A Proteomic Study on Seed Germination of Nitraria roborowskii Kom.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Experimental Instrumentation
2.3. Protein Extraction Quality Control
2.4. SDS–PAGE Electrophoresis
2.5. Trypsin Treatment and Desalting
2.6. Labeling Samples
2.7. Fractions
2.8. LC–MS/MS Mass Spectrometry Analysis
2.9. Data Processing
3. Functional Analysis of Differentially Expressed Proteins
3.1. GO Functional Annotation
3.2. KEGG Analysis
3.3. GO Function Enrichment Analysis and KEGG Annotation
4. Results and Analysis
4.1. Data Quality Control
4.1.1. Statistics of Protein Qualitative Results
4.1.2. Statistics of Protein Quantification Results
4.1.3. Evaluation and Statistical Analysis of Identified Peptides
4.1.4. Evaluation and Statistical Analysis of Identified Proteins
4.2. Bioinformatics Analysis
4.2.1. Global Analysis
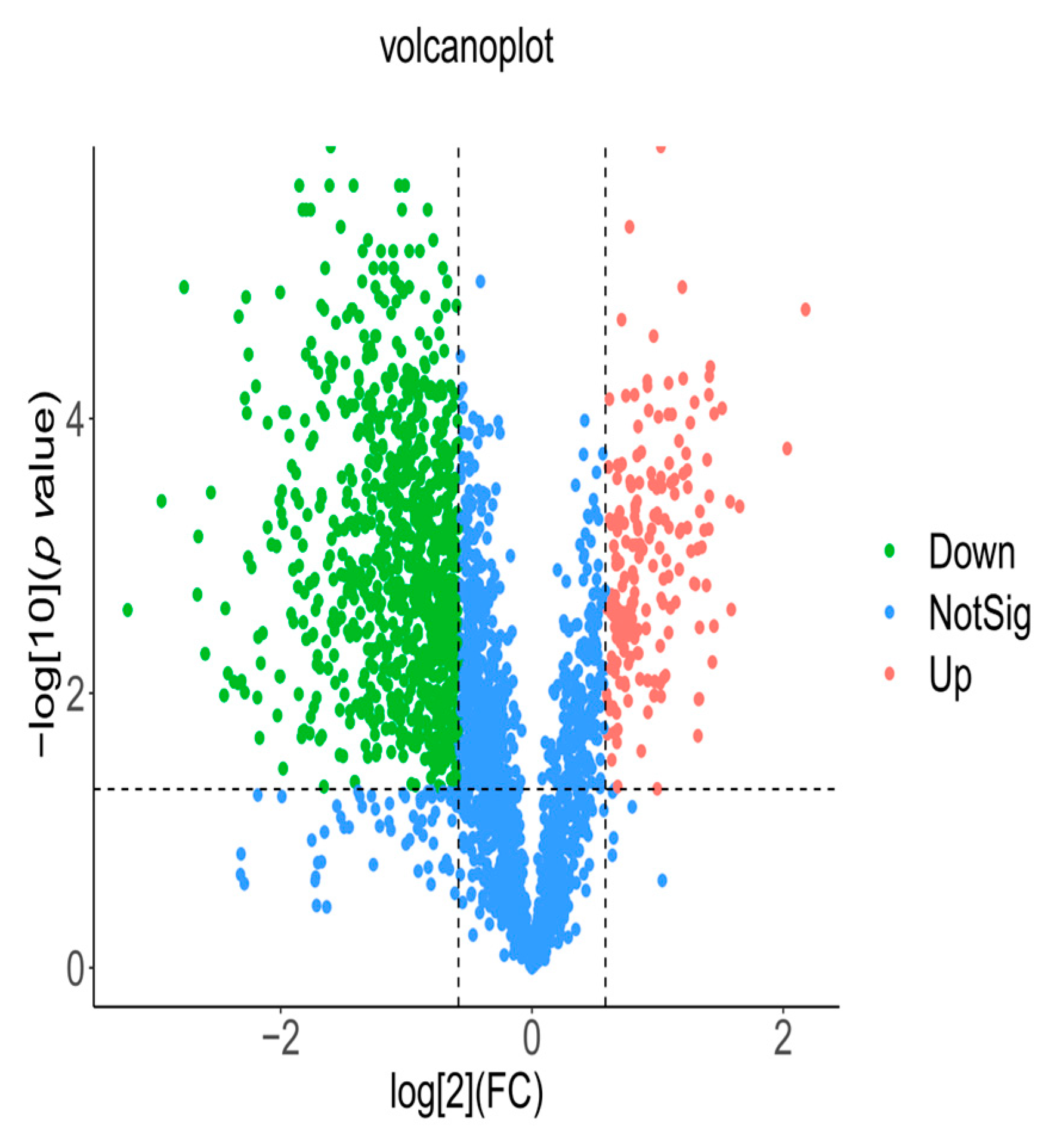
4.2.2. Statistics of the Differentially Expressed Proteins
4.3. Functional Analysis of Differentially Expressed Proteins
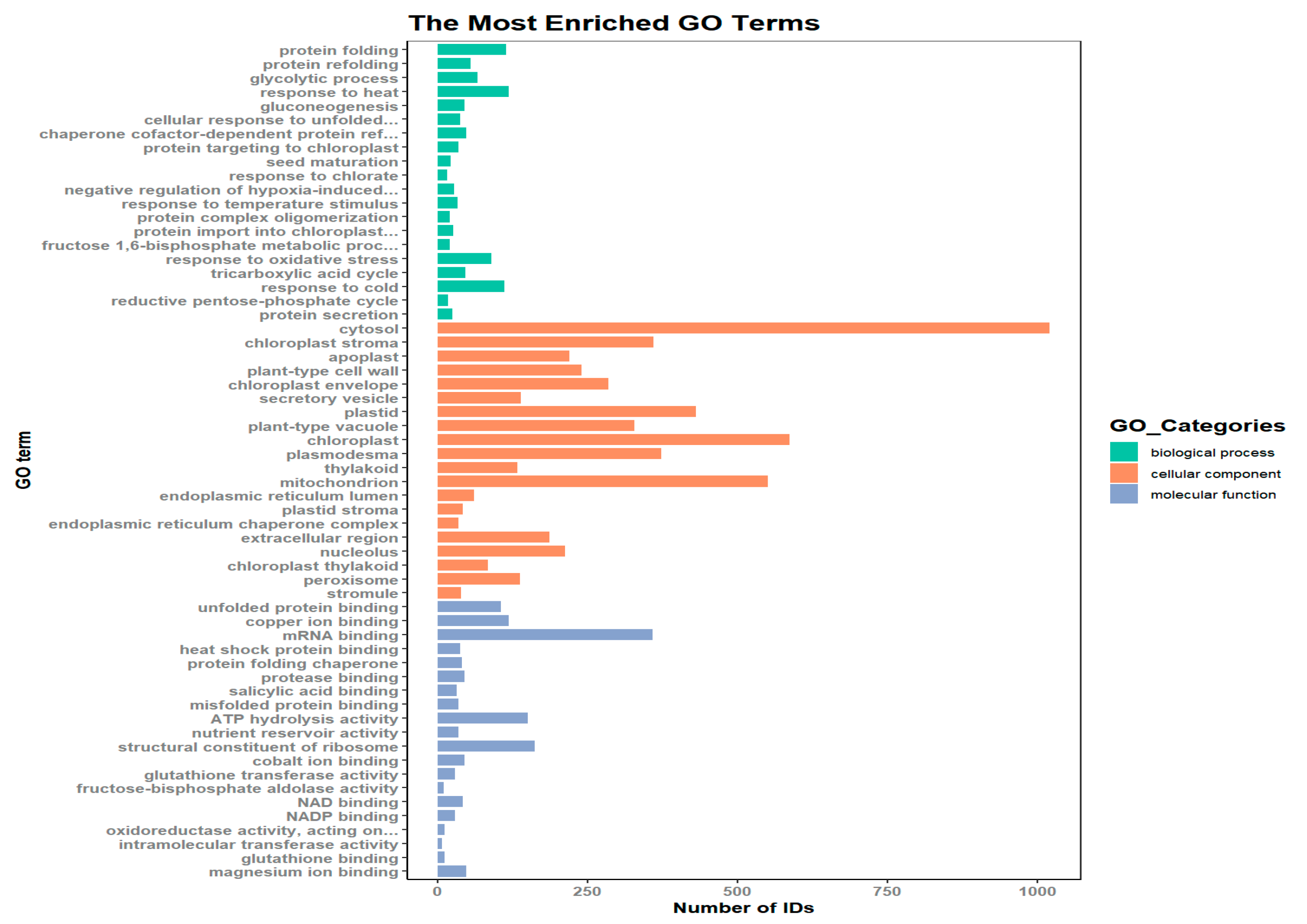
4.3.1. GO Functional Annotation of DEPs
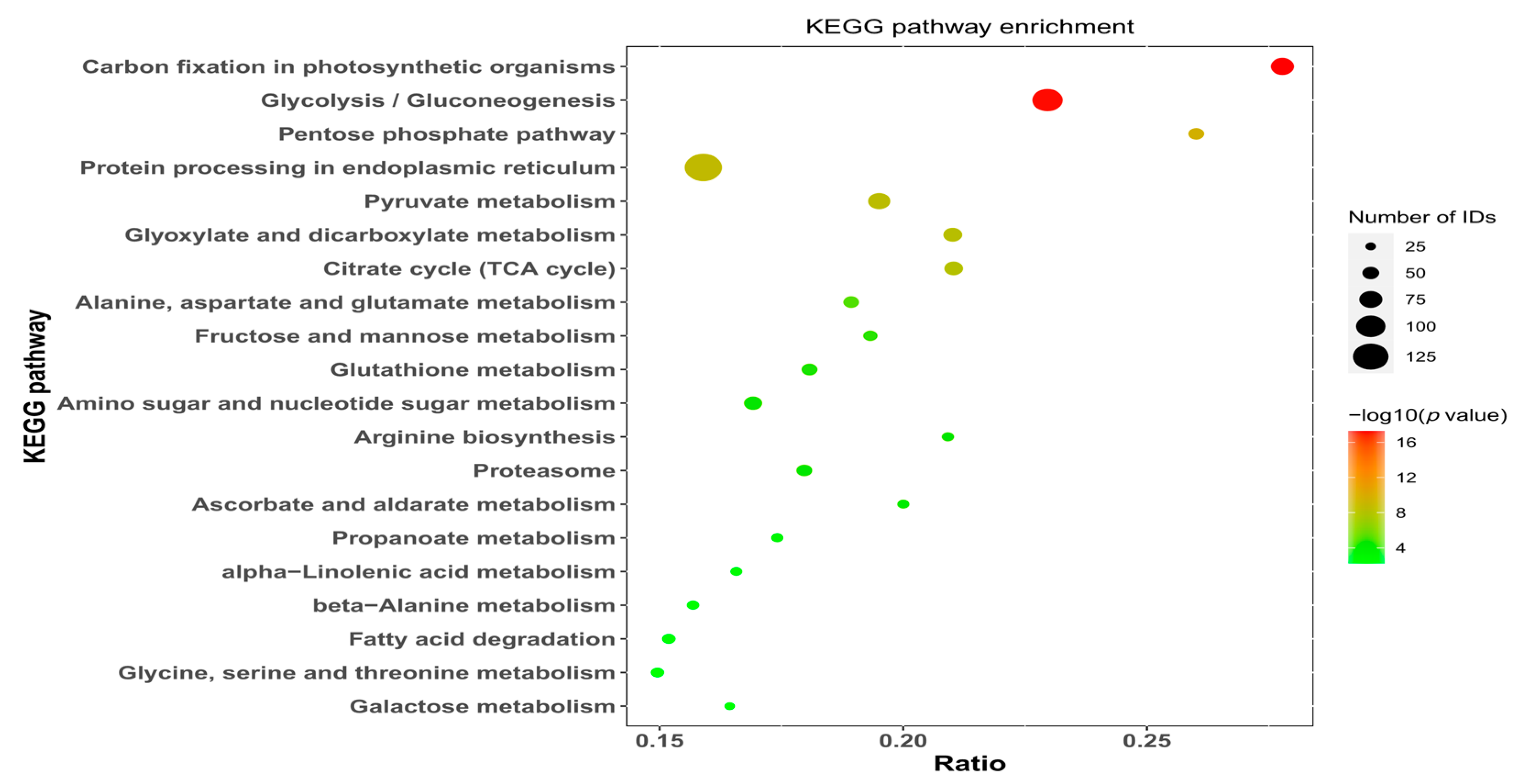
4.3.2. KEGG Analysis
4.3.3. COG Functional Annotation
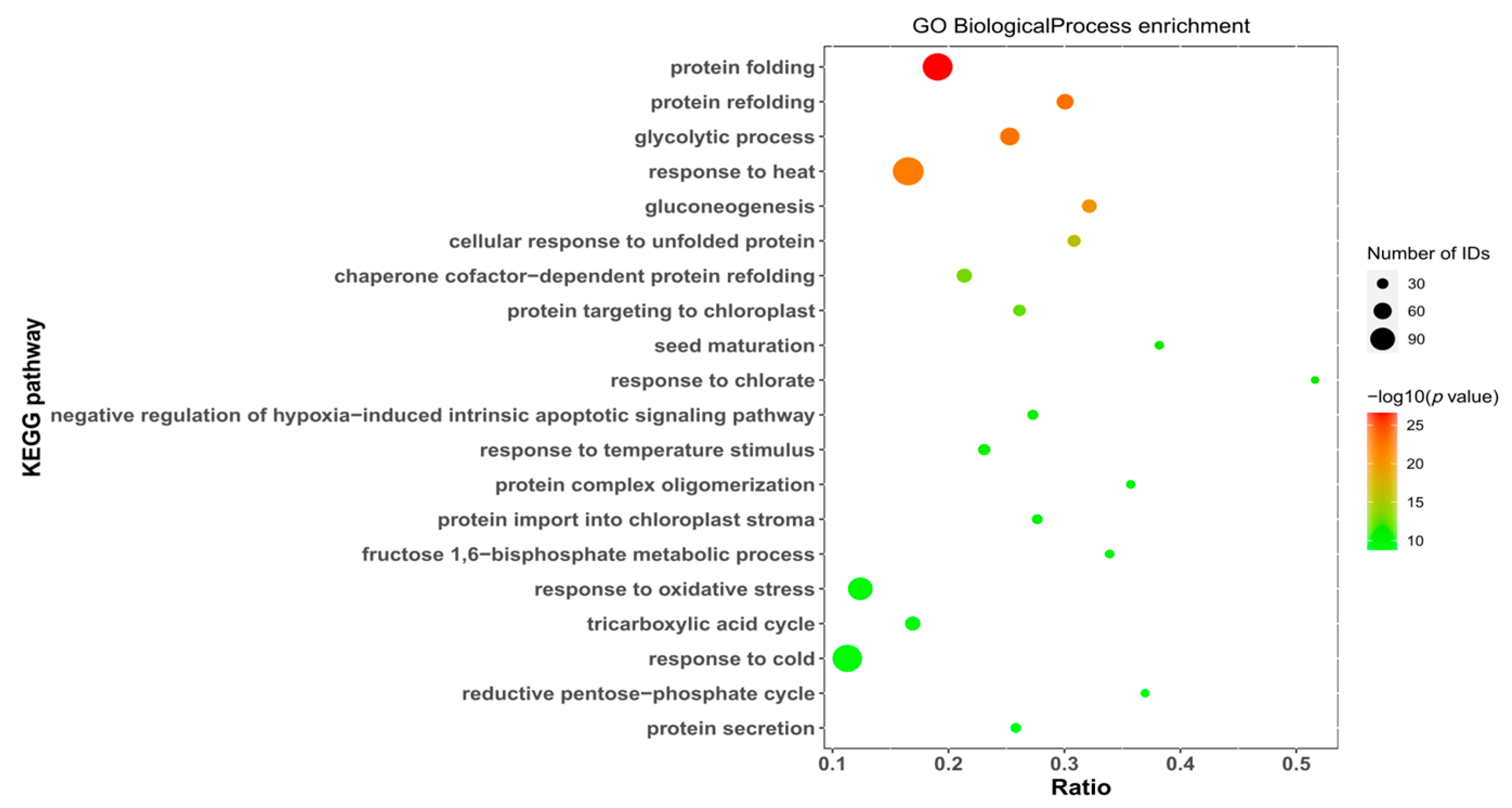
4.3.4. GO Enrichment Analysis
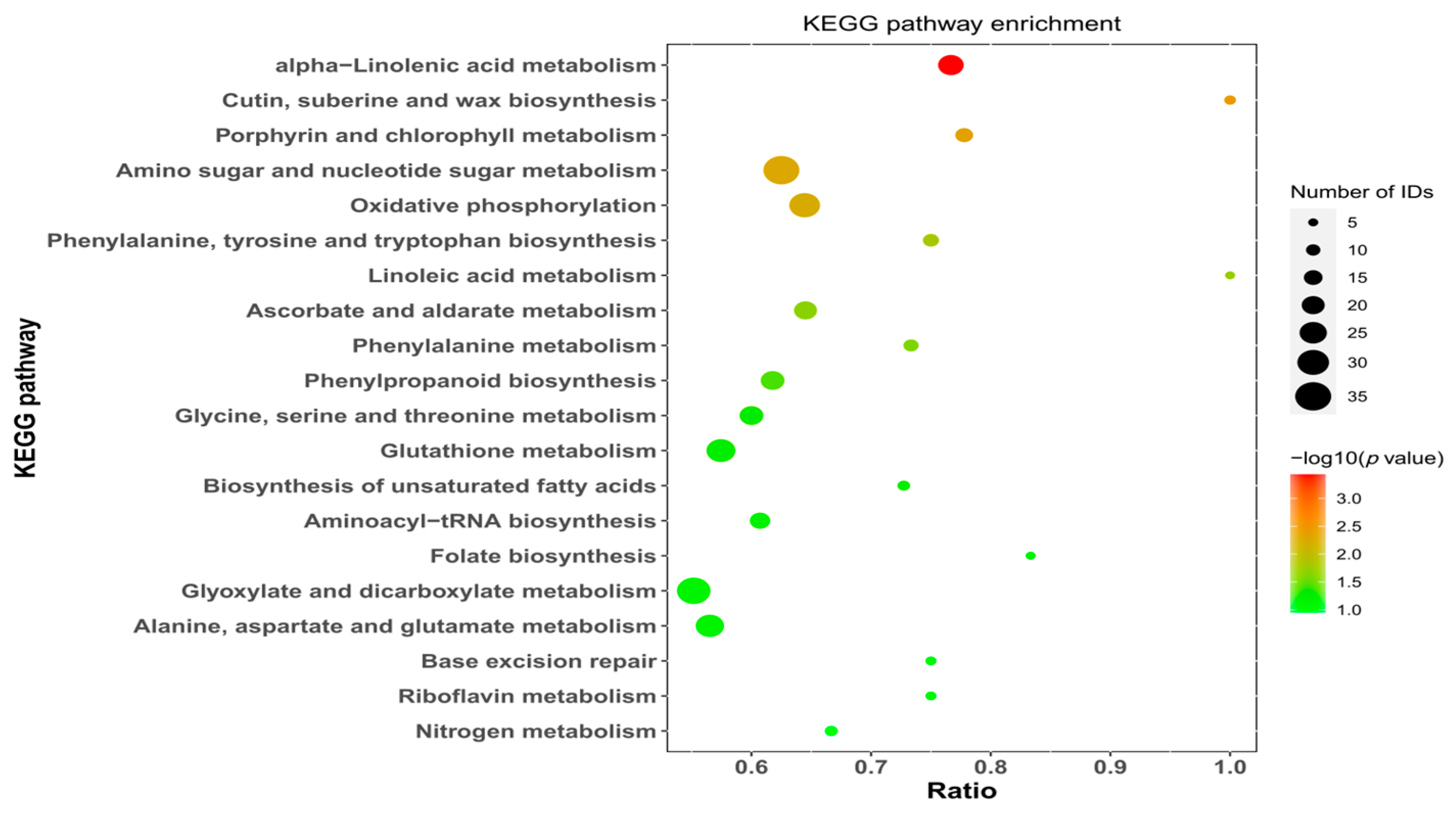
4.3.5. KEGG Pathway Annotation
4.3.6. Interaction Analysis
4.3.7. Analysis of Differentially Expressed Proteins
5. Discussion
5.1. Analysis of Differentially Expressed Proteins before and after the Release of Dormancy in N. roborowskii Seeds
