Fine Roots in Hemiboreal Forest Stands and Clearcut Areas with Nutrient-Rich Organic Soils in Latvia: Morphological Traits, Production and Carbon Input
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Fine Root Sampling
2.3. Determination of Fine Root Morphological Traits
2.4. Estimation of Fine Root Production and Carbon Input with Fine Root Litter
2.5. Soil Sampling and Analysis
2.6. Data Analysis
3. Results
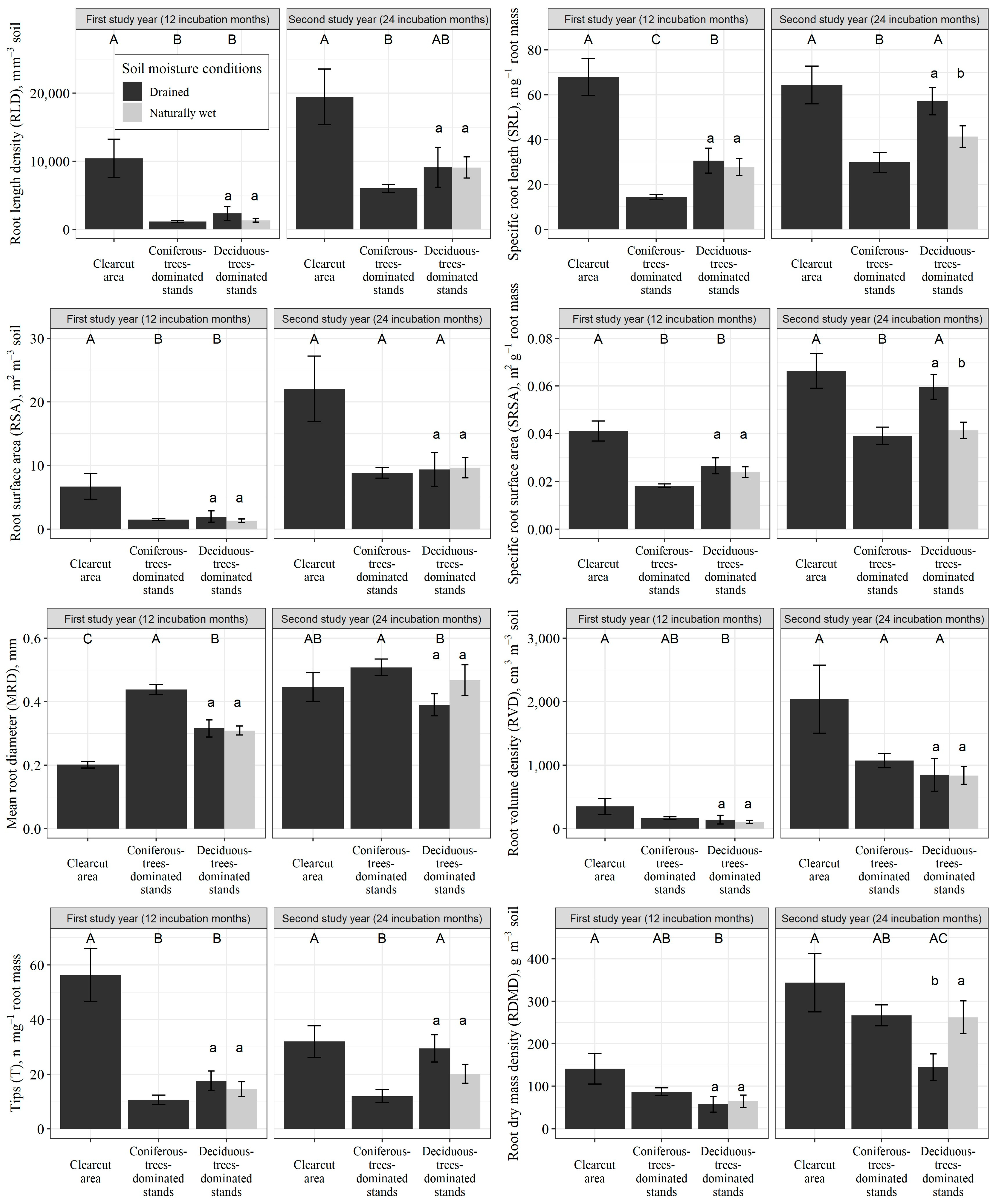
3.1. Morphological Traits of Fine Roots
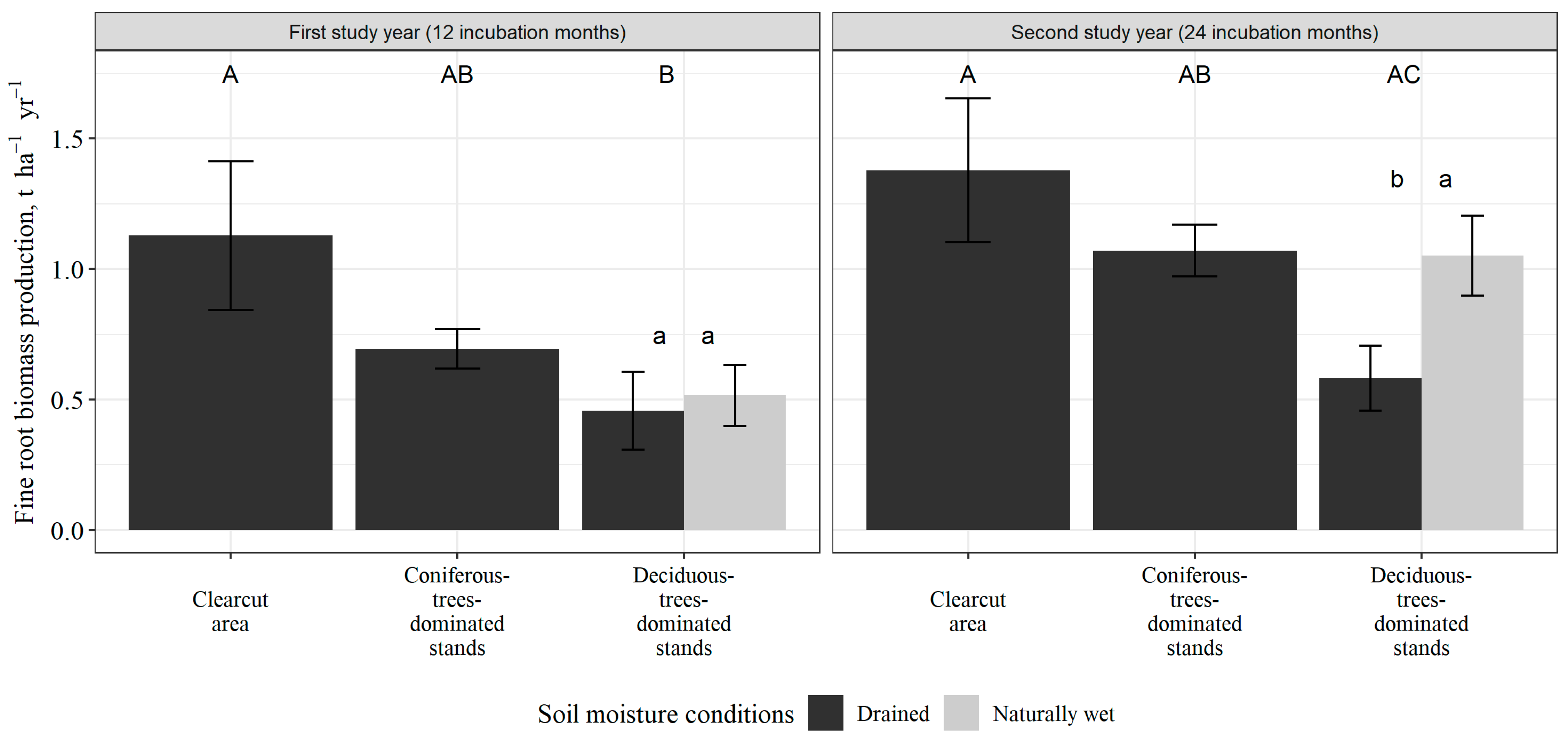
3.2. Fine Root Biomass Production
3.3. Carbon Input through Fine Root Litter
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Morphological Traits | Coniferous-Tree-Dominated Stands, Drained | Deciduous-Tree-Dominated Stands, Drained | Deciduous-Tree-Dominated Stands, Naturally Wet | Clearcut Area, Drained |
|---|---|---|---|---|
| Root length density | <0.001 | <0.001 | <0.001 | 0.068 |
| Specific root length | <0.001 | <0.001 | 0.017 | 0.894 |
| Root surface area | <0.001 | <0.001 | <0.001 | 0.010 |
| Specific root surface area | <0.001 | <0.001 | <0.001 | 0.005 |
| Average root diameter | 0.078 | 0.068 | 0.001 | <0.001 |
| Root volume density | <0.001 | <0.001 | <0.001 | 0.002 |
| Root dry mass density | <0.001 | 0.005 | <0.001 | 0.077 |
| Tips | 0.641 | 0.030 | 0.182 | 0.035 |
Appendix B
| Group of Study Sites | Variables of Soil General Chemistry, Unit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk Density, kg m3 | pH KCl | OC, g kg−1 | TN, g kg−1 | C/N ratio | P, g kg−1 | K, g kg−1 | Ca, g kg−1 | Mg, g kg−1 | |
| 0–20 cm soil layer (mean values were calculated from 0–10 cm and 10–20 cm soil layers) | |||||||||
| Coniferous-tree-dominated stands, drained | 240.9 ±29.0 | 4.2 ±0.1 | 478.8 ±19.9 | 23.6 ±1.0 | 20.5 ±0.6 | 0.93 ±0.08 | 0.35 ±0.03 | 17.1 ±1.4 | 1.56 ±0.17 |
| Deciduous-tree-dominated stands, drained | 285.4 ±47.1 | 5.5 ±0.2 | 365.8 ±40.6 | 24.9 ±2.9 | 14.8 ±0.4 | 1.31 ±0.14 | 0.81 ±0.13 | 27.5 ±3.7 | 2.52 ±0.32 |
| Deciduous-tree-dominated stands, naturally wet | 352.6 ±68.1 | 4.2 ±0.3 | 380.1 ±53.0 | 21.9 ±3.1 | 17.3 ±0.8 | 1.44 ±0.44 | 0.44 ±0.03 | 12.7 ±2.7 | 1.50 ±0.30 |
| Clearcut area, drained | 187.7 ±7.4 | 4.1 ±0.6 | 505.7 ±33.6 | 23.3 ±2.6 | 22.9 ±3.9 | 0.78 ±0.12 | 0.65 ±0.05 | 25.3 ±9.6 | 2.48 ±0.75 |
| 20–40 cm soil layer (mean values were calculated from 20–30 cm and 30–40 cm soil layers) | |||||||||
| Coniferous-tree-dominated stands, drained | 352.7 ±67.3 | 4.8 ±0.1 | 426.5 ±35.8 | 20.1 ±1.6 | 21.4 ±1.0 | 0.68 ±0.11 | 0.22 ±0.04 | 18.0 ±1.9 | 1.61 ±0.22 |
| Deciduous-tree-dominated stands, drained | 666.0 ±182.1 | 5.7 ±0.2 | 264.4 ±69.5 | 16.0 ±4.1 | 16.5 ±0.9 | 0.80 ±0.15 | 1.35 ±0.34 | 18.6 ±3.3 | 2.73 ±0.48 |
| Deciduous-tree-dominated stands, naturally wet | 516.4 ±152.0 | 4.8 ±0.6 | 351.8 ±71.6 | 17.8 ±3.6 | 18.7 ±1.4 | 1.19 ±0.39 | 0.29 ±0.04 | 15.0 ±3.0 | 1.54 ±0.25 |
