Preparing for the Worst: Enhancing Seedling Traits to Reduce Transplant Shock in Semi-Arid Regions †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Species and Nursery Practices
2.3. Experimental Design
2.4. Elevated CO2
2.5. Drought Hardening
2.6. Abscisic Acid
2.7. Control Group
2.8. Physiological and Morphological Measurements
2.8.1. Gas Exchange
2.8.2. Osmotic Adjustment
2.8.3. Stomatal Density
2.8.4. Non-Structural Carbohydrates
2.8.5. Root Metrics
2.8.6. Treatment Relative Growth Rates
2.9. Field Experiment
Field Survival and Growth
2.10. Data Analysis
3. Results
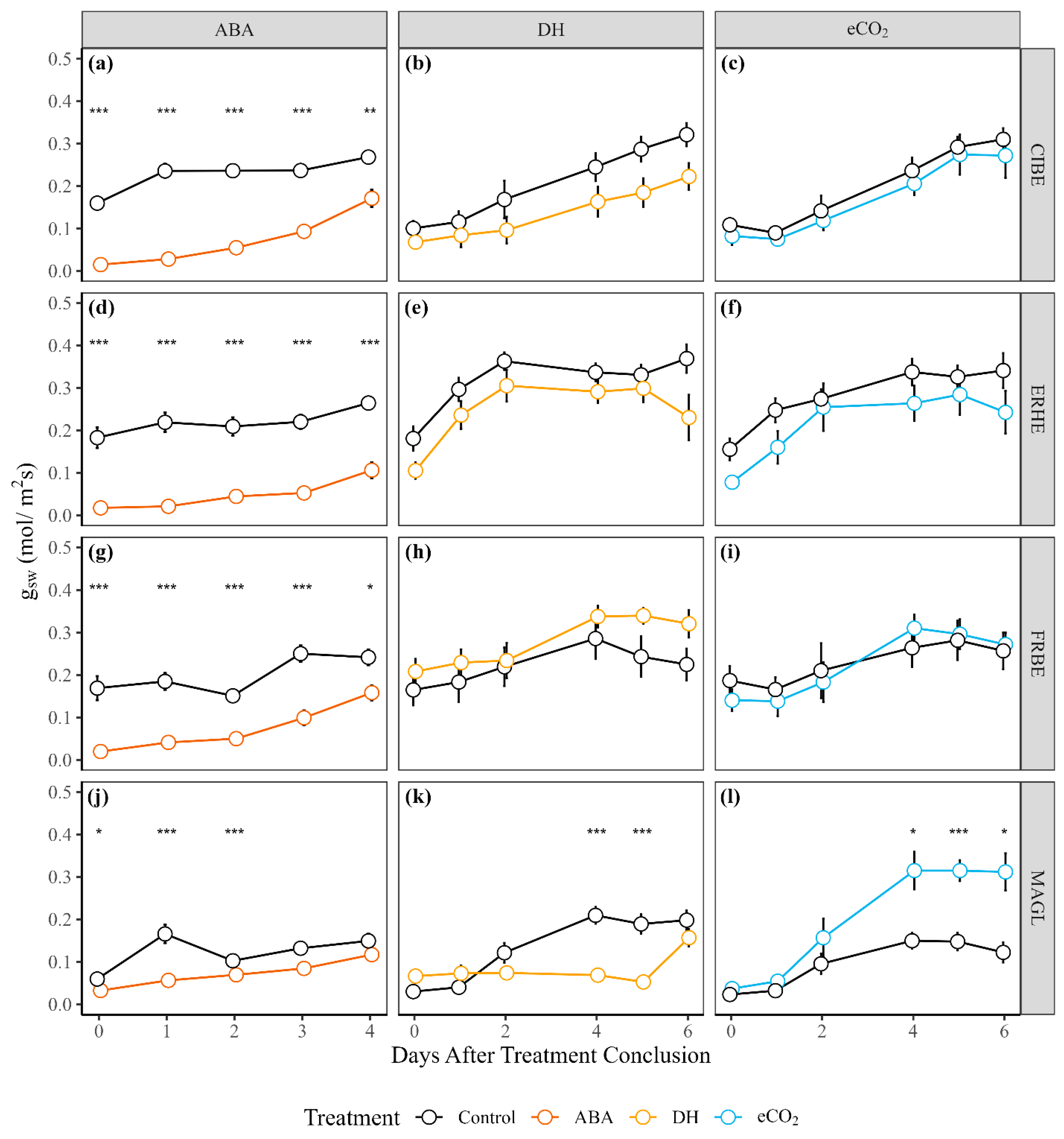
3.1. Photosynthesis and Gas Exchange Parameters
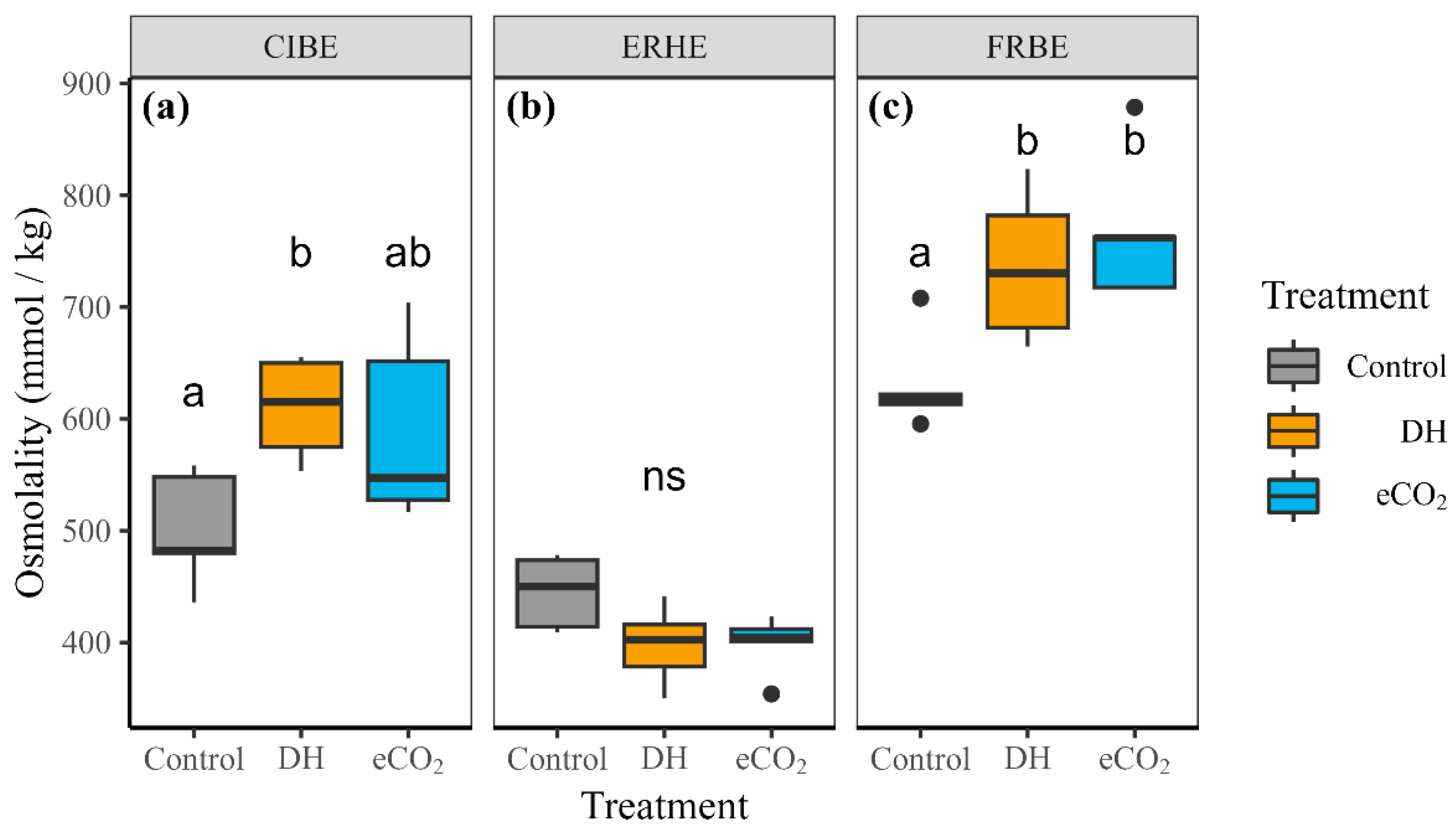
3.2. Leaf Osmolality and Non-Structural Carbohydrates
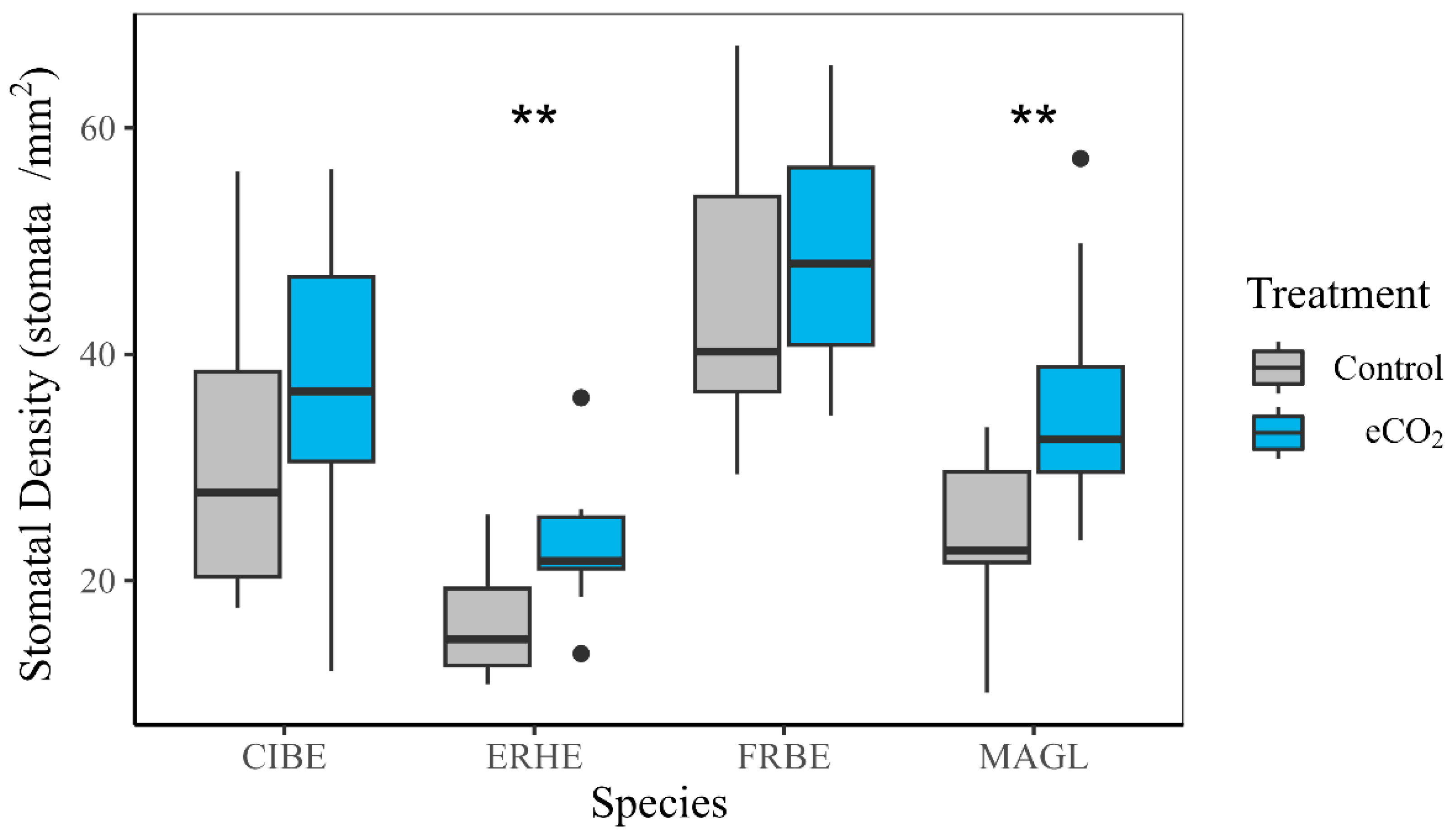
3.3. Root Morphology and Stomatal Density
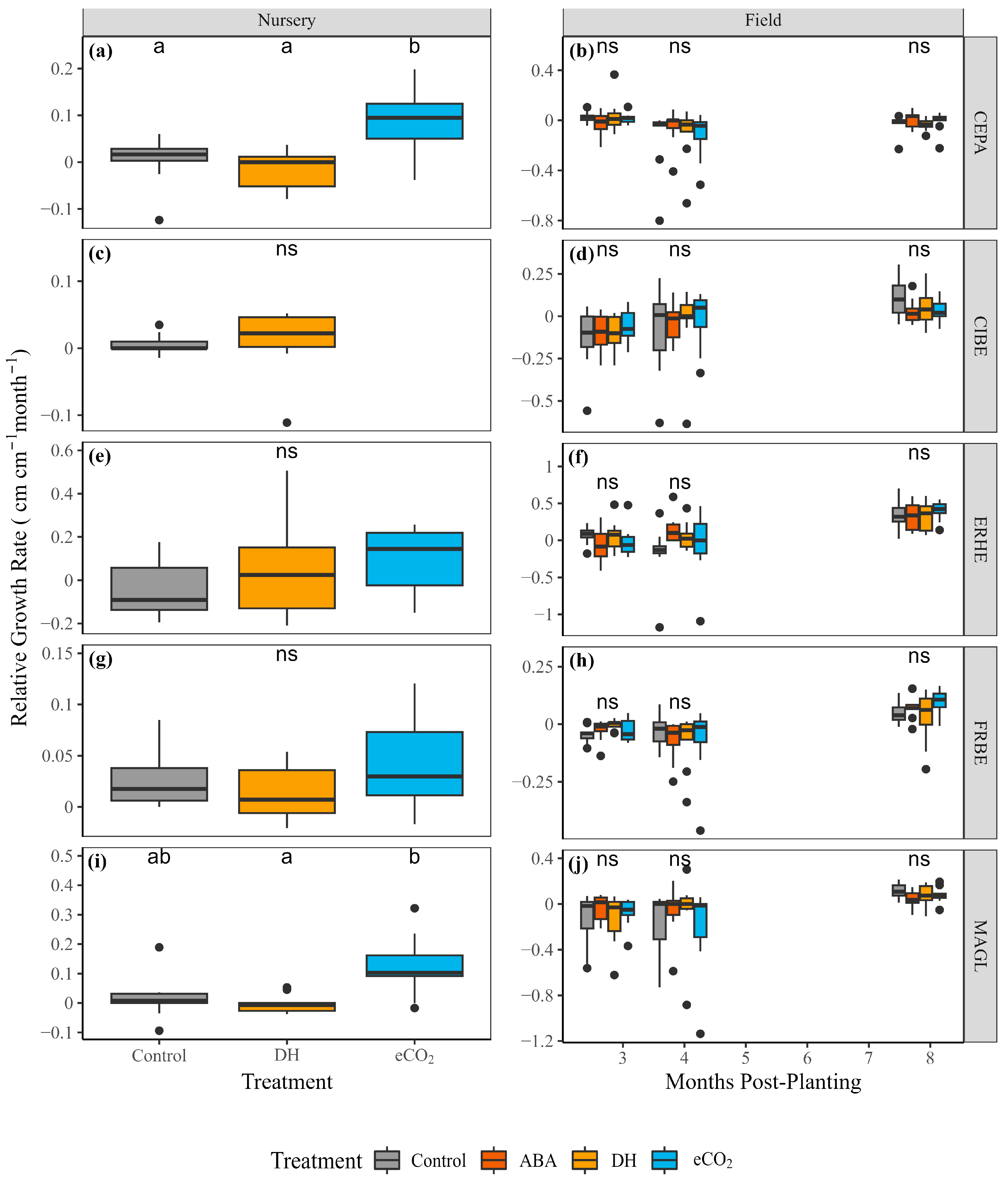
3.4. Field and Post-Treatment Growth and Mortality
4. Discussion
4.1. Response to Elevated CO2
4.2. Drought Hardening Increased Osmolality and Lowered Stomatal Conductance
4.3. Abscisic Acid Reliably Reduced gsw, E, and Asat
4.4. Modifications to Plant Traits Had Little Influence on Field Performance Due to Favorable Field Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derhé, M.A.; Murphy, H.; Monteith, G.; Menéndez, R. Measuring the Success of Reforestation for Restoring Biodiversity and Ecosystem Functioning. J. Appl. Ecol. 2016, 53, 1714–1724. [Google Scholar] [CrossRef]
- Evans, D.M.; Zipper, C.E.; Burger, J.A.; Strahm, B.D.; Villamagna, A.M. Reforestation Practice for Enhancement of Ecosystem Services on a Compacted Surface Mine: Path toward Ecosystem Recovery. Ecol. Eng. 2013, 51, 16–23. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Seedling Establishment on a Forest Restoration Site-An Ecophysiological Perspective. Reforesta 2018, 6, 110–139. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L.; Brown, P.H. The Physiological Basis of Containerised Tree Seedling ‘Transplant Shock’: A Review. Aust. For. 2005, 68, 112–120. [Google Scholar] [CrossRef]
- Duniway, M.C.; Petrie, M.D.; Peters, D.P.C.; Anderson, J.P.; Crossland, K.; Herrick, J.E. Soil Water Dynamics at 15 Locations Distributed across a Desert Landscape: Insights from a 27-Yr Dataset. Ecosphere 2018, 9, e02335. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Kiiskila, S.B.; Haase, D.L. Seedling Ecophysiology: Five Questions to Explore in the Nursery for Optimizing Subsequent Field Success. Tree Plant. Notes 2020, 63, 112–127. [Google Scholar]
- Villar-Salvador, P.; Puértolas, J.; Cuesta, B.; Peñuelas, J.L.; Uscola, M.; Heredia-Guerrero, N.; Rey Benayas, J.M. Increase in Size and Nitrogen Concentration Enhances Seedling Survival in Mediterranean Plantations. Insights from an Ecophysiological Conceptual Model of Plant Survival. New For. 2012, 43, 755–770. [Google Scholar] [CrossRef]
