Partial Organic Substitution Fertilization Improves Soil Fertility While Reducing N Mineralization in Rubber Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Soil Collection and Analysis
2.4. Soil N Mineralization Incubation and Analysis
2.5. Statistical Analysis
3. Results
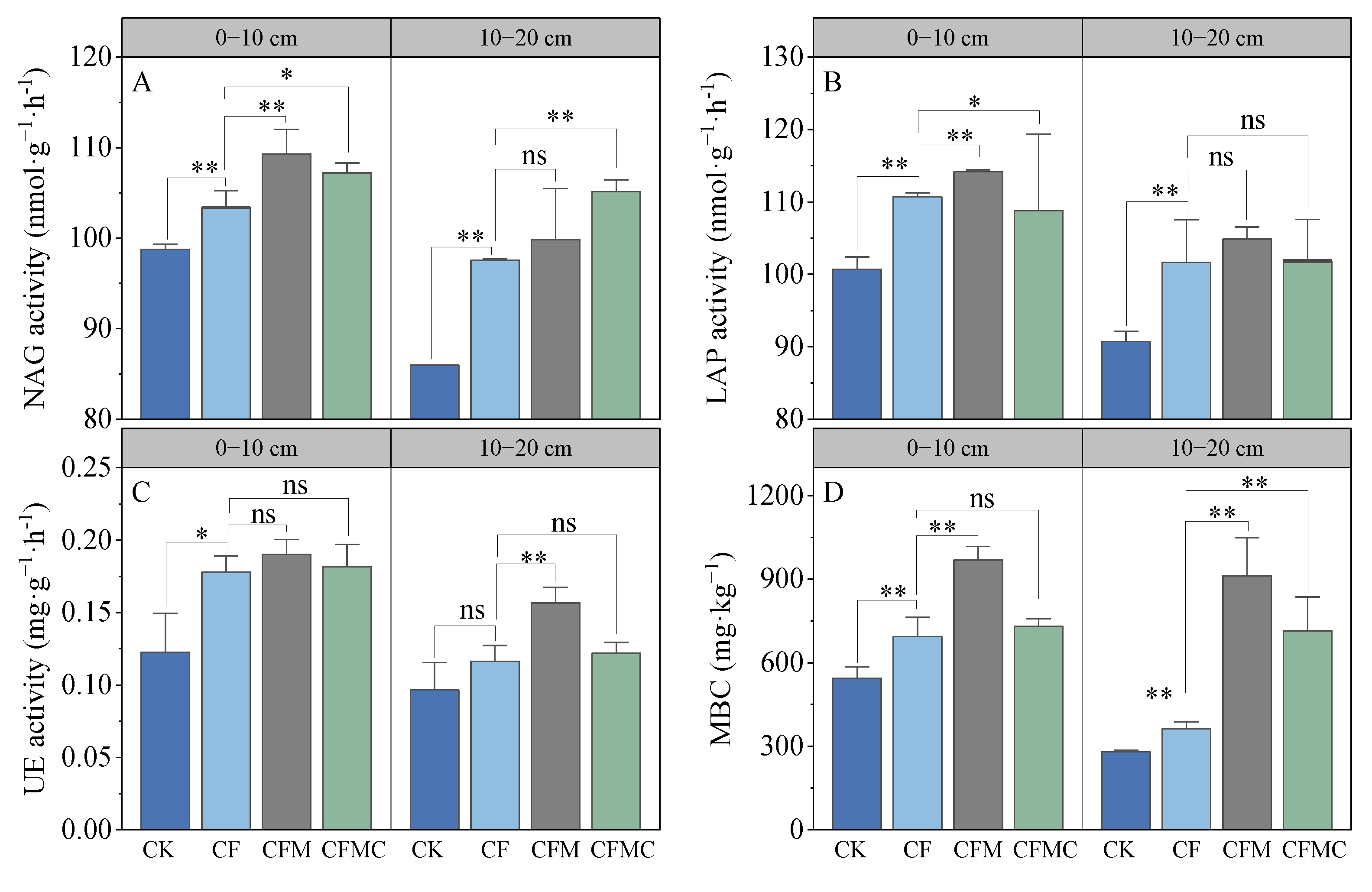
3.1. Soil Properties
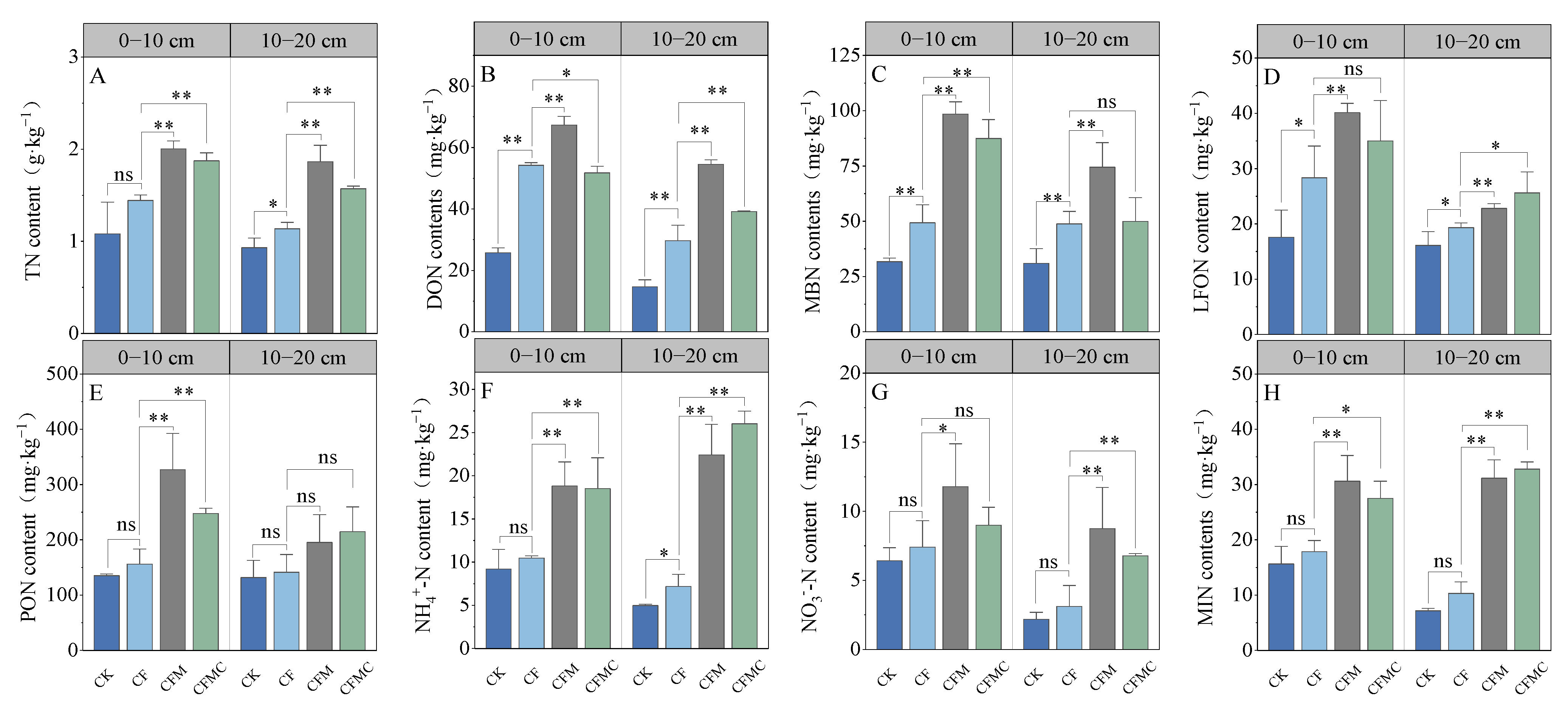
3.2. Soil TN and Labile N Components
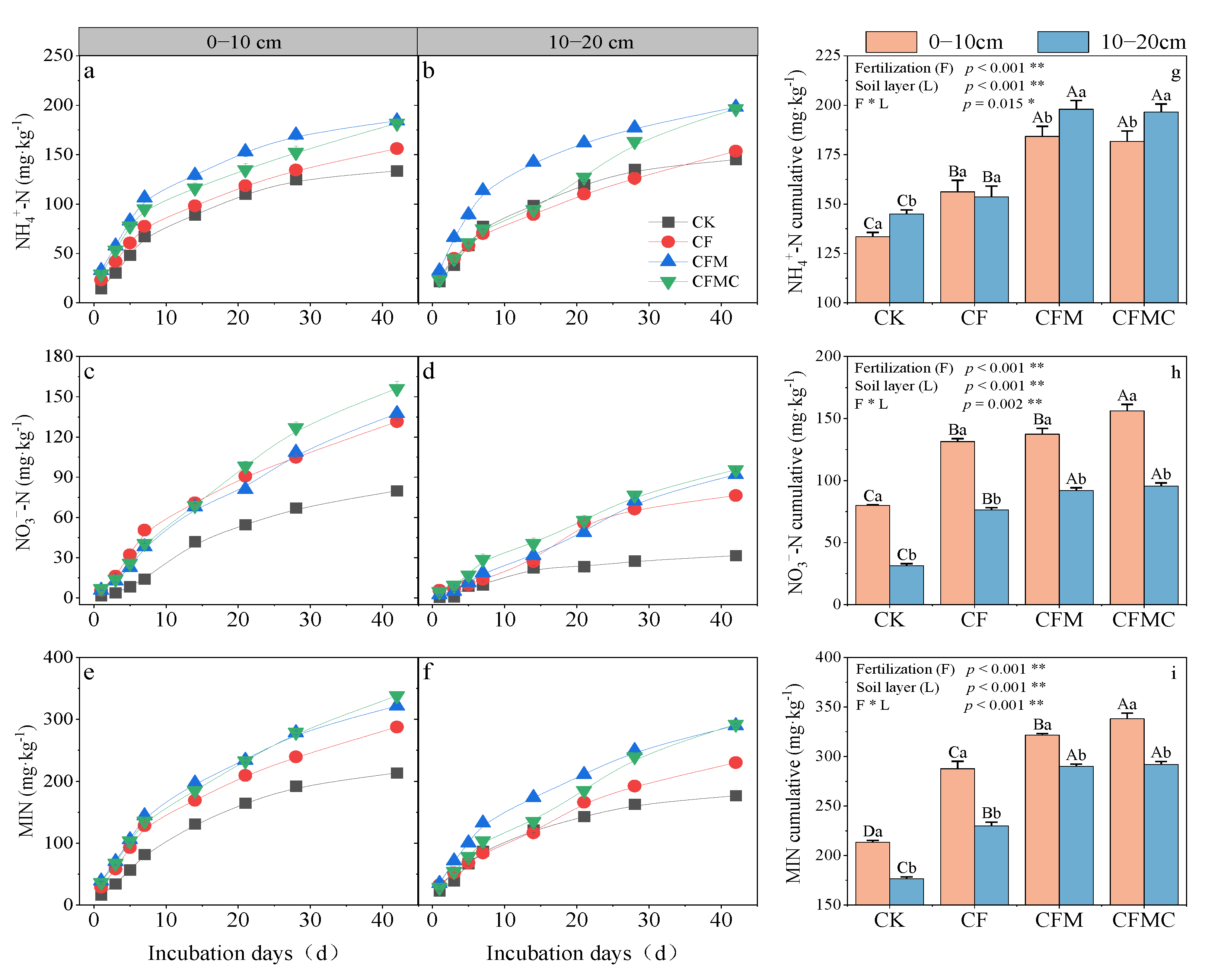
3.3. Soil N Mineralization Characteristic
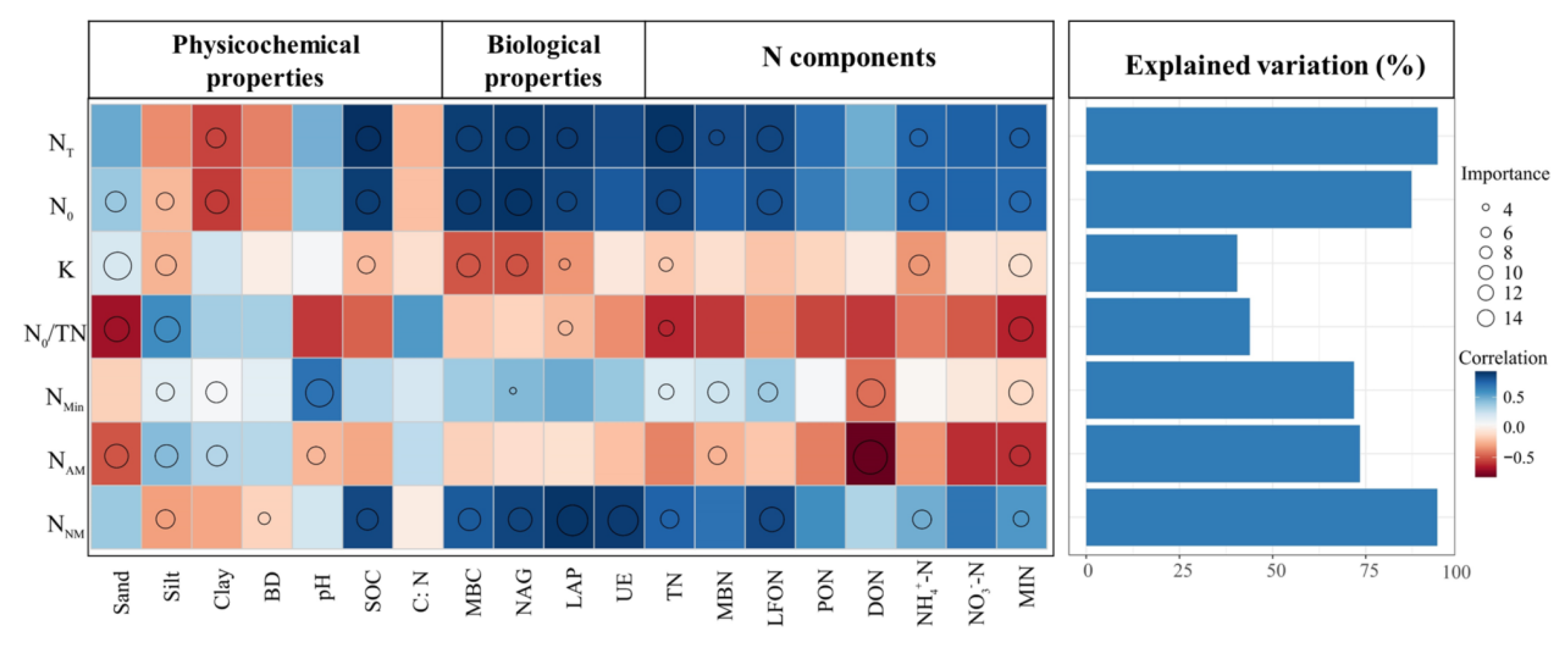
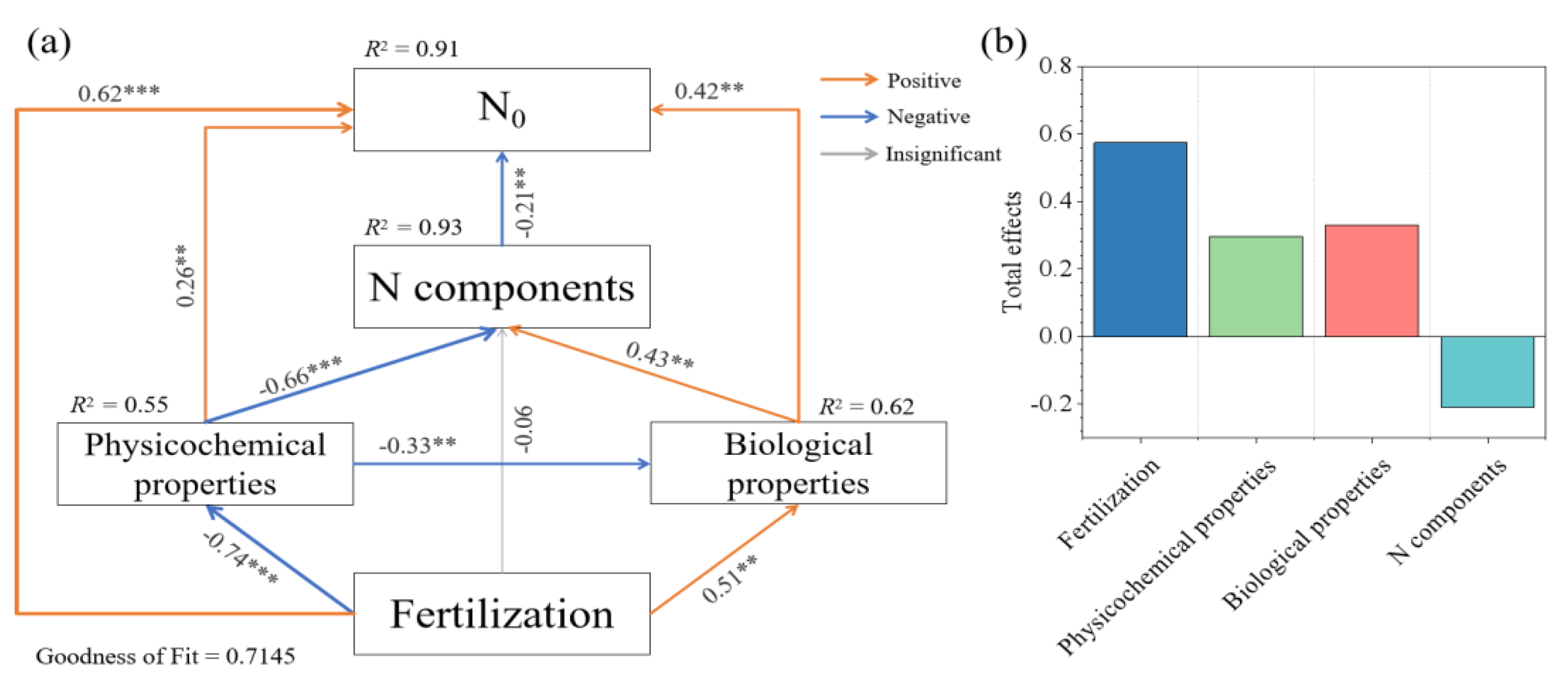
3.4. Relationship between Soil N Mineralization Parameters and Soil Factors and N Components
4. Discussion
4.1. Effect of Partial Organic Fertilizer Substitution on Contents of Soil TN and Labile N Components in Rubber Plantation
4.2. Effect of Partial Organic Fertilizer Substitution on Characteristics of Soil N Mineralization in Rubber Plantation
4.3. Mechanism Influencing Net Soil N Transformation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cheng, H.; Song, X.; Hu, Y.; Wu, T.; Yang, Q.; An, Z.; Feng, S.; Deng, Z.; Wu, W.; Zeng, X.; et al. Chromosome-level wild Hevea brasiliensis genome provides new tools for genomic-assisted breeding and valuable loci to elevate rubber yield. Plant Biotechnol. J. 2023, 21, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.X.; Liu, W.J.; Tang, S.R.; Yang, Q.; Meng, L.; Wu, Y.Z.; Wang, J.J.; Wu, L.; Wu, M.; Xue, X.X.; et al. Long-term partial substitution of chemical nitrogen fertilizer with organic fertilizers increased SOC stability by mediating soil C mineralization and enzyme activities in a rubber plantation of Hainan Island, China. Appl. Soil Ecol. 2023, 182, 104691. [Google Scholar] [CrossRef]
- Bai, R.; Wang, J.; Li, N. Climate change increases the suitable area and suitability degree of rubber tree powdery mildew in China. Ind. Crop. Prod. 2022, 189, 115888. [Google Scholar] [CrossRef]
- Cheng, C.M.; Wang, R.; Jiang, J.S. Variation of soil fertility and carbon sequestration by planting Hevea brasiliensis in Hainan Island, China. J. Environ. Sci. 2007, 19, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Ma, Y.X.; Cao, K.F.; Li, H.M.; He, J.F.; Liu, C.P.; Liu, H.P.; Li, B. Impacts of Long-term Rubber Plantation on Soil Carbon and Nitrogen Sequestration in Xishuangbanna. Chin. J. Soil Sci. 2015, 46, 412–419. [Google Scholar] [CrossRef]
- Rao, X.; Liu, C.A.; Tang, J.W.; Nie, Y.; Liang, M.Y.; Shen, W.J.; Siddique, K.H. Rubber-leguminous shrub systems stimulate soil N2O but reduce CO2 and CH4 emissions. Forest. Ecol. Manag. 2021, 480, 118665. [Google Scholar] [CrossRef]
- Garousi, F.; Shan, Z.J.; Ni, K.; Yang, H.; Shan, J.; Cao, J.H.; Jiang, Z.C.; Yang, J.L.; Zhu, T.B.; Müller, C. Decreased inorganic N supply capacity and turnover in calcareous soil under degraded rubber plantation in the tropical karst region. Geoderma 2021, 381, 114754. [Google Scholar] [CrossRef]
- Ouyang, Y.; Norton, J.M. Short-term nitrogen fertilization affects microbial community composition and nitrogen mineralization functions in an agricultural soil. Appl. Environ. Microbiol. 2020, 86, e02278-19. [Google Scholar] [CrossRef]
