Biomass Equations and Carbon Stock Estimates for the Southeastern Brazilian Atlantic Forest
Abstract
:1. Introduction
2. Material and Methods
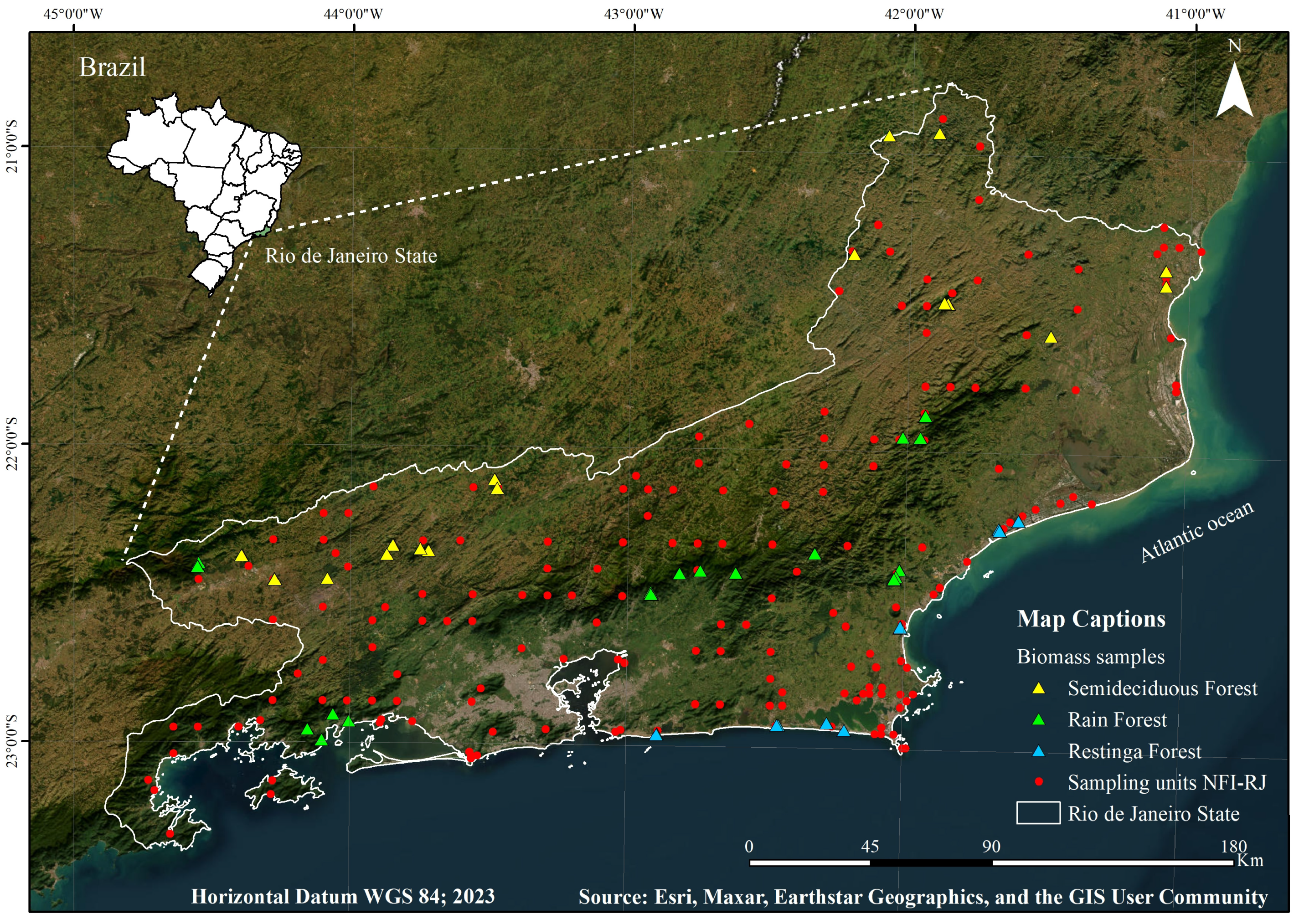
2.1. Study Area
2.2. Experimental Design
2.3. Sampling for the Biomass Equation Fitting
2.4. Non-Destructive Wood Volume Determination
2.5. Basic Wood Density and Carbon Content
2.6. Sampling for Biomass Estimation (Forest Inventory)
2.7. Data Analysis
2.7.1. Allometric Equation Fitting
