isotopic profiling, and spectrometric methods are limited in their capacity to identify the origin of timber due to variations among tissue types, individual sample age, individual and population genetic differences, and/or environmental influences on timber composition [17]. Thus, genetic methods that allow the correct identification of tree species and tracking of the timber origin are essential for controlling the legality of timber by public authorities, industry, and trading companies.
Here, we evaluate the use of a DNA fingerprinting method to track the intensively exploited and high-value wood of the Neotropical pioneer tree Jacaranda copaia (Aubl.) D. Don. (Bignonaceae). The genus Jacaranda has ca. 53 species, all Neotropical, and most are found in the Cerrado around the Amazon Forest. J. copaia is the only species of Jacaranda that is widely distributed in the Amazon Forest [19]. The species is found from Central America to western South America and from Belize to Bolivia, including Brazil, French Guiana, and Peru [19]. In Brazil, the species is found in the states of Acre, Amapá, Amazonas, Maranhão, Mato Grosso, Pará, Rondônia, and Roraima [19]. J. copaia is a fast-growing species, with an average annual increase of 2.64 cm in diameter at breast height (DBH) and 2.32 m in height at 9 years of age at a spacing of 3 m × 2 m [20]. Adult trees have straight stems, reaching 106 cm in DBH and 45 m in height [19,20]. Its wood is light and used for furniture [20,21]. The species is hermaphroditic and self-incompatible; thus, reproduction occurs mainly by outcrossing (>94%) [22,23,24]. About 40 species of bees, wasps, butterflies, and hummingbirds have been detected as potential pollinators, although Euglossa spp. and Centris spp. bees were identified as the main pollinators in the Tapajós National Forest, Brazil [22]. Each fruit can have up to 250 winged seeds that are dispersed by the wind [22]. Short-distance pollen and seed dispersal, up to 90 m and 100 m respectively, results in an intrapopulation spatial genetic distance that ranges from 50 to 500 m, which, in turn, causes mating between related individuals [24].
Here, samples of J. copaia collected at 43 sampling sites in Bolivia, Brazil, French Guiana, and Peru were used to characterize its genetic diversity and population structure and to test the ability of chloroplast, mitochondrial, and nuclear single nucleotide polymorphism (SNP) markers to determine the geographic origin of wood of the species harvested from the Amazon Forest.
2. Materials and Methods
2.1. Sampling
There is considerable confusion in the literature regarding the naming of samples and populations; therefore, we specify here our naming convention: “individual sample” corresponds to the biological material sampled from a single individual tree; “sampling sites” are locations where individual samples are collected; “population samples” are the groups of individuals sampled at a sampling site; “reporting groups” are the biological populations or genetic groups (i.e., groups of interbreeding individuals with a common ancestry) as estimated from population genetic analyses (i.e., STRUCTURE analyses) and that are used to assign individuals; “country” refers to all the population samples collected within the political borders of a country. Countries may include several reporting groups, and reporting groups may range across different countries.
Cambium or leaf samples were collected from 832 individual trees from 43 sampling sites (2–31 individuals) in the Amazon rainforest in four countries: Bolivia, Brazil, French Guiana, and Peru (Table 1). The minimum distance between individual trees was 50 m, and the distance between the sampling sites ranged from 23–2648 km. All the sampled trees were georeferenced using GPS, and the collected plant material (cambium or leaves) was stored in labeled plastic bags with silica gel, which absorbs moisture and prevents fungal growth and DNA degradation. In Bolivia, population samples were collected from 5 sampling sites on farms and forestry concessions. In Brazil, population samples were collected from 12 sampling sites in national forests, parks, extractive reserves, and ecological stations, with support from the Chico Mendes Institute of Biodiversity. In French Guiana, population samples were collected by the Institut national de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE) together with the forest authorities (Office National des Forêts, ONF) at 4 sampling sites. Samples from six additional individuals were collected at three additional sites. In Peru, population samples were collected from 19 sampling sites in national forests, parks, extractive reserves, ecological stations, farms, and forestry concessions. However, due to the low sample size at 5 sampling sites (2 trees), for the genetic analysis, these individuals were grouped with the closest sampling sites. We also divided the Brazilian samples into West (six population samples) and East (six population samples) origins and Peru into North (nine population samples) and South (eight population samples) origins. All individual samples were registered in a database at the Thünen Institute (SampleDataBase, Grosshansdorf, Germany).

Table 1.
Information on the population sample size (n), sampling site latitude and longitude, population sample abbreviation (Abbrev), and sample size after combining population samples with only two individuals with the closest population sample (n1).
2.2. DNA Extraction and SNP Analysis
Cambium and leaf samples collected in Brazil were sent to the Laboratory of Population Genetics and Forestry of São Paulo State University in Ilha Solteira, Brazil (UNESP-FEIS) for DNA isolation. Samples collected in French Guiana, Peru, and Bolivia were sent to Thünen Institute facilities in Großhansdorf, Germany, for DNA isolation. DNA isolation from leaf and cambium was carried out according to Dumolin et al. [25]. The individual samples were screened for 128 SNP and insertion–deletion INDEL markers (nCpMtSNPs) according to Sebbenn at al. [26], using the MassARRAY® iPLEXTM (Assay Design Suite v2.0 [Agena Bioscience™, San Diego, EUA, CA, USA]) genotyping assay. Three types of SNPs were used: 113 nuclear SNPs (nSNPs), 11 chloroplast SNPs (CpSNPs), and 4 mitochondrial SNPs (MtSNPs). The combined nuclear, chloroplast, and mitochondrial SNP markers will hereafter be referred to as nCpMtSNP markers.
2.3. Genetic Diversity Analysis
The genetic diversity of the nSNPs was determined for each population sample and country by the total number of alleles (), percentage of polymorphic loci (), and observed () and expected () heterozygosity. The mean fixation index () was estimated to quantify the inbreeding coefficient within each population sample or country. To test whether values of the population samples were statistically different from zero, alleles were randomly permuted among individuals within population samples. For CpMtSNPs, the genetic diversity was determined for each site by and the percentage of polymorphic loci (%). We also estimated the percentage of polymorphic loci (), observed for all the nCpMtSNP markers. These analyses were carried out using the GDANT 1b software [27].
2.4. Bayesian Clustering Analysis
The Bayesian clustering method implemented in the software STRUCTURE v.2.3.4 [28] was used for the 128 nCpMtSNP markers to check for the number of reporting groups (i.e., genetic populations). We set the length of burn-in and the Markov chain Monte Carlo to 10,000 and tested K values from 2–10 30 times. We used the admixture model with correlated allele frequencies. The optimal number of reporting groups was estimated with the ΔK method [29] using the software CLUMPAK v.1.1.2 [30]. For each ideal K, cluster membership coefficient (Q) data from the 30 STRUCTURE runs were permuted with the software CLUMPAK, and the results were presented on a map, grouping individuals by location sampled.
2.5. Genetic Differentiation among Populations, Countries, and Crusters
Overall and pairwise genetic differentiation () was estimated among all the population samples (locals), and clusters were determined by STRUCTURE analysis among countries and population samples within countries and for all nCpMtSNP, nSNP, and CpMtSNPs markers. These analyses were carried out using the GDANT 1b software [27]. Isolation by distance (IBD) gene dispersal, using the Spearman correlation coefficient () of the pairwise and geographical distance, was studied at two levels: (1) among population samples with a sample size higher than 16 individuals; and (2) among clusters determined by STRUCTURE analysis. We also assessed the IBD by the estimating the spatial genetic structure (SGS), based on calculation of the co-ancestry coefficient () described in Loiselle et al. [31], between mean pairs of individuals within 10 distance classes (0–198, 199–441, 442–655, 656–860, 861–1068, 1069–1409, 1410–1838, 1839–2770, 2771–2478, and 2479–2646 km), determined using the same number of pairs per class, and using the SPAGEDI 1.5 software [32]. The statistical significance of the average for each distance class was derived by comparing the limits of the confidence interval at 95% probability for the average for each distance class. Confidence intervals were estimated by randomly permuting (1000 times) the genotypes among the distance classes using the SPAGEDI software (ver 1.5).