- Luo, N.; Grossnickle, S.C.; Li, G. The Effect of Nursery Drought Preconditioning on Summer Plantation Performance of Pinus tabuliformis: High Winter Mortality Mediated by Trade off between Seedling Carbohydrate and Field Growth. New For. 2022, 53, 301–317. [Google Scholar] [CrossRef]
- Breton, V.; Crosaz, Y.; Rey, F. Effects of Wood Chip Amendments on the Revegetation Performance of Plant Species on Eroded Marly Terrains in a Mediterranean Mountainous Climate (Southern Alps, France). Solid. Earth 2016, 7, 599–610. [Google Scholar] [CrossRef]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Use of Shrubs as Nurse Plants: A New Technique for Reforestation in Mediterranean Mountains. Restor. Ecol. 2002, 10, 297–305. [Google Scholar] [CrossRef]
- Rajaud, A.; de Noblet-Ducoudré, N. Tropical Semi-Arid Regions Expanding over Temperate Latitudes under Climate Change. Clim. Chang. 2017, 144, 703–719. [Google Scholar] [CrossRef]
- Belda, M.; Holtanová, E.; Halenka, T.; Kalvová, J. Climate Classification Revisited: From Köppen to Trewartha. Clim. Res. 2014, 59, 1–13. [Google Scholar] [CrossRef]
- Chan, D.; Wu, Q. Significant Anthropogenic-Induced Changes of Climate Classes since 1950. Sci. Rep. 2015, 5, 13487. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Maps at 1-Km Resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Coopman, R.E.; Jara, J.C.; Bravo, L.A.; Sáez, K.L.; Mella, G.R.; Escobar, R. Changes in Morpho-Physiological Attributes of Eucalyptus globulus Plants in Response to Different Drought Hardening Treatments. Electron. J. Biotechnol. 2008, 11, 30–39. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Planelles, R.; Oliet, J.; Peñuelas-Rubira, J.L.; Jacobs, D.F.; González, M. Drought Tolerance and Transplanting Performance of Holm Oak (Quercus Ilex) Seedlings after Drought Hardening in the Nursery. Tree Physiol. 2004, 24, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Villar-Salvador, P.; Peñuelas, J.L.; Jacobs, D.F. Nitrogen Nutrition and Drought Hardening Exert Opposite Effects on the Stress Tolerance of Pinus pinea L. Seedlings. Tree Physiol. 2013, 33, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Valliere, J.M.; Zhang, J.; Sharifi, M.R.; Rundel, P.W. Can We Condition Native Plants to Increase Drought Tolerance and Improve Restoration Success? Ecol. Appl. 2019, 29, e01863. [Google Scholar] [CrossRef]
- Vilagrosa, A.; Cortina, J.; Gil-Pelegrín, E.; Bellot, J. Suitability of Drought-Preconditioning Techniques in Mediterranean Climate. Restor. Ecol. 2003, 11, 208–216. [Google Scholar] [CrossRef]
