Response of Live Oak Regeneration to Planting Density, Fertilizer, and Mulch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Plant Material
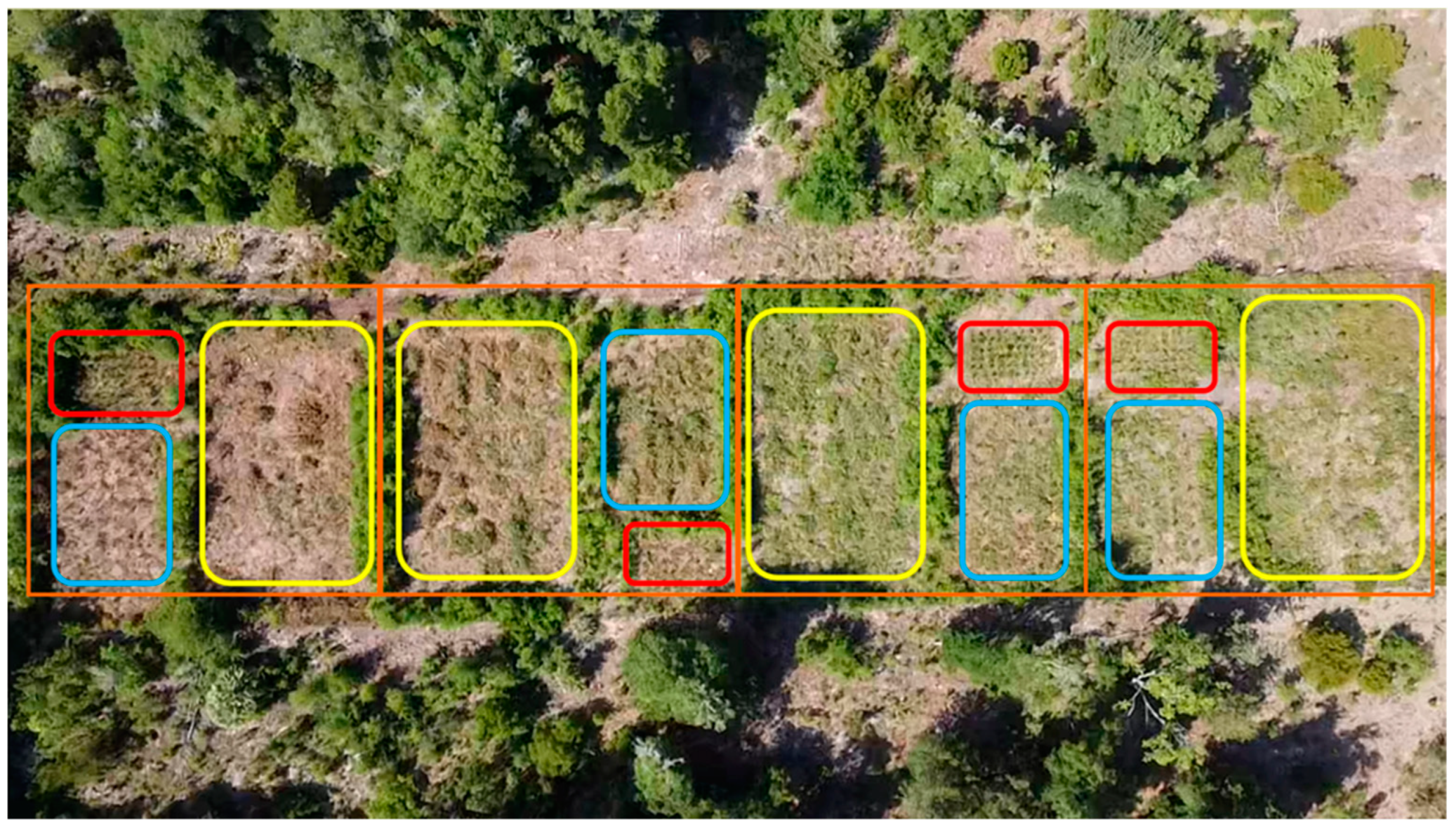
2.3. Experimental Design and Treatments
2.4. Measurements
