A Climate-Sensitive Mixed-Effects Individual Tree Mortality Model for Masson Pine in Hunan Province, South–Central China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data
2.2. Variable Selection
2.2.1. Individual Tree Size Variables
2.2.2. Competition Variables
2.2.3. Site Condition Variables
2.2.4. Structural Diversity Variables
2.2.5. Climate Variables
2.3. Generalized Linear Mixed-Effects Mortality Model Development
2.4. Model Evaluation and Validation
2.5. Mortality Implementation Methods
3. Results
3.1. Generalized Linear Mixed-Effects Mortality Model
3.2. Contributions of Tree Size, Competition, Structural Diversity, and Climate Factors to Tree Mortality
3.3. The Optimal Threshold
4. Discussion
4.1. Effects of Tree Size, Competition, and Structural Diversity on Tree Mortality
4.2. Effects of Climate Factors on Tree Mortality
4.3. Threshold Selection
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Titus, S.J.; Huang, S. Modeling individual tree mortality for white spruce in Alberta. Ecol. Model. 2003, 163, 209–222. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Chhin, S.; Wang, H.; Duan, A.; Zhang, J. Relative contributions of competition, stand structure, age, and climate factors to tree mortality of chinese fir plantations: Long-term spacing trials in southern China. For. Ecol. Manag. 2020, 465, 118103. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Q.; Sharma, R.; Wang, Y.; He, P.; Guo, J.; Lei, Y.; Fu, L. A climate sensitive mixed-effects diameter class mortality model for prince rupprecht larch (Larix gmelinii var. Principis-rupprechtii) in northern China. For. Ecol. Manag. 2021, 491, 119091. [Google Scholar] [CrossRef]
- Hawkes, C. Woody plant mortality algorithms: Description, problems and progress. Ecol. Model. 2000, 126, 225–248. [Google Scholar] [CrossRef]
- Brandl, S.; Paul, C.; Knoke, T.; Falk, W. The influence of climate and management on survival probability for Germany’s most important tree species. For. Ecol. Manag. 2020, 458, 117652. [Google Scholar] [CrossRef]
- Li, Y.; Kang, X.; Zhang, Q.; Guo, W. Modelling tree mortality across diameter classes using mixed-effects zero-inflated models. J. For. Res. 2018, 31, 131–140. [Google Scholar] [CrossRef]
- Qiu, S.; Xu, M.; Li, R.; Zheng, Y.; Clark, D.; Cui, X.; Liu, L.; Lai, C.; Zhang, W.; Liu, B. Climatic information improves statistical individual-tree mortality models for three key species of Sichuan Province, China. Ann. For. Sci. 2015, 72, 443–455. [Google Scholar] [CrossRef]
- Alenius, V.; Hökkä, H.; Salminen, H.; Jutras, S. Modelling forest systems. In Proceedings of the Workshop on the Interface between Reality, Modelling and the Parameter Estimation Processes, Sesimbra, Portugal, 2–5 June 2002; pp. 225–236. [Google Scholar]
- Adame, P.; Río, M.; Cañellas, I. Modeling individual-tree mortality in Pyrenean oak (Quercus pyrenaica willd.) stands. Ann. For. Sci. 2010, 67, 810. [Google Scholar] [CrossRef]
- Timilsina, N.; Staudhammer, C. Individual tree mortality model for slash pine in Florida: A mixed modeling approach. S. J. Appl. For. 2012, 36, 211–219. [Google Scholar]
- Pedersen, S. Models of Individual Tree Mortality for Trembling Aspen, Lodgepole Pine, Hybrid Spruce and Subalpine Fir in Northwestern British Columbia. Ph.D. Thesis, Sveriges lantbruksuniversitet, Umeå, Sweden, 2007. [Google Scholar]
- Rennolls, K.; Clutter, J.; Fortson, J.; Pienaar, L.; Brister, G.; Bailey, R. Timber management: A quantitative approach. Biometrics 1983, 40, 569. [Google Scholar] [CrossRef]
- Yao, X.; Titus, S.; Macdonald, S. A generalized logistic model of individual tree mortality for aspen, white spruce, and lodgepole pine in Alberta mixedwood forests. Can. J. For. Res. 2001, 31, 283–291. [Google Scholar] [CrossRef]