5.2. Analysis of Proteomic Metabolic Pathways before and after the Release of Dormancy in N. roborowskii Seeds
5.3. Combined Analysis of the Transcriptome and Proteome before and after Dormancy Release in N. roborowskii Seeds
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bewley, J. Derek. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Zhou, L.H. Nitariaceae, Peganaceae, Zygophyllaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2008; Volume 11, pp. 41–50. [Google Scholar]
- Liu, Y.K.; Li, Y.L.; Zhang, Z.J.; Wang, T.Q.; Wang, S.R.; Wang, Y. Adaptability and Soil Modification in Soda Salinized Soil by Lycium and Nitraria. J. Jilin Agric. Univ. 2024, 46, 1–11. [Google Scholar]
- Wu, D.; Jiang, S.; Wang, G.; Wang, L.; Wu, L.; Li, J.; Jia, W.; Liu, L.; Xu, J.; Zhang, D.; et al. Characterization of alkaloids and phenolics in Nitraria roborowskii Kom. fruit by UHPLC-triple-TOF-MS/MS and its sucrase and maltase inhibitory effects. Food Chem. 2024, 447, 138743. [Google Scholar] [CrossRef]
- Commander, L.; Merritt, D.; Rokich, D.; Dixon, K. Seed biology of Australian arid zone species: Germination of 18 species used for rehabilitation. J. Arid Environ. 2009, 73, 617–625. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Zeng, Y.; Wei, J.; Yu, L.; Wang, Y. Seed viability, germination and dormancy of Nitraria roborowskii (Nitrariaceae). Seed Sci. Technol. 2016, 44, 647–653. [Google Scholar] [CrossRef]