| Clearcut area, drained | 189.9 ±7.5 | 4.3 ±0.6 | 477.7 ±33.4 | 27.2 ±8.5 | 22.9 ±5.9 | 0.47 ±0.05 | 0.34 ±0.02 | 24.3 ±9.4 | 2.51 ±0.75 |
| 40–80 cm soil layer (mean values were calculated from 40–50 cm, 50–60 cm, 60–70 cm, and 70–80 cm soil layers) | |||||||||
| Coniferous-tree-dominated stands, drained | 511.9 ±77.4 | 5.3 ±0.1 | 371.1 ±33.2 | 14.8 ±1.2 | 24.9 ±1.5 | 0.65 ±0.15 | 0.31 ±0.05 | 17.4 ±1.4 | 2.12 ±0.34 |
| Deciduous-tree-dominated stands, drained | 1032.8 ±151.1 | 6.1 ±0.2 | 159.2 ±49.8 | 8.9 ±2.8 | 22.8 ±3.2 | 0.53 ±0.09 | 1.35 ±0.24 | 14.5 ±2.2 | 4.57 ±0.81 |
| Deciduous-tree-dominated stands, naturally wet | 720.4 ±124.7 | 5.2 ±0.1 | 260.7 ±54.1 | 13.0 ±2.7 | 16.8 ±1.1 | 0.43 ±0.06 | 0.38 ±0.07 | 11.8 ±2.2 | 1.51 ±0.19 |
| Clearcut area, drained | 492.3 ±182.3 | 5.3 ±0.3 | 360.6 ±83.8 | 16.7 ±5.1 | 21.9 ±4.0 | 0.50 ±0.09 | 0.42 ±0.08 | 20.9 ±4.5 | 2.96 ±0.48 |
Appendix C
| Soil Layer, cm | Variables of Soil General Chemistry | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Soil Bulk Density | pH KCl | OC | TN | C/N Ratio | P | K | Ca | Mg | |
| 0–10 | 0.066 | −0.307 | 0.097 | −0.208 | 0.424 * | −0.398 | −0.254 | −0.289 | −0.243 |
| 10–20 | 0.234 | −0.553 ** | −0.114 | −0.417 * | 0.413 * | −0.338 | −0.218 | −0.479 * | −0.567 ** |
| 20–30 | 0.201 | −0.743 *** | −0.040 | −0.315 | 0.482 * | −0.210 | −0.260 | −0.510 * | −0.552 ** |
| 30–40 | 0.062 | −0.705 *** | −0.003 | −0.321 | 0.569 ** | −0.236 | −0.381 | −0.417 * | −0.406 |
| 40–50 | 0.144 | −0.157 | −0.096 | −0.312 | 0.096 | −0.157 | −0.073 | −0.232 | −0.117 |
| 50–60 | 0.086 | −0.184 | −0.039 | −0.192 | 0.006 | −0.159 | −0.078 | −0.151 | −0.102 |
| 60–70 | 0.030 | −0.059 | 0.036 | −0.117 | 0.115 | −0.140 | −0.090 | −0.045 | −0.043 |
| 70–80 | −0.001 | −0.098 | 0.042 | −0.099 | −0.011 | −0.025 | −0.059 | −0.043 | −0.092 |
References
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Chen, H.Y.H. Global responses of fine root biomass and traits to plant species mixtures in terrestrial ecosystems. Global Ecol. Biogeogr. 2020, 30, 2s89–304. [Google Scholar] [CrossRef]
- Huang, L.; Xia, Z.; Cao, Y. A Bibliometric Analysis of Global Fine Roots Research in Forest Ecosystems during 1992–2020. Forests 2022, 13, 93. [Google Scholar] [CrossRef]
- Saha, S.; Huang, L.; Khoso, M.A.; Wu, H.; Han, D.; Ma, X.; Poudel, T.R.; Li, B.; Zhu, M.; Lan, Q.; et al. Fine root decomposition in forest ecosystems: An ecological perspective. Front. Plant Sci. 2023, 14, 1277510. [Google Scholar] [CrossRef]
- Lampela, M.; Minkkinen, K.; Straková, P.; Bhuiyan, R.; He, W.; Mäkiranta, P.; Ojanen, P.; Penttilä, T.; Laiho, R. Responses of fine-root biomass and production to drying depend on wetness and site nutrient regime in boreal forested peatland. Front. For. Glob. Chang. 2023, 6, 1190893. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.-D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef]