2.7.2. Biomass Stock Estimates
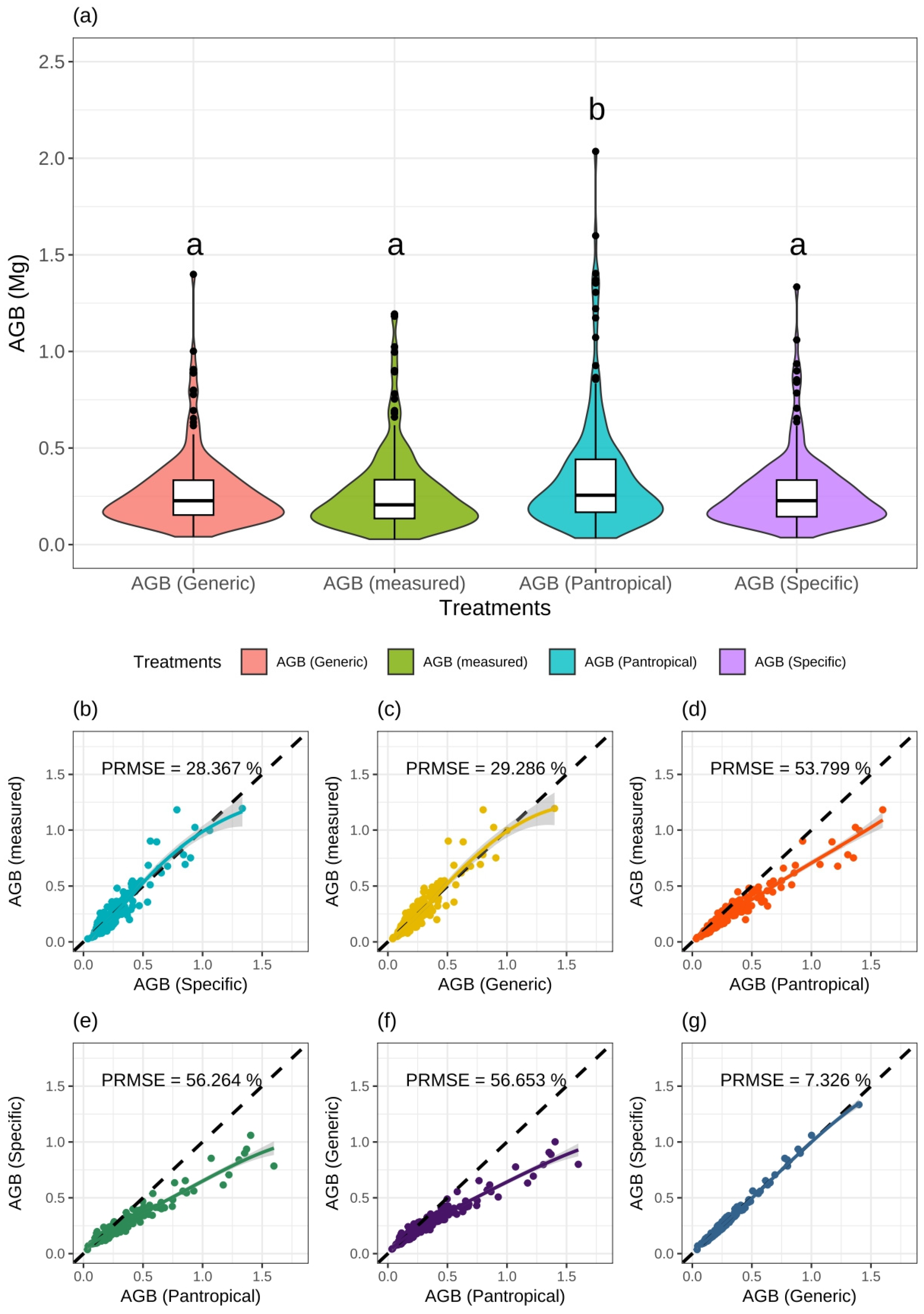
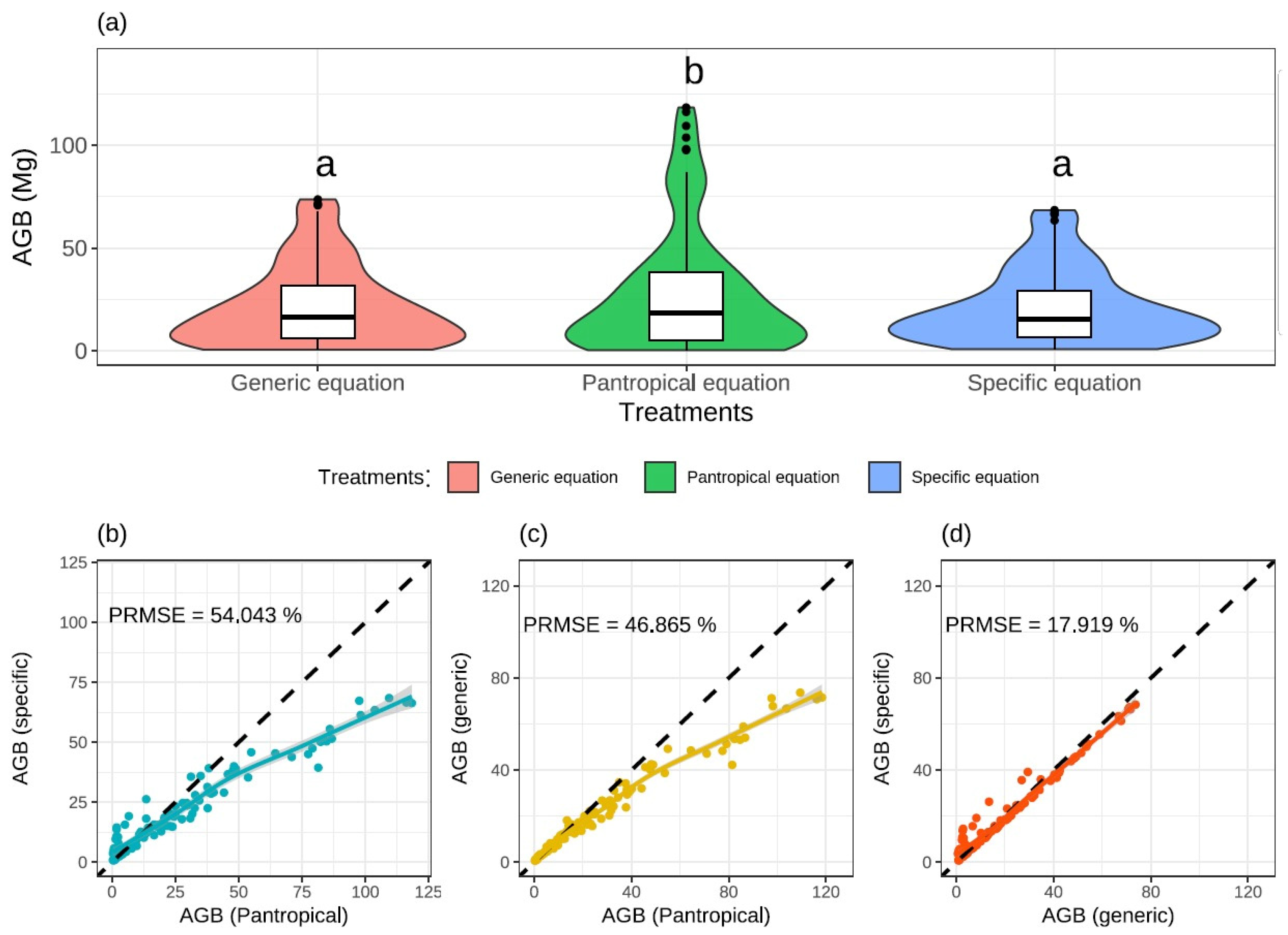
2.7.3. Model Validation and Hypothesis Testing
3. Results
3.1. Model Fitting and Selection
3.2. Estimates of the Biomass and Carbon Stocks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Rödenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial Gross Carbon Dioxide Uptake: Global Distribution and Covariation with Climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Wright, J. Spatial patterns and recent trends in the climate of tropical rainforest regions. R. Soc. 2004, 359, 311–329. [Google Scholar] [CrossRef] [PubMed]
- SOS MATA ATLÂNTICA; INPE. Atlas dos Remanescentes Florestais da Mata Atlântica Período 2019–2020. Fundação SOS Mata Atlântica and INPE: São Paulo, Brazil, 2021; p. 73. Available online: https://cms.sosma.org.br/wp-content/uploads/2021/05/SOSMA_Atlas-da-Mata-Atlantica_2019-2020.pdf (accessed on 13 December 2023).