- Lu, Y.; Lei, J.-Q.; Zeng, F.-J.; Xu, L.-S.; Liu, G.-J.; Peng, S.-L.; Huang, C.-B. Effects of salt stress on Nitraria roborowskii growth and physiological characteristics of stress resistance. Chin. J. Appl. Ecol. 2014, 25, 711–717. [Google Scholar]
- Lu, Y.; Lei, J.; Zeng, F. NaCl salinity-induced changes in growth, photosynthetic properties, water status and enzymatic antioxidant system of Nitraria roborowskii Kom. Pak. J. Bot 2016, 48, 843–851. [Google Scholar]
- Zhang, X.H.; Yan, L.; Zhang, W.B. Morphological and structural characteristics of Nitraria roborowskii Kom and its ecological adaptabilities. J. Arid Land Resour. Environ. 2013, 27, 74–79. [Google Scholar]
- Ren, S.F.; Jiang, L.M.; Lv, G.H. A Study on Endogenous Inhibitors of Nitraria roborowskii Kom. Seeds. Forests 2024, 15, 773. [Google Scholar] [CrossRef]
- Wasinger, V.C.; Cordwell, S.J.; Cerpa-Poljak, A.; Yan, J.X.; Gooley, A.A.; Wilkins, M.R.; Duncan, M.W.; Harris, R.; Williams, K.L.; Humphery-Smith, I. Progress with gene-product mapping of the Mollicutes: Mycoplasma genitalium. Electrophoresis 1995, 16, 1090–1094. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomic analysis of Arabidopsis seed germination and priming. Plant Physiol. 2001, 126, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Kamo, M.; Kawakami, T.; Miyatake, N.; Tsugita, A. Separation and characterization of Arabidopsis thaliana proteins by two-dimensional gel electrophoresis. Electrophoresis 1995, 16, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Chibani, K.; Ali-Rachedi, S.; Job, C.; Job, D.; Jullien, M.; Grappin, P. Proteomic analysis of seed dormancy in Arabidopsis. Plant Physiol. 