2.5. Statistical Analysis
3. Results
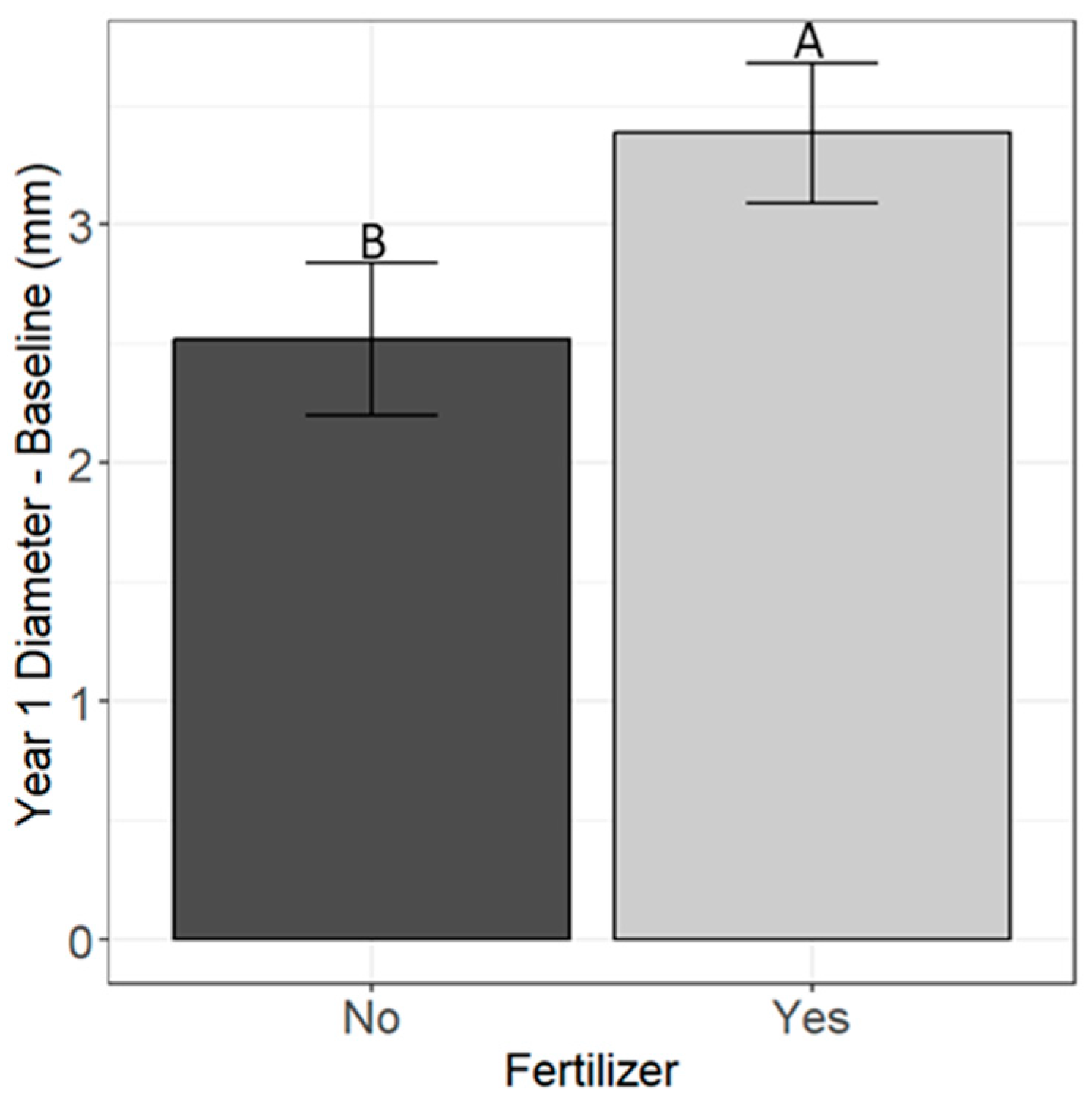
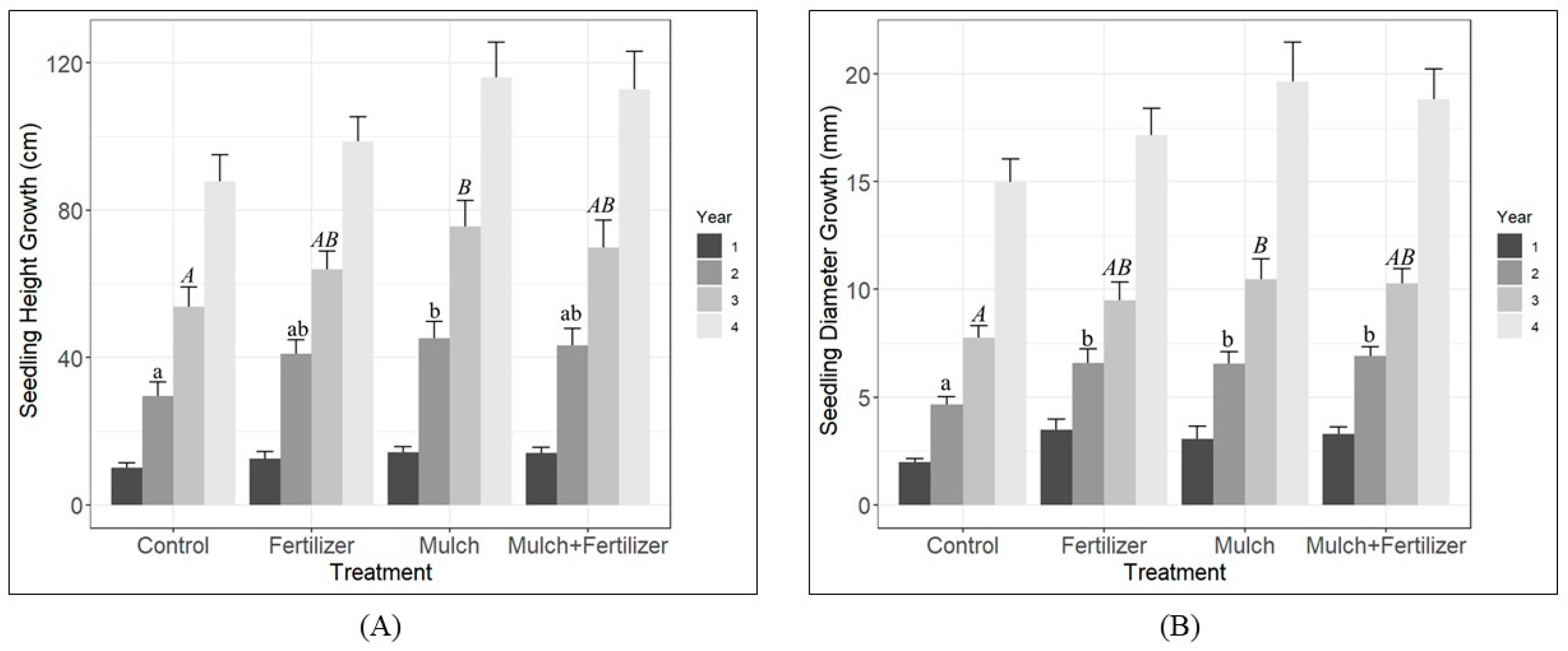
3.1. Seedling Survival and Growth
3.2. Foliar Nutrient Analysis N-P-K
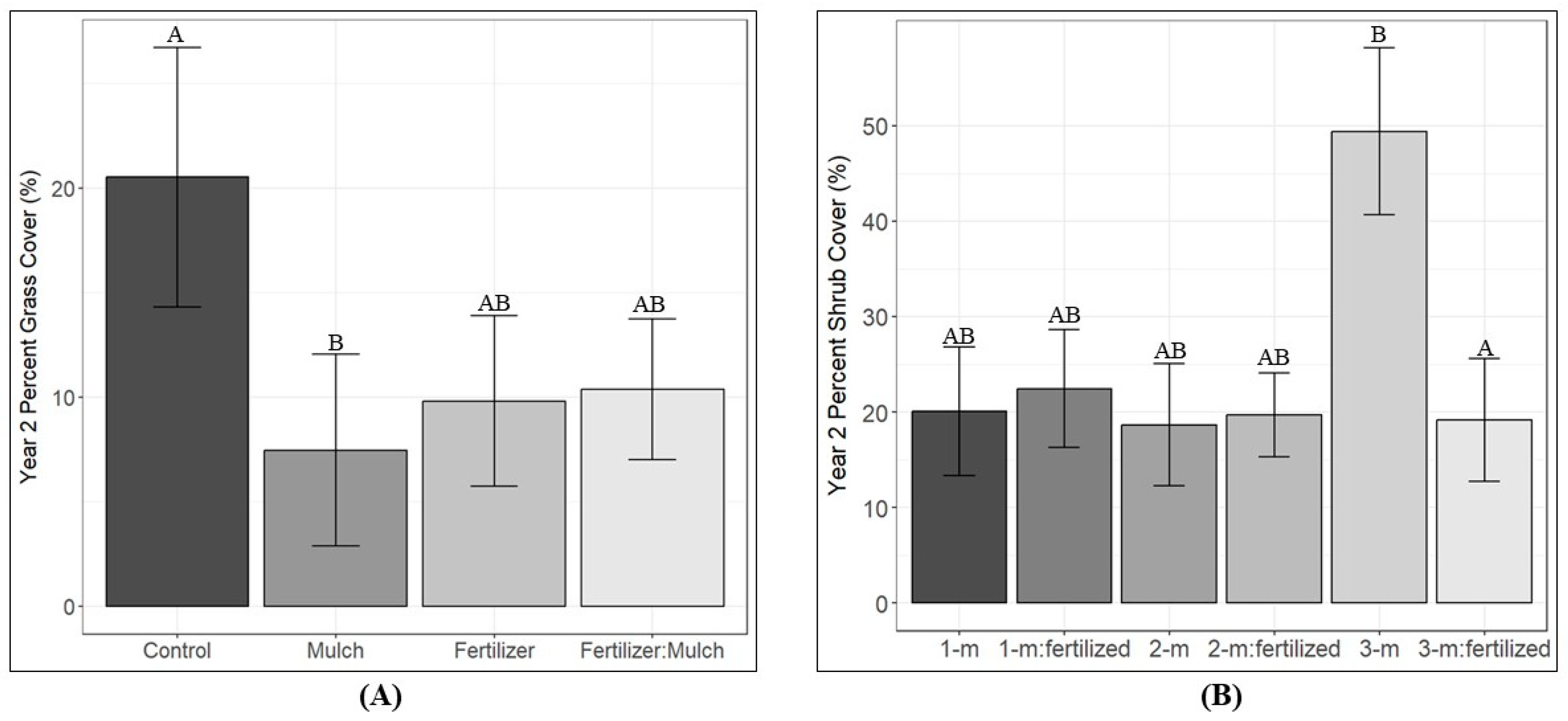
3.3. Competing Vegetation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellis, V.J. Ecology of Maritime Forests of the Southern Atlantic Coast: A Community Profile; U.S. Department of the Interior, National Biological Service: Washington, DC, USA, 1995.
