Heavy Metal Content in Medicinal Plants Grown in Hydroponics and Forest Soil in the Central Part of Western Siberia
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Plant Samples
2.3. Hydroponics
2.4. Sampling and Soil Analysis
2.5. Analysis of the Mineral Wool Substrate and Fertilizers
2.6. Collection of Plant Samples and Laboratory Analyses
2.7. Statistical Analysis
3. Results
3.1. Chemical Composition of the Soil Sample and Hydroponic Material
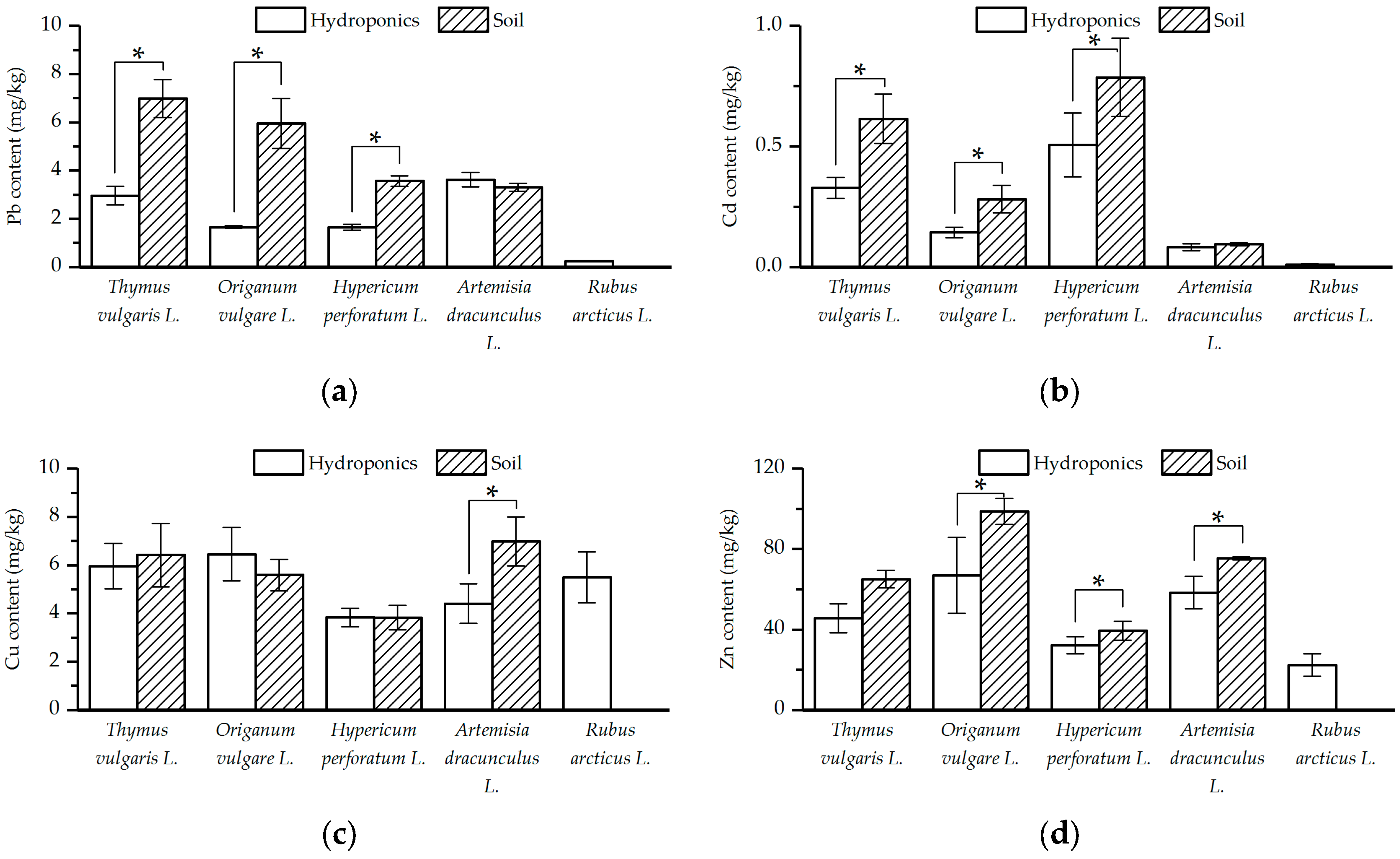
3.2. Chemical Composition of the Plants Grown in the Soil and Hydroponic System
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, L.A.; Douhovnikoff, V. Environmental Issues in Russia. Annu. Rev. Environ. Resour. 2008, 33, 437–460. [Google Scholar] [CrossRef][Green Version]
- Revich, B.A. Assessment of the effect produced by the fuel and energy complex on the environment and health. Stud. Russ. Econ. Dev. 2010, 21, 403–410. [Google Scholar] [CrossRef]
- Evseev, A.V.; Dushkova, D.O.; Goretskaya, A.G. Biomonitoring of aerothechnogenic contamination of ecosystems in the North of Russia by heavy metals. Arct. Ecol. Econ. 2020, 3, 23–33. [Google Scholar] [CrossRef]
- Wen, T.; Fu, W.; Li, X. Analysis of Spatial and Temporal Dynamics of Finland’s Boreal Forests and Types over the Past Four Decades. Forests 2024, 15, 786. [Google Scholar] [CrossRef]
- Bashkin, V.N.; Galiulin, R.V.; Galiulina, R.A.; Arabsky, A.K. Risk of soil contamination by heavy metals through gas-dust emissions. Probl. Risk Anal. 2019, 16, 42–49. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Petkovšek, S.A.; Pokaorny, B. Lead and cadmium in mushrooms from the vicinity of two large emission sources in Slovenia. Sci. Total Environ. 2013, 443, 944–954. [Google Scholar] [CrossRef]
- Kalhok, S.B.; Klint, M.; Olsen, S.M.; Mahonen, O.; Friðriksdóttir, S.; Kroglund, M.; Bulgakov, V.; Tsaturov, Y.; Lundeberg, T.; Turesson, A.; et al. AMAP Assessment 2021: Human Health in the Arctic. Arctic Monitoring and Assessment Programme; AMAP: Tromso, Norway, 2021; 240p. [Google Scholar]
- Zeiner, M.; Juranović Cindrić, I. Harmful Elements (Al, Cd, Cr, Ni, and Pb) in Wild Berries and Fruits Collected in Croatia. Toxics 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Nalbandyan-Schwarz, A.; Bondo Pedersen, K.; Evenset, A.; Heimstad, E.; Sandanger, T.M.; Myllynen, P.; Rautio, A. Combined Contaminant Levels from Local Harvested Food Items in the Norwegian–Finnish–Russian Border Region. Resources 2024, 13, 54. [Google Scholar] [CrossRef]