2006, 142, 1493–1510. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol. 2002, 129, 823–837. [Google Scholar] [CrossRef]
- Lane, B.G. Oxalate, germin, and the extracellular matrix of higher plants. FASEB J. 1994, 8, 294–301. [Google Scholar] [CrossRef]
- Machado, C.F. Gene Expression during Germination and Postgermination of Dried and Nondried Developing Seeds of Tomato. Diss. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2007. [Google Scholar]
- Xu, X.; Zheng, R.; Li, C.; Gai, J.Y.; Yu, D.Y. Differential Proteomic Analysis of Seed Germination in Soybean. Adv. Biochem. Biophys. 2006, 11, 1106–1112. [Google Scholar]
- Yamauchi, Y.; Ogawa, M.; Kuwahara, A.; Hanada, A.; Kamiya, Y.; Yamaguchi, S. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 2004, 16, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Bentsink, L.; Koornneef, M. Seed dormancy and germination. Arab. Book Am. Soc. Plant Biol. 2008, 6, e0119. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Khan, A.A. The Physiology and Biochemistry of Seed Dormancy and Germination; North-Holland Publishing Company: Amsterdam, The Netherlands; New York, NY, USA; Oxford, UK, 1977. [Google Scholar]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Zhang, C.C.; Gao, Z.; Luo, L.N.; Zhang, Z.X.; Wang, J.; Lu, F.H.; Xiang, Z.X. Transcriptome analysis of seed embryos before and after dormancy release state of Thesium chinense. Chin. J. Tradit. Chin. Med. 2020, 45, 3837–3843. [Google Scholar]