- Lima, R.A.F.; Oliveira, A.A.; Pitta, G.R.; Gasper, A.L.; Vibrans, A.C.; Chave, J.; ter Steege, H.; Prado, P.I. The erosion of biodiversity and biomass in the Atlantic Forest biodiversity hotspot. Nat. Commun. 2020, 11, 6347. [Google Scholar] [CrossRef] [PubMed]
- Metzger, J.P.; Martensen, A.C.; Dixo, M.; Bernacci, L.C.; Ribeiro, M.C.; Teixeira, A.M.G.; Pardini, R. Time-lag in biological responses to landscape changes in a highly dynamic Atlantic forest region. Biol. Conserv. 2009, 142, 1166–1177. [Google Scholar] [CrossRef]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G.; Carmona, A.; Arana, M.; Mercado-Gómez, J.D. Biogeographic regionalization of the Neotropical region: New map and shapefile. An. Acad. Bras. Ciências 2022, 94, e20211167. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Scolforo, J.R.; Oliveira, A.D.; Acerbi-Júnior, F.W. Inventário Florestal de Minas Gerais: Equações de Volume, peso de Matéria seca e Carbono para Diferentes Fisionomias da Flora Nativa, 1st ed.; Editora UFLA: Lavras, Brazil, 2008; p. 216. [Google Scholar]
- Souza, A.L.; Boina, A.; Soares, C.P.B.; Vital, B.R.; Gaspar, R.O.; Lana, J.M. Estrutura fitossociológica, estoques de volume, biomassa, carbono e dióxido de carbono em Floresta Estacional Semidecidual. Rev. Árvore 2012, 36, 169–179. [Google Scholar] [CrossRef]
- Figueiredo, L.T.M.; Soares, C.P.B.; Sousa, A.L.; Leite, H.G.; Silva, G.F. Dinâmica do estoque de carbono em fuste de árvores de uma Floresta Estacional Semidecidual. Cerne 2015, 21, 161–167. [Google Scholar] [CrossRef]
- Vibrans, A.C.; Moser, P.; Oliveira, L.Z.; Maçaneiro, J.P. Generic and specific stem volume models for three subtropical forest types in southern Brazil. Ann. For. Sci. 2015, 72, 865–874. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Souza, C.R.; Azevedo, C.P.; Rossi, L.M.B.; Silva, K.E.; Santos, J.; Higuchi, N. Dynamics and carbon storage in primary forest in the region of Manaus/AM. Acta Amazon. 2012, 42, 501–506. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Yanai, A.M.; Fonseca, F.O.R.; Fearnside, P.M. Carbon stock loss from deforestation through 2013 in Brazilian Amazonia. Glob. Chang. Biol. 2015, 21, 1271–1292. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.E.; Souza, C.R.; Azevedo, C.P.; Rossi, L.M.B. Dinâmica florestal, estoque de carbono e fitossociologia de uma floresta densa de terra-firme na Amazônia Central. Sci. For. 2015, 43, 193–201. [Google Scholar]
- Marra, D.M.; Higuchi, N.; Trumbore, S.E.; Ribeiro, G.H.P.M.; Santos, J.; Carneiro, V.M.C.; Lima, A.J.N.; Chambers, J.Q.; Negrón-Juárez, R.I.; Holzwarth, F.; et al. Predicting biomass of hyperdiverse and structurally complex central Amazonian forests—A virtual approach using extensive field data. Biogeosciences 2016, 13, 1553–1570. [Google Scholar] [CrossRef]
- Cysneiros, V.C.; Gaui, T.D.; Filho, T.B.S.; Pelissari, A.L.; Machado, S.A.; Carvalho, D.C.; Moura, T.A.; Amorim, H.B. Tree volume modeling for forest types in the Atlantic Forest: Generic and specific models. iForest 2020, 13, 417–425. [Google Scholar] [CrossRef]
- Vieira, S.A.; Alves, L.F.; Aidar, M.; Araújo, L.S.; Baker, T.; Batista, J.L.F.; Campos, M.C.; Camargo, P.B.; Chave, J.; Delitti, W.B.C.; et al. Estimation of biomass and carbon stocks: The case of the Atlantic Forest. Biota Neotrop. 2008, 8, 21–29. [Google Scholar] [CrossRef]
- Colmanetti, M.A.A.; Weiskittel, A.; Scolforo, H.F.; Sotomayor, J.F.M.; Couto, H.T.Z. Calibrating individual tree biomass models for contrasting tropical species at an uneven-aged site in the native Atlantic Forest of Brazil: A direct comparison of alternative approaches, sample sizes, and sample selection methods. For. Ecol. Manag. 2020, 473, 118306. [Google Scholar] [CrossRef]
- Vorster, A.G.; Evangelista, P.H.; Stovall, A.E.L.; Ex, S. Variability and uncertainty in forest biomass estimates from the tree to landscape scale: The role of allometric equations. Carbon Balance Manag. 2020, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- SFB—Serviço Florestal Brasileiro. Inventário Florestal Nacional: Principais Resultados: Rio de Janeiro, 1st ed.; MMA: Brasília, Brazil, 2018; p. 83. Available online: https://snif.florestal.gov.br/images/pdf/publicacoes/periodo_eleitoral/publicacoes_ifn/relatorios/IFN_RJ_2018_periodo_eleitoral.pdf (accessed on 13 January 2024).
