Vitality and Inhibition Parameters in the Analysis of Dual Fungal Cultures as an Effective Tool in the Bio-Protection of Forest Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculation
2.2. Calibration of the Pathogen Growth in Monocultures
2.3. The Analyses of H. fraxineus Growth Inhibition in Dual Cultures
2.4. Software
3. Results
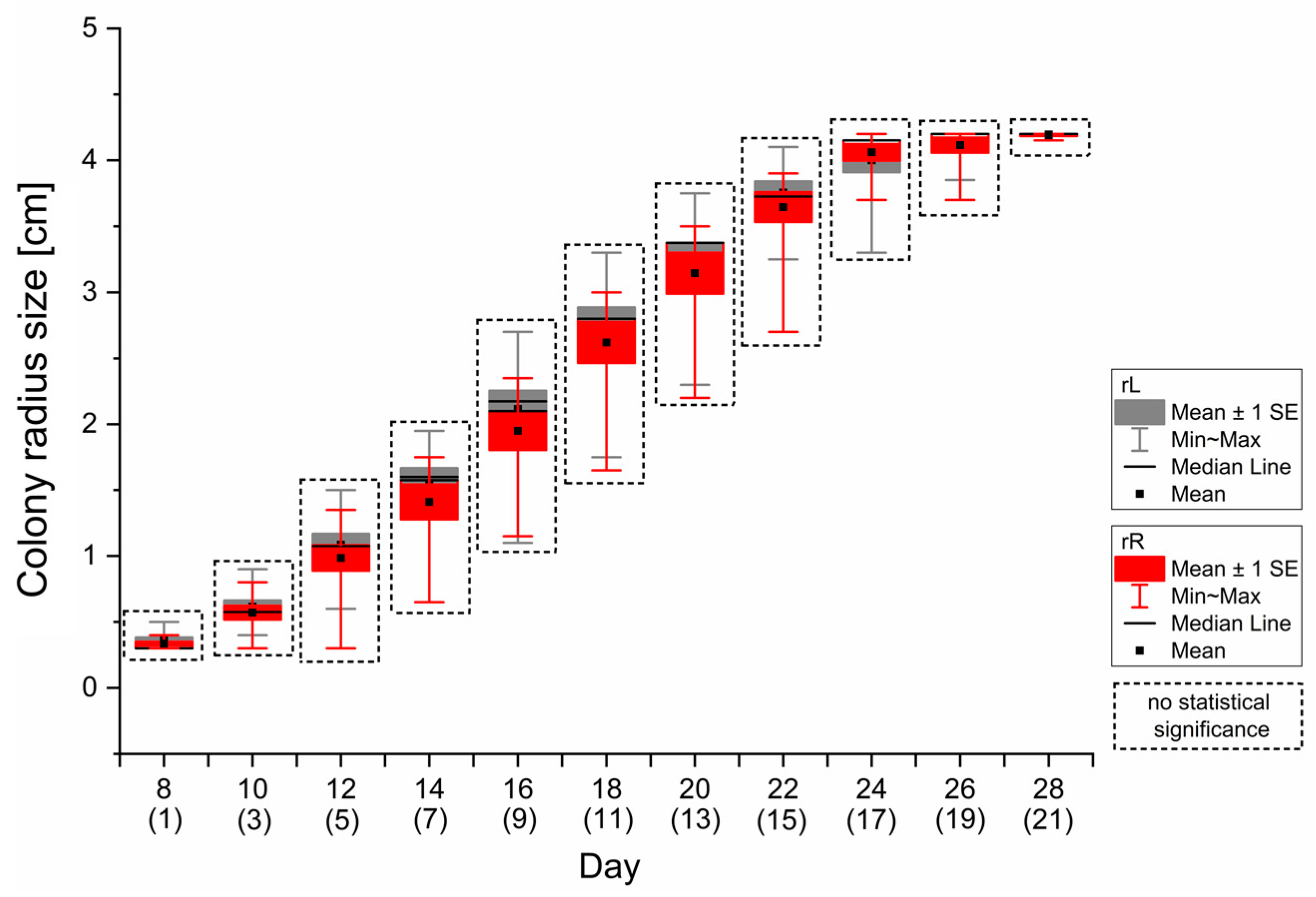
3.1. Pathogen’s Growth Measurement Calibration
3.2. Vitality Parameter
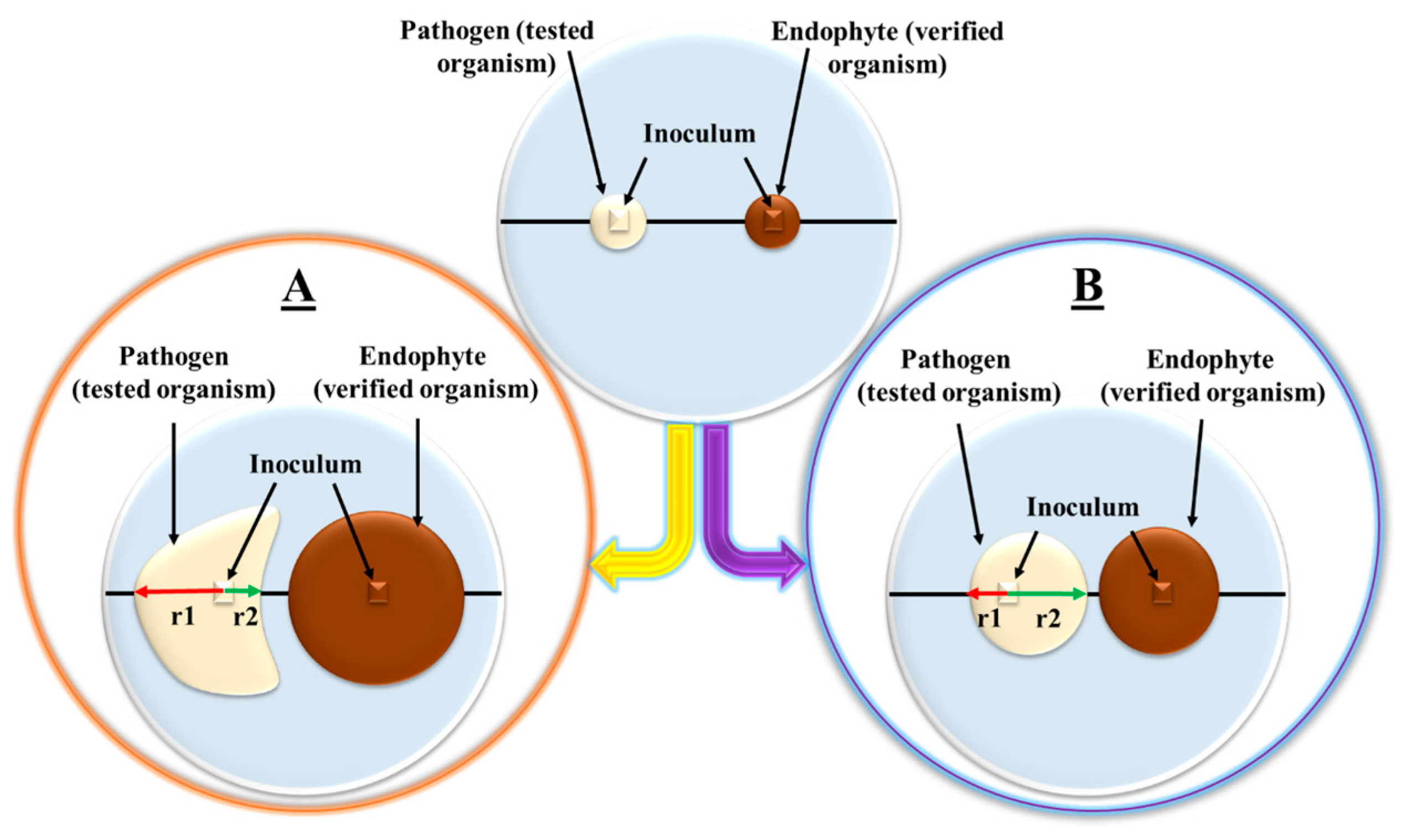
3.3. Inhibition Parameter as a Summary Factor in the Interaction between Organisms in Dual Cultures
4. Discussion
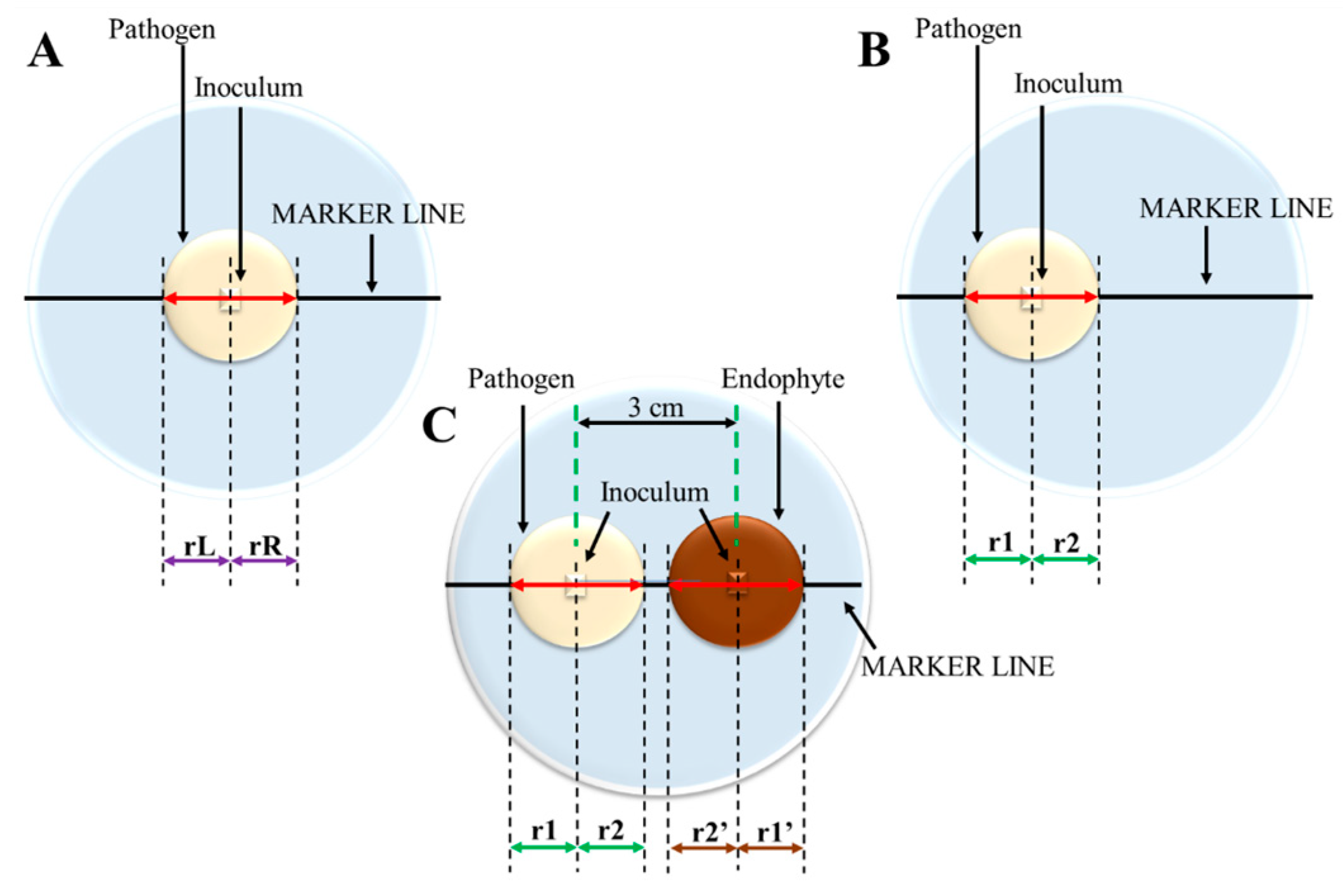
- Inocula in dual cultures should be placed at the same distance from each other as from the plate edge to ensure that fungus will have the same distance to both physical barriers. The distances are strongly dependent on the Petri dish diameter; therefore, the calibration control must be carried out on a plate with exactly the same parameters as the dual cultures.