- Luo, L.N.; Xiang, Z.X. The Relative Difference Genes in the Process of Dormancy Release of Polygonatum sibiricum Red Based on the Transcriptome Sequencing Analysis. Chin. Agron. Bull. 2021, 37, 1–8. [Google Scholar]
- Seo, M.; Hanada, A.; Kuwahara, A.; Endo, A.; Okamoto, M.; Yamauchi, Y.; North, H.; Marion-Poll, A.; Sun, T.; Koshiba, T.; et al. Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006, 48, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Piskurewicz, U.; Jikumaru, Y.; Kinoshita, N.; Nambara, E.; Kamiya, Y.; Lopez-Molina, L. The Gibberellic Acid Signaling Repressor RGL2 Inhibits Arabidopsis Seed Germination by Stimulating Abscisic Acid Synthesis and ABI5 Activity. Plant Cell 2008, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.P. Study on the Physiological and Biochemical Changes and Molecular Mechanisms in Cercis Chinensis Seeds during Dormancy Releasing; Nanjing Forestry University: Nanjing, China, 2020. [Google Scholar]
- Pawłowski, T.A. Proteome analysis of Norway maple (Acer platanoides L.) seeds dormancy breaking and germination: Influence of abscisic and gibberellic acids. BMC Plant Biol. 2009, 9, 48. [Google Scholar] [CrossRef]
- Miernyk, J.A.; Hajduch, M. Seed proteomics. J. Proteom. 2011, 74, 389–400. [Google Scholar] [CrossRef]