- Haya, B.K.; Alford-Jones, K.; Anderegg, W.R.L.; Beymer-Farris, B.; Blanchard, L.; Bomfim, B.; Chin, D.; Evans, S.; Hogan, M.; Holm, J.A.; et al. Quality Assessment of REDD+ Carbon Credit Projects; Berkeley Public Policy: Berkeley, CA, USA, 2023; p. 12. Available online: https://gspp.berkeley.edu/assets/uploads/page/Quality-Assessment-of-REDD+-Carbon-Crediting-EXECUTIVE-SUMMARY.pdf (accessed on 10 January 2024).
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Pereira, M.G.; Anjos, L.H.C.; Neto, E.C.S.; Junior, C.R.P. Solos do Rio de Janeiro: Gênese, Classificação e Limitações ao Uso Agrícola, 1st ed.; Atena: Ponta Grossa, Brazil, 2023; p. 231. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; p. 356. Available online: https://www.agroapi.cnptia.embrapa.br/portal/assets/docs/SiBCS-2018-ISBN-9788570358004.pdf (accessed on 13 January 2024).
- IUSS—International Union of Soil Sciences Working Group WRB. World Reference Base for Soil Resources: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences: Vienna, Austria, 2022; p. 236. Available online: https://www.isric.org/sites/default/files/WRB_fourth_edition_2022-12-18.pdf (accessed on 13 January 2024).
- APG IV. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- REFLORA—Flora e Funga do Brasil. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 1 June 2024).
- Avery, T.E.; Burkhart, H.E. Forest Measurements, 5th ed.; Waveland Press: Long Grove, IL, USA, 2015; p. 456. [Google Scholar]
- IPCC—Intergovernmental Panel on Climate Change. Climate Change 2007: Climate Change Impacts, Adaption and Vulnerability, 1st ed.; Cambridge University Press: New York, NY, USA, 2007; p. 976. Available online: https://www.ipcc.ch/site/assets/uploads/2018/03/ar4_wg2_full_report.pdf (accessed on 10 January 2024).
- Clutter, J.; Fortson, J.C.; Pienaar, L.V.; Brister, G.H.; Bailey, R.L. Timber Management: A Quantitative Approach, 1st ed.; John Wiley and Sons: New York, NY, USA, 1983; p. 333. [Google Scholar]
- Loetsch, F.; Zöhrer, F.; Haller, K.E. Forest Inventory, 2nd ed.; BLV Verlagsgesellschaft: Munich, Germany, 1973; p. 469. [Google Scholar]
- R Core Team. Available online: http://www.R-project.org (accessed on 3 December 2023).
- Husch, B.; Beers, T.W.; Kershaw, J.A., Jr. Forest Mensuration, 4th ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2003; p. 443. [Google Scholar]
- Spurr, S.H. Forestry Inventory, 1st ed.; Ronald Press: New York, NY, USA, 1952; p. 476. [Google Scholar]
- Schumacher, F.; Hall, F. Logarithmic expression of timber-tree volume. J. Agric. Res. 1933, 47, 719–734. [Google Scholar]
- Sileshi, G.W. A critical review of forest biomass estimation models, common mistakes and corrective measures. For. Ecol. Manag. 2014, 329, 237–254. [Google Scholar] [CrossRef]
- Aabeyir, R.; Adu-Bredu, S.; Agyare, W.A.; Weir, M.J.C. Allometric models for estimating aboveground biomass in the tropical woodlands of Ghana, West Africa. For. Ecosyst. 2020, 7, 41. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Huang, Z.; Xiang, W.; Yan, W.; Lei, P.; Zhou, X.; Peng, C. Development and evaluation of models for the relationship between tree height and diameter at breast height for Chinese-fir plantations in Subtropical China. PLoS ONE 2015, 10, e0125118. [Google Scholar] [CrossRef]
- Draper, N.R.; Smith, H. Applied Regression Analysis, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1998; p. 736. [Google Scholar] [CrossRef]
- Queiroz, W.T. Amostragem em Inventário Florestal, 1st ed.; Edufra: Belém, Brazil, 2012; p. 441. [Google Scholar]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Data from: Global Wood Density Database. Dryad Digit. Repos. 2009. Available online: https://doi.org/10.5061/dryad.234 (accessed on 6 December 2023).