- Siemens, J.; Kaupenjohann, M. Contribution of dissolved organic nitrogen to N leaching from four German agricultural soils. J. Plant Nutr. Soil Sci. 2002, 165, 675–681. [Google Scholar] [CrossRef]
- Huang, L.N.; Cheng, S.M.; Liu, H.L.; Zhao, Z.X.; Wei, S.X.; Sun, S.L. Effects of nitrogen reduction combined with organic fertilizer on growth and nitrogen fate in banana at the seedling stage. Environ. Res. 2022, 214, 113826. [Google Scholar] [CrossRef]
- Fujii, K.; Hayakawa, C.; Panitkasate, T.; Maskhao, I.; Funakawa, S.; Kosaki, T.; Nawata, E. Acidification and buffering mechanisms of tropical sandy soil in northeast Thailand. Soil Tillage Res. 2017, 165, 80–87. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2017, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Fallah, S.; Sourki, A.A. Organic and inorganic fertilizer effect on soil CO2 flux, microbial biomass, and growth of Nigella sativa L. Int. Agrophys. 2017, 31, 103–116. [Google Scholar] [CrossRef]
- Han, Z.; Hou, H.; Yao, X.; Qian, X.; Zhou, M. Substituting Partial Chemical Fertilizers with Bio-Organic Fertilizers to Reduce Greenhouse Gas Emissions in Water-Saving Irrigated Rice Fields. Agronomy 2024, 14, 544. [Google Scholar] [CrossRef]
- Jin, S.Q.; Zhou, F. Zero growth of chemical fertilizer and pesticide use: China’s objectives, progress and challenges. J. Res. Ecol. 2018, 9, 50–58. [Google Scholar] [CrossRef]
- Zeng, Y.; He, K.; Zhang, J.; Li, P. Adoption and ex-post impacts of sustainable manure management practices on income and happiness: Evidence from swine breeding farmers in rural Hubei, China. Ecol. Econ. 2023, 208, 107809. [Google Scholar] [CrossRef]
- Ros, G.H.; Hoffland, E.; Kessel, C.V.; Temminghoff, E.J.M. Extractable and dissolved soil organic nitrogen–a quantitative assessment. Soil Biol. Biochem. 2009, 41, 1029–1039. [Google Scholar] [CrossRef]
- Wu, H.Q.; Du, S.Y.; Zhang, Y.L.; An, J.; Zou, H.T.; Zhang, Y.L.; Yu, N. Effects of irrigation and nitrogen fertilization on greenhouse soil organic nitrogen fractions and soil-soluble nitrogen pools. Agric. Water Manag. 2019, 216, 415–424. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zhang, Y.Q.; Yan, C.R.; Liu, E.K.; Chen, B.Q. Soil nitrogen and its fractions between long-term conventional and no-tillage systems with straw retention in dryland farming in northern China. Geoderma 2016, 269, 138–144. [Google Scholar] [CrossRef]
- Hu, R.; Wang, X.P.; Pan, Y.X.; Zhang, Y.F.; Zhang, H.; Chen, N. Seasonal variation of net N mineralization under different biological soil crusts in Tengger Desert, North China. Catena 2015, 127, 9–16. [Google Scholar] [CrossRef]