- Khalil-Ur-Rehman, M.; Wang, W.; Dong, Y.; Faheem, M.; Xu, Y.; Gao, Z.; Shen, Z.G.; Tao, J. Comparative transcriptomic and proteomic analysis to deeply investigate the role of hydrogen cyanamide in grape bud dormancy. Int. J. Mol. Sci. 2019, 20, 3528. [Google Scholar] [CrossRef]
- Catusse, J.; Job, C.; Job, D. Transcriptome-and proteome-wide analyses of seed germination. Comptes Rendus Biol. 2008, 331, 815–822. [Google Scholar] [CrossRef]
- Wu, H.Z.; Jin, G. A Reasearch on the Interaction mechanism for Cystatin and Polyphenol Oxidase from Nicotaina Tobacum. J. Hefei Univ. (Nat. Sci.) 2006, 1, 45–48. [Google Scholar]
- Zhao, W.J.; Zhang, H.Y.; Xu, Y.N.; Luo, J.; Liu, M.J. Effects of the Alternative Oxidase and Cytochrome Oxidase Pathways on the Germination of Seeds and Growth of Roots of Alfalfa Seedlings Under Different Temperatures. J. Grassl. Sci. 2022, 30, 84–92. [Google Scholar]
- Roberts, E.H. Oxidative processes and the control of seed germination. Seed Ecol. 1973, 28, 189–218. [Google Scholar]
- Yang, P.; Li, X.; Wang, X.; Chen, H.; Chen, F.; Shen, S. Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics 2007, 7, 3358–3368. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Zhen, S.; Cheng, Z.; Cao, H.; Ge, P.; Yan, Y. Proteomic analysis reveals key proteins and phosphoproteins upon seed germination of wheat (Triticum aestivum L.). Front. Plant Sci. 2015, 6, 1017. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhang, L.Y.; Xia, K.; Jiang, M.; Wei, X. Transcriptomic Analysis of the Seed of Garcinia paucinervis, a Rare and Endangered Plant Species, During Dormancy Releasing. For. Sci. Res. 2021, 34, 35–46. [Google Scholar]