- Van Den Driessche, R. Influence of Container Nursery Regimes on Drought Resistance of Seedlings Following Planting. I. Survival and Growth. Can. J. For. Res. 1991, 21, 555–565. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Francis Salifu, K.; Davis, A.S. Drought Susceptibility and Recovery of Transplanted Quercus rubra Seedlings in Relation to Root System Morphology. Ann. Sci. 2009, 66, 504. [Google Scholar] [CrossRef]
- Van Den Driessche, R. Influence of Container Nursery Regimes on Drought Resistance of Seedlings Following Planting: II. Stomatal Conductance, Specific Leaf Area, and Root Growth Capacity. Can. J. For. Res. 1991, 21, 566–572. [Google Scholar] [CrossRef]
- Rietveld, W.J. Transplanting Stress in Bareroot Conifer Seedlings: Its Development and Progression to Establishment. North. J. Appl. For. 1989, 6, 99–107. [Google Scholar] [CrossRef]
- Galvez, D.A.; Landhäusser, S.M.; Tyree, M.T. Low Root Reserve Accumulation during Drought May Lead to Winter Mortality in Poplar Seedlings. New Phytol. 2013, 198, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M. Effects of Water Deficits on Carbon Assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Grossnickle, S. Seedling Size and Reforestation Success. How Big Is Big Enough? In Proceedings of the Thin Green Line: A Symposium on the State-of-the-Art in Reforestation, Thunder Bay, ON, Canada, 26–28 July 2005; pp. 138–143. [Google Scholar]
- Burdett, A.N. Physiological Processes in Plantation Establishment and the Development of Specifications for Forest Planting Stock. Can. J. For. Restor. 1990, 20, 415–427. [Google Scholar] [CrossRef]
- Kozlowski, T.; Pallardy, S. Acclimation and Adaptive Responses of Woody Plants to Environmental Stresses. Bot. Rev. 2002, 68, 270–334. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Why Seedlings Survive: Influence of Plant Attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Folk, R.S.; Grossnickle, S.C. Stock-Type Patterns of Phosphorus Uptake, Retranslocation, Net Photosynthesis and Morphological Development in Interior Spruce Seedlings. New For. 2000, 19, 27–49. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.C.; Domingo, R.; Torrecillas, A.; Pérez-Pastor, A. Water Stress Preconditioning to Improve Drought Resistance in Young Apricot Plants. Plant Sci. 2000, 156, 245–251. [Google Scholar] [CrossRef]
- Thomas, D.S. Survival and Growth of Drought Hardened Eucalyptus pilularis Sm. Seedlings and Vegetative Cuttings. New For. 2009, 38, 245–259. [Google Scholar] [CrossRef]
- Zhang, S.H.; Xu, X.F.; Sun, Y.M.; Zhang, J.L.; Li, C. zhou Influence of Drought Hardening on the Resistance Physiology of Potato Seedlings under Drought Stress. J. Integr. Agric. 2018, 17, 336–347. [Google Scholar] [CrossRef]
- Ritchie, G.A. Carbohydrate Reserves and Root Growth Potential in Douglas-Fir Seedlings before and after Cold Storage. Can. J. For. Res. 1982, 12, 905–912. [Google Scholar] [CrossRef]