- Asymmetrical monoculture (calibration variant 2 shown in Figure 1B) is essential to properly analyze data from dual-culture experiments.
- In calibration monocultures, fungal inocula must be inoculated at the same distance from the plate edge as fungi in the dual-culture experiments to determine the end time of measurements in co-cultures.
- In dual-culture experiments, radii measurements should be terminated at the last day with unaffected mycelial growth in both directions in calibration monocultures—in H. fraxineus growth studies, using the proposed scheme, it was the 16th day from the start of pathogen cultivation, i.e., the 9th day of the potential dual-culture experiment.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmermann, V.; Børja, I.; Hietala, A.M.; Kirisits, T.; Solheim, H. Ash Dieback: Pathogen Spread and Diurnal Patterns of Ascospore Dispersal, with Special Emphasis on Norway. EPPO Bull. 2011, 41, 14–20. [Google Scholar] [CrossRef]
- Brasier, C.M. Ophiostoma Novo-Ulmi Sp. Nov., Causative Agent of Current Dutch Elm Disease Pandemics. Mycopathologia 1991, 115, 151–161. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Meijer, A.; Abel, J.; Colinas, C.; Aletà, N.; Guàrdia, M. Evaluation of Chestnut Susceptibility to Cryphonectria parasitica: Screening under Controlled Conditions. Agriculture 2021, 11, 1158. [Google Scholar] [CrossRef]
- Moricca, S.; Linaldeddu, B.T.; Ginetti, B.; Scanu, B.; Franceschini, A.; Ragazzi, A. Endemic and Emerging Pathogens Threatening Cork Oak Trees: Management Options for Conserving a Unique Forest Ecosystem. Plant Dis. 2016, 100, 2184–2193. [Google Scholar] [CrossRef] [PubMed]
- Warwell, M.V.; McDonald, G.I.; Hanna, J.W.; Kim, M.-S.; Lalande, B.M.; Stewart, J.E.; Hudak, A.T.; Klopfenstein, N.B. Armillaria altimontana Is Associated with Healthy Western White Pine (Pinus monticola): Potential in Situ Biological Control of the Armillaria Root Disease Pathogen, A. solidipes. Forests 2019, 10, 294. [Google Scholar] [CrossRef]
- Queloz, V.; Grünig, C.R.; Berndt, R.; Kowalski, T.; Sieber, T.N.; Holdenrieder, O. Cryptic Speciation in Hymenoscyphus albidus. For. Pathol. 2011, 41, 133–142. [Google Scholar] [CrossRef]
- Sieber, T.N. The Phyllosphere Mycobiome of Woody Plants. In Forest Microbiology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 111–132. ISBN 978-0-12-822542-4. [Google Scholar]
- Coghlan, A. Are Europe’s Ash Trees Finished? Available online: http://www.newscientist.com/article/dn22449-are-europes-ash-trees-finished.html (accessed on 31 October 2012).