- Wang, X.; Davanture, M.; Zivy, M.; Bailly, C.; Nambara, E.; Corbineau, F. Label-Free Quantitative Proteomics Reveal the Involvement of PRT6 in Arabidopsis thaliana Seed Responsiveness to Ethylene. Int. J. Mol. Sci. 2022, 23, 9352. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef]
- Kumar, D.; Bansal, G.; Narang, A.; Basak, T.; Abbas, T.; Dash, D. Integrating transcriptome and proteome profiling: Strategies and applications. Proteomics 2016, 16, 2533–2544. [Google Scholar] [CrossRef]



| Samples | Labeling | Labeling Information |
|---|---|---|
| CK | CK1 | 128 C |
| CK | CK2 | 129 N |
| CK | CK3 | 129 C |
| GA3 | GA3_SC30 d1 | 130 N |
| GA3 | GA3_SC30 d2 | 130 C |
| GA3 | GA3_SC30 d3 | 131 N |
| Sample | Concentration (μg/μL) | Volumes (μL) | Total (μg) | Grade |
|---|---|---|---|---|
| CK1 | 9.58 | 900 | 8624.54 | A2 |
| CK2 | 9.35 | 900 | 8411.07 | A2 |
| CK3 | 9.6 | 800 | 7678.2 | A2 |
| GA3_SC30_d1 | 2.9 | 1000 | 2899.51 | A1 |
| GA3_SC30_d2 | 3.57 | 1000 | 3569.07 | A1 |
| GA3_SC30_d3 | 2.78 | 1000 | 2782.51 | A1 |
| Parameters | Value |
|---|---|
| Enzyme | Trypsin |
| Static modification | Carbamidomethyl (C) |
| Dynamic modification | M oxidation (15.995 Da); TMT-16plex (K, N-terminal) |
| Acetyl (protein N-terminal); | |
| Precursor ion mass tolerance | ±15 ppm |
| Fragment ion mass tolerance | ±0.02 Da |
| Max missed cleavages | 2 |
| Type | GO_BP | Protein Number |