- Woods, N.N.; Tuley, P.A.; Zinnert, J.C. Long-Term Community Dynamics Reveal Different Trajectories for Two Mid-Atlantic Maritime Forests. Forests 2021, 12, 1063. [Google Scholar] [CrossRef]
- Arkema, K.K.; Guannel, G.; Verutes, G.; Wood, S.A.; Guerry, A.; Ruckelshaus, M.; Kareiva, P.; Lacayo, M.; Silver, J.M. Coastal Habitats Shield People and Property from Sea-Level Rise and Storms. Nat. Clim. Chang. 2013, 3, 913–918. [Google Scholar] [CrossRef]
- Costanza, R.; Pérez-Maqueo, O.; Martinez, M.L.; Sutton, P.; Anderson, S.J.; Mulder, K. The Value of Coastal Wetlands for Hurricane Protection. Ambio 2008, 37, 241–248. [Google Scholar] [CrossRef]
- Gómez-Baggethun, E.; Barton, D.N. Classifying and Valuing Ecosystem Services for Urban Planning. Ecol. Econ. 2013, 86, 235–245. [Google Scholar] [CrossRef]
- Tibbetts, J. Coastal Cities: Living on the Edge. Environ. Health Perspect. 2002, 110, A674–A681. [Google Scholar] [CrossRef]
- NOAA 2022 Sea Level Rise Technical Report. Available online: https://oceanservice.noaa.gov/hazards/sealevelrise/sealevelrise-tech-report.html#step1 (accessed on 14 June 2024).
- Lopazanski, M.J.; Evans, J.P.; Shaw, R.E. An Assessment of Maritime Forest Resources on the North Carolina Coast; North Carolina Department of Natural Resources and Community Development, Division of Coastal Management: Raleigh, NC, USA, 1988.
- Conner, W.H.; Mixon, W.D.; Wood, G.W. Maritime Forest Habitat Dynamics on Bulls Island, Cape Romain National Wildlife Refuge, SC, Following Hurricane Hugo. For. Ecol. Manag. 2005, 212, 127–134. [Google Scholar] [CrossRef]
- Kurtz, C.M. Consequences of Salinity and Freezing Stress for Two Populations of Quercus virginiana Mill. (Fagaceae) Grown in a Common Garden. J. Torrey Bot. Soc. 2013, 140, 145–156. [Google Scholar] [CrossRef]
- Gresham, C.A.; Williams, T.M.; Lipscomb, D.J. Hurricane Hugo Wind Damage to Southeastern U.S. Coastal Forest Tree Species. Biotropica 1991, 23, 420–426. [Google Scholar] [CrossRef]
- Albers, G.; Alber, M. A Vegetative Survey of Back-Barrier Islands near Sapelo Island, Georgia; University of Georgia: Athens, Greece, 2003. [Google Scholar]
- Taggart, J.; Long, Z. Effects of White-Tailed Deer (Odocoileus virginianus) on the Maritime Forest of Bald Head Island, North Carolina. Am. Midl. Nat. 2015, 173, 283–293. [Google Scholar] [CrossRef]
- Baldwin, N.A.; Crosby, M.K. The Allelopathic Influence of Post Oak (Quercus stellata) on Plant Species in Southern U.S. Forests. In Proceedings of the 18th Biennal Southern Silvicultural Research Conference, Knoxville, TN, USA, 2–5 March 2015; United States Department of Agriculture (USDA), Forest Service: Asheville, NC, USA, 2015. [Google Scholar]