2.6. Genetic Assignment Analysis
No wood DNA samples were used here. The purpose of our study was to determine if the population structure and genetic differentiation among J. copaia across the species range is enough to allow for the correct determination of the origin of an individual sample. Provided that DNA can be extracted from the target material, the same analytical procedures can be used for DNA collected from wood stored by timber companies, sawn wood, musical instruments, furniture, etc. However, it is important to highlight that extracting genomic DNA from wood samples is more difficult and less successful, as the wood DNA is typically degraded. Similar procedures have been used in other studies for the same purpose [15,16]. The Bayesian method [33] implemented in GeneClass 2.0 [34] was used to assign population samples (group of individuals collected in a sampling site) and individuals to their reporting group and country. The genetic grouping determined by the STRUCTURE analysis was used to group individuals into reporting groups. Both population sample and individual sample assignments were caried out for all nCpMtSNP markers, and the most likely reporting group determined by the highest score according to the Bayesian criteria was used as an indicator of the power of the markers to compute the proportion of correctly assigned population samples or individuals in self-assignments tests [35]. Here, the individual samples from the population samples (reference data) were self-classified to the reporting groups and countries using the leave-one-out approach [36]. We also estimated the overall, ≥80%, and ≥95% score rate of population samples and individual samples assigned to the correct origin reporting group.
3. Results
3.1. Genetic Diversity
The rate of missing data among loci ranged from 0.1 to 47.1%, with an average of 4.4% (Table S1), and among population samples, it ranged from 0.8 to 32.3%, with an average of 4.6% (Table 2). Across population samples, the total number of alleles () for 113 nSNPs ranged from 122–226 alleles (mean of 183), and for 15 chloroplast and mitochondrial loci (CpMtSNPs) from 14–19 alleles, it had a mean of 15.9 alleles (Table 2).

Table 2.
Genetic diversity of Jacaranda copaia in different population samples and countries for 113 nuclear (nSNP), 15 chloroplast and mitochondrial (CpMtSNP), and 128 nuclear, chloroplast, and mitochondrial (nCpMtSNP) markers. * denotes p < 0.05; n is the sample size; % is the percentage of missing data; is the percentage of polymorphic loci; is the percentage of polymorphic loci for all 128 nCpMtSNPs; is the total number of alleles; and are the observed and expected heterozygosity, respectively; is the fixation index, where bold values represent the average fixation index among populations () within countries.