- Ding, Y.; Leppälammi-Kujansuu, J.; Helmisaari, H.-S. Fine root longevity and below- and aboveground litter production in a boreal Betula pendula forest. For. Ecol. Manag. 2019, 431, 17–25. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in boreal Forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Abiotic and biotic factors controlling fine root biomass, carbon and nutrients in closed-canopy hybrid poplar stands on post-agricultural land. Sci. Rep. 2019, 9, 6296. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, N.; Yu, K.; Zhao, C. The Effects of Fine Roots and Arbuscular Mycorrhizal Fungi on Soil Macropores. Soil Tillage Res. 2023, 225, 105528. [Google Scholar] [CrossRef]
- Freschet, G.T.; Roumet, C. Sampling roots to capture plant and soil functions. Funct. Ecol. 2017, 31, 1506–1518. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhao, J.; Gong, L. The morphological and chemical properties of fine roots respond to nitrogen addition in a temperate Schrenk’s spruce (Picea schrenkiana) forest. Sci. Rep. 2021, 11, 3839. [Google Scholar] [CrossRef]
- Tan, J.; Yu, W.; Liu, Y.; Guo, Y.; Liu, N.; Fu, H.; Di, N.; Duan, J.; Li, X.; Xi, B. Response of Fine-Root Traits of Populus tomentosa to Drought in Shallow and Deep Soil. Forests 2023, 14, 951. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, M.; Dao, J.; Lin, L.; Chen, C.; Chen, Y.; Wang, Z. Fine-Root Morphology of Woody and Herbaceous Plants Responds Differently to Altered Precipitation: A Meta-Analysis. For. Ecol. Manag. 2024, 552, 121570. [Google Scholar] [CrossRef]
- Kwatcho Kengdo, S.; Peršoh, D.; Schindlbacher, A.; Heinzle, J.; Tian, Y.; Wanek, W.; Borken, W. Long-term soil warming alters fine root dynamics and morphology, and their ectomycorrhizal fungal community in a temperate forest soil. Glob. Chang. Biol. 2022, 28, 1803–1819. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, M.; Shao, J.; Zhou, G.; Liu, R.; Zhou, L.; Liu, H.; He, Y.; Chen, Y.; Zhou, X. Fine Root Trait-Function Relationships Affected by Mycorrhizal Type and Climate. Geoderma 2021, 394, 115011. [Google Scholar] [CrossRef]
- Fujita, S.; Noguchi, K.; Tange, T. Different Waterlogging Depths Affect Spatial Distribution of Fine Root Growth for Pinus thunbergii Seedlings. Front. Plant Sci. 2021, 12, 614764. [Google Scholar] [CrossRef] [PubMed]
- Vogt, K.A.; Vogt, D.J.; Palmiotto, P.A.; Boon, B.; ÓHara, J.; Asbjornsen, H. Review of Root Dynamics in Forest Ecosystems Grouped by Climate, Climatic Forest Type, and Species. Plant Soil 1996, 187, 159–219. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A Global Analysis of Root Distributions for Terrestrial Biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- Leuschner, C.; Hertel, D. Fine root biomass of temperate forests in relation to soil acidity and fertility, climate, age, and species. Prog. Bot. 2003, 64, 405–438. [Google Scholar]