- Guang De, L.; Jia, L.; Li, X. Research Advances in Allelopathy of Quercus L. For. Stud. China 2007, 9, 287–294. [Google Scholar] [CrossRef]
- Saha, S.; Kuehne, C.; Bauhus, J. Intra- and Interspecific Competition Differently Influence Growth and Stem Quality of Young Oaks (Quercus robur L. and Quercus petraea (Mattuschka) Liebl.). Ann. For. Sci. 2014, 71, 381–393. [Google Scholar] [CrossRef]
- Waring, R.H.; Schlesinger, W.H. Forest Ecosystems, Concepts and Management; Acad. Press. Inc.: Orlando, FL, USA, 1985; Volume 340. [Google Scholar]
- Yang, X.; Zhang, W.; He, Q. Effects of Intraspecific Competition on Growth, Architecture and Biomass Allocation of Quercus liaotungensis. J. Plant Interact. 2019, 14, 284–294. [Google Scholar] [CrossRef]
- Wolf, A.; Møller, P.F.; Bradshaw, R.H.W.; Bigler, J. Storm Damage and Long-Term Mortality in a Semi-Natural, Temperate Deciduous Forest. For. Ecol. Manag. 2004, 188, 197–210. [Google Scholar] [CrossRef]
- Spector, T.; Putz, F.E. Crown Retreat of Open-Grown Southern Live Oaks (Quercus virginiana) Due to Canopy Encroachment in Florida, USA. For. Ecol. Manag. 2006, 228, 168–176. [Google Scholar] [CrossRef]
- Vince, S.W.; Humphrey, S.R.; Simons, R.W. The Ecology of Hydric Hammocks: A Community Profile; U.S. Department of the Interior, Fish and Wildlife Service, Research and Development: Washington, DC, USA, 1989.
- Wang, X.; Rahman, M.A.; Mokroš, M.; Rötzer, T.; Pattnaik, N.; Pang, Y.; Zhang, Y.; Da, L.; Song, K. The Influence of Vertical Canopy Structure on the Cooling and Humidifying Urban Microclimate during Hot Summer Days. Landsc. Urban Plan. 2023, 238, 104841. [Google Scholar] [CrossRef]
- Greenly, K.; Rakow, D. The Effect of Wood Mulch Type and Depth on Weed and Tree Growth and Certain Soil Parameters. AUF 1995, 21, 225–232. [Google Scholar] [CrossRef]
- Harris, R.W. Arboriculture: Integrated Management of Landscape Trees, Shrubs, and Vines; Prentice-Hall International: Engelwood Cliffs, NJ, USA, 1992. [Google Scholar]
- Chakraborty, D.; Nagarajan, S.; Aggarwal, P.; Gupta, V.K.; Tomar, R.K.; Garg, R.N.; Sahoo, R.N.; Sarkar, A.; Chopra, U.K.; Sarma, K.S.S.; et al. Effect of Mulching on Soil and Plant Water Status, and the Growth and Yield of Wheat (Triticum aestivum L.) in a Semi-Arid Environment. Agric. Water Manag. 2008, 95, 1323–1334. [Google Scholar] [CrossRef]
- NOAA Climate-Brunswick, GA. Available online: https://www.weather.gov/wrh/Climate?wfo=jax (accessed on 14 June 2023).
- Soil Survey Staff Web Soil Survey. Available online: https://www.nrcs.usda.gov/resources/data-and-reports/web-soil-survey (accessed on 16 June 2023).