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 5, 1–26. [Google Scholar] [CrossRef]
- Robinson, A.P.; Duursma, R.A.; Marshall, J.D. A regression-based equivalence test for model validation: Shifting the burden of proof. Tree Physiol. 2005, 25, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Weiskittel, A.R.; Hann, D.W.; Kershaw, J.A.; Vanclay, J.K. Forest Growth and Yield Modeling, 1st ed.; John Wiley and Sons: New York, NY, USA, 2011; p. 432. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Fontes, M.A.L. Patterns of Floristic Differentiation among Atlantic Forests in Southeastern Brazil and the Influence of Climate. Biotropica 2000, 32, 793–810. [Google Scholar] [CrossRef]
- Scarano, F.R. Structure, Function and Floristic Relationships of Plant Communities in Stressful Habitats Marginal to the Brazilian Atlantic Rainforest. Ann. Bot. 2002, 90, 517–524. [Google Scholar] [CrossRef]
- Assis, M.A.; Prata, B.E.M.; Pedroni, F. Florestas de restinga e de terras baixas na planície costeira do sudeste do Brasil: Vegetação e heterogeneidade ambiental. Biota Neotrop. 2011, 11, 103–121. [Google Scholar] [CrossRef]
- Vibrans, A.C.; Oliveira, L.Z.; Gasper, A.L.; Lingner, D.V.; Schorn, L.A.; Silva, D.A. Unprecedented large-area turnover estimates for the subtropical Brazilian Atlantic Forest based on systematically-gathered data. For. Ecol. Manag. 2022, 505, 119902. [Google Scholar] [CrossRef]
- Chave, J.; Piponiot, C.; Maréchaux, I.; Foresta, H.; Larpin, D.; Fischer, F.J.; Derroire, G.; Vincent, G.; Hérault, B. Slow rate of secondary forest carbon accumulation in the Guianas compared with the rest of the Neotropics. Ecol. Appl. 2020, 30, e02004. [Google Scholar] [CrossRef] [PubMed]
- Chazdon, R.L.; Broadbent, E.N.; Rozendaal, D.M.A.; Bongers, F.; Zambrano, A.M.A.; Aide, T.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; et al. Carbon sequestration potential of second-growth forest regeneration in the Latin American tropics. Sci. Adv. 2016, 2, e1501639. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Bongers, F.; Aide, T.; Zambrano, A.M.A.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; Broadbent, E.N.; Chazdon, R.L.; et al. Biomass resilience of Neotropical secondary forests. Nature 2016, 530, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Brando, P.M.; Balch, J.K.; Nepstad, D.C.; Morton, D.C.; Putz, F.E.; Coe, M.T.; Silvério, D.; Macedo, M.N.; Davidson, E.A.; Nóbrega, C.C.; et al. Abrupt increases in Amazonian tree mortality due to drought–fire interactions. Proc. Natl. Acad. Sci. USA 2014, 111, 6347–6352. [Google Scholar] [CrossRef] [PubMed]
- Scaranello, M.A.S.; Alves, L.F.; Vieira, S.A.; Camargo, P.B.; Joly, C.A.; Martinelli, L.A. Height-diameter relationships of tropical Atlantic moist forest trees in southeastern Brazil. Sci. Agric. 2012, 69, 26–37. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Feldpausch, T.R.; Lloyd, J.; Lewis, S.L.; Brienen, R.J.W.; Gloor, M.; Mendoza, A.M.; Lopez-Gonzalez, G.; Banin, L.; Salim, K.A.; Affum-Baffoe, K.; et al. Tree height integrated into pantropical forest biomass estimates. Biogeosciences 2012, 9, 3381–3403. [Google Scholar] [CrossRef]