- Finér, L.; Helmisaari, H.S.; Lõhmus, K.; Majdi, H.; Brunner, I.; Børja, I.; Eldhuset, E.; Godbold, D.; Grebenc, T.; Konopka, B.; et al. Variation in fine root biomass of three European tree species: Beech (Fagus sylvatica L.), Norway spruce (Picea abies L. Karst.), and Scots pine (Pinus sylvestris L.). Plant Biosyst. 2007, 141, 394–405. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Zhang, B.; Wu, D.; Zhu, D.; Zhang, W.; Ye, Q.; Yan, J.; Fu, J.; Fang, C.; et al. Nitrogen deposition and increased precipitation interact to affect fine root production and biomass in a temperate forest: Implications for carbon cycling. Sci. Total Environ. 2021, 765, 144497. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, R.; Minkkinen, K.; Helmisaari, H.S.; Ojanen, P.; Penttilä, T.; Laiho, R. Estimating fine-root production by tree species and understorey functional groups in two contrasting peatland forests. Plant Soil 2017, 412, 299–316. [Google Scholar] [CrossRef]
- Du, H.; Liu, L.; Su, L.; Zeng, F.; Wang, K.; Peng, W.; Zhang, H.; Song, T. Seasonal changes and vertical distribution of fine root biomass during vegetation restoration in a karst area, Southwest China. Front. Plant Sci. 2019, 9, 2001. [Google Scholar] [CrossRef] [PubMed]
- Konôpka, B. Differences in Fine Root Traits between Norway Spruce (Picea abies [L.] Karst.) and European Beech (Fagus sylvatica L.)—A Case Study in the Kysucké Beskydy Mts. J. For. Sci. 2009, 55, 556–566. [Google Scholar] [CrossRef]
- Jaeger, F.C.; Handa, I.T.; Paquette, A.; Parker, W.C.; Messier, C. Young temperate tree species show different fine root acclimation capacity to growing season water availability. Plant Soil 2024, 496, 485–504. [Google Scholar] [CrossRef]
- Vanninen, P.H.; Ylitalo, H.; Sievänen, R.; Mäkelä, A. Effects of age and site quality on the distribution of biomass in Scots pine (Pinus sylvestris L.). Trees 1996, 10, 231–238. [Google Scholar] [CrossRef]
- Helmisaari, H.S.; Makkonen, K.; Kellomäki, S.; Valtonen, E.; Mälkönen, E. Below- and above-ground biomass, production and nitrogen use in Scots pine stand in eastern Finland. Forest Ecol. Manag. 2002, 165, 317–326. [Google Scholar] [CrossRef]
- Claus, A.; George, E. Effect of stand age on fine-root biomass and biomass distribution in three European forest chronosequences. Can. J. Forest Res. 2005, 35, 1617–1625. [Google Scholar] [CrossRef]
- Olesinski, J.; Lavigne, M.B.; Kershaw, J.A.; Krasowski, M.J. Fine-root dynamics change during stand development and in response to thinning in balsam fir (Abies balsamea L. Mill.) forests. Forest Ecol. Manag. 2012, 286, 48–58. [Google Scholar] [CrossRef]