The percentage of polymorphic loci () for 113 nSNPs was higher (ranging among populations from 23%–88.5%) than for the 15 CpMtSNPs (0%–26.7%) and 128 nCpMtSNP markers (20.3%–78.1%; mean of 55.9%). The observed heterozygosity () ranged from 0.023–0.343 (mean of 0.178), and the expected heterozygosity () for the nSNPs ranged among populations from 0.028–0.348 (mean of 0.204). The values of for the nSNPs were high (≥50% of the maximum value for biallelic loci; 0.5) for 21 populations, where the highest values were observed for the Brazilian and Bolivian populations. The mean intrapopulation fixation index () ranged from −0.086-0.295 and was significantly (p < 0.05) higher than zero in 6 of the 38 populations, suggesting inbreeding. At a country level, and were highest in Brazil for the nSNPs (226 and 100%, respectively) and CpMtSNPs (30 and 100%, respectively) and lowest in French Guiana (nSNPs = 200 and 76.1%, respectively; CpMtSNPs = 18; 20%, respectively). Bolivia presented the highest (0.319) and (0.359) values and the lowest value (0.113), while French Guiana presented the lowest (0.095) and (0.192) values and the highest value (0.506).
3.2. Bayesian Cluster
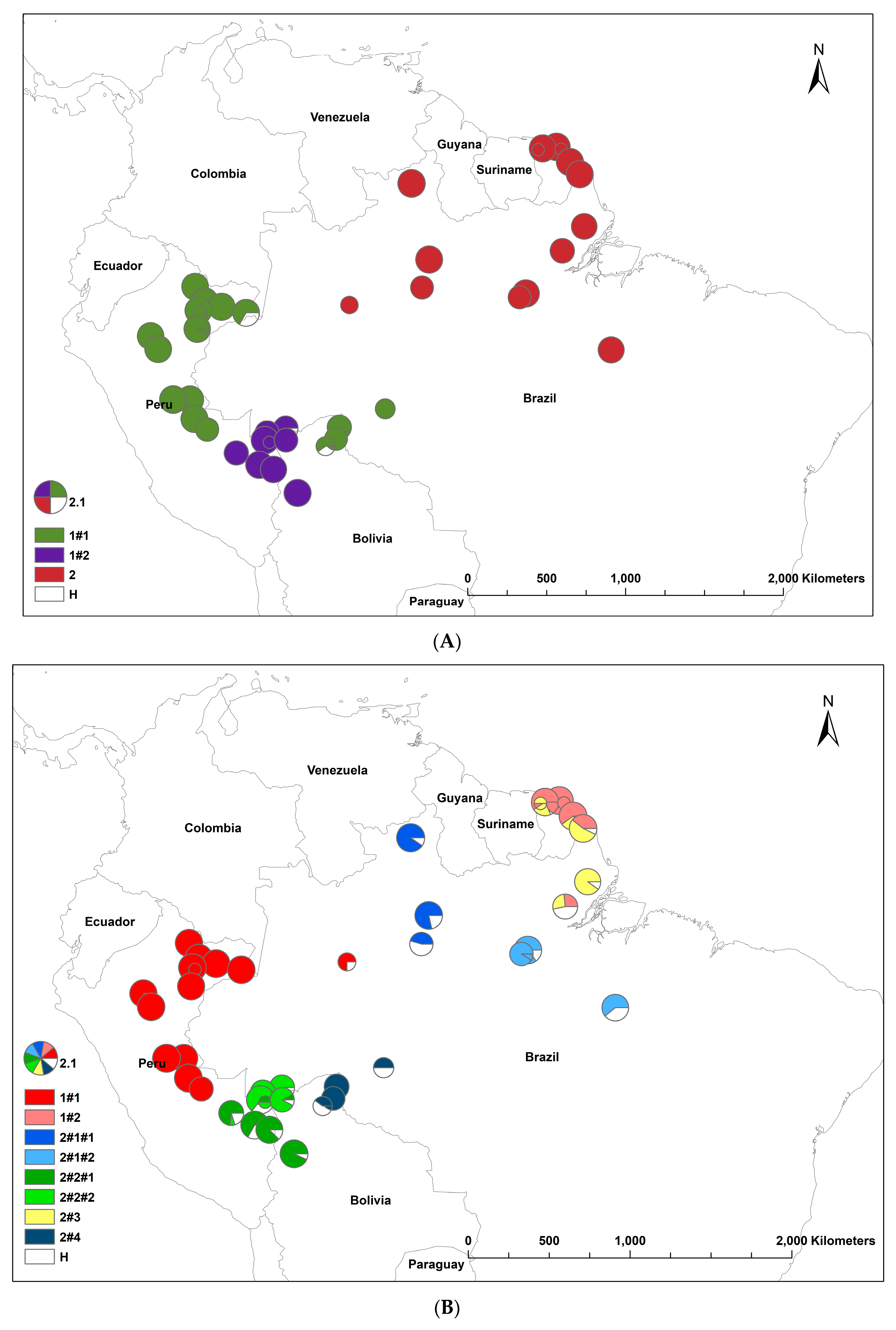
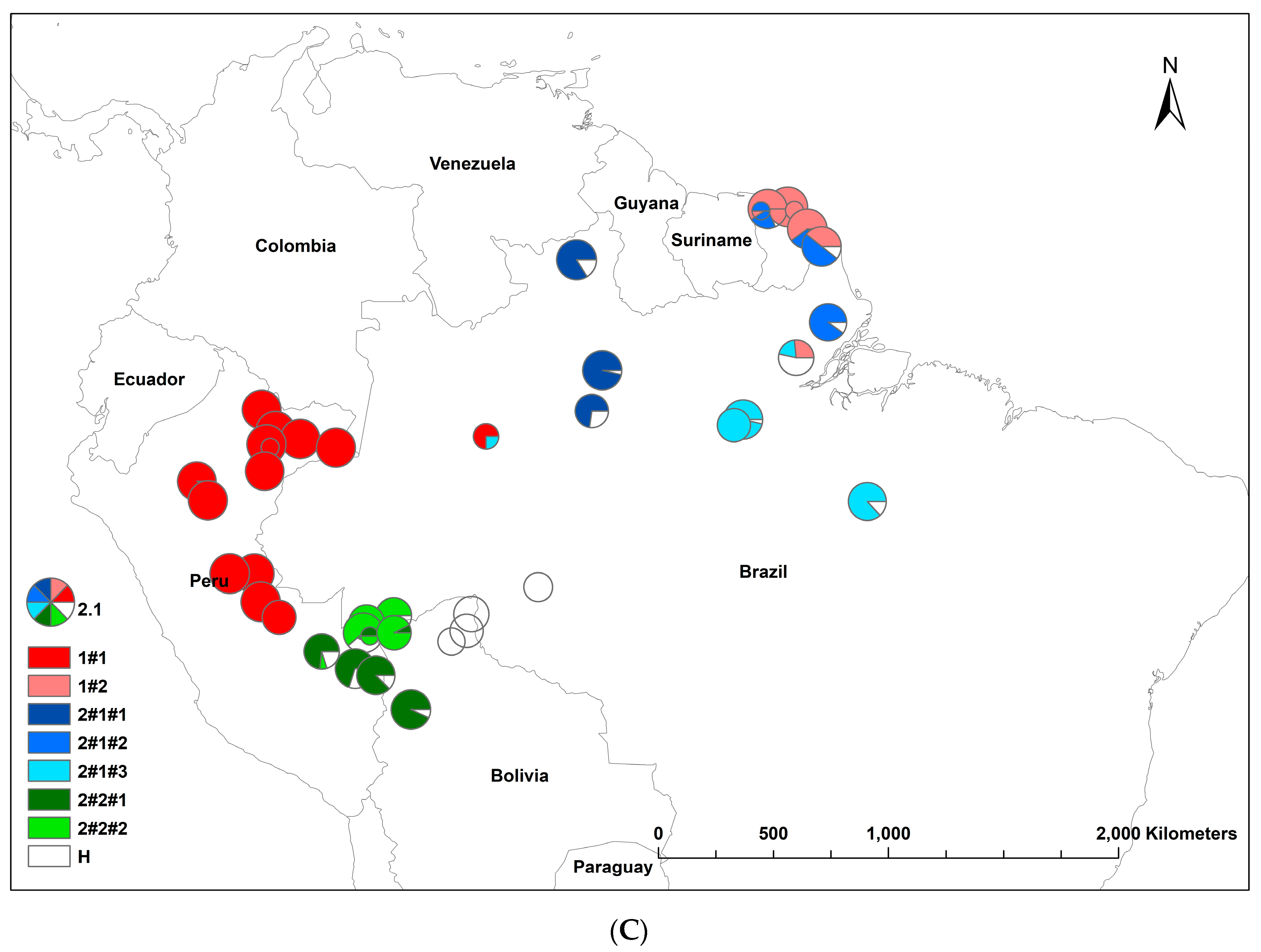
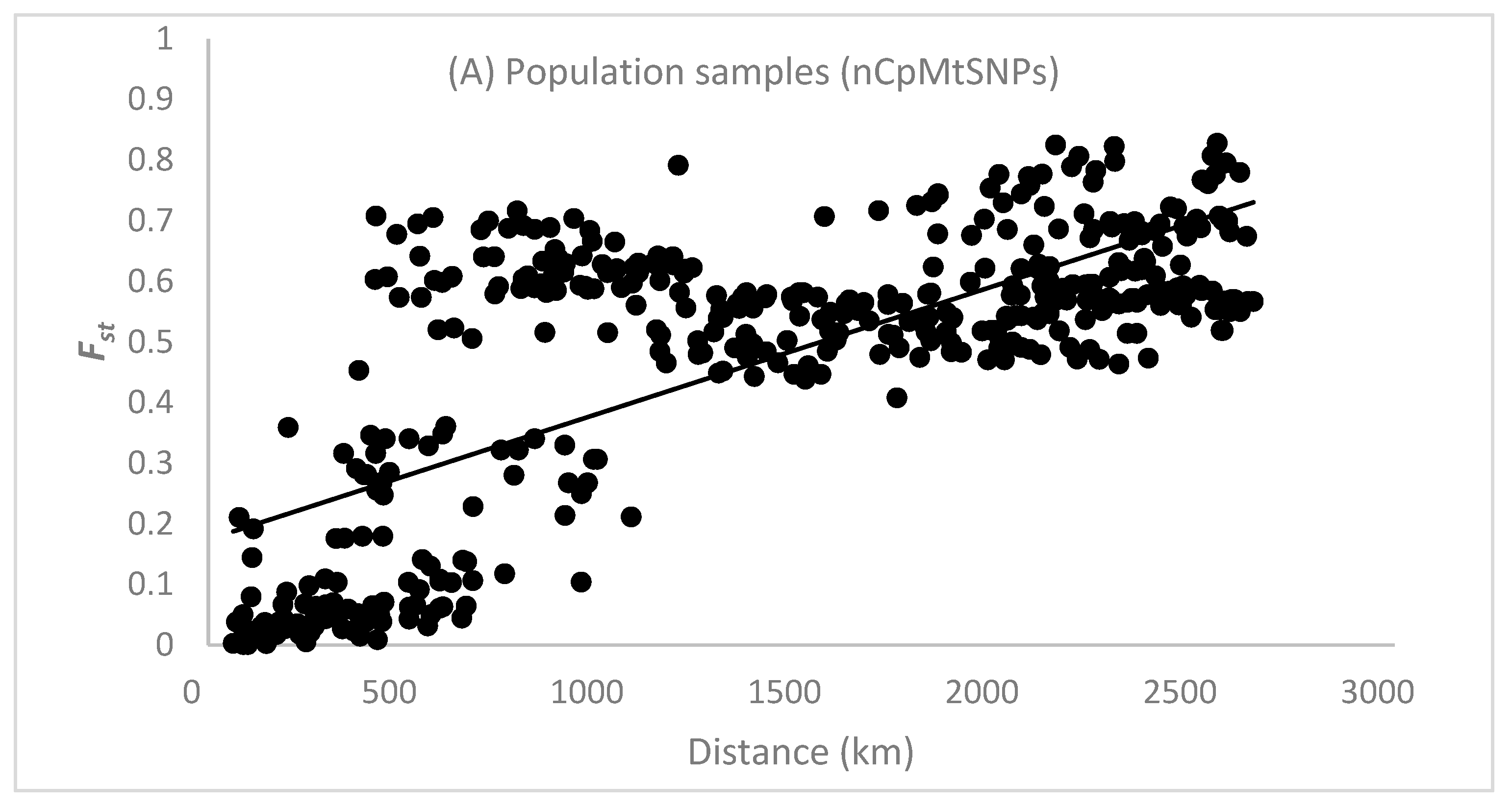
The results of the STRUCTURE analysis showed that based on ΔK, the best representation of the data was obtained with a K of four or nine different reporting groups (i.e., genetic populations) (Figure 1). With five groups, there was less mixing of gene pools within individuals. With K = 5, 85% of all individuals had a gene pool membership coefficient above 0.9, while for K = 4, only 76% of all individuals had gene pool membership coefficients above 0.9. The STRUCTURE analysis based on CpMtSNPs showed a clear distinction between the French Guiana and North Brazil sampling sites (red) from those from North Peru, South Brazil (6BW-An; 7B-Ja), and East Bolivia (34Bo-Ri, 35Bo-De) (green and white) and those from South Peru (29P-In; 30P-Md, 31P-Ca, 32P-Am, 33P-Ta), Southwest Brazil (9BW-Xa, 10B-Co), and Bolivia (36Bo-Vi, 37Bo-Ch; 38Bo-Ma) (blue) (Figure 1A). The STRUCTURE analysis based on nSNP markers revealed that there were a few population samples with mixtures of individuals from different gene pools (e.g., Flona do Jamari (7BW-Ja), Resex Tapajos-Arapins (14BE-Ar), and ESEC Maraca (5BW-Ma) in Brazil and Piste de Paul Isnard (2FG-Is), Route de Cacao (3FG-Ro), and Saut Maripa (4FG-Ma) in French Guiana in Figure 1B). We found one genetic group (K3) that was only found in two Brazilian sampling sites (8BW-Jr. 11BE-Am), and another genetic group (K1) was distributed more in the middle of the Amazon basin (Figure 1B).