- Mabvurira, D.; Miina, J. Individual-tree growth and mortality models for Eucalyptus grandis (hill) maiden plantations in Zimbabwe. For. Ecol. Manag. 2002, 161, 231–245. [Google Scholar] [CrossRef]
- Eid, T.; Tuhus, E. Models for individual tree mortality in Norway. For. Ecol. Manag. 2001, 154, 69–84. [Google Scholar] [CrossRef]
- Monserud, R.; Sterba, H. Modeling individual tree mortality for Austrian forest species. For. Ecol. Manag. 1999, 113, 109–123. [Google Scholar] [CrossRef]
- Allison, P. Logistic Regression Using the SAS System: Theory and Application; SAS Publishing: New York, NY, USA, 1999. [Google Scholar]
- Grégoire, T.; Schabenberger, O.; Barrett, J. Linear modelling of irregularly spaced, unbalanced, longitudinal data from permanent-plot measurements. Can. J. For. Res. 2011, 25, 137–156. [Google Scholar] [CrossRef]
- Ma, Z.; Peng, C.; Li, W.; Zhu, Q.; Wang, W.; Song, X.; Liu, J. Modeling individual tree mortality rates using marginal and random effects rgression models. Nat. Resour. Model. 2013, 26, 131–153. [Google Scholar] [CrossRef]
- Groom, J.; Hann, D.; Temesgen, H. Evaluation of mixed-effects models for predicting douglas-fir mortality. For. Ecol. Manag. 2012, 276, 139–145. [Google Scholar] [CrossRef]
- Wang, W.; Bai, Y.; Jiang, C.; Yang, H.; Meng, J. Development of a linear mixed-effects individual-tree basal area increment model for masson pine in Hunan Province, south-central China. J. Sustain. For. 2020, 39, 526–541. [Google Scholar] [CrossRef]
- Fu, L.; Sharma, R.; Hao, K.; Tang, S. A generalized interregional nonlinear mixed-effects crown width model for prince rupprecht larch in northern China. For. Ecol. Manag. 2017, 389, 364–373. [Google Scholar] [CrossRef]
- Xu, H.; Sun, Y.; Wang, X.; Wang, J.; Fu, Y. Linear mixed-effects models to describe individual tree crown width for China-fir in Fujian province, southeast China. PLoS ONE 2015, 10, e0122257. [Google Scholar]
- Crecente-Campo, F.; Tome, M.; Soares, P.; Dieguez-Aranda, U. A generalized nonlinear mixed-effects height-diameter model for Eucalyptus globulus L. In northwestern Spain. For. Ecol. Manag. 2010, 259, 943–952. [Google Scholar] [CrossRef]
- Sharma, M.; Parton, J. Height–diameter equations for boreal tree species in Ontario using a mixed-effects modeling approach. For. Ecol. Manag. 2007, 249, 187–198. [Google Scholar] [CrossRef]
- Hamilton, D. A logistic model of mortality in thinned and unthinned mixed conifer stands of northern Idaho. For. Sci. 1986, 32, 989–1000. [Google Scholar] [CrossRef]
- Wyckoff, P.; Clark, J. Predicting tree mortality from diameter growth: A comparison of maximum likelihood and Bayesian approaches. Can. J. For. Res. 2000, 30, 156–167. [Google Scholar] [CrossRef]
- Allen, C.; Macalady, A.; Chenchouni, H.; Bachelet, D.; Mcdowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.; Hogg, E.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Mantgem, P.; Stephenson, N.; Byrne, J.; Daniels, L.; Franklin, J.; Fule, P.; Harmon, M.; Larson, A.; Smith, J.; Taylor, A.; et al. Widespread increase of tree mortality rates in the western united states. Science 2009, 323, 521–524. [Google Scholar] [CrossRef]
- Neumann, M.; Mues, V.; Moreno, A.; Hasenauer, H.; Seidl, R. Climate variability drives recent tree mortality in Europe. Glob. Chang. Biol. 2017, 23, 4788–4797. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ma, Z.; Lei, X.; Zhu, Q.; Chen, H.; Wang, W.; Liu, S.; Li, W.; Fang, X.; Zhou, X. A drought-induced pervasive increase in tree mortality across Canada’s boreal forests. Nat. Clim. Chang. 2011, 1, 467–471. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. Report of Forest Resources in China (2014–2018); China Forestry Publishing House: Beijing, China, 2019; pp. 1–2.