- Cuesta, B.; Vega, J.; Villar-Salvador, P.; Rey-Benayas, J.M. Root Growth Dynamics of Aleppo Pine (Pinus halepensis Mill.) Seedlings in Relation to Shoot Elongation, Plant Size and Tissue Nitrogen Concentration. Trees Struct. Funct. 2010, 24, 899–908. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Burslem, D.F.R.P.; Caduff, A.; Tay, J.; Hector, A. Contrasting Nonstructural Carbohydrate Dynamics of Tropical Tree Seedlings under Water Deficit and Variability. New Phytol. 2015, 205, 1083–1094. [Google Scholar] [CrossRef]
- Mexal, J.G.; Landis, T.D. Target Seedling Concepts: Height and Diameter. In Proceedings of the Western Forest Nursery Associations; Rose, R., Campbell, S.J., Landis, T.D., Eds.; Gen. Tech. Rep RM-200; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 1990; pp. 17–36. [Google Scholar]
- Leskovar, D.I.; Goreta, S.; Jifon, J.L.; Agehara, S.; Shinohara, T.; Moore, D. ABA to Enhance Water Stress Tolerance of Vegetable Transplants. Acta Hortic. 2008, 782, 253–263. [Google Scholar] [CrossRef]
- Habermann, E.; Dias de Oliveira, E.A.; Contin, D.R.; San Martin, J.A.B.; Curtarelli, L.; Gonzalez-Meler, M.A.; Martinez, C.A. Stomatal Development and Conductance of a Tropical Forage Legume Are Regulated by Elevated [CO2] under Moderate Warming. Front. Plant Sci. 2019, 10, 609. [Google Scholar] [CrossRef]
- Li, W.; Hartmann, H.; Adams, H.D.; Zhang, H.; Jin, C.; Zhao, C.; Guan, D.; Wang, A.; Yuan, F.; Wu, J. The Sweet Side of Global Change-Dynamic Responses of Non-Structural Carbohydrates to Drought, Elevated CO2 and Nitrogen Fertilization in Tree Species. Tree Physiol. 2018, 38, 1706–1723. [Google Scholar] [CrossRef]
- Landis, T.D. Chapter 2—Irrigation and Water Management. In The Container Tree Nursery Manual; US Department of Agriculture Forest Service: Washington, DC, USA, 1989; Volume 4, pp. 69–119. [Google Scholar]
- Lin, J.; Jach, M.E.; Ceulemans, R. Stomatal Density and Needle Anatomy of Scots Pine (Pinus sylvestris) Are Affected by Elevated CO2. New Phytol. 2001, 150, 665–674. [Google Scholar] [CrossRef]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 Induces Physiological, Biochemical and Structural Changes in Leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef]
- Habermann, E.; San Martin, J.A.B.; Contin, D.R.; Bossan, V.P.; Barboza, A.; Braga, M.R.; Groppo, M.; Martinez, C.A. Increasing Atmospheric CO2 and Canopy Temperature Induces Anatomical and Physiological Changes in Leaves of the C4 Forage Species Panicum maximum. PLoS ONE 2019, 14, e0212506. [Google Scholar] [CrossRef] [PubMed]