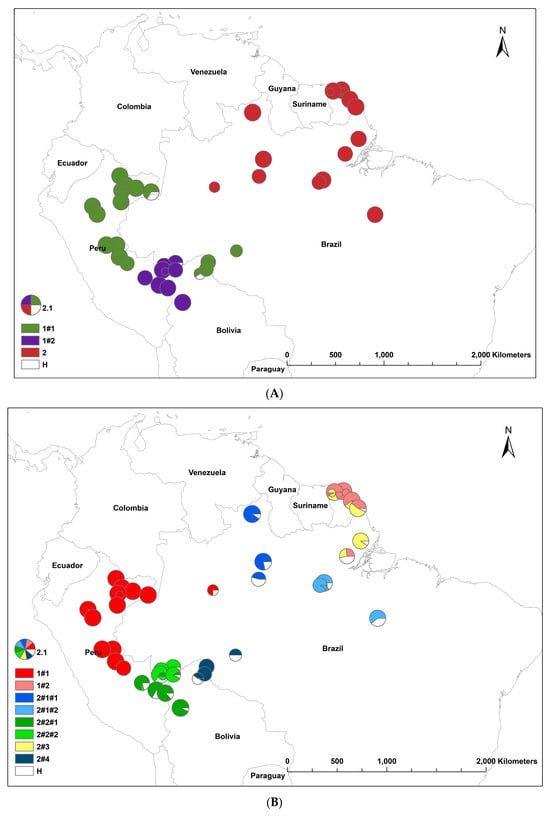
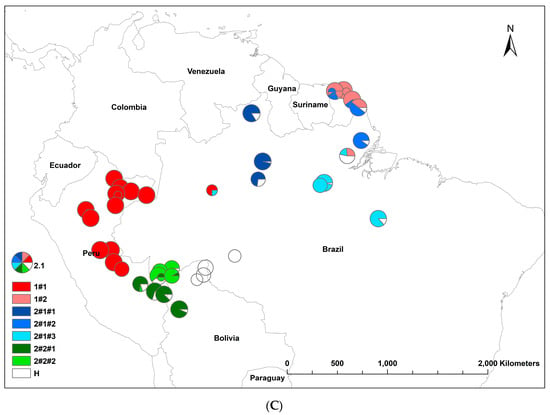
Figure 1.
Population structure of Jacaranda copaia in South America, showing the spatial distribution of reporting groups (i.e., genetic clusters) based on CpMtSNP (K = 4) (A), nSNP (K = 9) (B), and nCpMtSNP (K = 8) (C) markers estimated by STRUCTURE (K = 4 to 9).
3.3. Population Differentiation
To determine differences between nuclear SNPs (nSNPs) and chloroplast and mitochondrial markers (CpMtSNPs) in estimating the genetic differentiation () among population samples, we compared the results among all 128 nCpMtSNPs, 113 nSNPs, and 15 CpMtSNPs (Table 3). The results showed that the among all the population samples and countries was higher for the CpMtSNPs (0.942 and 0.695, respectively) than for all the nCpMtSNPs (0.484 and 0.295, respectively) and nSNPs (0.415 and 0.233, respectively). The was also higher among clusters determined by STRUCTURE analysis for the CpMtSNPs (0.896) than for the CpMtSNPs (0.401) and nSNPs (0.315). Among population samples within Brazil, Peru, and Bolivia, the was also higher for the CpMtSNPs (0.974, 0.741, and 0.735, respectively) than for all the nCpMtSNPs (0.3, 0.466, and 0.142, respectively) and nSNPs (0.224, 0.456, and 0.107, respectively). In contrast, among the population samples within French Guiana, the was higher for the nCpMtSNPs (0.12) and nSNPs (0.117) than the CpMtSNPs (0.004). The results also showed that the estimates of for nCpMtSNPs, nSNPs, and CpMtSNPs were generally highest within Peru and Brazil, where the number of sampling sites was highest (Peru = 17, Brazil = 12), indicating that the number of sampling sites is important for detecting high population differentiation within countries. The higher for CpMtSNPs and nCpMtSNPs than for nSNPs indicated that the combination of the 113 nuclear and 15 cytoplasmatic SNP markers increases the capacity to detect genetic differences among populations.

Table 3.
Genetic differentiation () among all population samples, genetic groups, countries, and all population samples within countries for all the nCpMtSNPs (128), nSNPs (113), and CpMtSNPs (15).