- Wang, T.; Wang, G.; Innes, J.; Seely, B.; Chen, B. Climateap: An application for dynamic local downscaling of historical and future climate data in Asia pacific. Front. Agric. Sci. Eng. 2017, 4, 448–458. [Google Scholar] [CrossRef]
- Hamilton, D. Extending the range of applicability of an individual tree mortality model. Can. J. For. Res. 1990, 20, 1212–1218. [Google Scholar] [CrossRef]
- Jutras, S.; Hokka, H.; Alenius, V.; Salminen, H. Modeling mortality of individual trees in drained peatland sites in Finland. Silva Fenn. 2003, 37, 235–251. [Google Scholar] [CrossRef]
- Silvertown, J. Introduction to plant population ecology. Vegetatio 1984, 56, 86. [Google Scholar]
- Goff, F.; West, D. Canopy-understory interaction effects on forest population structure. For. Sci. 1975, 21, 98–108. [Google Scholar]
- Weiner, J. Asymmetric competition in plant populations. Trends Ecol. Evol. 1990, 5, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Cannell, M.; Rothery, P.; David, F. Competition within stands of Picea sitchensis and Pinus contorta. Ann. Bot. 1984, 53, 349–362. [Google Scholar] [CrossRef]
- Weiner, S. Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia 1998, 113, 447–455. [Google Scholar]
- Soares, P.; Tomé, M. Globtree: An Individual Tree Growth Model for Eucalyptus globulus in Portugal; CABI Publishing: Wallingford, UK, 2003; pp. 97–110. [Google Scholar]
- Barclay, H.; Layton, C. Growth and mortality in managed douglas fir: Relation to a competition index. For. Ecol. Manag. 1990, 36, 187–204. [Google Scholar] [CrossRef]
- Casper, B.; Jackson, R. Plant competition underground. Annu. Rev. Ecol. Syst. 1997, 28, 545–570. [Google Scholar] [CrossRef]
- Fridman, J.; Goran, S. A three-step approach for modelling tree mortality in Swedish forests. Scand. J. For. Res. 2001, 16, 455–466. [Google Scholar] [CrossRef]
- Stage, A. An expression for the effect of aspect, slope, and habitat type on tree growth. For. Sci. 1976, 22, 457–460. [Google Scholar]