- Shirish, P.; Kelkar, S.; Bhalerao, S. Mulching: A Soil and Water Conservation Practice. Res. J. Agric. For. Sci. 2013, 1, 2320–6063. [Google Scholar]
- Benigno, S.M.; Dixon, K.W.; Stevens, J.C. Increasing Soil Water Retention with Native-Sourced Mulch Improves Seedling Establishment in Postmine Mediterranean Sandy Soils. Restor. Ecol. 2013, 21, 617–626. [Google Scholar] [CrossRef]
- Percival, G.C.; Gklavakis, E.; Noviss, K. Influence of Pure Mulches on Survival, Growth and Vitality of Containerized and Field Planted Trees. J. Environ. Hortic. 2009, 27, 200–206. [Google Scholar] [CrossRef]
- Rakow, D.A. Types and Uses of Mulch in Landscape; Cornell University Cooperative Extension: Ithaca, NY, USA, 1989; pp. 1–4. [Google Scholar]
- Gilman, E.F.; Yeager, T.H.; Kent, D. Fertilizer Rate and Type Impacts Magnolia and Oak Growth in Sandy Landscape Soil. J. Arboric. 2000, 26, 177–182. [Google Scholar] [CrossRef]
- Thyroff, E.C.; Burney, O.T.; Oliet, J.A.; Redick, C.H.; Jacobs, D.F. Toward Identifying Alternatives to Fencing for Forest Restoration: Tube Shelters Outperform Mesh Shelters for Deer Browse Protection of Live Oak, Quercus virginiana. Land 2022, 11, 966. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Salifu, K.F.; Seifert, J.R. Growth and Nutritional Response of Hardwood Seedlings to Controlled-Release Fertilization at Outplanting. For. Ecol. Manag. 2005, 214, 28–39. [Google Scholar] [CrossRef]
- Dey, D.C.; Jacobs, D.; McNabb, K.; Miller, G.; Baldwin, V.; Foster, G. Artificial Regeneration of Major Oak (Quercus) Species in the Eastern United States—A Review of the Literature. For. Sci. 2008, 54, 77–106. [Google Scholar] [CrossRef]
- Struve, D.K.; Joly, R.J. Transplanted Red Oak Seedlings Mediate Transplant Shock by Reducing Leaf Surface Area and Altering Carbon Allocation. Can. J. For. Res. 1992, 22, 1441–1448. [Google Scholar] [CrossRef]
- Gale Encyclopedia of World Biography: 2004 Supplement. Available online: https://www.gale.com/ebooks/9780787693459/encyclopedia-of-world-biography-2004-supplement (accessed on 26 November 2023).
- Georgia Historical Society Cannon’s Point Plantation. Available online: https://www.georgiahistory.com/ghmi_marker_updated/cannons-point-plantation/ (accessed on 26 November 2023).
- St Simons Land Trust Archaeology. Available online: https://sslt.org/protected-properties-2/cannons-point-preserve/research/archaeology/ (accessed on 26 November 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference, R Package Version 1.10.0. 2009. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 26 November 2023).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots, R Package Version 0.6.0. 2023. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 26 November 2023).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation, R Package Version 1.1.4. 2023. Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 26 November 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, Migration or Extirpation: Climate Change Outcomes for Tree Populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef]
- Balduman, L.M.; Aitken, S.N.; Harmon, M.; Adams, W.T. Genetic Variation in Cold Hardiness of Douglas-Fir in Relation to Parent Tree Environment. Can. J. For. Res. 1999, 29, 62–72. [Google Scholar] [CrossRef]
- Dudley, S.A. Differing Selection on Plant Physiological Traits in Response to Environmental Water Availability: A Test of Adaptive Hypotheses. Evolution 1996, 50, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Linhart, Y.B.; Grant, M.C. Evolutionary Significance of Local Genetic Differentiation in Plants. Annu. Rev. Ecol. Syst. 1996, 27, 237–277. [Google Scholar] [CrossRef]
- Park, I.; DeWalt, S.J.; Siemann, E.; Rogers, W.E. Differences in Cold Hardiness between Introduced Populations of an Invasive Tree. Biol. Invasions 2012, 14, 2029–2038. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Sánchez-Gómez, D.; Aranda, I.; Valladares, F. Phenotypic Plasticity and Local Adaptation in Leaf Ecophysiological Traits of 13 Contrasting Cork Oak Populations under Different Water Availabilities. Tree Physiol. 2010, 30, 618–627. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Davis, A.S.; Dumroese, R.K.; Burney, O.T. Nursery Cultural Techniques Facilitate Restoration of Acacia koa Competing with Invasive Grass in a Dry Tropical Forest. Forests 2020, 11, 1124. [Google Scholar] [CrossRef]
- Mariotti, B.; Maltoni, A.; Jacobs, D.F.; Tani, A. Container Effects on Growth and Biomass Allocation in Quercus robur and Juglans regia Seedlings. Scand. J. For. Res. 2015, 30, 401–415. [Google Scholar] [CrossRef]
- Davis, A.S.; Jacobs, D.F. Quantifying Root System Quality of Nursery Seedlings and Relationship to Outplanting Performance. New Forest 2005, 30, 295–311. [Google Scholar] [CrossRef]
- Haase, D.L. Understanding Forest Seedling Quality: Measurements and Interpretation. Tree Plant. Notes 2008, 52, 24–30. [Google Scholar]
- Schroth, G.; Zech, W.; Heimann, G. Mulch Decomposition under Agroforestry Condition in Sub-Humid Tropical Savanna Processes and Influence of Perennial Plants. Plant Soil 1992, 147, 1–11. [Google Scholar] [CrossRef]
- Bell, N.; Sullivan, D.M.; Cook, T. Mulching Woody Ornamentals with Organic Materials. Available online: https://extension.oregonstate.edu/catalog/pub/ec-1629-mulching-woody-ornamentals-organic-materials (accessed on 26 January 2024).