- Børja, I.; de Wit, H.A.; Steffenrem, A.; Majdi, H. Stand age and fine root biomass, distribution, and morphology in a Norway spruce chronosequence in southeast Norway. Tree Physiol. 2008, 28, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, R.L.; Pregitzer, K.S. The dynamics of fine root length, biomass, and nitrogen content in two northern hardwood ecosystems. Can. J. For. Res. 1993, 23, 2507–2520. [Google Scholar] [CrossRef]
- Jentschke, G.; Drexhage, M.; Fritz, H.W.; Fritz, E.; Schella, B.; Lee, D.H.; Gruber, F.; Heimann, J.; Kuhr, M.; Schmidt, J.; et al. Does Soil Acidity Reduce Subsoil Rooting in Norway Spruce (Picea abies)? Plants 2001, 237, 91–108. [Google Scholar] [CrossRef]
- Godbold, D.L.; Fritz, H.-W.; Jentschke, G.; Meesenburg, H.; Rademacher, P. Root Turnover and Root Necromass Accumulation of Norway Spruce (Picea abies) Are Affected by Soil Acidity. Tree Physiol. 2003, 23, 915–921. [Google Scholar] [CrossRef]
- Świątek, B.; Pietrzykowski, M. Soil Factors Determining the Fine-Root Biomass in Soil Regeneration after a Post-Fire and Soil Reconstruction in Reclaimed Post-Mining Sites under Different Tree Species. Catena 2021, 204, 105449. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. TAMM Review: Continuous Root Forestry—Living Roots Sustain the Belowground Ecosystem and Soil Carbon in Managed Forests. Forest Ecol. Manag. 2023, 532, 120848. [Google Scholar] [CrossRef]
- James, J.; Harrison, R. The Effect of Harvest on Forest Soil Carbon: A Meta-Analysis. Forests 2016, 7, 308. [Google Scholar] [CrossRef]
- Vårdal, M. Effects of Forest Management History on Fine Roots and Mycorrhizal Colonization in Norwegian Boreal Spruce Forests. Master’s Thesis, Faculty of Environmental Sciences and Natural Resource Management, Norwegian University of Life Sciences, Ås, Norway, 10 May 2023. [Google Scholar]
- Martinović, T.; Kohout, P.; López-Mondéjar, R.; Algora Gallardo, C.; Starke, R.; Tomšovský, M.; Baldrian, P. Bacterial Community in Soil and Tree Roots of Picea abies Shows Little Response to Clearcutting. FEMS Microbiol. Ecol. 2022, 98, fiac118. [Google Scholar] [CrossRef]
- Welke, S.E.; Hope, G.D.; Hunt, G.A. Effects of harvesting on fine root biomass and decomposition in an Engelmann spruce–subalpine fir forest. Can. J. For. Res. 2003, 33, 847–853. [Google Scholar] [CrossRef]
- Bardule, A.; Polmanis, K.; Krumšteds, L.L.; Bardulis, A.; Lazdinš, A. Fine root morphological traits and production in coniferous- and deciduous-tree forests with drained and naturally wet nutrient-rich organic soils in hemiboreal Latvia. iForest 2023, 16, 165–173. [Google Scholar] [CrossRef]
- Bušs, K. Forest Ecology and Typology; Zinatne: Riga, Latvia, 1981; 68p. [Google Scholar]
- Kārkliņš, A.; Gemste, I.; Mežals, H.; Nikodemus, O.; Skujāns, R. Latvijas Augšņu Noteicējs; Latvia University of Agriculture: Jelgava, Latvia, 2009; 240p. [Google Scholar]
- LVGMC Klimata Portals. Available online: https://klimats.meteo.lv/ (accessed on 30 May 2024).