- Liang, J.; Buongiorno, J.; Monserud, R.; Kruger, E.; Zhou, M. Effects of diversity of tree species and size on forest basal area growth, recruitment, and mortality. For. Ecol. Manag. 2007, 243, 116–127. [Google Scholar] [CrossRef]
- Pretzsch, H. Analysis and modeling of spatial stand structures. Methodological considerations based on mixed beech-larch stands in lower saxony. For. Ecol. Manag. 1997, 97, 237–253. [Google Scholar] [CrossRef]
- Lei, X.; Wang, W.; Peng, C. Relationships between stand growth and structural diversity in spruce-dominated forests in New Brunswick, Canada. Can. J. For. Res. 2009, 39, 1835–1847. [Google Scholar] [CrossRef]
- Meng, J.; Li, S.; Wang, W.; Liu, Q.; Xie, S.; Wu, M. Estimation of forest structural diversity using the spectral and textural information derived from spot-5 satellite images. Remote Sens. 2006, 8, 125. [Google Scholar] [CrossRef]
- Ozdemir, I.; Karnieli, A. Predicting forest structural parameters using the image texture derived from worldview-2 multispectral imagery in a dryland forest, Israel. Int. J. Appl. Earth Obs. 2011, 13, 701–710. [Google Scholar] [CrossRef]
- Mccullagh, P. Generalized linear models. Eur. J. Oper. Res. 1989, 16, 285–292. [Google Scholar] [CrossRef]
- Hosmer, D.; Lemeshow, S. Applied Logistic Regression; Wiley: New York, NY, USA, 2000; pp. 352–353. [Google Scholar]
- Saveland, J.; Neuenschwander, L. A signal detection framework to evaluate models of tree mortality following fire damage. For. Sci. 1990, 36, 66–76. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, Y.; Cao, Q.; Chen, X.; Liu, X. Improving tree survival prediction with forecast combination and disaggregation. Can. J. For. Res. 2011, 41, 1928–1935. [Google Scholar] [CrossRef]
- Schabenberger, O.; Pierce, F. Contemporary Statistical Models for the Plant and Soil Sciences; CRC Press: New York, NY, USA, 2001. [Google Scholar]
- Mac Nally, R.; Walsh, C. Hierarchical partitioning public-domain software. Biodivers. Conserv. 2004, 13, 659–660. [Google Scholar] [CrossRef]
- Weber, L.; Ek, A.; Droessler, T. Comparison of stochastic and deterministic mortality estimation in an individual tree based stand growth model. Can. J. For. Res. 1986, 16, 1139–1141. [Google Scholar] [CrossRef]