- Annan, K.; Dickson, R.A.; Amponsah, I.K.; Nooni, I.K. The heavy metal contents of some selected medicinal plants sampled from different geographical locations. Pharmacogn. Res. 2013, 5, 103–108. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, C.-I. Black Goji Berry (Lycium ruthenicum Murray): A Review of Its Pharmacological Activity. Nutrients 2023, 15, 4181. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, T.; Shabani, L.; Sabzalian, M.R. LED light sources improved the essential oil components and antioxidant activity of two genotypes of lemon balm (Melissa officinalis L.). Bot. Stud. 2021, 9, 62. [Google Scholar] [CrossRef]
- Bespal’ko, L.V.; Pinchuk, E.V.; Ushakova, I.T. Lemon balm (Melissa officinalis L.) is a valuable aromatic culture. Veg. Crops Russ. 2019, 3, 57–61. [Google Scholar] [CrossRef]
- Balashova, I.T.; Bespal’ko, L.V.; Molchanova, A.V.; Sirota, S.M.; Kharchenko, V.A.; Soldatenko, A.V. Biochemical composition of some aromatic and medicinal plants after cultivation on the multi circle hydroponic installations. Plant Biol. Hortic. Theory Innov. 2021, 4, 67–77. [Google Scholar] [CrossRef]
- Sachir, E.E.; Puscasu, C.G.; Caraiane, A.; Raftu, G.; Badea, F.C.; Mociu, M.; Albu, C.M.; Sachelarie, L.; Hurjui, L.L.; Bartok-Nicolae, C. Studies Regarding the Antibacterial Effect of Plant Extracts Obtained from Epilobium parviflorum Schreb. Appl. Sci. 2022, 12, 2751. [Google Scholar] [CrossRef]
- Krasnoborov, I.M.; Lomonosova, M.N.; Tupitsyna, N.N. Flora of Siberia. Asteraceae (Compositae); Krasnoborov, I.M., Lomonosova, M.N., Tupitsyna, N.N., Eds.; Nauka: Novosibirsk, Russia, 1997; Volume 13, 472p. [Google Scholar]
- Yarkova, N.N.; Fedorova, V.M. Seed Science of Agricultural Plants; IPC “Prokrost”: Perm, Russia, 2016; 116p. [Google Scholar]
- Pimenov, M.G.; Vlasova, N.V.; Zuev, V.V. Flora of Siberia. Geraniaceae–Cornaceae; Malyschev, L.I., Ed.; Nauka: Novosibirsk, Russia, 1996; Volume 10, 254p. [Google Scholar]
- Krasnoborov, I.M.; Shaulo, D.N.; Lomonosova, M.N.; Vibe, E.I.; Vasina, A.L.; Tupitsyna, N.N. Key to Plants of the Khanty-Mansiysk Autonomous Okrug; Krasnoborov, I.M., Ed.; Basko Publication: Novosibirsk, Russia; Yekaterinburg, Russia, 2006; 304p. [Google Scholar]