- Kowalski, T.; Bilański, P.; Holdenrieder, O. Virulence of Hymenoscyphus albidus and H. fraxineus on Fraxinus excelsior and F. pennsylvanica. PLoS ONE 2015, 10, e0141592. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.E.; Niemi, M.; Sierota, Z.H. Comparison of Three Products Based on Phlebiopsis gigantea for the Control of Heterobasidion annosum in Europe. Biocontrol Sci. Technol. 2000, 10, 467–477. [Google Scholar] [CrossRef]
- Grabka, R.; d’Entremont, T.W.; Adams, S.J.; Walker, A.K.; Tanney, J.B.; Abbasi, P.A.; Ali, S. Fungal Endophytes and Their Role in Agricultural Plant Protection against Pests and Pathogens. Plants 2022, 11, 384. [Google Scholar] [CrossRef]
- Akram, S.; Ahmed, A.; He, P.; He, P.; Liu, Y.; Wu, Y.; Munir, S.; He, Y. Uniting the Role of Endophytic Fungi against Plant Pathogens and Their Interaction. J. Fungi. 2023, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Pukalski, J.; Marcol, N.; Wolan, N.; Płonka, P.M.; Ryszka, P.; Kowalski, T.; Latowski, D. Detection of a Pheomelanin-like Pigment by EPR Spectroscopy in the Mycelium of Plenodomus biglobosus. Acta Biochim. Pol. 2020, 67, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, T.; Bilański, P. Fungi Detected in the Previous Year’s Leaf Petioles of Fraxinus excelsior and Their Antagonistic Potential against Hymenoscyphus fraxineus. Forests 2021, 12, 1412. [Google Scholar] [CrossRef]
- Bilański, P.; Kowalski, T. Fungal Endophytes in Fraxinus excelsior Petioles and Their in Vitro Antagonistic Potential against the Ash Dieback Pathogen Hymenoscyphus fraxineus. Microbiol. Res. 2022, 257, 126961. [Google Scholar] [CrossRef]
- Nawrot-Chorabik, K.; Marcol-Rumak, N.; Latowski, D. Investigation of the Biocontrol Potential of Two Ash Endophytes against Hymenoscyphus fraxineus Using In Vitro Plant–Fungus Dual Cultures. Forests 2021, 12, 1750. [Google Scholar] [CrossRef]
- Diner, A.M.; Karnosky, D.F. Differential Responses of Two Conifers to in Vitro Inoculation with Agrobacterium rhizogenes. Eur. J. For. Pathol. 1987, 17, 211–216. [Google Scholar] [CrossRef]
- do Nascimento, J.S.; Silva, F.M.; Magallanes-Noguera, C.A.; Kurina-Sanz, M.; Dos Santos, E.G.; Caldas, I.S.; Luiz, J.H.H.; Silva, E.d.O. Natural Trypanocidal Product Produced by Endophytic Fungi through Co-Culturing. Folia Microbiol. 2020, 65, 323–328. [Google Scholar] [CrossRef]
- Palyzová, A.; Svobodová, K.; Sokolová, L.; Novák, J.; Novotný, Č. Metabolic Profiling of Fusarium oxysporum f. Sp. Conglutinans Race 2 in Dual Cultures with Biocontrol Agents Bacillus amyloliquefaciens, Pseudomonas aeruginosa, and Trichoderma harzianum. Folia Microbiol. 2019, 64, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.L. Concerning the Characters of Certain Fungi as Exhibited by Their Growth in the Presence of Other Fungi. Am. J. Bot. 1924, 11, 168–188. [Google Scholar] [CrossRef]
- Fokkema, N.J. The Role of Saprophytic Fungi in Antagonism against Drechslera sorokiniana (Helminthosporium sativum) on Agar Plates and on Rye Leaves with Pollen. Physiol. Plant Pathol. 1973, 3, 195–205. [Google Scholar] [CrossRef]
- Royse, D.J.; Ries, S.M. The Influence of Fungi Isolated from Peach Twigs on the Pathogenicity of Cytospora cincta. Phytopathology 1978, 68, 603–607. [Google Scholar] [CrossRef]
- Manandhar, J.B.; Thapliyal, P.N.; Cavanaugh, K.J.; Sinclair, J.B. Interaction between Pathogenic and Saprobic Fungi Isolated from Soybean Roots and Seeds. Mycopathologia 1987, 98, 69–75. [Google Scholar] [CrossRef]
- Hajieghrari, B.; Torabi Giglou, M.; Mohammadi, M.R.; Davari, M. Biological Potantial of Some Iranian Trichoderma Isolates in the Control of Soil Borne Plant Pathogenic Fungi. Afr. J. Biotechnol. 2008, 7, 967–972. [Google Scholar]