- Li, Z.L.; Zeng, Z.Q.; Tian, D.S.; Wang, J.S.; Fu, Z.; Wang, B.X.; Tang, Z.; Chen, W.N.; Chen, H.Y.H.; Wang, C.H.; et al. The stoichiometry of soil microbial biomass determines the metabolic quotient of nitrogen mineralization. Environ. Res. Lett. 2020, 15, 034005. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U. Translocation of metals by trees and fungi regulates pH, soil organic matter turnover, and nitrogen availability in acidic forest soil. Soil Biol. Biochem. 2013, 63, 142–153. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Hu, C.; Xu, X.R.; Aziz, T.; Wu, L.; Waqas, M.A.; Farooq, M.; Hu, X.; Zhang, W.J.; Xu, M.G. Long-term manure application increased soil organic carbon and nitrogen mineralization through the accumulation of unprotected and physically protected carbon fractions. Pedosphere 2023, 33, 343–354. [Google Scholar] [CrossRef]
- Yu, S.Q.; Sayer, E.J.; Li, Z.A.; Mo, Q.F.; Wang, M.; Li, Y.W.; Li, Y.X.; Xu, G.L.; Hu, Z.M.; Wang, F.M. Delayed wet season increases soil net N mineralization in a seasonally dry tropical forest. Sci. Total Environ. 2022, 823, 153314. [Google Scholar] [CrossRef]
- Thomas, B.W.; Sharifi, M.; Whalen, J.K.; Chantigny, M.H. Mineralizable nitrogen responds differently to manure type in contrasting soil textures. Soil Sci. Soc. Am. J. 2020, 79, 1396–1405. [Google Scholar] [CrossRef]
- Yang, Z.L.; Chen, X.H.; Hou, J.T.; Liu, H.Y.; Tan, W.F. Soil texture and pH exhibit important effects on biological nitrogen fixation in paddy soil. Appl. Soil Ecol. 2022, 178, 104571. [Google Scholar] [CrossRef]
- Zhang, L.G.; Chen, X.; Xu, Y.J.; Jin, M.C.; Ye, X.X.; Gao, H.J.; Chu, W.Y.; Mao, J.D.; Thompson, M.L. Soil labile organic carbon fractions and soil enzyme activities after 10 years of continuous fertilization and wheat residue incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef]
- Fan, B.; Yin, L.M.; Dijkstra, F.A.; Lu, J.Y.; Shao, S.; Wang, P.; Wang, Q.K.; Cheng, W.X. Potential gross nitrogen mineralization and its linkage with microbial respiration along a forest transect in eastern China. Appl. Soil Ecol. 2022, 171, 104347. [Google Scholar] [CrossRef]
- Watts, D.B.; Torbert, H.A.; Prior, S.A.; Huluka, G. Long-term tillage and poultry litter impacts soil carbon and nitrogen mineralization and fertility. Soil Sci. Soc. Ame. J. 2010, 74, 1239–1247. [Google Scholar] [CrossRef]
- Maitlo, A.A.; Zhang, S.Q.; Ahmed, W.; Jangid, K.; Ali, S.; Yang, H.B.; Bhatti, S.M.; Duan, Y.H.; Xu, M.G. Potential nitrogen mineralization and its availability in response to long-term fertilization in a Chinese Fluvo-Aquic Soil. Agronomy 2022, 12, 1260. [Google Scholar] [CrossRef]
- Lan, G.Y.; Li, Y.W.; Lesueur, D.; Wu, Z.X.; Xie, G.S. Seasonal changes impact soil bacterial communities in a rubber plantation on Hainan Island, China. Sci. Total Environ. 2018, 626, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.F.; Ren, C.Q.; Luo, X.H.; Xue, X.X.; Zhao, C.M.; Ma, J.Y.; Geng, J.M.; Wang, W.B. Transport of Inorganic Nitrogen in the Soil of Rubber Plantation under Different Nitrogen Application Rates at Early Stage. Chin. J. Trop. Crop. 2021, 42, 3514–3520. [Google Scholar] [CrossRef]
- Li, J.L.; Liang, Y.Y.; Liu, W.J.; Yang, Q.; Xu, W.X.; Tang, S.R.; Wang, J.J. Effects of manure substituting chemical nitrogen fertilizer on rubber seedling growth and soil environment. Chin. J. Appl. Ecol. 2022, 33, 431–438. [Google Scholar] [CrossRef]