- Hunter, M.O.; Keller, M.; Victoria, D.; Morton, D.C. Tree height and tropical forest biomass estimation. Biogeosciences 2013, 10, 8385–8399. [Google Scholar] [CrossRef]
- Fearnside, P.M. Wood density for estimating forest biomass in Brazilian Amazonia. For. Ecol. Manag. 1997, 90, 59–87. [Google Scholar] [CrossRef]
- Minini, D.; Silva, J.G.M.; Paula, R.R.; Rocha, S.M.G.; Filho, T.B.S.; Gaui, T.D.; Dias, H.M.; Nabais, C.; Vidaurre, G.B. Insights about wood density in Atlantic Forest ecosystems: Spatial variability and alternative measurement. Can. J. For. Res. 2022, 52, 1212–1223. [Google Scholar] [CrossRef]
- Salimon, C.I.; Putz, F.E.; Menezes-Filho, L.; Anderson, A.; Silveira, M.; Brown, I.F.; Oliveira, L.C. Estimating state-wide biomass carbon stocks for a REDD plan in Acre, Brazil. For. Ecol. Manag. 2011, 262, 555–560. [Google Scholar] [CrossRef]
- Asner, G.P.; Powell, G.V.N.; Mascaro, J.; Knapp, D.E.; Clark, J.K.; Jacobson, J.; Kennedy-Bowdoin, T.; Balaji, A.; Paez-Acosta, G.; Victoria, E.; et al. High-resolution forest carbon stocks and emissions in the Amazon. Proc. Natl. Acad. Sci. USA 2010, 107, 16738–16742. [Google Scholar] [CrossRef] [PubMed]
- Saatchi, S.S.; Harris, N.L.; Brown, S.; Lefsky, M.; Mitchard, E.T.A.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S.; et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed]
- Baccini, A.; Goetz, S.J.; Walker, W.S.; Laporte, N.T.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.S.A.; Dubayah, R.; Friedl, M.A.; et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Chang. 2012, 2, 182–185. [Google Scholar] [CrossRef]
- Clark, D.B.; Kellner, J.R. Tropical forest biomass estimation and the fallacy of misplaced concreteness. J. Veg. Sci. 2012, 23, 1191–1196. [Google Scholar] [CrossRef]
- Yuen, J.Q.; Fung, T.; Ziegler, A.D. Review of allometric equations for major land covers in SE Asia: Uncertainty and implications for above- and below-ground carbon estimates. For. Ecol. Manag. 2016, 360, 323–340. [Google Scholar] [CrossRef]
- Ruggiero, P.G.C.; Metzger, J.P.; Tambosi, L.R.; Nichols, E. Payment for ecosystem services programs in the Brazilian Atlantic Forest: Effective but not enough. Land Use Policy 2019, 82, 283–291. [Google Scholar] [CrossRef]
- Mamedes, I.; Guerra, A.; Rodrigues, D.B.B.; Garcia, L.C.; Godoi, R.F.; Oliveira, P.T.S. Brazilian payment for environmental services programs emphasize water-related services. Int. Soil Water Conserv. Res. 2023, 11, 276–289. [Google Scholar] [CrossRef]
- Mota, P.K.; Costa, A.M.; Prado, R.B.; Fernandes, L.F.S.; Pissarra, T.C.T.; Pacheco, F.A.L. Payment for Environmental Services: A critical review of schemes, concepts, and practice in Brazil. Sci. Total Environ. 2023, 899, 165639. [Google Scholar] [CrossRef]

| Model Number | Author | Mathematical Model | Input Variables |
|---|---|---|---|
| 1 | Husch [34] | y = β0 dβ1 + εi | Single (dbh) |
| 2 | Spurr [35] | y = β0 + β 1 . (d2 .h) + εi | Multiple (dbh, Ht) |
| 3 | Schumacher e Hall [36] | y = β 0 dβ1 . hβ2 + εi |
| Equation | Model | B0 | B1 | B2 | Syx% | Ryy | AIC | VarPower |
|---|---|---|---|---|---|---|---|---|