- Raich, J.W.; Riley, R.H.; Vitousek, P.M. Use of root-ingrowth cores to assess nutrient limitations in forest ecosystems. Can. J. For. Res. 1994, 24, 2135–2138. [Google Scholar] [CrossRef]
- Majdi, H. Root sampling methods—Applications and limitations of the minirhizotron technique. Plant Soil 1996, 185, 255–258. [Google Scholar] [CrossRef]
- Vogt, K.A.; Vogt, D.J.; Bloomfield, J. Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant Soil 1998, 200, 71–89. [Google Scholar] [CrossRef]
- Liu, L.; Fan, Y.; Wang, Y.; Shen, X.; Janssens, I.; Guenet, B.; Xiao, C. Fine-root turnover, litterfall and soil microbial community of three mixed coniferous-deciduous forests dominated by Korean pine (Pinus koraiensis) along a latitudinal gradient. Front. Plant Sci. 2019, 10, 317. [Google Scholar] [CrossRef]
- Byrne, K.M. Technical Note: A Rapid Method to Estimate Root Production in Grasslands, Shrublands, and Forests. Rangeland Ecol. Manag. 2021, 76, 74–77. [Google Scholar] [CrossRef]
- Norby, R.J.; Ledford, J.; Reilly, C.D.; Miller, N.E.; O’Neill, E.G. Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. Proc. Natl. Acad. Sci. USA 2004, 101, 9689–9693. [Google Scholar] [CrossRef]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. Forest Ecol. Manag. 2011, 262, 2008–2023. [Google Scholar] [CrossRef]
- Laiho, R.; Bhuiyan, R.; Straková, P.; Mäkiranta, P.; Badorek, T.; Penttilä, T. Modified ingrowth core method plus infrared calibration models for estimating fine root production in peatlands. Plant Soil 2014, 385, 311–327. [Google Scholar] [CrossRef]
- Leppälammi-Kujansuu, J.; Aro, L.; Salemaa, M.; Hansson, K.; Kleja, D.B.; Helmisaari, H.S. Fine root longevity and carbon input into soil from below- and aboveground litter in climatically contrasting forests. Forest Ecol. Manag. 2014, 326, 79–90. [Google Scholar] [CrossRef]
- LVS ISO 10694:2006; Soil Qualitsy–Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). Latvian Standards: Riga, Latvia, 2006.
- LVS ISO 11464:2005; Soil Quality–Pretreatment of Samples for Physic–Chemical Analysis. Latvian Standards: Riga, Latvia, 2005.
- LVS ISO 11272:2017; Soil Quality—Determination of Dry Bulk Denssity. Latvian Standards: Riga, Latvia, 2017.
- LVS EN ISO 10390:2022; Soil, Treated Biowaste and Sludge–Determination of pH (ISO 10390:2021). Latvian Standards: Riga, Latvia, 2022.
- LVS ISO 13878:1998; Soil Quality. Determination of Total Nitrogen Content by Dry Combustion (“Elemental Analysis”). Latvian Standards: Riga, Latvia, 1999.
- Huo, C.; Gu, J.; Yu, L.; Zhang, X.; Xu, Y.; Liang, C.; Wang, Q.; Zhang, X. Temporal Dynamics of Fine Root Production, Mortality and Turnover Deviate Across Branch Orders in a Larch Stand. Oecologia 2022, 199, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Majasalmi, T.; Rautiainen, M. The Impact of Tree Canopy Structure on Understory Variation in a Boreal Forest. For. Ecol. Manag. 2020, 466, 118100. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L. Population Biology of Plants; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Kotowski, W.; van Andel, J.; van Diggelen, R.; Hogendorf, J. Responses of fen plant species to groundwater level and light intensity. Plant Ecol. 2001, 155, 147–156. [Google Scholar] [CrossRef]
- Sakamaki, Y.; Ino, Y. Influence of shade timing on an Equisetum arvense L. population. Ecol. Res. 2002, 17, 673–686. [Google Scholar]
- Butlers, A.; Laiho, R.; Soosaar, K.; Jauhiainen, J.; Schindler, T.; Bārdule, A.; Kamil-Sardar, M.; Haberl, A.; Samariks, V.; Vahter, H.; et al. Soil and forest floor carbon balance in drained and undrained hemiboreal peatland forests. EGUsphere 2024, preprint. [Google Scholar]
- Murphy, M.; Laiho, R.; Moore, T. Effects of Water Table Drawdown on Root Production and Aboveground Biomass in a Boreal Bog. Ecosystems 2009, 12, 1268–1282. [Google Scholar] [CrossRef]