- Drake, B.G.; Gonzàlez-Meler, M.A.; Long, S.P. More Efficient Plants: A Consequence of Rising Atmospheric CO2? Annu. Rev. Plant Biol. 1997, 48, 609–639. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Carbon Limitation in Trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon Dynamics in Trees: Feast or Famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of Non-Structural Carbohydrates in Terrestrial Plants: A Global Synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Mphande, W.; Kettlewell, P.S.; Grove, I.G.; Farrell, A.D. The Potential of Antitranspirants in Drought Management of Arable Crops: A Review. Agric. Water Manag. 2020, 236, 106143. [Google Scholar] [CrossRef]
- Kim, J.; Malladi, A.; van Iersel, M.W. Physiological and Molecular Responses to Drought in Petunia: The Importance of Stress Severity. J. Exp. Bot. 2012, 63, 6335–6345. [Google Scholar] [CrossRef]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of Abscisic Acid-Mediated Control of Stomatal Aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef]
- Hebert, C.J.; LeBude, A.V.; Campbell, C. Effects of S-ABA on the Physiology and Marketability of Various Container-Grown Taxa During Short-Term Desiccation. J. Environ. Hortic. 2010, 28, 202–208. [Google Scholar] [CrossRef]
- Waterland, N.L.; Campbell, C.A.; Finer, J.J.; Jones, M.L. Abscisic Acid Application Enhances Drought Stress Tolerance in Bedding Plants. HortScience 2010, 45, 409–413. [Google Scholar] [CrossRef]
- Racsko, J.; Marmor, F.; Hopkins, C.R.; Petracek, P.; Silverman, F.P.; Fritts, R.; Liu, X.; Woolard, D.; Lopez, J.; Leep, D.; et al. Use of S-Abscisic Acid (ConTegoTM SL) in Vegetable Production. Acta Hortic. 2014, 1042, 243–253. [Google Scholar] [CrossRef]
- Shinohara, T.; Leskovar, D.I. Effects of ABA, Antitranspirants, Heat and Drought Stress on Plant Growth, Physiology and Water Status of Artichoke Transplants. Sci. Hortic. 2014, 165, 225–234. [Google Scholar] [CrossRef]
- Park, S.; Mills, S.A.; Moon, Y.; Waterland, N.L. Evaluation of Antitranspirants for Enhancing Temporary Water Stress Tolerance in Bedding Plants. Horttechnology 2016, 26, 444–452. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Liu, X.; Shao, L.; Sun, H.; Chen, S. Improving Winter Wheat Performance by Foliar Spray of ABA and FA Under Water Deficit Conditions. J. Plant Growth Regul. 2016, 35, 83–96. [Google Scholar] [CrossRef]
- Huang, X.; Lin, S.; He, S.; Lin, X.; Liu, J.; Chen, R.; Li, H. Characterization of Stomata on Floral Organs and Scapes of Cut ‘Real’ Gerbaras and Their Involvement in Postharvest Water Loss. Postharvest Biol. Technol. 2018, 142, 39–45. [Google Scholar] [CrossRef]
- Leslie, D.M., Jr. An International Borderland of Concern: Conservation of Biodiversity in the Lower Rio Grande Valley Scientific Investigations Report, 2016-5078; US Geological Survey: Reston, VA, USA, 2016. [Google Scholar]
- Ruiz, M. Personal Communication; The Nature Conservancy: Brownsville, TX, USA, 2021. [Google Scholar]
- Soil Survey Staff Web Soil Survey. Available online: https://www.nrcs.usda.gov/resources/data-and-reports/web-soil-survey (accessed on 14 August 2024).