- Duryea, M.; English, R.J.; Hermansen, L.A. A Comparison of Landscape Mulches: Chemical, Allelopathic, and Decomposition Properties. AUF 1999, 25, 88–97. [Google Scholar] [CrossRef]
- Meentemeyer, V. Macroclimate and Lignin Control of Litter Decomposition Rates. Ecology 1978, 59, 465–472. [Google Scholar] [CrossRef]
- Maggard, A.O.; Will, R.E.; Hennessey, T.C.; McKinley, C.R.; Cole, J.C. Tree-Based Mulches Influence Soil Properties and Plant Growth. HortTechnology 2012, 22, 353–361. [Google Scholar] [CrossRef]
- Scharenbroch, B.; Watson, G.W. Wood Chips and Compost Improve Soil Quality and Increase Growth of Acer rubrum and Betula nigra in Compacted Urban Soil. Arboric. Urban For. 2014, 40, 319–331. [Google Scholar] [CrossRef]
- Andrzejczyk, T.; Liziniewicz, M.; Drozdowski, S. Effect of Spacing on Growth and Quality Parameters in Sessile Oak (Quercus petraea) Stands in Central Poland: Results 7 Years after Planting. Scand. J. For. Res. 2015, 30, 710–718. [Google Scholar] [CrossRef]
- Gauthier, M.-M.; Zellers, K.E.; Löf, M.; Jacobs, D.F. Inter- and Intra-Specific Competitiveness of Plantation-Grown American chestnut (Castanea dentata). For. Ecol. Manag. 2013, 291, 289–299. [Google Scholar] [CrossRef]
- Jensen, A.M.; Löf, M. Effects of Interspecific Competition from Surrounding Vegetation on Mortality, Growth and Stem Development in Young Oaks (Quercus robur). For. Ecol. Manag. 2017, 392, 176–183. [Google Scholar] [CrossRef]
- Oliver, C.; Larson, B. Forest Stand Dynamics, Update Edition; Yale School of the Environment Other Publications: New York, NY, USA, 1996; ISBN 978-0-471-13833-4. [Google Scholar]
- Huang, J.; Hammerbacher, A.; Weinhold, A.; Reichelt, M.; Gleixner, G.; Behrendt, T.; van Dam, N.M.; Sala, A.; Gershenzon, J.; Trumbore, S.; et al. Eyes on the Future–Evidence for Trade-Offs between Growth, Storage and Defense in Norway Spruce. New Phytol. 2019, 222, 144–158. [Google Scholar] [CrossRef]
- Canham, C.D.; Berkowitz, A.R.; Kelly, V.R.; Lovett, G.M.; Ollinger, S.V.; Schnurr, J. Biomass Allocation and Multiple Resource Limitation in Tree Seedlings. Can. J. For. Res. 1996, 26, 1521–1530. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Salifu, K.F.; Seifert, J.R. Relative Contribution of Initial Root and Shoot Morphology in Predicting Field Performance of Hardwood Seedlings. New For. 2005, 30, 235–251. [Google Scholar] [CrossRef]
- Rebbeck, J.; Gottschalk, K.; Scherzer, A. Do Chestnut, Northern Red, and White Oak Germinant Seedlings Respond Similarly to Light Treatments? Growth and Biomass. Can. J. For. Res. 2011, 41, 2219–2230. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Puértolas, J.; Cuesta, B.; Peñuelas, J.L.; Uscola, M.; Heredia-Guerrero, N.; Rey Benayas, J.M. Increase in Size and Nitrogen Concentration Enhances Seedling Survival in Mediterranean Plantations. Insights from an Ecophysiological Conceptual Model of Plant Survival. New For. 2012, 43, 755–770. [Google Scholar] [CrossRef]
- Collet, C.; Löf, M.; Pagès, L. Root System Development of Oak Seedlings Analysed Using an Architectural Model. Effects of Competition with Grass. Plant Soil 2006, 279, 367–383. [Google Scholar] [CrossRef]
- Clatterbuck, W.K. The Potential of Using Coppice Growth as Training Trees in Plantations for the Production of High-Quality Oak Boles. In Proceedings of the 18th Biennial Southern Silvicultural Research Conference, Knoxville, TN, USA, 2–5 March 2015; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2015. [Google Scholar]
- Saha, S.; Kuehne, C.; Bauhus, J. Tree Species Richness and Stand Productivity in Low-Density Cluster Plantings with Oaks (Quercus robur L. and Q. petraea (Mattuschka) Liebl.). Forests 2013, 4, 650–665. [Google Scholar] [CrossRef]