- Samariks, V.; Ķēniņa, L.; Kitenberga, M.; Aun, K.; Varik, M.; Liepiņa, A.A.; Jansons, Ā. The effect of drainage on fine-root biomass, production, and turnover in hemiboreal old-growth forests on organic soils. Ecosphere 2024, 15, e4849. [Google Scholar] [CrossRef]
- Neumann, M.; Godbold, D.L.; Hirano, Y.; Finér, L. Improving models of fine root carbon stocks and fluxes in European forests. J. Ecol. 2020, 108, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, K.; Helmisaari, H.S. Fine root biomass and production in Scots pine stands in relation to stand age. Tree Physiol. 2001, 21, 193–198. [Google Scholar] [CrossRef]
- Kriiska, K.; Frey, J.; Asi, E.; Kabral, N.; Uri, V.; Aosaar, J.; Varik, M.; Napa, Ü.; Apuhtin, V.; Timmusk, T.; et al. Variation in Annual Carbon Fluxes Affecting the SOC Pool in Hemiboreal Coniferous Forests in Estonia. For. Ecol. Manag. 2019, 433, 419–430. [Google Scholar] [CrossRef]
- Weemstra, M.; Sterck, F.J.; Visser, E.J.W.; Kuyper, T.W.; Goudzwaard, L.; Mommer, L. Fine-root trait plasticity of beech (Fagus sylvatica) and spruce (Picea abies) forests on two contrasting soils. Plant Soil 2017, 415, 175–188. [Google Scholar] [CrossRef]
- Cools, N.; Vesterdal, L.; De Vos, B.; Vanguelova, E.; Hansen, K. Tree species is the major factor explaining C ratios in European forest soils. Forest Ecol. Manag. 2014, 311, 3–16. [Google Scholar] [CrossRef]
- Gao, W.; Chen, D.; Hu, X.; Fang, X.; Li, Q.; Huang, Q.; Sun, F.; Zhou, J.; Bai, Y.; Zhang, J.; et al. Nitrogen deposition drives the intricate changes of fine root traits. Global Ecol. Conserv. 2023, 43, e024432023. [Google Scholar] [CrossRef]
- Gower, S.T.; Vogt, K.A.; Grier, C.C. Carbon dynamics of Rocky Mountain Douglas-fir: Influence of water and nutrient availability. Ecol. Monogr. 1992, 62, 43–65. [Google Scholar] [CrossRef]
- Peng, Y.F.; Guo, D.L.; Yang, Y.H. Global patterns of root dynamics under nitrogen enrichment. Glob. Ecol. Biogeogr. 2016, 26, 102–114. [Google Scholar] [CrossRef]
- He, W.; Mäkiranta, P.; Straková, P.; Ojanen, P.; Penttilä, T.; Bhuiyan, R.; Minkkinen, K.; Laiho, R. Fine-Root Production in Boreal Peatland Forests: Effects of Stand and Environmental Factors. For. Ecol. Manag. 2023, 550, 121503. [Google Scholar] [CrossRef]
- Janssens, I.; Dieleman, W.; Luyssaert, S.; Subke, J.-A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nature Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y. A global analysis of fine root production as affected by soil nitrogen and phosphorus. Proc. Biol. Sci. 2012, 279, 3796–3802. [Google Scholar] [CrossRef]
- Lehtonen, A.; Palviainen, M.; Ojanen, P.; Kalliokoski, T.; Nöjd, P.; Kukkola, M.; Penttilä, T.; Mäkipää, R.; Leppälämi-Kujansuu, J.; Helmisaari, H.S. Modelling fine root biomass of boreal tree stands using site and stand variables. For. Ecol. Manag. 2016, 359, 361–369. [Google Scholar] [CrossRef]
- Ding, Y.; Leppälammi-Kujansuu, J.; Salemaa, M.; Schiestl-Aalto, P.; Kulmala, L.; Ukonmaanaho, L.; Nöjd, P.; Minkkinen, K.; Makita, N.; Železnik, P.; et al. Distinct patterns of below- and aboveground growth phenology and litter carbon inputs along a boreal site type gradient. For. Ecol. Manag. 2021, 489, 119081. [Google Scholar] [CrossRef]
- Ostonen, I.; Lõhmus, K.; Helmisaari, H.S.; Truu, J.; Meel, S. Fine root morphological adaptations in Scots pine, Norway spruce and silver birch along a latitudinal gradient in boreal forests. Tree Physiol. 2007, 27, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Ostonen, I.; Lõhmus, K.; Alama, S.; Truu, J.; Kaar, E.; Vares, A.; Uri, V.; Kurvits, V. Morphological adaptations of fine roots in Scots pine (Pinus sylvestris L.), silver birch (Betula pendula Roth.) and black alder (Alnus glutinosa (L.) Gaertn.) stands in recultivated areas of oil shale mining and semicoke hills. Oil Shale 2006, 23, 187–202. [Google Scholar] [CrossRef]