- Fu, X.; Wei, K.; Luo, W.; Zhang, Q.; Liu, J. Effect of Organic Fertilizer Replacing Chemical Fertilizer on Growth of Rubber Cutting Tree. Spec. Econ. Anim. Plant 2022, 25, 23–28. [Google Scholar]
- Tang, Q.; Wu, B.; Cao, Q.; Guo, P.; Qin, J.; Liu, Z. Fertility evaluation and spatial distribution characteristics of soil nutrients of rubber plantations in Hainan Wushi farm. Guizhou Agric. Sci. 2007, 41, 116–121. [Google Scholar]
- Zhang, H.; Zhang, G.; Zhao, Y.; Zhao, W.; Qi, Z. Chemical degradation of a Ferralsol (Oxisol) under intensive rubber (Hevea brasiliensis) farming in tropical China. Soil Tillage Res. 2007, 93, 109–116. [Google Scholar] [CrossRef]
- Zhou, W.J.; Ji, H.L.; Zhu, J.; Zhang, Y.P.; Sha, L.Q.; Liu, Y.T.; Zhang, X.; Zhao, W.; Dong, Y.X.; Bai, X.L.; et al. The effects of nitrogen fertilization on N2O emissions from a rubber plantation. Sci. Rep. 2016, 6, 28230. [Google Scholar] [CrossRef]
- Lu, R.K. Chemical Analysis of Agricultural Soil; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Wu, J.S.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Tang, S.; Cheng, W.; Hu, R.; Guigue, J.; Hattori, S.; Tawaraya, K.; Tokida, T.; Fukuoka, M.; Yoshimoto, M.; Sakai, H.; et al. Five-year soil warming changes soil C and N dynamics in a single rice paddy field in Japan. Sci. Total Environ. 2021, 756, 143845. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate soil organic matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Ellert, B.H. Light fraction and macro organic matter in mineral soil. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 397–407. [Google Scholar]
- DeForest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l–DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Guan, S.Y. Soil Enzyme Research Methods; Agricultural Press: Beijing, China, 1986. [Google Scholar]
- Marzi, M.; Shahbazi, K.; Kharazi, N.; Rezaei, M. The influence of organic amendment sources on carbon and nitrogen mineralization in different soils. J. Soil Sci. Plant Nut. 2020, 20, 177–191. [Google Scholar] [CrossRef]
- Ali, S.; Li, D.C.; Huang, J.; Ahmed, W.; Abbas, M.; Qaswar, M.; Anthonio, C.K.; Zhang, L.; Wang, B.R.; Xu, Y.M.; et al. Soil microbial biomass and extracellular enzymes regulate nitrogen mineralization in a wheat-maize cropping system after three decades of fertilization in a Chinese Ferrosol. J. Soil Sediment. 2020, 21, 281–294. [Google Scholar] [CrossRef]
- Feng, N.; Liu, D.D.; Li, Y.; Liu, P. Soil net N mineralization and hydraulic properties of carbonate-derived laterite under different vegetation types in Karst forests of China. Sci. Total Environ. 2023, 856, 159116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qin, J.; Yao, W.; Bi, L.; Lai, T.; Yu, X. Effect of long-term application of manure and mineral fertilizers on nitrogen mineralization and microbial biomass in paddy soil during rice growth stages. Plant Soil Environ. 2009, 55, 101–109. [Google Scholar] [CrossRef]
- Cai, A.D.; Xu, H.; Shao, X.F.; Zhu, P.; Zhang, W.J.; Xu, M.G.; Murphy, D.V. Carbon and Nitrogen Mineralization in Relation to Soil Particle-Size Fractions after 32 Years of Chemical and Manure Application in a Continuous Maize Cropping System. PLoS ONE 2016, 11, e0152521. [Google Scholar] [CrossRef][Green Version]