3.4. Isolation by Distance
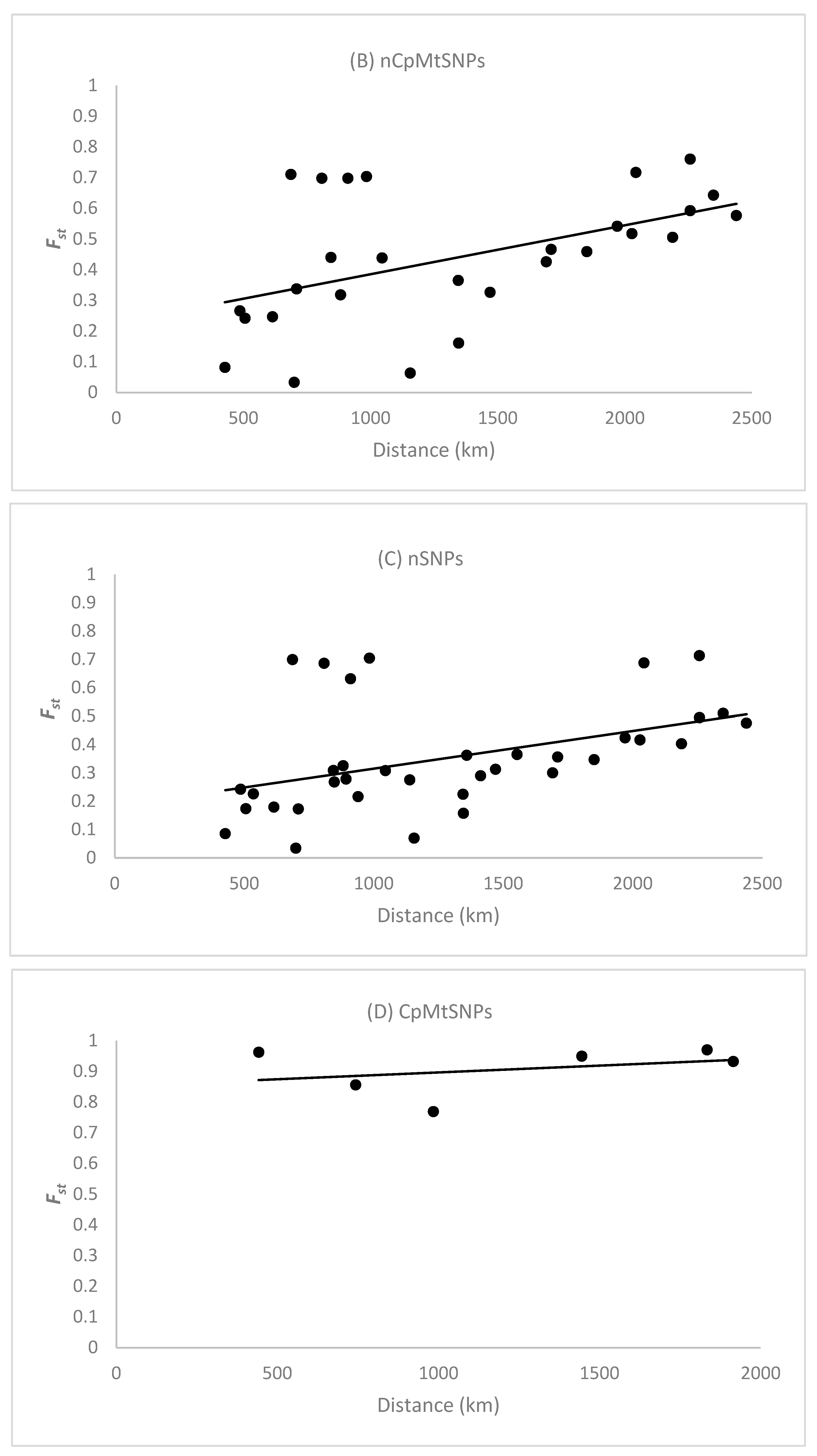
The Spearman correlation coefficient () between the pairwise and the spatial distance between population samples (n > 16 individuals) based on the 128 nCpMtSNP markers was significantly (p < 0.001) higher than zero ( = 0.626), indicating an isolation by distance (IBD) genetic pattern (Figure 2A). The Spearman correlation coefficient () between the pairwise and the spatial distance between clustered locals, as determined by STRUCTURE analysis, was also significantly (p < 0.001) greater than zero for nCpMtSNPs ( = 0.521; Figure 2B) and nSNPs ( = 0.552; Figure 2C), reinforcing the occurrence of an isolation-by-distance genetic pattern. The estimate of pairwise co-ancestry for the nSNP and nCpMtSNP markers between individuals within ten distance classes was significantly higher than zero up to the distance of 655 km, whereas for the CpMtSNP markers, this was significant up to 1068 km and non-significant or significantly lower than zero in the other distance classes (Figure S1).
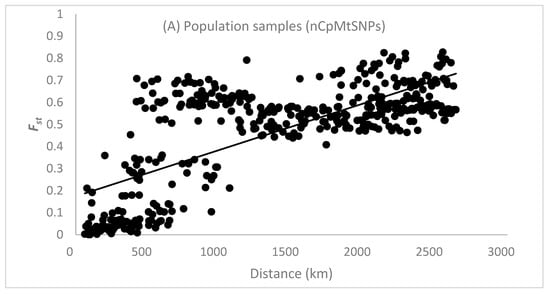
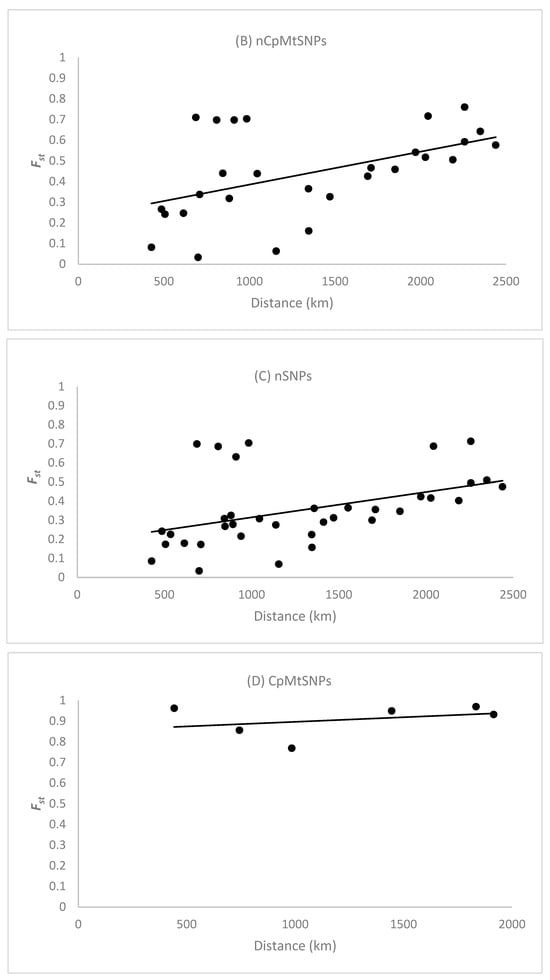
Figure 2.
Pattern of isolation by distance in Jacaranda copaia population samples (A) and genetic groups determined by cluster analysis using STRUCTURE analysis (B–D). is the pairwise genetic differentiation between population samples (n > 16 individuals) for all 128 nCpMtSNP markers (A) and the pairwise genetic differentiations between clustered population samples as determined by STRUCTURE analysis for all 128 nCpMtSNP (B), 113 nSNP (C), and 15 CpMtSNP (D) markers. The Spearman correlation coefficient () was significantly higher than zero (p < 0.01) between the pairwise and the geographical distance among populations (A) for all 128 nCpMtSNP markers (0.645), as well as between and the spatial distance between clustered population samples as determined by STRUCTURE analysis (B–D) for the nCpMtSNPs (0.521) and nSNPs (0.552), whereas non-significant correlation was observed for the CpMtSNPs (0.142).
3.5. Genetic Assignment
We performed self-assignment tests based on the seven main genetic clusters determined in the STRUCTURE analysis (Figure 1C) using all 128 nCpMtSNP markers. Due to the genetic similarity and small sample size of the 15BE-Te site (Figure 1A,B), it was grouped with the 12 population samples from Northern Peru for statistical analyses. Self-assignment tests were carried out to assign or exclude the seven clusters as the origin of individuals or groups of individuals (locations grouped by cluster) from our sample. The reporting group assignment test was able to self-assign 100% of the genetic population (genetic clusters) samples (score of 100%) to the correct origin for seven reporting groups and four countries (Table 4). The self-assignment test of individuals was able to self-assign 94.5%–100% (mean of 98.4%) of individuals to the correct origin reporting group (score ranging from 91.7%–100%; mean of 97.5%). The rates of correct individual assignments for scores ≥ 80% and ≥95% were high (95.5% and 89.9%, respectively). The individual sample assignment test was able to self-assign from 87.6% of individuals from French Guiana to 100% of individuals from Bolivia to the correct origin country (scores ranging from 93.4%–99.9%). The rate of correct individual assignment to the correct country source with scores of ≥ 80% and ≥ 95% ranged from 87.8%–100% and 72.8%–100%, respectively.

Table 4.
Corrected genetic population sample (self-assignment test of grouped individuals) and individual self-assignment (self-assignment test of individuals) rate and scores for reporting groups and countries (mean score) for all 128 nCpMtSNP markers. “Rate” means the percentage of individuals assigned to the correct source reporting group or country; rates > 80% and >95% are the rates of individuals assigned to the correct source reporting group or country with score values ≥ 80% and 95%, respectively.
4. Discussion
4.1. Genetic Diversity
The illegal timber trade, both species-specific and illegally sourced, has become a major problem for tropical forests. Control of the chain of custody for timber originating from the Amazonian Forest, as well as timber from other biomes and regions of the planet, has created an urgency to verify the species, countries, and place of origin declared in transport and export documentation and to restrict, or even eradicate, illegal logging.