- Connell, J. Apparent versus “Real” Competition in Plants; Academic Press: New York, NY, USA, 1990; pp. 9–26. [Google Scholar]
- Shifley, S.; Fan, Z.; Kabrick, J.; Jensen, R. Oak mortality risk factors and mortality estimation. For. Ecol. Manag. 2006, 229, 16–26. [Google Scholar] [CrossRef]
- Thomas, B.; Thomas, C.; Georges, K.; Christian, P.; Guillaume, L.; Benoit, C.; Dusan, G. Tree size inequality reduces forest productivity: An analysis combining inventory data for ten European species and a light competition model. PLoS ONE 2016, 11, e0151852. [Google Scholar]
- Nykaenen, M.; Peltola, H.; Quine, C.; Kellomaeki, S.; Broadgate, M. Factors affecting snow damage of trees with particular reference to European conditions. Silva Fenn. 1997, 31, 193–213. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Q.; Xu, M.; Chi, Y.; Shen, R.; Li, P.; Dai, H. Responses of Pinus massoniana and Pinus taeda to freezing in temperate forests in central China. Can. J. For. Res. 2012, 27, 520–531. [Google Scholar]
- Bae, J.; Choo, Y.; Ono, K.; Sumida, A.; Hara, T. Photoprotective mechanisms in cold-acclimated and nonacclimated needles of Picea glehnii. Photosynthetica 2010, 48, 110–116. [Google Scholar] [CrossRef]
- Bravo, L.; Saavedra-Mella, F.; Vera, F.; Guerra, A.; Cavieres, L.; Ivanov, A.; Huner, N.; Corcuera, L. Effect of cold acclimation on the photosynthetic performance of two ecotypes of Colobanthus quitensis (kunth) bartl. J. Exp. Bot. 2007, 58, 3581. [Google Scholar] [CrossRef]
- Pearce, R. Plant freezing and damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Vogel, J.; Zarka, D.; Buskirk, H.; Fowler, S.; Thomashow, M. Roles of the cbf2 and zat12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 2005, 41, 195–211. [Google Scholar] [CrossRef]
- Lines, E.; Zavala, M.; Purves, D.; Coomes, D. Predictable changes in aboveground allometry of trees along gradients of temperature, aridity and competition. Glob. Ecol. Biogeogr. 2012, 21, 1017–1028. [Google Scholar] [CrossRef]