- Vasin, A.M.; Vasina, A.L. The Red Book of the Khanty-Mansiysk Autonomous Okrug-Yugra: Animals, Plants and Fungi, 2nd ed.; Basko Publication: Yekaterinburg, Russia, 2013; 460p. [Google Scholar]
- Doron’kin, V.M.; Kovtonyuk, N.K.; Zuev, V.V. Flora of Siberia. Pyrolaceae-Lamiaceae (Labiatae); Doron’kin, V.M., Kovtonyuk, N.K., Zuev, V.V., Eds.; Nauka: Novosibirsk, Russia, 1997; Volume 11, 296p. [Google Scholar]
- Vydrina, S.N.; Kurbatskiy, V.I.; Polozhiy, A.V. Flora of Siberia. Rosaceae; Vydrina, S.N., Kurbatskiy, V.I., Polozhiy, A.V., Eds.; Nauka: Novosibirsk, Russia, 1988; Volume 8, 200p. [Google Scholar]
- GOST 17.4.4.02-84; Nature Conservation. The Soil. Method of Sampling and Preparation of Samples for Chemical, Bacteriological, Helminthological Analysis. Standartinform: Moscow, Russia, 2008; 8p.
- GOST R ISO 11464:2006; Soil Quality. Preliminary Preparation of Samples for Physical and Chemical Analysis. Standartinform: Moscow, Russia, 2019; 11p.
- Tache, A.M.; Dinu, L.D.; Vamanu, E. Novel Insights on Plant Extracts to Prevent and Treat Recurrent Urinary Tract Infections. Appl. Sci. 2022, 12, 2635. [Google Scholar] [CrossRef]
- GOST 30178-96; Raw Materials and Food Products. Atomic Absorption Method for Determining Toxic Substances. Standartinform: Moscow, Russia, 2010; pp. 24–32.
- Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods. Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF. Rotterdam, The Netherlands, 4–8 April 2016; CF/10 INF/1, March 2016. [Google Scholar]
- GB 2762-2022; National Food Safety Standard-Maximum Levels of Contaminants in Foods. National Health Commission (NHC) and the State Administration of Market Regulation (SAMR): China, 2023; 21p.
- Khan, C.S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollution. 2008, 3, 686–692. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC; European Commission: Brussels, Belgium, 2005; pp. 1–16. [Google Scholar]
- Commission Regulation (EU) 2023/915—Publications Office. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915 (accessed on 9 September 2024).
- SanPiN 2.3.2.1078-01. (1.10.7) Hygienic Safety Requirements and Food Products (as Amended and Supplemented on 6 July 2011), Standartinform: Moscow, Russia, 2002; 269p.