- Landum, M.C.; do Rosário Félix, M.; Alho, J.; Garcia, R.; Cabrita, M.J.; Rei, F.; Varanda, C.M.R. Antagonistic Activity of Fungi of Olea europaea L. against Colletotrichum acutatum. Microbiol. Res. 2016, 183, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Hewedy, O.A.; Abdel Lateif, K.S.; Seleiman, M.F.; Shami, A.; Albarakaty, F.M.; El-Meihy, R.M. Phylogenetic Diversity of Trichoderma Strains and Their Antagonistic Potential against Soil-Borne Pathogens under Stress Conditions. Biology 2020, 9, 189. [Google Scholar] [CrossRef]
- Lahlali, R.; Hijri, M. Screening, Identification and Evaluation of Potential Biocontrol Fungal Endophytes against Rhizoctonia solani AG3 on Potato Plants. FEMS Microbiol. Lett. 2010, 311, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Steindorff, A.S.; Ramada, M.H.S.; Coelho, A.S.G.; Miller, R.N.G.; Pappas, G.J.; Ulhoa, C.J.; Noronha, E.F. Identification of Mycoparasitism-Related Genes against the Phytopathogen Sclerotinia sclerotiorum through Transcriptome and Expression Profile Analysis in Trichoderma harzianum. BMC Genom. 2014, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-B.; Sun, M.-H.; Li, S.-D. Identification of Mycoparasitism-Related Genes in Clonostachys rosea 67-1 Active against Sclerotinia sclerotiorum. Sci. Rep. 2015, 5, 18169. [Google Scholar] [CrossRef]
- Moreno-Ruiz, D.; Lichius, A.; Turrà, D.; Di Pietro, A.; Zeilinger, S. Chemotropism Assays for Plant Symbiosis and Mycoparasitism Related Compound Screening in Trichoderma atroviride. Front. Microbiol. 2020, 11, 601251. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a Mechanism of Trichoderma-Mediated Suppression of Plant Diseases. Fungal. Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Hasan, R.; Lv, B.; Uddin, M.J.; Chen, Y.; Fan, L.; Sun, Z.; Sun, M.; Li, S. Monitoring Mycoparasitism of Clonostachys rosea against Botrytis cinerea Using GFP. J. Fungi. 2022, 8, 567. [Google Scholar] [CrossRef]
- Geiger, A.; Karácsony, Z.; Geml, J.; Váczy, K.Z. Mycoparasitism Capability and Growth Inhibition Activity of Clonostachys rosea Isolates against Fungal Pathogens of Grapevine Trunk Diseases Suggest Potential for Biocontrol. PLoS ONE 2022, 17, e0273985. [Google Scholar] [CrossRef] [PubMed]
- Embacher, J.; Seehauser, M.; Kappacher, C.; Stuppner, S.; Zeilinger, S.; Kirchmair, M.; Neuhauser, S. Serpula lacrymans Reacts with a General, Unspecialized Chemical Response during Interaction with Mycoparasitic Trichoderma Spp. and Bacteria. Fungal. Ecol. 2023, 63, 101230. [Google Scholar] [CrossRef]
- Haňáčková, Z.; Havrdová, L.; Černý, K.; Zahradnik, D.; Koukol, O. Fungal endophytes in ash shoots–diversity and inhibition of Hymenoscyphus fraxineus. Balt. For. 2017, 23, 89–106. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pukalski, J.; Olchawa-Pajor, M.; Jedynak, P.; Nawrot-Chorabik, K.; Latowski, D. Vitality and Inhibition Parameters in the Analysis of Dual Fungal Cultures as an Effective Tool in the Bio-Protection of Forest Ecosystems. Forests 2024, 15, 1510. https://doi.org/10.3390/f15091510
Pukalski J, Olchawa-Pajor M, Jedynak P, Nawrot-Chorabik K, Latowski D. Vitality and Inhibition Parameters in the Analysis of Dual Fungal Cultures as an Effective Tool in the Bio-Protection of Forest Ecosystems. Forests. 2024; 15(9):1510. https://doi.org/10.3390/f15091510
Chicago/Turabian StylePukalski, Jan, Monika Olchawa-Pajor, Paweł Jedynak, Katarzyna Nawrot-Chorabik, and Dariusz Latowski. 2024. "Vitality and Inhibition Parameters in the Analysis of Dual Fungal Cultures as an Effective Tool in the Bio-Protection of Forest Ecosystems" Forests 15, no. 9: 1510. https://doi.org/10.3390/f15091510