- Vygodskaya, N.; Schulze, E.; Tchebakova, N.; Karpachevskii, L.; Kozlov, D.; Sidorov, K.; Panfyorov, M.; Abrazko, M.; Shaposhnikov, E.; Solnzeva, O.; et al. Climate control of stand thinning in unmanaged spruce forests of the southern taiga in European Russia. Tellus B 2002, 54, 443–461. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, L.; Cao, Q.; Duan, A.; Zhang, J. Climate-sensitive self-thinning trajectories of chinese fir plantations in south China. Can. J. For. Res. 2018, 48, 1388–1397. [Google Scholar] [CrossRef]
- Balakhnina, T. Plant Responses to Soil Flooding; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Das, A.; Battles, J.; Stephenson, N.; van Mantgem, P. The relationship between tree growth patterns and likelihood of mortality: A study of two tree species in the Sierra Nevada. Can. J. For. Res. 2007, 37, 580–597. [Google Scholar] [CrossRef]
- Flewelling, J.; Monserud, R. Published Comparing methods for modelling tree mortality. In Proceedings of the Second Forest Vegetation Simulator Conference; USDA for Serv Proceedings RMRS-P-25; USDA Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2002; pp. 168–175. [Google Scholar]
- Wykoff, W.; Crookston, N.; Stage, A. User’s Guide to the Stand Prognosis Model; U.S. Dept. of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1982.
- Fortin, M.; Bédard, S.; DeBlois, J.; Meunier, S. Predicting individual tree mortality in northern hardwood stands under uneven-aged management in southern Québec, Canada. Ann. For. Sci. 2008, 65, 205. [Google Scholar] [CrossRef]
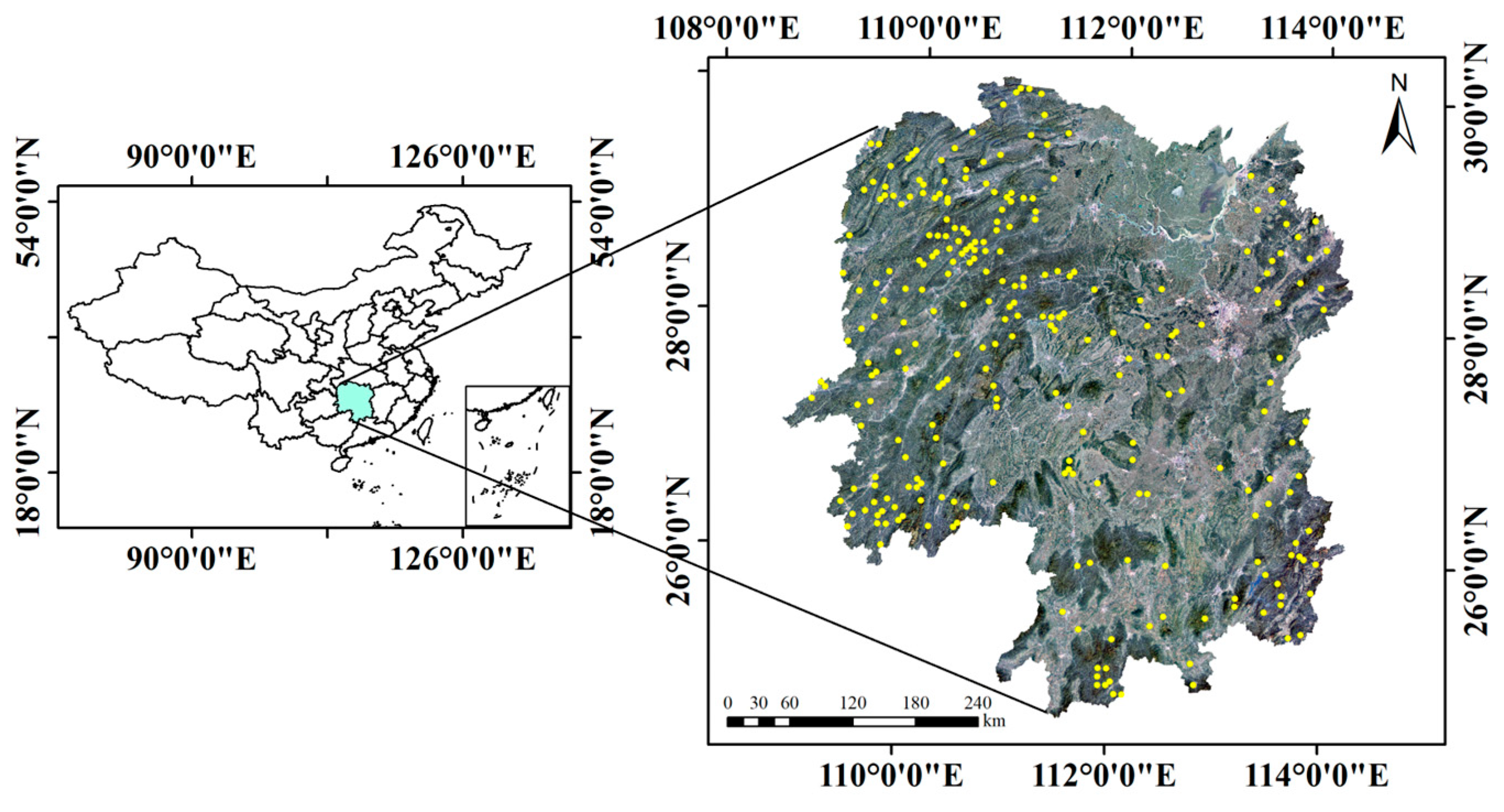
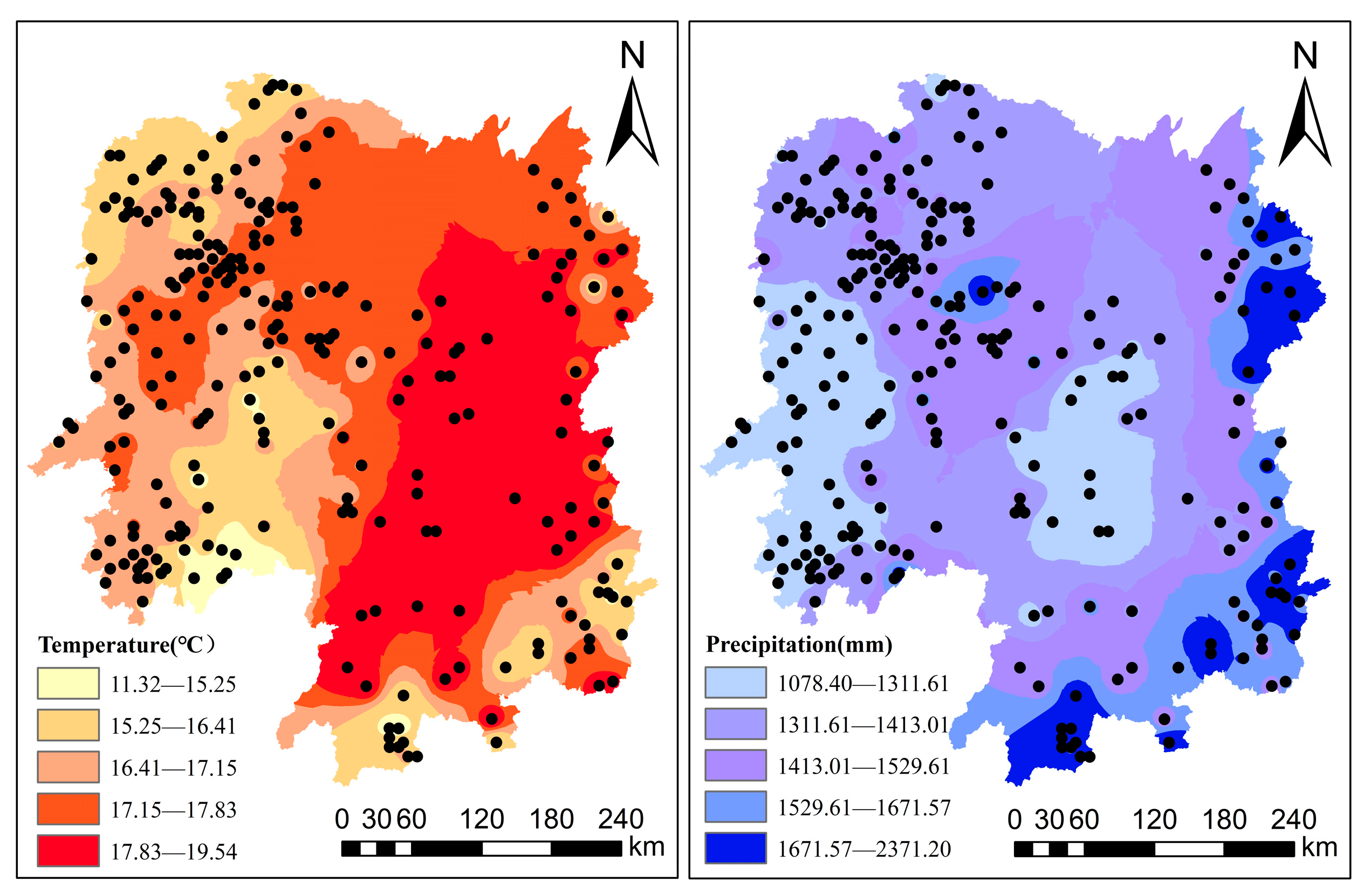


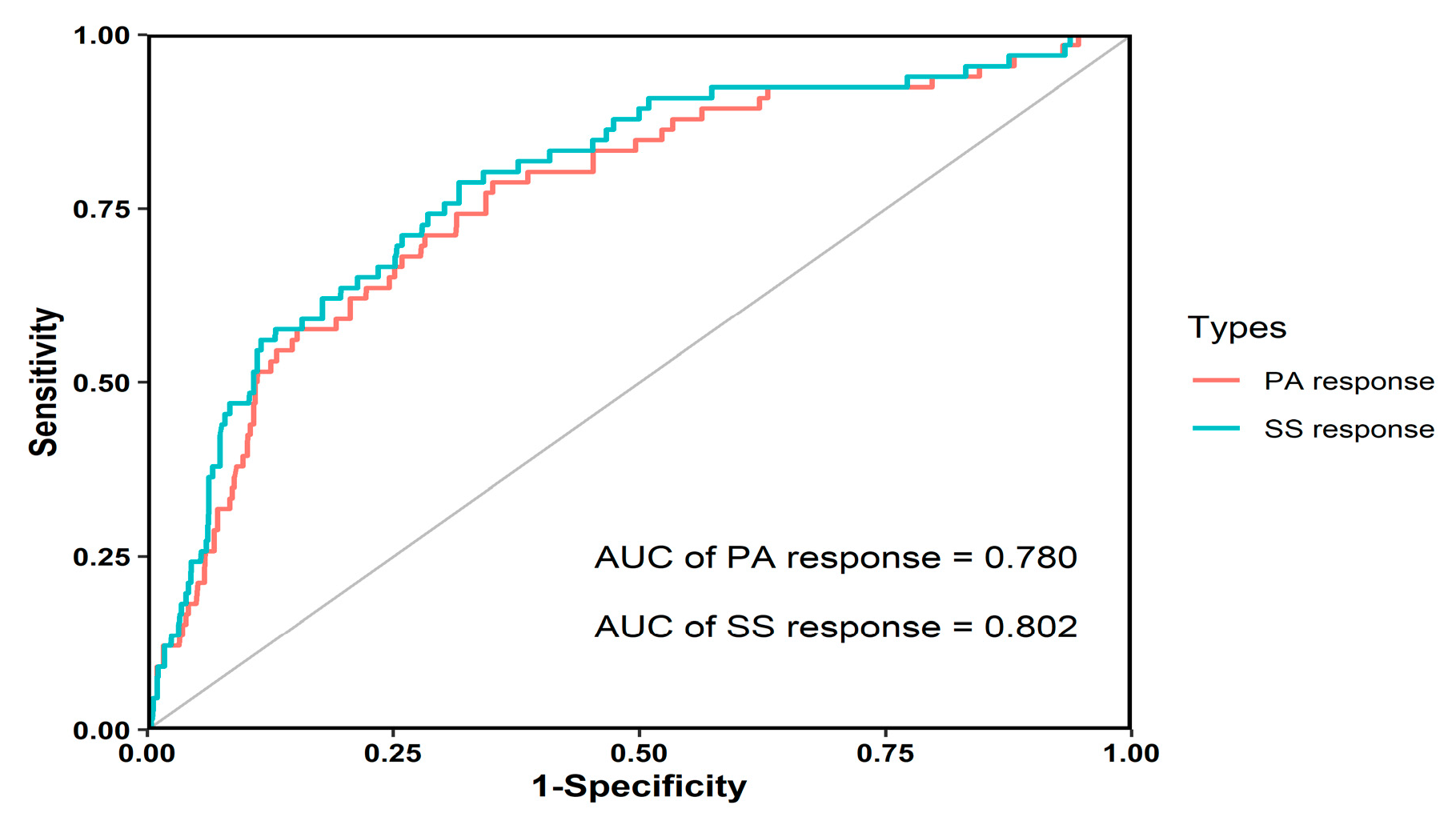

| Variable | Model Development Data | Model Validation Data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alive (4439) | Dead (221) | Alive (1203) | Dead (58) | |||||||||
| Mean (SD.) | Max. | Min. | Mean (SD.) | Max. | Min. | Mean (SD.) | Max. | Min. | Mean (SD.) | Max. | Min. | |
| DBH (cm) | 12.14 (6.16) | 48.7 | 5.0 | 9.69 (3.94) | 24.5 | 5.1 | 12.43 (6.25) | 76.9 | 5.0 | 11.12 (4.56) | 21.2 | 5.7 |
| BAL (m2) | 0.44 (0.34) | 1.99 | 0 | 0.66 (0.39) | 2.00 | 0 | 0.60 (0.40) | 1.71 | 0 | 0.96 (0.42) | 1.68 | 0 |