- Thornthwaite, C.W. An Approach toward a Rational Classification of Climate. Geogr. Rev. 1948, 38, 55. [Google Scholar] [CrossRef]
- Stowe, L.G.; Brown, J.L. A Geographic Perspective on the Ecology of Compound Leaves. Evolution 1981, 35, 818–821. [Google Scholar] [CrossRef]
- Xu, F.; Guo, W.; Xu, W.; Wei, Y.; Wang, R. Leaf Morphology Correlates with Water and Light Availability: What Consequences for Simple and Compound Leaves. Prog. Nat. Sci. 2009, 19, 1789–1798. [Google Scholar] [CrossRef]
- Givnish, T.J. Comparative Studies of Leaf Form: Assessing the Relative Roles of Selective Pressures and Phylogenetic Constraints. New Phytol. 1987, 106, 131–160. [Google Scholar] [CrossRef]
- Mohsin, F.; Arias, M.; Albrecht, C.; Wahl, K.; Fierro-Cabo, A.; Christoffersen, B. Species-Specific Responses to Restoration Interventions in a Tamaulipan Thornforest. Ecol. Manag. 2021, 491, 119–154. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Ardy, R.; Zhang, Y.; Sun, S.; Cao, K.; Sack, L. Rapid Determination of Comparative Drought Tolerance Traits: Using an Osmometer to Predict Turgor Loss Point. Methods Ecol. Evol. 2012, 3, 880–888. [Google Scholar] [CrossRef]
- Albrecht, C.; Contreras, Z.; Wahl, K.; Sternberg, M.; Christoffersen, B. Winners and Losers in Dryland Reforestation: Species Survival, Growth, and Recruitment along a 33-Year Planting Chronosequence. Restor. Ecol. 2022, 30, e13559. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Landhäusser, S.M.; Chow, P.S.; Turin Dickman, L.; Furze, M.E.; Kuhlman, I.; Schmid, S.; Wiesenbauer, J.; Wild, B.; Gleixner, G.; Hartmann, H.; et al. Standardized Protocols and Procedures Can Precisely and Accurately Quantify Non-Structural Carbohydrates. Tree Physiol. 2018, 38, 1764–1778. [Google Scholar] [CrossRef]
- Hunt, R.; Causton, D.R.; Shipley, B.; Askew, A.P. A Modern Tool for Classical Plant Growth Analysis. Ann. Bot. 2002, 90, 485–488. [Google Scholar] [CrossRef]
- Sheil, D.; May, R.M. Mortality and Recruitment Rate Evaluations in Heterogenous Tropical Forests. J. Ecol. 1996, 84, 91–100. [Google Scholar] [CrossRef]
- R, version 4.3.1; A Language and Environment for Statistical Computing; Windows; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Rstatix, version 0.7.2, Pipe-Friendly Framework for Basic Statistical Tests; R Package; Datanovia: Marseille, France, 2023. [CrossRef]
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships. R. News 2002, 2, 7–10. [Google Scholar]
- Fox, J. Regression Diagnostics: An Introduction; Sage Publications Inc.: Thousand Oaks, CA, USA, 1991. [Google Scholar]
- Rcompanion, version 2.4.36, Functions to Support Extension Education Program Evaluation; R package; Rutgers Cooperative Extension: New Brunswick, NJ, USA, 2024.
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Seemann, J.R.; Downton, W.J.S.; Berry, J.A. Temperature and Leaf Osmotic Potential as Factors in the Acclimation of Photosynthesis to High Temperature in Desert Plants. Plant Physiol. 1986, 80, 926–930. [Google Scholar] [CrossRef]
- Iba, K. Acclimative Response to Temperature Stress in Higher Plants: Approaches of Gene Engineering for Temperature Tolerance. Annu. Rev. Plant Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What Have We Learned from 15 Years of Free-Air CO2 Enrichment (FACE)? A Meta-Analytic Review of the Responses of Photosynthesis, Canopy Properties and Plant Production to Rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Doheny-Adams, T.W.; Britton-Harper, Z.J.; Gray, J.E. Increasing Water-Use Efficiency Directly through Genetic Manipulation of Stomatal Density. New Phytol. 