- World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; World Health Organization: Geneva, Switzerland, 2007; 118p. [Google Scholar]
- Pietrelli, L.; Menegoni, P.; Papetti, P. Bioaccumulation of Heavy Metals by Herbaceous Species Grown in Urban and Rural Sites. Water Air Soil Pollut. 2022, 233, 19. [Google Scholar] [CrossRef]
- Siromlya, T.; Zagurskaya, Y. Bayandina, Irina The elemental composition of Hypericum perforatum plants sampled in environmentally different habitats by the example of West Siberia. Bot. Pacifica 2020, 9, 127–132. [Google Scholar] [CrossRef]
- Glavač, N.K.; Djogo, S.; Ražić, S.; Kreft, S.; Veber, M. Accumulation of Heavy Metals from Soil in Medicinal Plants. Arch. Ind. Hyg. Toxicol. 2017, 68, 236–244. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S. Essential Oils Yield and Heavy Metals Content of Some Aromatic Medicinal Plants Grown in Ash-Shoubak Region, South of Jordan. Adv. Environ. Biol. 2009, 3, 296–301. [Google Scholar]
- Lovkova, M.Y.; Buzuk, G.N. Medicinal plants–concentrators and superconcentrators of microelements, perspectives for their use in medicine. Vopr. Biol. Med. I Farm. Him. 2013, 11, 43–49. [Google Scholar]
- Jurca, T.; Marian, E.; Vicas, L.; Gatea, D. Simultaneous determination of metals in Hypericum perforatum L. by ICP-OES. Rev. De Chim. 2011, 62, 1154–1156. [Google Scholar]
- Ufimtseva, M.D. The patterns in accumulation of chemical elements by higher plants and their responses in biogeochemical provinces. Geochem. Int. 2015, 53, 441–455. [Google Scholar] [CrossRef]
- Medvedev, I.F.; Derevyagin, S.S. Heavy Metals in Ecosystems; Racurs Publication: Saratov, Russia, 2017; 178p. [Google Scholar]
- Barbeş, L.; Bărbulescu, A.; Stanciu, G. Statistical Analysis of Mineral Elements Content in Different Melliferous Plants from the Dobrogea Region, România. Rom. Rep. Phys. 2020, 72, 705. [Google Scholar]
- Pavlova, D.; Karadjova, I.; Krasteva, I. Essential and Toxic Element Concentrations in Hypericum Perforatum. Aust. J. Bot. 2015, 63, 152–158. [Google Scholar] [CrossRef]
- Marinescu, E.; Elisei, A.M.; Aprotosoaie, A.N.A.C.; Cioancă, O.; Trifan, A.; Miron, A.; Robu, S.; Ifrim, C.; Hăncianu, M. Assessment of Heavy Metals Content in Some Medicinal Plants and Spices Commonly Used In Romania. Farmacia 2020, 68, 1099–1105. [Google Scholar] [CrossRef]
- Kostić, D.; Mitić, S.; Zarubica, A.; Mitić, M.; Veličković, J.; Randjelović, S. Content of Trace Metals in Medicinal Plants and Their Extracts. Hem. Ind. 2011, 65, 165–170. [Google Scholar] [CrossRef]
- Slađana, Č.A.; Snežana, B.T.; Mile, D.D.; Milan, M.A.; Maja, M.N. Assessment of the quality of polluted areas based on the content of heavy metals in different organs of the grapevine (Vitis vinifera) cv Tamjanika. Environ. Sci. Pollut. Res. 2015, 22, 7155–7175. [Google Scholar] [CrossRef]
- Kudrevatykh, I.Y.; Geraskina, A.P. Comparison of structure and chemical composition of ground cover and soils of fir-spruce forests in Pechora-Ilych state nature reserve, Northern Urals. Nat. Conserv. Res. 2021, 6, 80–92. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar]
- EFSA Scientific Committee; More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; et al. Re-evaluation of the existing health-based guidance values for copper and exposure assessment from all sources. EFSA J. 2023, 21, e07728. [Google Scholar]