Our study, based on fresh samples of cambium and leaves, shows the potential of DNA fingerprinting to track the origin (reporting groups) of J. copaia timber. Our study also suggests that this method has the potential to monitor and verify the chain of custody of J. copaia timber products, provided that the extraction of genomic data from wood samples containing degraded DNA (furniture, musical instruments, and other timber products) is successful. Although the extraction of genomic material from dried samples of timber and its derivatives is more difficult, recent studies have successfully developed specific DNA extraction protocols for wood samples containing degraded DNA [37,38,39].
Our study shows only moderate levels of genetic diversity for nSNPs and low levels of genetic diversity for CpMtSNPs for J. copaia. However, the observed () and expected () heterozygosity are generally moderate and high, respectively, and are within the reported pattern identified for other Neotropical trees using nSNP markers. For example, in Carapa sp., has been observed to range from 0.017–0.262 among populations, and ranges from 0.02–0.251 [40] in Dipteryx sp. has been observed to range from 0.03–0.39 and from 0.03–0.29 among populations [16], and in Handroanthus sp., has been observed to range from 0.15–0.276 and from 0.071–0.371 among populations [41]. The genetic diversity of J. copaia was especially low in some Peru population samples due to the low percentage of polymorphic loci () for all the nCpMtSNP markers. The estimate of the intrapopulation fixation index () indicated inbreeding in some populations. Inbreeding has also been observed for other tropical trees [1,2,40,41,42,43,44,45]. However, because J. copaia is self-incompatible [22], and our population samples were taken from geographically distant trees, the observed inbreeding is likely an artefact of the Wahlund effect [46] due to the mixing of samples from different subpopulations, as previously shown for J. copaia [47].
4.2. Population Genetic Differentiation
The presence of population genetic differentiation and intrapopulation spatial genetic structure (SGS), or the occurrence of isolation by distance (IBD) patterns, is key for the assignment of timber to different origins [36], including country, population, and regions within countries. High genetic differentiation among the different reporting groups increases the success of genetic assignment [36]. Our results showed strong genetic differentiation among all reporting groups and between countries, as well as SGS and a pattern of IBD. The for all the nCpMtSNP, nSNPs, and CpMtSNPs was generally high, especially for CpMtSNPs. The was higher among reporting groups than among countries. The mean assignment test of individuals to the correct reporting groups of origin was also higher than assignments test among countries of origin.
Within countries for all the nCpMtSNP and CpMtSNP markers, was lowest among the French Guiana population samples. In agreement, the lowest self-assignment of individuals was found in French Guiana (87.6%). Brazil, Peru, and Bolivia showed a high rate of self-assignment of individuals to the correct country of origin (>98%). The results showed that the among all population samples, reporting groups, and countries was higher for the CpMtSNPs than for the nSNPs. This can be attributed to the fact that CpMtSNPs have lower variability due to uniparental inheritance, while nuclear SNPs have higher genetic diversity due to biparental inheritance and recombination. Thus, CpMtSNPs can show low variation within or among closely neighboring reporting groups, where nSNPs can present high variation and share many alleles among reporting groups, as can be observed in Figure 1. Furthermore, the percentage of polymorphic loci was lower for the CpMtSNPs than the nSNPs, where the highest polymorphism was observed, and many population samples shared the same alleles, which reduces the genetic differences between population samples. These results indicate the importance of using both nSNP and CpMtSNP markers to better detect population genetic structures and thus increase the potential for assigning wood origins to population samples, reporting groups, and countries.
4.3. Genetic Assignment and Practical Applications
The results indicate a high capacity to correctly assign grouped individuals at two levels: among countries and among reporting groups (100%). Our results confirm that this approach offers the possibility of high levels of success when grouping timber samples based on their sampling site of origin. This success can be attributed to the general wide genetic differentiation among countries and reporting groups. Even when the differentiation was only moderate ( < 0.2) (French Guiana, Bolivia), differences in allele frequencies between analyzed groups were enough to produce high scores for the determination of the correct origin. However, it is important to emphasize that this is true only if the reference data set (the collection of samples of individuals from sampling sites encompassing the species range) contains genetic data of the timber’s original population. Therefore, success in determining the origin of J. copaia timber, as well as of other species, greatly depends on the reference data set developed by the authorities involved in controlling the origin of timber (reference population samples). The reference data set should include genetic data from all sites where harvesting is legally practiced.
The results of the assignment tests of individuals for all the nCpMtSNPs were lower than from assignment tests of the groups of individuals, but they also showed a high power of correct assignment among countries and reporting groups. In general, the results for the assignment testing of individuals indicate that this approach has the power to determine the reporting group of origin and, in some cases, estimated the specific site of the timber origin. When using CpMtSNP markers, it is necessary to develop a large number of loci to improve both population sample and individual assignment tests. Similar results from better self-assignment tests at the population sample level rather that at the individual were reported for Hymenaea sp. [15]. For Swietenia macrophylla, based on nuclear microsatellite loci (SSRs), the assignment test was higher at the country (82%) than at the population sample (71%) level [1]. For the Malaysian Gonystylus bancanus tree, the self-assignment rate using a set of 16 nSSRs was lower (55%) than that observed here at the population sample level [43]. For SNP markers of Entandrophragma cylindricum, the assignment at the country level ranged from 66%–74%, depending on the assignment method [14]. Many other studies on tropical and temperate tree species have been developed using microsatellite and SNP markers to track timber; these show that, thanks to its ability to detect spatial genetic structure and genetic differentiation, DNA fingerprinting is the most effective tool to track the country and population of origin of timber [48,49,50].
Finally, we recommend that the timber sector add such genetic controls as an independent audit beyond the paper-based proof of the chain of custody. It is important to note that the power of the genetic reference data to detect false declarations reaches 100% if more than one sample with the same declaration is tested.
5. Conclusions
The genetic differentiation () among all population samples, reporting groups, and countries was generally high, especially when based on CpMtSNP markers. Furthermore, there is a strong isolation-by-distance pattern among population samples, favoring the tracking of population samples or individual samples to the correct site. For self-assignment tests, we were able to obtain 100% accuracy at the country and reporting group level for all samples using all 128 nCpMtSNPs and 113 nSNPs. Our results show that the use of all the nCpMtSNP markers is suitable to correctly determine the country and reporting group of origin for J. copaia timber, thus offering a very useful tool for customs and local and international police. The J. copaia reference database presented here represents a robust assignment tool available to timber companies or regulating agencies to test and validate declarations of timber origin. We recommend the use of the method described herein for other native tropical species, since it is highly effective in identifying the origin of wood, thus helping the police and other relevant agencies in defining illegally deforested areas as well as unsustainable extraction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15081478/s1, Figure S1. Spatial genetic structure (SGS) of Jacaranda copaia for all sampled individuals in all populations for all 128 nCpMtSNP, 113 nSNP, and 15 CpMtSNP markers. Unfilled circles indicate mean (Coancestry coefficient) values that are significantly different than zero (p < 0.05) for the hypothesis of the absence of SGS (H0: = 0); vertical bars show the standard error at 95% probability; Table S1. Population sample size (n), missing data (%), genetic diversity, and differentiation among population samples () for each locus and the mean nCpMtSNP markers: k = number of alleles; = observed heterozygosity; = expected heterozygosity.