- Bhattacharyya, R.; Kundu, S.; Prakash, V.; Gupta, H.S. Sustainability under combined application of mineral and organic fertilizers in a rainfed soybean–wheat system of the Indian Himalayas. Eur. J. Agron. 2008, 28, 33–46. [Google Scholar] [CrossRef]
- Yang, L.J.; Zhang, L.L.; Geisseler, D.; Wu, Z.J.; Gong, P.; Xue, Y.; Yu, C.X.; Juan, Y.H.; Horwath, W.R. Available C and N affect the utilization of glycine by soil microorganisms. Geoderma 2016, 283, 32–38. [Google Scholar] [CrossRef]
- Kundu, S.; Bhattacharyya, R.; Prakash, V.; Gupta, H.S.; Pathak, H.; Ladha, J.K. Long-term yield trend and sustainability of rainfed soybean–wheat system through farmyard manure application in a sandy loam soil of the Indian Himalayas. Biol. Fertil. Soil. 2007, 43, 271–280. [Google Scholar] [CrossRef]
- Ahmad, R.; Naveed, M.; Aslam, M.; Zahir, Z.A.; Arshad, M.; Jilani, G. Economizing the use of nitrogen fertilizer in wheat production through enriched compost. Renew. Agric. Food Syst. 2008, 23, 243–249. [Google Scholar] [CrossRef]
- Liu, C.A.; Nie, Y.; Zhang, Y.M.; Tang, J.W.; Siddique, K.H.M. Introduction of a leguminous shrub to a rubber plantation changed the soil carbon and nitrogen fractions and ameliorated soil environments. Sci. Rep. 2018, 8, 17324. [Google Scholar] [CrossRef]
- Muhdi, H.D.S.; Butar-Butar, R.D. Diversity, biomass, and carbon stock of understorey plants in the rubber agroforestry and rubber monoculture systems in Central Tapanuli District, North Sumatra, Indonesia. Biodiversitas 2020, 21, 3508–3518. [Google Scholar] [CrossRef]
- Xu, S.; Sayer, E.J.; Eisenhauer, N.; Lu, X.; Wang, J.; Liu, C. Aboveground litter inputs determine carbon storage across soil profiles: A meta-analysis. Plant Soil 2021, 462, 429–444. [Google Scholar] [CrossRef]
- Hou, Q.; Ni, Y.M.; Huang, S.; Zuo, T.; Wang, J.; Ni, W.Z. Effects of substituting chemical fertilizers with manure on rice yield and soil labile nitrogen in paddy fields of China: A meta-analysis. Pedosphere 2023, 33, 172–184. [Google Scholar] [CrossRef]
- Hossain, M.E.; Mei, X.R.; Zhang, W.Y.; Dong, W.Y.; Yan, Z.X.; Liu, X.; Rachit, S.; Gopalakrishnan, S.; Liu, E.K. Substitution of chemical fertilizer with organic fertilizer affects soil total nitrogen and its fractions in northern China. Int. J. Environ. Res. Public Health 2021, 18, 12848. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Z.; Gao, J.R.; Wang, H.Q.; Liu, J.; Yu, N.; Zhang, Y.L. Effects of combined application of nitrogen fertilizer and manure on the dynamics of net mineralized nitrogen in greenhouse soil. Chin. J. Soil Sci. 2021, 52, 1173–1181. [Google Scholar] [CrossRef]
- Chen, Y.C.; Liu, Z.M.; Xu, M.G.; Li, X.Q.; Ding, H.P.; Yang, C.X.; Li, C.L. Spatial distribution of soil nitrogen of rubber tree plantation and its relationship with rubber tree growth in Xishuangbanna. Southwest Chin. J. Agric. Sci. 2019, 32, 584–589. [Google Scholar] [CrossRef]