- Isaac, M.E.; Martin, A.R.; de Melo Virginio Filho, E.; Rapidel, B.; Roupsard, O.; Van den Meersche, K. Intraspecific trait variation and coordination: Root and leaf economics spectra in coffee across environmental gradients. Front. Plant Sci. 2017, 8, 1196. [Google Scholar] [CrossRef]
- Rezapour, A.; Truu, M.; Maddison, M.; Rohula-Okunev, G.; Tullus, A.; Uri, V.; Mander, Ü.; Ostonen, I. Morphological Variation in Absorptive Roots in Downy Birch (Betula pubescens) and Norway Spruce (Picea abies) Forests Growing on Drained Peat Soils. Forests 2022, 13, 112. [Google Scholar] [CrossRef]
- Brunner, I.; Pannatier, E.G.; Frey, B.; Rigling, A.; Landolt, W.; Zimmermann, S.; Dobbertin, M. Morphological and physiological responses of Scots pine fine roots to water supply in a dry climatic region in Switzerland. Tree Physiol. 2009, 29, 541–550. [Google Scholar] [CrossRef]
- Schwieger, S.; Blume-Werry, G.; Ciesiolka, F.; Anadon-Rosell, A. Root biomass and root traits of Alnus glutinosa show size-dependent and opposite patterns in a drained and a rewetted forest peatland. Ann. Bot. 2021, 127, 337–346. [Google Scholar] [CrossRef]
- Frymark-Szymkowiak, A.; Kieliszewska-Rokicka, B. The Fine Root Distribution and Morphology of Mature White Poplar in Natural Temperate Riverside Forests under Periodically Flooded or Dry Hydrological Conditions. Forests 2023, 14, 223. [Google Scholar] [CrossRef]



| Dominant Tree Species | Soil Drainage Status | Value | Forest Stand Parameters, Unit | |||||
|---|---|---|---|---|---|---|---|---|
| Stand Age, years | Basal Area, m2 ha−1 | Tree Density, Tree Number ha | Growing Stock, m3 ha−1 | Tree Diameter at Breast Height, cm | Tree Height, m | |||
| Deciduous (n = 13) including silver birch (n = 7) and black alder (n = 6) | drained (n = 6) | mean | 38 | 20.0 | 1523 | 172 | 16.6 | 16.4 |
| S.E. | 7 | 3.3 | 353 | 38 | 1.7 | 1.7 | ||
| median | 34 | 18.8 | 1780 | 141 | 17.2 | 16.5 | ||
| range | 19–62 | 11.0–33.7 | 340–2660 | 58–307 | 10.3–21.8 | 10.0–22.0 | ||
| naturally wet (n = 7) | mean | 44 | 24.1 | 3700 | 272 | 12.9 | 14.1 | |
| S.E. | 10 | 5.3 | 1456 | 104 | 2.9 | 2.8 | ||
| median | 56 | 27.9 | 2000 | 247 | 13.0 | 15.0 | ||
| range | 10–73 | 2.5–39.9 | 1160–11,700 | 8–809 | 3.3–23.0 | 4.6–25.6 | ||
| Coniferous (n = 10), Norway spruce (n = 10) | drained (n = 10) | mean | 48 | 24.1 | 912 | 264 | 19.2 | 18.3 |
| S.E. | 6 | 3.3 | 120 | 40 | 1.8 | 1.8 | ||
| median | 50 | 27.4 | 780 | 291 | 20.3 | 20.5 | ||
| range | 10–74 | 2.0–38.5 | 362–1700 | 7–462 | 4.2–25.4 | 3.2–23.0 | ||
| Group | C Concentration, g kg−1 | N Concentration, g kg−1 | C/N Ratio |
|---|---|---|---|
| Coniferous-tree-dominated stands, drained | 474.3 ± 2.1 b | 15.8 ± 0.6 a | 30.7 ± 1.6 a |
| Deciduous-tree-dominated stands, drained | 481.3 ± 2.3 b | 18.9 ± 1.2 a | 26.0 ± 1.8 a |
| Deciduous-tree-dominated stands, naturally wet | 503.0 ± 4.0 a | 19.0 ± 1.3 a | 27.3 ± 1.9 a |
| Clearcut area, drained | 490.5 ± 6.0 ab | 19.0 ± 1.6 a | 26.7 ± 2.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazdiņš, A.; Petaja, G.; Bārdule, A.; Polmanis, K.; Kalēja, S.; Maliarenko, O.; Melnik, N. Fine Roots in Hemiboreal Forest Stands and Clearcut Areas with Nutrient-Rich Organic Soils in Latvia: Morphological Traits, Production and Carbon Input. Forests 2024, 15, 1500. https://doi.org/10.3390/f15091500
Lazdiņš A, Petaja G, Bārdule A, Polmanis K, Kalēja S, Maliarenko O, Melnik N. Fine Roots in Hemiboreal Forest Stands and Clearcut Areas with Nutrient-Rich Organic Soils in Latvia: Morphological Traits, Production and Carbon Input. Forests. 2024; 15(9):1500. https://doi.org/10.3390/f15091500
Chicago/Turabian StyleLazdiņš, Andis, Guna Petaja, Arta Bārdule, Kaspars Polmanis, Santa Kalēja, Oksana Maliarenko, and Nadiia Melnik. 2024. "Fine Roots in Hemiboreal Forest Stands and Clearcut Areas with Nutrient-Rich Organic Soils in Latvia: Morphological Traits, Production and Carbon Input" Forests 15, no. 9: 1500. https://doi.org/10.3390/f15091500