Author Contributions
Conceptualization, L.F.M.C., A.M.S., B.D., C.B.-J. and S.C.; Methodology, A.M.S. and C.B.-J.; Data validation, M.M., V.T., A.D. and C.B.-J.; Formal Analysis, A.M.S. and C.B.-J.; Investigation, A.M.S. and C.B.-J.; Resources, B.D.; Writing—Original Draft Preparation, L.F.M.C. and A.M.S.; Writing—Review and Editing, L.F.M.C., A.M.S. and N.T.; Supervision, B.D.; Project Administration, B.D. and C.B.-J.; Sampling, B.R.V.M.-S., K.P.-V., E.N.H.C., C.R.G.-D., N.T., V.T. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the German Federal Ministry of Food and Agriculture within the framework of the “Large scale project on genetic timber verification” (Project No. 28I-001-01). Genotyping was performed at the Genomic and Sequencing Facility of Bordeaux (grants from the Conseil Regional d’Aquitaine, n° 20030304002FA and 20040305003FA; the European Union, FEDER n° 2003227; and Investissements d’avenir, n° ANR-10-EQPX-16-01). Support for the UMR EcoFoG was provided by CEBA: ANR-10-LABX-25-01.
Data Availability Statement
The data presented in this study are available on request from the corresponding author to prevent data from being used by private companies to make a profit.
Acknowledgments
Permits ICMBIO n° 47960, 49369, registration CGen n° A16E4DA (Brazil), MMAYA/VMABCCGDF/DGBAP/MEG n° 0280/2016 (Bolivia), R.D. n° 001-2016-SERNANP-DGANP, R.D. n° 001A-2015-SERFOR-DGGSPFFS-DGSPF, and Contract n° 001-2016-SERFOR-DGGSPFFS-DGSPF (Peru) were granted for the collection, transport, and genetic analyses of samples. We are grateful to colleagues from IIAP, INRAE French Guiana, CIRAD Paracou Station, Office National des Forêts, and Thünen Institute for their assistance during field work and laboratory work. In Bolivia, samples were identified by Museo de Historia Natural Noel Kempff Mercado.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Degen, D.; Ward, S.E.; Lemes, M.R.; Navarro, C.; Cavers, S.; Sebbenn, A.M. Verifying the geographic origin of mahogany (Swietenia macrophylla King) with DNA-fingerprints. Forensic Sci. Int. Genet. 2013, 7, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.; Lin, C.; Ju, L. Tracking the geographical origin of timber by DNA fingerprinting: A study of the endangered species Cinnamomum kanehirae in Taiwan. Holzforschung 2017, 71, 853–862. [Google Scholar] [CrossRef]
- CITES. Comércio Internacional das Espécies da Flora e Fauna Selvagens em Perigo de Extinção. 2021. Available online: https://cites.org/esp/app/appendices.php (accessed on 1 June 2023).
- Lescuyer, G.; Ndotit, S.; Ndong, L.B.B.; Tsanga, R.; Cerutti, P.O. Policy Options for Improved Integration of Domestic Timber Markets under the Voluntary Partnership Agreement (VPA) Regime in Gabon; Center for International Forestry Research (CIFOR): Bogor, Indonesia, 2014. [Google Scholar]
- Federal Police Brazil. GOV. 2022. Available online: https://www.gov.br/pf/pt-br/search?SearchableText=madeira%20ilegal%20extra%C3%ADda%20da%20Amaz%C3%B4nia (accessed on 17 May 2022).
- Farias, E. Amazônia em Chamas: 90% da Madeira Exportada São Ilegais, Diz Polícia Federal. Amazônia Real, September 13. 2019. Available online: https://amazoniareal.com.br/amazonia-em-chamas-90-da-madeira-exportada-sao-ilegais-diz-policia-federal/67 (accessed on 31 July 2023).
- Perez Coelho, M.; Sanz, J.; Cabezudo, M. Analysis of volatile components of oak wood by solvent extraction and direct thermal desorption-gas chromatography-mass spectrometry. J. Chromat. 1997, 778, 427–434. [Google Scholar] [CrossRef]
- Deklerck, V.; Finch, K.; Gasson, P.; Van den Bulcke, J.; Van Acker, J.; Beeckman, H.; Espinoza, E. Comparison of species classification models of mass spectrometry data: Kernel discriminant analysis vs. random forest; A case study of Afrormosia (Pericopsis elata (Harms) Meeuwen). Rapid Commun. Mass Spectrom. 2017, 31, 1582–1588. [Google Scholar] [CrossRef]
- Kagawa, A.; Leavitt, S.W. Stable carbon isotopes of tree rings as a tool to pinpoint the geographic origin of timber. J. Wood Sci. 2010, 56, 175–183. [Google Scholar] [CrossRef]
- Bergo, M.C.J.; Pastore, T.C.M.; Coradin, V.T.R.; Wiedenhoeft, A.C.; Braga, J.W.B. NIRS identification of Swietenia macrophylla is robust across specimens from 27 countries. IAWA J. 2016, 37, 420–430. [Google Scholar] [CrossRef]
- Gasson, P.; Baas, P.; Wheeler, E. Wood anatomy of Cites-listed tree species. IAWA J. 2011, 32, 155–198. [Google Scholar] [CrossRef]
- Moya, R.; Wiemann, M.C.; Olivares, C. Identification of endangered or threatened Costa Rican tree species by wood anatomy and fluorescence activity. Rev. Biol. Trop. 2013, 61, 1113–1156. [Google Scholar]
- Tnah, L.H.; Lee, S.L.; Ng, K.K.S.; Tani, N.; Bhassu, S.; Othman, R.Y. Geographical traceability of an important tropical timber (Neobalanocarpus heimii) inferred from chloroplast DNA. For. Ecol. Manag. 2009, 258, 1918–1923. [Google Scholar] [CrossRef]
- Jolivet, C.; Degen, D. Use of DNA fingerprints to control the origin of sapelli timber (Entandrophragma cylindricum) at the forest concession level in Cameroon. Forensic Sci. Int. Genet. 2012, 6, 487–493. [Google Scholar] [CrossRef]
- Chaves, C.L.; Degen, B.; Pakull, B.; Mader, M.; Honorio, E.; Ruas, P.; Tysklind, N.; Sebbenn, A.M. Assessing the ability of chloroplast and nuclear DNA gene markers to verify the geographic origin of Jatoba (Hymenaea courbaril L.) timber. J. Hered. 2018, 109, 543–552. [Google Scholar] [CrossRef]
- Coronado, E.N.H.; Blanc-Jolivet, C.; Mader, M.; García-Dávila, C.R.; Gomero, D.A.; del Castillo, D.T.; Llampazo, G.F.; Pizango, G.H.; Sebbenn, A.M.; Meyer-Sand, B.R.V.; et al. SNP markers as a successful molecular tool for assessing species identity and geographic origin of trees in the economically important South American legume Genus. Dipteryx. J. Hered. 2020, 111, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Vlam, M.; de Groot, G.A.; Boom, A.; Copini, P.; Laros, I.; Veldhuijzen, K.; Zakamdi, D.; Zuidema, P.A. Developing forensic tools for an African timber: Regional origin is revealed by genetic characteristics, but not by isotopic signature. Biol. Conserv. 2018, 220, 262–271. [Google Scholar] [CrossRef]
- Abeele, S.V.; Hardy, O.J.; Beeckman, H.; Ilondea, B.A.; Janssens, S.B. Genetic markers for species conservation and timber tracking: Development of microsatellite primers for the Tropical African tree species Prioria balsamifera and Prioria oxyphylla. Forests 2019, 10, 1037. [Google Scholar] [CrossRef]
- Loureiro, A.A.; Silva, M.F.; Alencar, J.C. Essências Madeireiras da Amazonia; INPA: Bergen op Zoom, The Netherlands, 1979; Volume 1. [Google Scholar]