- Ren, C.Q.; Zhang, Y.F.; Wang, S.; Luo, X.H.; Xue, X.X.; Zhao, C.M.; Wang, W.B. Effects of different nitrogen application and ratios on the transportion Ccharacteristics of soil inorganic nitrogen in rubber plantations. Chin. J. Trop. Crop. 2023, 44, 1605–1614. [Google Scholar] [CrossRef]
- Wei, X.; Reich, P.B.; Hobbie, S.E.; Kazanski, C.E. Disentangling species and functional group richness effects on soil N cycling in a grassland ecosystem. Glob. Chang. Biol. 2017, 23, 4717–4727. [Google Scholar] [CrossRef]
- Zhang, J.B.; Zhu, T.B.; Cai, Z.C.; Qin, S.W.; Müller, C. Effects of long-term repeated mineral and organic fertilizer applications on soil nitrogen transformations. Eur. J. Soil Sci. 2012, 63, 75–85. [Google Scholar] [CrossRef]
- Ahmed, W.; Liu, K.L.; Qaswar, M.; Huang, J.; Huang, Q.H.; Xu, Y.M.; Ali, S.; Mehmood, S.; Asghar, R.M.; Mahmood, M.; et al. Long-term mineral fertilization improved the grain yield and phosphorus use efficiency by changing soil P fractions in ferralic cambisol. Agronomy 2019, 9, 784. [Google Scholar] [CrossRef]
- Wu, H.; Cai, A.; Xing, T.; Huai, S.; Zhu, P.; Xu, M.; Lu, C. Fertilization enhances mineralization of soil carbon and nitrogen pools by regulating the bacterial community and biomass. J. Soil Sediment. 2021, 21, 1633–1643. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.L.; Zhao, Y.Y.; Wang, D.X.; Xie, T.Q.; Lv, J.G.; Jin, D.F.; Shi, H.Z. Effects of organic fertilizers on nitrogen mineralization, soil enzyme activities and microbial communities in tobacco-planting soil. Soil 2023, 55, 1025–1034. [Google Scholar]
- Benedetti, A.; Sebastiani, G. Determination of potentially mineralizable nitrogen in agricultural soil. Biol. Fertil. Soils 1996, 21, 114–120. [Google Scholar] [CrossRef]
- Lv, Z.Z.; Wu, X.D.; Liu, Y.R.; Lan, J.X.; Hou, H.Q.; Ji, J.H.; Liu, X.M. Nitrogen mineralization characteristics of red paddy soil under long-term different proportions of organic and inorganic fertilization. Soil Fertr. Sci. Chin. 2021, 21, 47–53. [Google Scholar] [CrossRef]
- Mohanty, S.; Nayak, A.K.; Kumar, A.; Tripathi, R.; Shahid, M.; Bhattacharyya, P.; Raja, R.; Panda, B.B. Carbon and nitrogen mineralization kinetics in soil of rice–rice system under long term application of chemical fertilizers and farmyard manure. Eur. J. Soil Biol. 2013, 58, 113–121. [Google Scholar] [CrossRef]
- Luce, M.S.; Whalen, J.K.; Ziadi, N.; Zebarth, B.J. Net nitrogen mineralization is enhanced with the addition of nitrogen-rich particulate organic matter. Geoderma 2016, 262, 112–118. [Google Scholar] [CrossRef]
- Zhu, T.B.; Meng, T.Z.; Zhang, J.B.; Yin, Y.F.; Cai, Z.C.; Yang, W.Y.; Zhong, W.H. Nitregon mineralization, immobilization turnover, heterotrophic nitrification, and microbial groups in acid forest soils of subtropical China. Biol. Fertil. Soil 2013, 49, 323–331. [Google Scholar] [CrossRef]
- Huang, T.T.; Yang, N.; Lu, C.; Qin, X.L.; Siddique, K.H. Soil organic carbon, total nitrogen, available nutrients, and yield under different straw returning methods. Soil Tillage Res. 2021, 214, 105171. [Google Scholar] [CrossRef]