2015, 207, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S.; Pandey, G.P. Impact of Elevated Temperatures on Specific Leaf Weight, Stomatal Density, Photosynthesis and Chlorophyll Fluorescence in Soybean. Photosynth. Res. 2017, 131, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, H.; Cao, Y.; Li, J.; Zhu, Q.; Yao, J.; Xu, C. Effect of Simulated Warming on Leaf Functional Traits of Urban Greening Plants. BMC Plant Biol. 2020, 20, 139. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, R.; van Praet, L.; Jiang, X.N. Effects of CO2 Enrichment, Leaf Position and Clone on Stomatal Index and Epidermal Cell Density in Poplar (Populus). New Phytol. 1995, 131, 99–107. [Google Scholar] [CrossRef]
- Poole, I.; Lawson, T.; Weyers, J.D.B.; Raven, J.A. Effect of Elevated CO2 on the Stomatal Distribution and Leaf Physiology of Alnus glutinosa. New Phytol. 2000, 145, 511–521. [Google Scholar] [CrossRef]
- Feeley, K.; Martinez-Villa, J.; Perez, T.; Silva Duque, A.; Triviño Gonzalez, D.; Duque, A. The Thermal Tolerances, Distributions, and Performances of Tropical Montane Tree Species. Front. For. Glob. Chang. 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Kullberg, A.T.; Feeley, K.J. Limited Acclimation of Leaf Traits and Leaf Temperatures in a Subtropical Urban Heat Island. Tree Physiol. 2022, 42, 2266–2281. [Google Scholar] [CrossRef]
- Lu, N.; Nukaya, T.; Kamimura, T.; Zhang, D.; Kurimoto, I.; Takagaki, M.; Maruo, T.; Kozai, T.; Yamori, W. Control of Vapor Pressure Deficit (VPD) in Greenhouse Enhanced Tomato Growth and Productivity during the Winter Season. Sci. Hortic. 2015, 197, 17–23. [Google Scholar] [CrossRef]
- Herbert, T.J. Axial Rotation of Erythrina herbacea Leaflets. Am. J. Bot. 1984, 71, 76–79. [Google Scholar] [CrossRef]
- Ostonen, I.; Püttsepp, Ü.; Biel, C.; Alberton, O.; Bakker, M.R.; Lõhmus, K.; Majdi, H.; Metcalfe, D.; Olsthoorn, A.F.M.; Pronk, A.; et al. Specific Root Length as an Indicator of Environmental Change. Plant Biosyst. 2007, 141, 426–442. [Google Scholar] [CrossRef]
- Abrams, M.D. Sources of Variation in Osmotic Potentials with Special Reference to North American Tree Species. For. Sci. 1988, 34, 1030–1046. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Garbowski, M.; Avera, B.; Bertram, J.H.; Courkamp, J.S.; Gray, J.; Hein, K.M.; Lawrence, R.; McIntosh, M.; McClelland, S.; Post, A.K.; et al. Getting to the Root of Restoration: Considering Root Traits for Improved Restoration Outcomes under Drought and Competition. Restor. Ecol. 2020, 28, 1384–1395. [Google Scholar] [CrossRef]
- Saliendra, N.Z.; Meinzer, F.C. Symplast Volume, Turgor, Stomatal Conductance and Growth in Relation to Osmotic and Elastic Adjustment in Droughted Sugarcane. J. Exp. Bot. 1991, 42, 1251–1259. [Google Scholar] [CrossRef]
- Sanders, G.J.; Arndt, S.K. Osmotic Adjustment under Drought Conditions. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; pp. 199–229. ISBN 9783642326530. [Google Scholar]
- Martins, J.; Neves, M.; Canhoto, J. Drought-Stress-Induced Changes in Chloroplast Gene Expression in Two Contrasting Strawberry Tree (Arbutus unedo L.) Genotypes. Plants 2023, 12, 4133. [Google Scholar] [CrossRef]
- Von Moler, E.R.; Nelson, A.S. Perspectives on Drought Preconditioning Treatments With a Case Study Using Western Larch. Front. Plant Sci. 2021, 12, 741027. [Google Scholar] [CrossRef]
- Zhou, S.X.; Medlyn, B.E.; Prentice, I.C. Long-Term Water Stress Leads to Acclimation of Drought Sensitivity of Photosynthetic Capacity in Xeric but Not Riparian Eucalyptus Species. Ann. Bot. 2016, 117, 133–144. [Google Scholar] [CrossRef]
- Von Moler, E.R.; Kolb, T.; Brady, A.; Palmiero, B.N.; Wallace, T.R.; Waring, K.M.; Whipple, A.V. Plant Developmental Stage Influences Responses of Pinus strobiformis Seedlings to Experimental Warming. Plant-Environ. Interact. 2021, 2, 148–164. [Google Scholar] [CrossRef]