- Sampaio, P.T.B.; Barbosa, A.P.; Fernandes, N.P. Ensaio de espaçamento com caroba—Jacaranda copaia (AUBL.) D. Don. Bignonaceae. Acta Amaz. 1989, 9, 383. [Google Scholar] [CrossRef][Green Version]
- Gentry, A.H. Bignoniaceae—Part II (Tribe Tecomeae). Flora Neotrop. 1992, 25, 1–370. [Google Scholar]
- Maues, M.M.; Oliveira, P.E.A.M.; Kanashiro, M. Pollination biology in Jacaranda copaia (Aubl.) D. Don. (Bignoniaceae) at the “Floresta Nacional do Tapajós”, Central Amazon. Rev. Brasil. Bot. 2008, 31, 517–527. [Google Scholar] [CrossRef]
- James, T.; Veges, S.; Aldrich, P.; Hamrick, J.L. Mating systems of three tropical dry Forest tree species. Biotropica 1998, 30, 587–594. [Google Scholar] [CrossRef]
- Vinson, C.C.; Kanashiro, M.; Harris, S.A.; Boshier, D.H. Impacts of selective logging on inbreeding and gene flow in two Amazonian timber species with contrasting ecological and reproductive characteristics. Mol. Ecol. 2015, 24, 38–53. [Google Scholar] [CrossRef]
- Dumolin, S.; Demesure, B.; Petit, R.J. Inheritance of chloroplast and mitochondrial genomes in pediculate oak investigated with an efficient PCR method. Theor. Appl. Genet. 1995, 91, 1253–1256. [Google Scholar] [CrossRef]
- Sebbenn, A.M.; Blanc-Jolivet, C.; Mader, M.; Meyer-Sand, B.R.V.; Paredes-Villanueva, K.; Coronado, E.N.H.; Garcia-Davila, C.; Tysklind, N.; Troispoux, V.; Delcamp, A.; et al. Nuclear and plastidial SNP and INDEL markers for genetic tracking studies of Jacaranda copaia. Conserv. Genet. Resour. 2019, 11, 341–343. [Google Scholar] [CrossRef]
- Degen, B. Application. GDA-NT 2021—A computer program for population genetic data analysis and assignment. Conserv. Genet. Resour. 2022, 14, 347–350. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. CLUMPAK: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef]
- Loiselle, B.A.; Sork, V.L.; Nason, J.; Graham, C. Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae). Am. J. Bot. 1995, 82, 1420–1425. [Google Scholar] [CrossRef]
- Hardy, O.J.; Vekemans, X. SPAGeDI: A versatile computer program to analyses spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2002, 2, 618–620. [Google Scholar] [CrossRef]
- Rannala, B.; Mountain, J.L. Detecting immigration by using multilocus genotypes. Proc. Natl. Acad. Sci. USA 1997, 94, 9197–9201. [Google Scholar] [CrossRef]
- Piry, S.; Alapetite, A.; Cornuet, J.M.; Paetkau, D.; Baudouin, L.; Estoup, A. GENECLASS2: A software for genetic assignment and first-generation migrant detection. J. Hered. 2004, 95, 536–539. [Google Scholar] [CrossRef]
- Efron, B. Estimating the error rate of a prediction rule—Improvement on cross validation. J. Am. Stat. Assoc. 1983, 78, 316–331. [Google Scholar] [CrossRef]
- Cornuet, J.M.; Piry, S.; Luikart, G.; Estoup, A.; Solignac, M. New methods employing multilocus genotypes to select or exclude populations as origins of individuals. Genetics 1999, 153, 1989–2000. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Srivastava, A.; Hanur, V.S.; Rao, S.M. An effective wood DNA extraction protocol for three economic important timber species of India. Am. J. Plant Sci. 2018, 9, 139–149. [Google Scholar] [CrossRef][Green Version]
- Méndez-Cea, B.; Cobo-Simón, I.; Pérez-González, A.; García-García, I.; Linares, J.C.; Rodríguez, F.J.G. DNA extraction and amplification from Pinaceae dry wood. Silvae Genet. 2019, 68, 55–57. [Google Scholar] [CrossRef]
- Murillo-Sánchez, I.E.; López-Albarrán, P.; Santoyo-Pizano, G.; Martínez-Pacheco, M.M.; Velázquez-Becerra, C. Molecular identification of timber species from sawn timber and roundwood. Conserv. Genet. Resour. 2021, 13, 191–200. [Google Scholar] [CrossRef]
- Tysklind, N.; Blanc-Jolivet, C.; Mader, M.; Meyer-Sand, B.R.V.; Paredes-Villanueva, K.; Coronado, E.N.H.; Garcia-Davila, C.; Sebbenn, A.M.; Caron, K.; Troispoux, V.; et al. Development of nuclear and plastid SNP and INDEL markers for population genetic studies and timber traceability of Carapa species. Conserv. Genet. Resour. 2019, 11, 337–339. [Google Scholar] [CrossRef]
- Meyer-Sand, B.R.V.; Blanc-Jolivet, C.; Mader, M.; Paredes-Villanueva, K.; Tysklind, N.; Sebbenn, A.M.; Guichoux, E.; Degen, D. Development of a set of SNP markers for population genetics studies of Ipe (Handroanthus sp.), a valuable tree genus from Latin America. Conserv. Genet. Resour. 2018, 10, 779–781. [Google Scholar] [CrossRef]
- Tnah, L.H.; Lee, S.L.; Ng, K.K.S.; Faridah, Q.-Z.; Faridah-Hanum, I. Forensic DNA profiling of tropical timber species in Peninsular Malaysia. For. Ecol. Manag. 2010, 259, 1436–1446. [Google Scholar] [CrossRef]
- Ng, K.K.; Lee, S.L.; Tnah, L.H.; Nurul-Farhanah, Z.; Ng, C.H.; Lee, C.T.; Tani, N.; Diway, B.; Lai, P.S.; Khoo, E. Forensic timber identification: A case study of a cites listed species, Gonystylus bancanus (Thymelaeaceae). Forensic Sci. Int. Genet. 2016, 23, 197–209. [Google Scholar] [CrossRef]
- Ng, C.H.; Lee, S.L.; Tnah, L.H.; Ng, K.K.S.; Lee, C.T.; Diway, B.; Khoo, E. Geographic origin and individual assignment of Shorea platyclados (Dipterocarpaceae) for forensic identification. PLoS ONE 2017, 12, e0176158. [Google Scholar] [CrossRef]
- Ng, C.H.; Ng, K.K.S.; Lee, S.L.; Zakari, N.-F.; Lee, C.T.; Tnah, L.H. DNA databases of an important tropical timber tree species Shorea leprosula (Dipterocarpaceae) for forensic timber identification. Sci. Rep. 2022, 12, 9546. [Google Scholar] [CrossRef]
- Wahlund, S. Composition of populations and correlation appearances viewed in relation to the studies of inheritance. Hereditas 1928, 11, 65–106. [Google Scholar] [CrossRef]
- Scotti-Saintagne, C.; Dick, C.W.; Caron, H.; Vendramin, G.G.; Troispoux, V.; Sire, P.; Casalis, M.; Buonamici, A.; Valencia, R.; Lemes, M.R.; et al. Amazon diversification and cross-Andean dispersal of the widespread Neotropical tree species Jacaranda copaia (Bignoniaceae). J. Biogeogr. 2013, 40, 707–719. [Google Scholar] [CrossRef]
- Roman, G.; Gangitano, D.; Figueroa, A.; Solano, J.; Anabalón, L.; Houston, R. Use of Eucalyptus DNA profiling in a case of illegal logging. Sci. Justice 2020, 60, 487–494. [Google Scholar] [CrossRef]
- Huang, C.; Chu, F.H.; Huang, Y.-S.; Hung, Y.M.; Tseng, Y.-S.; Pu, C.-E.; Chao, C.-H.; Chou, S.-H.; Liu, S.-H.; You, Y.T.Y.; et al. Development and technical application of SSR-based individual identifcation system for Chamaecyparis taiwanensis against illegal logging convictions. Sci. Rep. 2020, 10, 22095. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Ding, X.; Feng, T.Y.; Chen, T.; Ye, J. Genetic diversity and population structure of Bursaphelenchus xylophilus in central China based on SNP Markers. Forests 2023, 14, 1443. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).