| BA (m2 ha−1) | 11.59 (6.54) | 32.23 | 0.71 | 13.56 (6.24) | 31.63 | 1.54 | 14.56 (6.71) | 29.43 | 1.29 | 17.68 (4.83) | 25.55 | 3.74 |
| NT (trees ha−1) | 1177.36 (575.22) | 2985 | 104 | 1488.69 (614.37) | 2806 | 239 | 1352.07 (491.26) | 2687 | 164 | 1254.50 (312.75) | 2269 | 552 |
| QMD (cm) | 11.21 (2.79) | 21.11 | 6.10 | 10.78 (2.19) | 18.22 | 6.10 | 11.61 (2.44) | 19.68 | 6.99 | 13.44 (2.15) | 17.43 | 8.26 |
| EL (m) | 391.14 (253.82) | 1560 | 80 | 643.13 (335.87) | 1560 | 85 | 524.02 (259.77) | 1195 | 100 | 616.66 (255.30) | 1020 | 170 |
| SL (°) | 23.85 (10.05) | 50 | 2 | 28.67 (7.93) | 48 | 7 | 21.87 (12.10) | 46 | 5 | 24.38 (11.58) | 40 | 5 |
| ASP (°) | 147.36 (97.65) | 315 | 0 | 150.68 (84.46) | 315 | 0 | 178.84 (105.62) | 315 | 0 | 250.60 (83.49) | 315 | 90 |
| SHI | 0.51 (0.47) | 2.25 | 0 | 1.04 (0.43) | 1.88 | 0 | 0.56 (0.51) | 2.00 | 0 | 0.98 (0.42) | 1.78 | 0.49 |
| SII | 0.26 (0.24) | 0.88 | 0 | 0.52 (0.20) | 0.83 | 0 | 0.29 (0.25) | 0.83 | 0 | 0.49 (0.18) | 0.79 | 0.23 |
| PI | 0.44 (0.27) | 1.00 | 0.03 | 0.62 (0.19) | 0.98 | 0.05 | 0.52 (0.25) | 0.96 | 0.14 | 0.60 (0.18) | 0.94 | 0.30 |
| GC | 0.36 (0.10) | 0.71 | 0.15 | 0.40 (0.08) | 0.71 | 0.20 | 0.40 (0.08) | 0.71 | 0.27 | 0.43 (0.03) | 0.50 | 0.37 |
| SDDBH | 3.74 (1.78) | 10.65 | 0.94 | 3.95 (1.42) | 10.23 | 1.16 | 4.22 (1.58) | 9.64 | 1.75 | 5.26 (0.93) | 6.56 | 3.02 |
| MAT (°C) | 17.31 (0.93) | 19.54 | 11.32 | 16.65 (1.18) | 18.86 | 11.32 | 16.80 (0.98) | 18.98 | 12.42 | 16.70 (1.09) | 18.64 | 15.34 |
| MAP (mm) | 1378.89 (172.60) | 2087.2 | 1078.4 | 1501.05 (234.80) | 2087.2 | 1128.6 | 1436.43 (193.82) | 2371.2 | 1170.0 | 1592.00 (268.99) | 2371.2 | 1276.4 |
| MSP (mm) | 160.96 (21.71) | 245.40 | 127.68 | 176.77 (25.79) | 245.28 | 127.68 | 168.53 (24.30) | 261.04 | 122.84 | 190.80 (25.15) | 261.04 | 137.68 |
| MP1 (mm) | 49.77 (18.69) | 96.4 | 15.6 | 52.84 (26.07) | 103.2 | 19.2 | 48.80 (15.29) | 77.0 | 16.8 | 55.16 (17.77) | 87.2 | 20.6 |
| MP7 (mm) | 150.79 (42.65) | 313.2 | 89.6 | 154.82 (33.07) | 291.2 | 89.6 | 155.49 (46.63) | 259.0 | 86.2 | 197.66 (56.08) | 269.0 | 106.4 |