- Zhao, H.T.; Zhang, X.L.; Xu, S.T.; Zhao, X.G.; Xie, Z.B.; Wang, Q.B. Effect of freezing on soil N mineralization under different plant communities in a semi-arid area during a non-growing season. Appl. Soil Ecol. 2010, 45, 187–192. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, S.; Fang, H.; Xu, M.; Yang, Y.; Li, Y.; Zhang, J.; Müller, C. Organic nitrogen addition causes decoupling of microbial nitrogen cycles by stimulating gross nitrogen transformation in a temperate forest soil. Geoderma 2021, 385, 114886. [Google Scholar] [CrossRef]
- Chen, D.; Xing, W.; Lan, Z.; Saleem, M.; Wu, Y.; Hu, S.; Bai, Y. Direct and indirect effects of nitrogen enrichment on soil organisms and carbon and nitrogen mineralization in a semi-arid grassland. Funct. Ecol. 2019, 33, 175–187. [Google Scholar] [CrossRef]
- Leogrande, R.; Vitti, C.; Castellini, M.; Garofalo, P.; Samarelli, I.; Lacolla, G.; Montesano, F.F.; Spagnuolo, M.; Mastrangelo, M.; Stellacci, A.M. Residual effect of compost and biochar amendment on soil chemical, biological, and physical properties and durum wheat response. Agronomy 2024, 14, 749. [Google Scholar] [CrossRef]

| Treatments | Soil Layer | Sand (%) | Silt (%) | Clay (%) | BD (g·cm−3) | pH | SOC (g·kg−1) | C:N Ratio |
|---|---|---|---|---|---|---|---|---|
| CK | 0–10 cm | 42.87 ± 1.29 Ca | 46.67 ± 1.40 Bb | 10.46 ± 0.31 Ba | 1.68 ± 0.03 Aa | 4.98 ± 0.02 Bb | 13.58 ± 0.25 Ca | 11.41 ± 1.05 Aa |
| CF | 49.68 ± 0.75 Ba | 37.33 ± 0.56 Cb | 12.98 ± 0.19 Aa | 1.66 ± 0.06 Aa | 5.13 ± 0.03 Aa | 16.58 ± 0.12 Ba | 11.49 ± 0.25 Aa | |
| CFM | 64.29 ± 0.36 Aa | 29.27 ± 0.29 Da | 6.46 ± 0.06 Cb | 1.50 ± 0.16 Aa | 5.23 ± 0.13 Ab | 19.04 ± 1.47 Aa | 9.50 ± 0.86 Ba | |
| CFMC | 41.16 ± 0.62 Ca | 53.33 ± 0.80 Aa | 5.50 ± 0.08 Cb | 1.63 ± 0.04 Aa | 5.18 ± 0.07 Ab | 19.62 ± 0.10 Aa | 10.48 ± 0.30 ABa | |
| CK | 10–20 cm | 36.03 ± 0.54 Db | 54.58 ± 0.75 Aa | 9.45 ± 0.32 Bb | 1.67 ± 0.03 Aa | 5.03 ± 0.01 Da | 9.46 ± 0.89 Db | 10.19 ± 0.94 Aa |
| CF | 45.19 ± 0.18 Bb | 43.97 ± 0.18 Ca | 10.84 ± 0.04 Ab | 1.60 ± 0.11 ABa | 5.15 ± 0.04 Ca | 12.21 ± 0.23 Cb | 10.72 ± 0.23 Ab | |
| CFM | 62.88 ± 1.07 Aa | 28.63 ± 0.57 Da | 8.63 ± 0.15 Ca | 1.49 ± 0.06 Ba | 5.68 ± 0.05 Aa | 18.59 ± 0.98 Aa | 10.02 ± 1.07 Aa | |
| CFMC | 40.74 ± 1.22 Ca | 52.61 ± 1.45 Ba | 6.75 ± 0.19 Da | 1.55 ± 0.08 ABa | 5.33 ± 0.02 Ba | 15.18 ± 0.71 Bb | 9.66 ± 0.36 Ab | |
| ANOVA results (F-values) | ||||||||
| Fertilization (F) | 1029.45 ** | 957.87 ** | 1032.87 ** | 4.75 * | 63.54 ** | 111.40 ** | 4.46 * | |
| Soil layer (L) | 91.51 ** | 86.81 ** | 0.72 ns | 1.39 ns | 49.32 ** | 117.90 ** | 3.80 ns | |
| F × L | 18.16 ** | 42.64 ** | 155.39 ** | 0.24 ns | 17.23 ** | 9.91 ** | 1.63 ns | |
| Treatments | Soil Layer | LFON/TN | PON/TN | DON/TN | MBN/TN | NH4+-N/TN | NO3−-N/TN | MIN/TN |
|---|---|---|---|---|---|---|---|---|
| (%) | ||||||||
| CK | 0–10 cm | 1.63 ± 0.10 Ba | 12.95 ± 3.02 ABa | 2.44 ± 0.43 Ba | 3.04 ± 0.76 Ba | 0.86 ± 0.04 ABa | 0.60 ± 0.07 Aa | 1.46 ± 0.12 Aa |
| CF | 1.96 ± 0.23 ABa | 10.80 ± 1.15 Ba | 3.76 ± 0.08 Aa | 3.42 ± 0.39 Bb | 0.73 ± 0.03 Ba | 0.52 ± 0.10 Aa | 1.24 ± 0.13 Ba | |
| CFM | 2.00 ± 0.01 Aa | 16.30 ± 2.20 Aa | 3.36 ± 0.18 Aa | 4.91 ± 0.17 Aa | 0.94 ± 0.11 Ab | 0.59 ± 0.11 Aa | 1.53 ± 0.19 Aa | |