| General | 1 | 5.45 × 10−4 | 1.9435 | 30.84 | 0.92 | −392 | 1.8148 | |
| 2 | 0.0736 | 1.74 × 10−5 | 29.78 | 0.92 | −378 | 1.4865 | ||
| 3 | 2 × 10−4 | 1.519 | 0.8251 | 27.65 | 0.92 | −426 | 2.2998 | |
| RAF | 1 | 7.2 × 10−4 | 1.8586 | 33.27 | 0.89 | −164 | 1.7630 | |
| 2 | 0.0787 | 1.63 × 10−5 | 31.85 | 0.91 | −165 | 1.4508 | ||
| 3 | 1.52 × 10−4 | 1.465 | 0.9627 | 29.73 | 0.91 | −184 | 2.2274 | |
| SF | 1 | 4.61 × 10−4 | 2.0109 | 24.71 | 0.93 | −138 | 1.3700 | |
| 2 | 0.0834 | 1.85 × 10−5 | 25.27 | 0.96 | −126 | 1.2381 | ||
| 3 | 3 × 10−4 | 1.6954 | 0.5059 | 23.35 | 0.95 | −140 | 1.6086 | |
| RF | 1 | 4.76 × 10−4 | 1.928 | 25.31 | 0.83 | −142 | 2.3081 | |
| 2 | 0.05 | 1.83 × 10−5 | 25.21 | 0.80 | −130 | 1.2925 | ||
| 3 | 0.0004 | 1.5896 | 0.4580 | 24.22 | 0.82 | −137 | 2.3529 |
| Vegetation Type | Area (ha) | UAs | Biomass (Mg ha−1) | CI (Mg ha−1) | CV (%) | Sampling Error (%) |
|---|---|---|---|---|---|---|
| DF | 5868.4 | 13 | 32.8 | ±9.4 | 16.0 | 28.55 |
| SF | 452,922.5 | 63 | 76.4 | ±8.4 | 6.63 | 11.06 |
| RAF | 895,278.8 | 68 | 85.5 | ±10.0 | 7.02 | 11.71 |
| MF | 18,936.6 | 19 | 53.0 | ±10.2 | 11.10 | 19.25 |
| RF | 37,561.3 | 22 | 48.7 | ±11.7 | 13.96 | 24.02 |
| TOTAL | 1,410,567.6 | 185 | 70.7 | ±5.4 | 4.64 | 7.67 |
| Vegetation Type | Area (ha) | UAs | Biomass (Mg ha−1) | CI (Mg ha−1) | CV (%) | Sampling Error (%) |
|---|---|---|---|---|---|---|
| DF | 5868.4 | 13 | 32.8 | ±9.4 | 16.02 | 28.55 |
| SF | 452,922.5 | 63 | 66.7 | ±7.9 | 7.15 | 11.93 |
| RAF | 895,278.8 | 68 | 92.4 | ±10.6 | 6.86 | 11.44 |
| MF | 18,936.6 | 19 | 53.0 | ±10.2 | 11.10 | 19.25 |
| RF | 37,561.3 | 22 | 27.7 | ±10.8 | 22.84 | 39.29 |
| TOTAL | 1,410,567.6 | 185 | 67.5 | ±5.9 | 5.29 | 8.74 |
| Vegetation Type | Area (ha) | UAs | Biomass (Mg ha−1) | CI (Mg ha−1) | CV (%) | Sampling Error (%) |
|---|---|---|---|---|---|---|
| DF | 5868.4 | 13 | 29.5 | ±11.2 | 21.21 | 37.81 |
| SF | 452,922.5 | 63 | 74.3 | ±11.5 | 9.30 | 15.53 |
| RAF | 895,278.8 | 68 | 115.7 | ±18.5 | 9.58 | 15.97 |
| MF | 18,936.6 | 19 | 58.9 | ±15.2 | 14.92 | 25.88 |
| RF | 37,561.3 | 22 | 27.2 | ±13.0 | 27.75 | 47.75 |
| TOTAL | 1,410,567.6 | 185 | 79.0 | ±9.3 | 7.14 | 11.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaui, T.D.; Cysneiros, V.C.; de Souza, F.C.; de Souza, H.J.; Silveira Filho, T.B.; Carvalho, D.C.d.; Pace, J.H.C.; Vidaurre, G.B.; Miguel, E.P. Biomass Equations and Carbon Stock Estimates for the Southeastern Brazilian Atlantic Forest. Forests 2024, 15, 1568. https://doi.org/10.3390/f15091568
Gaui TD, Cysneiros VC, de Souza FC, de Souza HJ, Silveira Filho TB, Carvalho DCd, Pace JHC, Vidaurre GB, Miguel EP. Biomass Equations and Carbon Stock Estimates for the Southeastern Brazilian Atlantic Forest. Forests. 2024; 15(9):1568. https://doi.org/10.3390/f15091568
Chicago/Turabian StyleGaui, Tatiana Dias, Vinicius Costa Cysneiros, Fernanda Coelho de Souza, Hallefy Junio de Souza, Telmo Borges Silveira Filho, Daniel Costa de Carvalho, José Henrique Camargo Pace, Graziela Baptista Vidaurre, and Eder Pereira Miguel. 2024. "Biomass Equations and Carbon Stock Estimates for the Southeastern Brazilian Atlantic Forest" Forests 15, no. 9: 1568. https://doi.org/10.3390/f15091568