| MWMT (°C) | 28.15 (1.37) | 30.44 | 21.88 | 27.15 (1.61) | 30.14 | 21.88 | 27.51 (1.66) | 30.18 | 22.94 | 27.14 (1.22) | 29.96 | 24.72 |
| MCMT (°C) | 4.74 (0.77) | 7.62 | −1.26 | 4.47 (1.12) | 6.38 | −1.26 | 4.47 (0.91) | 6.98 | −0.02 | 4.40 (1.29) | 6.98 | 3.06 |
| PAS (mm) | 4.78 (3.06) | 69.0 | 1.8 | 6.83 (10.06) | 69.0 | 1.8 | 5.43 (3.39) | 40.6 | 2.0 | 6.84 (3.97) | 14.8 | 2.2 |
| Variable | Parameter | Estimate | Standard Error | z | p | VIF 1 |
|---|---|---|---|---|---|---|
| Intercept | −3.32 × 101 | 1.29 × 101 | −2.56 × 100 | 1.00 × 10−2 | - | |
| DBH | −1.13 × 10−1 | 3.90 × 10−2 | −2.88 × 100 | 4.00 × 10−3 | 1.10 | |
| GC | 2.98 × 100 | 3.13 × 100 | 9.50 × 10−1 | 3.42 × 10−1 | 1.33 | |
| BAL | 2.10 × 100 | 6.18 × 10−1 | 3.40 × 100 | 6.52 × 10−4 | 1.17 | |
| MCMT | −1.48 × 10−1 | 3.09 × 10−1 | −4.78 × 10−1 | 6.33 × 10−1 | 1.06 | |
| Log (MSP) | 5.38 × 100 | 2.43 × 100 | 2.21 × 100 | 2.70 × 10−2 | 1.21 | |
| AIC = 1029; BIC = 1074, −2LL = 1015 (df = 7) | ||||||
| = 9.769, p = 0.282 | ||||||
| Method | ACR (%) | Sensitivity (%) | Specificity (%) | Predicted Dead Trees (%) |
|---|---|---|---|---|
| (0.25) | 96.65 | 65.61 | 98.20 | 4.83 |
| (0.06) | 90.86 | 91.86 | 90.80 | 13.11 |
| Random number | 95.67 | 76.92 | 96.60 | 6.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, N.; He, Y.; Chen, K.; Lv, Y.; Wang, J.; Zhang, Z. A Climate-Sensitive Mixed-Effects Individual Tree Mortality Model for Masson Pine in Hunan Province, South–Central China. Forests 2024, 15, 1543. https://doi.org/10.3390/f15091543
Yan N, He Y, Chen K, Lv Y, Wang J, Zhang Z. A Climate-Sensitive Mixed-Effects Individual Tree Mortality Model for Masson Pine in Hunan Province, South–Central China. Forests. 2024; 15(9):1543. https://doi.org/10.3390/f15091543
Chicago/Turabian StyleYan, Ni, Youjun He, Keyi Chen, Yanjie Lv, Jianjun Wang, and Zhenzhong Zhang. 2024. "A Climate-Sensitive Mixed-Effects Individual Tree Mortality Model for Masson Pine in Hunan Province, South–Central China" Forests 15, no. 9: 1543. https://doi.org/10.3390/f15091543