| CFMC | 1.87 ± 0.23 ABa | 13.23 ± 0.70 ABa | 2.77 ± 0.11 Ba | 4.68 ± 0.43 Aa | 0.99 ± 0.15 Ab | 0.48 ± 0.04 Aa | 1.47 ± 0.14 Ab | |
| CK | 10–20 cm | 1.73 ± 0.07 Aa | 14.26 ± 2.76 Aa | 1.58 ± 0.08 Cb | 3.34 ± 0.51 BCa | 0.54 ± 0.03 Cb | 0.24 ± 0.05 Bb | 0.77 ± 0.08 Cb |
| CF | 1.70 ± 0.10 Aa | 12.38 ± 1.45 ABa | 2.61 ± 0.39 ABb | 4.28 ± 0.23 Aa | 0.63 ± 0.06 Ca | 0.27 ± 0.10 Bb | 0.90 ± 0.12 Cb | |
| CFM | 1.23 ± 0.10 Bb | 10.43 ± 1.20 Bb | 2.93 ± 0.18 Ab | 3.99 ± 0.17 ABb | 1.20 ± 0.06 Ba | 0.47 ± 0.12 Aa | 1.67 ± 0.07 Ba | |
| CFMC | 1.63 ± 0.17 Aa | 13.66 ± 1.79 Aa | 2.49 ± 0.03 Bb | 3.18 ± 0.49 Cb | 1.66 ± 0.04 Aa | 0.43 ± 0.01 Aa | 2.09 ± 0.03 Aa | |
| ANOVA results (F-values) | ||||||||
| Fertilization (F) | 2.45 ns | 1.43 ns | 33.57 ** | 8.38 ** | 96.29 ** | 3.07 ns | 52.53 ** | |
| Soil layer (L) | 24.37 ** | 0.64 ns | 51.29 ** | 3.12 ns | 15.60 ** | 31.62 ** | 1.93 ns | |
| F × L | 9.07 ** | 4.94 * | 4.50 * | 9.26 ** | 46.36 ** | 4.12 * | 34.79 ** | |
| Treatments | Soil Layer (cm) | Fitting Parameters | N0/TN (%) | ||
|---|---|---|---|---|---|
| N0 (mg·kg−1) | k (d−1) | R2 | |||
| CK | 0–10 | 235.58 ± 4.49 Da | 0.058 ± 0.002 Ab | 0.998 | 19.80 ± 1.81 Aa |
| CF | 289.43 ± 16.4 Ca | 0.069 ± 0.009 Aa | 0.983 | 20.04 ± 0.57 Aa | |
| CFM | 323.24 ± 17.19 Ba | 0.073 ± 0.009 Aa | 0.983 | 16.14 ± 1.26 Ba | |
| CFMC | 354.40 ± 8.02 Aa | 0.058 ± 0.009 Aa | 0.976 | 18.95 ± 0.97 ABa | |
| CK | 10–20 | 176.60 ± 5.30 Db | 0.089 ± 0.007 Aa | 0.992 | 19.03 ± 0.87 Ba |
| CF | 230.21 ± 20.57 Cb | 0.059 ± 0.011 Ba | 0.967 | 20.27 ± 2.50 Aa | |
| CFM | 286.21 ± 17.28 Bb | 0.077 ± 0.011 ABa | 0.975 | 15.40 ± 1.51 Ca | |
| CFMC | 312.73 ± 7.92 Ab | 0.041 ± 0.009 Ca | 0.971 | 19.91 ± 0.35 Ab | |
| ANOVA results (F-values) | |||||
| Fertilization (F) | 131.322 ** | 9.562 ** | 15.135 ** | ||
| Soil layer (L) | 37.878 ** | 3.063 ns | 2.473 ns | ||
| F × L | 12.327 ** | 7.563 ** | 5.566 ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Yang, Q.; Liu, W.; Jiang, Y.; Guo, X.; Sun, R.; Luo, W.; Fang, M.; Wu, Z. Partial Organic Substitution Fertilization Improves Soil Fertility While Reducing N Mineralization in Rubber Plantations. Forests 2024, 15, 1521. https://doi.org/10.3390/f15091521
Xu W, Yang Q, Liu W, Jiang Y, Guo X, Sun R, Luo W, Fang M, Wu Z. Partial Organic Substitution Fertilization Improves Soil Fertility While Reducing N Mineralization in Rubber Plantations. Forests. 2024; 15(9):1521. https://doi.org/10.3390/f15091521
Chicago/Turabian StyleXu, Wenxian, Qiu Yang, Wenjie Liu, Yamin Jiang, Xinwei Guo, Rui Sun, Wei Luo, Mengyang Fang, and Zhixiang Wu. 2024. "Partial Organic Substitution Fertilization Improves Soil Fertility While Reducing N Mineralization in Rubber Plantations" Forests 15, no. 9: 1521. https://doi.org/10.3390/f15091521
APA StyleXu, W., Yang, Q., Liu, W., Jiang, Y., Guo, X., Sun, R., Luo, W., Fang, M., & Wu, Z. (2024). Partial Organic Substitution Fertilization Improves Soil Fertility While Reducing N Mineralization in Rubber Plantations. Forests, 15(9), 1521. https://doi.org/10.3390/f15091521