- Neyland, R. Wildflowers of the Coastal Plain; LSU Press: Baton Rouge, LA, USA, 2009. [Google Scholar]
- Tanimoto, E. Regulation of Root Growth by Plant Hormones—Roles for Auxin and Gibberellin. CRC Crit. Rev. Plant Sci. 2005, 24, 249–265. [Google Scholar] [CrossRef]
- Dickinson, A.J.; Lehner, K.; Mi, J.; Jia, K.P.; Mijar, M.; Dinneny, J.; Al-Babili, S.; Benfey, P.N. β-Cyclocitral Is a Conserved Root Growth Regulator. Proc. Natl. Acad. Sci. USA 2019, 116, 10563–10567. [Google Scholar] [CrossRef] [PubMed]
- Ewing, K.; Best, C. South Texas Tamaulipan Thornscrub Restoration Experiment Measures Growth of Planted Woody Vegetation. Ecol. Restor. 2004, 22, 11–17. [Google Scholar] [CrossRef]
- Dick, K.; Alexander, H.D.; Moczygemba, J.D. Use of Shelter Tubes, Grass-Specific Herbicide, and Herbivore Exclosures to Reduce Stressors and Improve Restoration of Semiarid Thornscrub Forests. Restor. Ecol. 2016, 24, 785–793. [Google Scholar] [CrossRef]
- Zirbel, C.R.; Brudvig, L.A. Trait–Environment Interactions Affect Plant Establishment Success during Restoration. Ecology 2020, 101, e02971. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Duration (n) | Duration (f) | Total Exposure (n) | Total Exposure (f) | Response Variables (n) | Response Variables (f) |
|---|---|---|---|---|---|---|
| ABA (abscisic acid) | Applied Dec. 13 | Applied Nov. 14 | NA | NA | gsw, E, Asat, WUE | mortality and growth |
| DR (drought hardening) | Oct. 14–Nov. 29 | Oct. 14–Nov. 14 | NA | NA | gsw, E, Asat, WUE, osmleaf, growth, SRL | mortality and growth |
| eCO2 (elevated CO2) | Oct. 14–Nov. 29 | Oct. 14–Nov. 14 | 747.3 h | 547.4 h | gsw, E, Asat, WUE, osmleaf, growth, SRL, SD, NSCs | mortality and growth |
| Response | Treatment | CIBE | ERHE | FRBE | MAGL | CEPA |
|---|---|---|---|---|---|---|
| gsw | DH | ns | ns | ns | ↓ | - |
| eCO2 | ns | ns | ns | ↑ | - | |
| ABA | ↓ | ↓ | ↓ | ↓ | - | |
| T | DH | ns | ns | ns | - | - |
| eCO2 | - | ns | ns | - | - | |
| ABA | ↓ | ↓ | ↓ | - | - | |
| Asat | DH | ns | ns | ns | - | - |
| eCO2 | - | ↓ | ns | - | - | |
| ABA | ns | ↓ | ↓ | - | - | |
| WUE | DH | ns | ns | ns | - | - |
| eCO2 | - | ns | ns | - | - | |
| ABA | ns | ns | ns | - | - | |
| SD | eCO2 | ns | ↑ | ns | ↑ | - |
| osmleaf | DH | ↑ | ns | ↑ | - | - |
| eCO2 | ns | ns | ↑ | - | - | |
| SRL * | DH | ns | ns | ns | ns | ns |
| eCO2 | ns | ns | ns | ns | ns | |
| RL * | DH | ns | ns | ns | ns | ns |
| eCO2 | ns | ns | ↑ | ns | ns | |
| RGRn | DH | ns | ns | ns | ns | ns |
| eCO2 | ns | ns | ns | ↑ | ↑ | |
| RGRf | DH | ns | ns | ns | ns | ns |
| eCO2 | ns | ns | ns | ns | ns | |
| ABA | ns | ns | ns | ns | ns | |
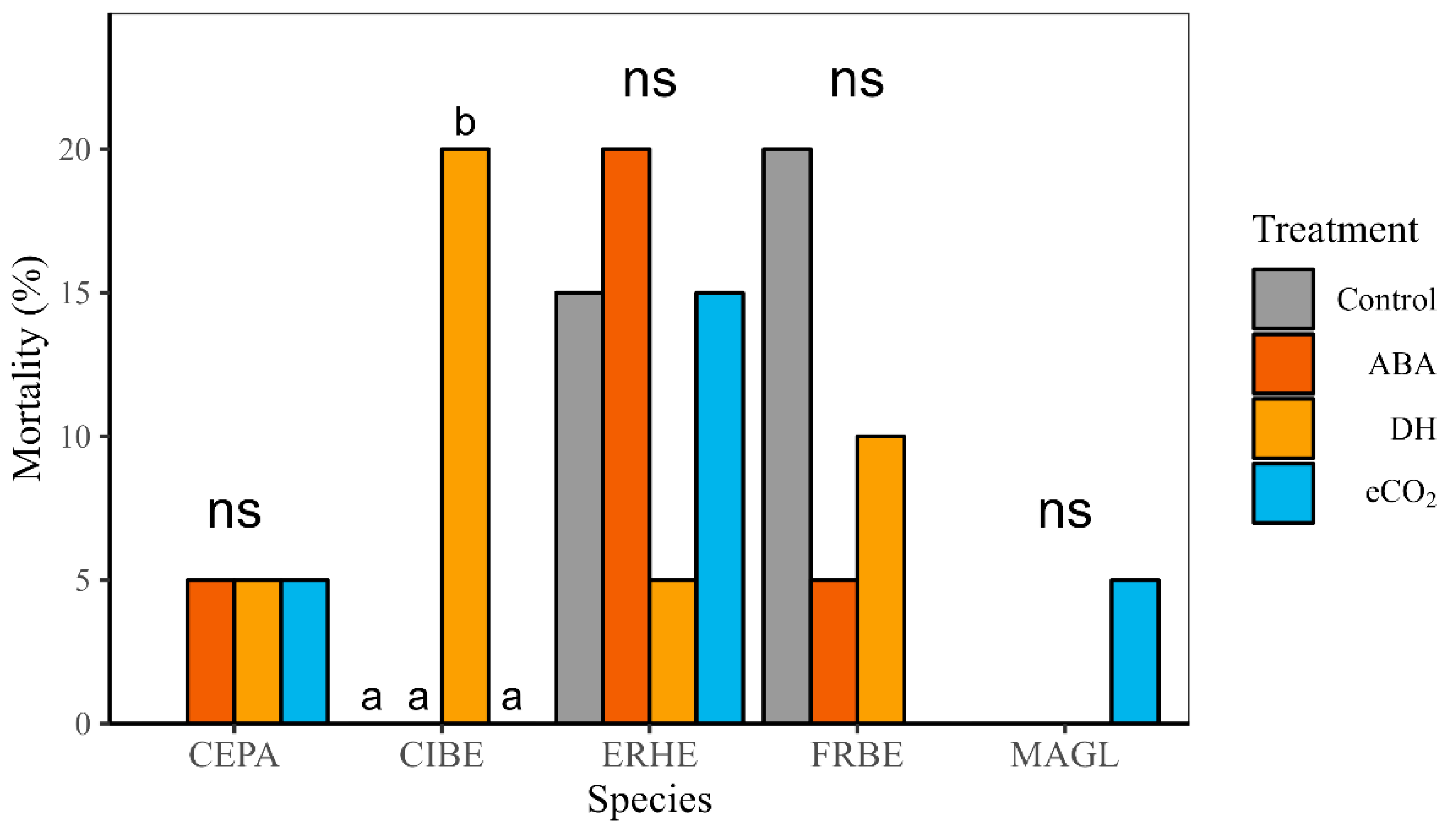
| Mortality | DH | ↑ | ns | ns | ns | ns |
| eCO2 | ns | ns | ns | ns | ns | |
| ABA | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mainhart, D.E.; Christoffersen, B.O.; Thompson, R.A.; Reemts, C.M.; Fierro-Cabo, A. Preparing for the Worst: Enhancing Seedling Traits to Reduce Transplant Shock in Semi-Arid Regions. Forests 2024, 15, 1607. https://doi.org/10.3390/f15091607
Mainhart DE, Christoffersen BO, Thompson RA, Reemts CM, Fierro-Cabo A. Preparing for the Worst: Enhancing Seedling Traits to Reduce Transplant Shock in Semi-Arid Regions. Forests. 2024; 15(9):1607. https://doi.org/10.3390/f15091607
Chicago/Turabian StyleMainhart, Douglas E., Bradley O. Christoffersen, R. Alexander Thompson, Charlotte M. Reemts, and Alejandro Fierro-Cabo. 2024. "Preparing for the Worst: Enhancing Seedling Traits to Reduce Transplant Shock in Semi-Arid Regions" Forests 15, no. 9: 1607. https://doi.org/10.3390/f15091607