- Thyroff, E.C.; Burney, O.T.; Jacobs, D.F. Herbivory and Competing Vegetation Interact as Site Limiting Factors in Maritime Forest Restoration. Forests 2019, 10, 950. [Google Scholar] [CrossRef]
- Kissel, D.E.; Sonon, L.S. Soil Test Handbook for Georgia: Fertilizer Recommendations by Crops; University of Georgia Cooperative Extension: Athens, GA, USA, 2008. [Google Scholar]
- Sambeek, J.W.V.; Kabrick, J.M.; Dey, D.C. Foliar Nutrient Responses of Oak Saplings to Nitrogen Treatments on Alkaline Soils within the Missouri River Floodplain. In Proceedings of the 20th Central Hardwood Forest Conference, Columbia, MO, USA, 28 March–1 April 2016; USDA US Forest Service: Newtown Square, PA, USA, 2017. [Google Scholar]
- Miller, E.M.; Seastedt, T.R. Impacts of Woodchip Amendments and Soil Nutrient Availability on Understory Vegetation Establishment Following Thinning of a Ponderosa Pine Forest. For. Ecol. Manag. 2009, 258, 263–272. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-Term Effects of Mineral Fertilizers on Soil Microorganisms—A Review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Southern Trees Fact Sheets. IFAS Extension University of Florida. Available online: https://edis.ifas.ufl.edu/collections/envhort-trees (accessed on 28 January 2024).
- Thyroff, E.C.; Burney, O.T.; Mickelbart, M.V.; Jacobs, D.F. Unraveling Shade Tolerance and Plasticity of Semi-Evergreen Oaks: Insights From Maritime Forest Live Oak Restoration. Front. Plant Sci. 2019, 10, 1526. [Google Scholar] [CrossRef]
- Mylavarapu, R.; Li, Y.; Silveira, M.; Mackowiak, C.; McCray, M. Soil-Test-Based Phosphorus Recommendations for Commercial Agricultural Production in Florida. Available online: https://edis.ifas.ufl.edu/publication/SS699 (accessed on 14 December 2023).
- Fajardo, A.; McIntire, E.J.B. Under Strong Niche Overlap Conspecifics Do Not Compete but Help Each Other to Survive: Facilitation at the Intraspecific Level. J. Ecol. 2011, 99, 642–650. [Google Scholar] [CrossRef]
- Meilan, R. Planning the Tree Planting Operation. In Planting and Care of Fine Hardwood Seedlings; Purdue University Cooperative Extension Service: West Lafayette, IN, USA, 2006. [Google Scholar]
- Padilla, F.M.; Pugnaire, F.I. The Role of Nurse Plants in the Restoration of Degraded Environments. Front. Ecol. Environ. 2006, 4, 196–202. [Google Scholar] [CrossRef]
- Callaway, R.M. Direct Mechanisms for Facilitation. In Positive Interactions and Interdependence in Plant Communities; Springer: Dordrecht, The Netherlands, 2007; pp. 15–116. ISBN 978-1-4020-6223-0. [Google Scholar]
- Hanberry, B.B.; Nowacki, G.J. Oaks Were the Historical Foundation Genus of the East-Central United States. Quat. Sci. Rev. 2016, 145, 94–103. [Google Scholar] [CrossRef]
- Reyer, C.P.O.; Rammig, A.; Brouwers, N.; Langerwisch, F. Forest Resilience, Tipping Points and Global Change Processes. J. Ecol. 2015, 103, 1–4. [Google Scholar] [CrossRef]
- Butler, B.J.; Butler, S.M. Family Forest Ownerships with 10+ Acres in Georgia, 2011–2013; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2016; 2p. [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Innusa, B.N.; Burney, O.T.; Jacobs, D.F. Response of Live Oak Regeneration to Planting Density, Fertilizer, and Mulch. Forests 2024, 15, 1594. https://doi.org/10.3390/f15091594
Innusa BN, Burney OT, Jacobs DF. Response of Live Oak Regeneration to Planting Density, Fertilizer, and Mulch. Forests. 2024; 15(9):1594. https://doi.org/10.3390/f15091594
Chicago/Turabian StyleInnusa, Brianne N., Owen T. Burney, and Douglass F. Jacobs. 2024. "Response of Live Oak Regeneration to Planting Density, Fertilizer, and Mulch" Forests 15, no. 9: 1594. https://doi.org/10.3390/f15091594
APA StyleInnusa, B. N., Burney, O. T., & Jacobs, D. F. (2024). Response of Live Oak Regeneration to Planting Density, Fertilizer, and Mulch. Forests, 15(9), 1594. https://doi.org/10.3390/f15091594