- Araya, M.; Olivares, M.; Pizarro, F. Copper in human health. Int. J. Environ. Health 2007, 1, 608–620. [Google Scholar] [CrossRef]
- World Health Organization. Safety Evaluation of Certain Food Additives and Contaminants/Prepared by The Sixty-First Meeting of The Joint FAO/WHO Expert Committee on Food Additives (JEFCA); WHO: Geneva, Switzerland, 1991; Voluem 776, p. 1. [Google Scholar]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
| Element | Content, mg/g | Element | Content, mg/g | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Soil | Substrate | Fertilizers | Soil | Substrate | Fertilizers | ||||
| Yara Ferticare Hydro | Yara Liva Calcinit | Yara Ferticare Hydro | Yara Liva Calcinit | ||||||
| Ca | 15.6 | 234.2 | – | 351.1 | Cr | 0.2 | 1.8 | <2.0 × 10−3 | |
| Si | 504.2 | 175.5 | – | 1.6 | Mn | 0.5 | 2.0 | 3.4 | <1.0 × 10−2 |
| Al | 97.4 | 59.7 | – | – | Cu | <1.0 × 10−2 | 0.4 | 0.8 | 0.8 |
| Fe | 28.6 | 48.3 | 2.0 | – | V | <1.0 × 10−2 | 0.4 | <1.0 × 10−2 | |
| P | 2.5 | – | 40.5 | – | Sr | <5.0 × 10−3 | 0.4 | <5.0 × 10−3 | 10.1 |
| Mg | 6.4 | 39.1 | 12.7 | 1.9 | Zn | 0.1 | 0.1 | 0.5 | <5.0 × 10−3 |
| K | 30.7 | 2.1 | 329.1 | 1.6 | Ni | <4.0 × 10−2 | 0.1 | <1.0 × 10−3 | |
| S | 1.7 | 1.6 | 42.5 | 2.0 | Rb | <5.0 × 10−3 | 0.1 | <5.0 × 10−3 | |
| Na | 6.9 | – | – | – | Pb | <1.5 × 10−2 | |||
| Ti | 9.0 | 2.8 | – | – | Cd | <8.0 × 10−3 | |||
| Reference | Pb (mg/kg) | Cd (mg/kg) | Zn (mg/kg) | Cu (mg/kg) |
|---|---|---|---|---|
| FAO/WHO 2016 [28] | 0.05–1.5 | 0.05–4.0 | - | - |
| GB 2762-2022. China [29] | 5 | 0.2 | - | - |
| Khan C.S., 2008 [30] | 9 | 0.2 | 100 | 20 |
| Regulation (EC) 396/2005 [31] | 3.0 | 1.0 | - | |
| Commission Regulation (EU) 915/2023 (for dried spices and cereals) [32] | 0.9–2.0 | 0.05–0.2 | - | - |
| MPC SanHS 2.3.2.1078-01 (1.10.7) [33] | 6 | 1 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulyukin, M.A.; Sutormin, O.S.; Samoylenko, Z.A.; Kravchenko, I.V.; Bulatova, E.V.; Gulakova, N.M.; Baranenko, D.A.; Petrova, Y.Y. Heavy Metal Content in Medicinal Plants Grown in Hydroponics and Forest Soil in the Central Part of Western Siberia. Forests 2024, 15, 1606. https://doi.org/10.3390/f15091606
Mulyukin MA, Sutormin OS, Samoylenko ZA, Kravchenko IV, Bulatova EV, Gulakova NM, Baranenko DA, Petrova YY. Heavy Metal Content in Medicinal Plants Grown in Hydroponics and Forest Soil in the Central Part of Western Siberia. Forests. 2024; 15(9):1606. https://doi.org/10.3390/f15091606
Chicago/Turabian StyleMulyukin, Maksim A., Oleg S. Sutormin, Zoya A. Samoylenko, Inessa V. Kravchenko, Elena V. Bulatova, Natalia M. Gulakova, Denis A. Baranenko, and Yuliya Yu. Petrova. 2024. "Heavy Metal Content in Medicinal Plants Grown in Hydroponics and Forest Soil in the Central Part of Western Siberia" Forests 15, no. 9: 1606. https://doi.org/10.3390/f15091606
APA StyleMulyukin, M. A., Sutormin, O. S., Samoylenko, Z. A., Kravchenko, I. V., Bulatova, E. V., Gulakova, N. M., Baranenko, D. A., & Petrova, Y. Y. (2024). Heavy Metal Content in Medicinal Plants Grown in Hydroponics and Forest Soil in the Central Part of Western Siberia. Forests, 15(9), 1606. https://doi.org/10.3390/f15091606