|---|---|---|
| Germination | Seed germination | 63 |
| Positive regulation of seed germination | 4 | |
| Regulation of seed germination | 42 | |
| Negative regulation of seed germination | 29 | |
| Dormancy | Seed dormancy | 83 |
| Release of seed from dormancy | 1 | |
| Regulation of seed dormancy process | 4 | |
| Embryo development ending in seed dormancy | 75 | |
| Antioxidase | Peroxidase | 4 |
| Superoxide dismutase | 8 | |
| Catalase | 4 | |
| Phytohormone | Gibberellin | 11 |
| Positive regulation of gibberellin biosynthetic process | 1 | |
| Gibberellic acid-mediated signaling pathway | 5 | |
| Response to abscisic acid | 83 | |
| Regulation of abscisic acid-activated signaling pathway | 12 | |
| Auxin-activated signaling pathway | 13 | |
| Response to ethylene | 19 | |
| Response to cytokinin | 7 | |
| Salicylic acid biosynthetic process | 27 | |
| Response to jasmonic acid | 35 | |
| Metabolism | Glyoxylate and dicarboxylate metabolism | 58 |
| Amino sugar and nucleotide sugar metabolism | 56 | |
| Starch and sucrose metabolism | 36 | |
| Sphingolipid metabolism | 6 | |
| Glycerophospholipid metabolism | 14 | |
| Glycerolipid metabolism | 16 | |
| Cyanoamino acid metabolism | 12 | |
| Folate biosynthesis | 6 | |
| Galactose metabolism | 24 | |
| Glutathione metabolism | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Lv, G. A Proteomic Study on Seed Germination of Nitraria roborowskii Kom. Forests 2024, 15, 1661. https://doi.org/10.3390/f15091661
Ren S, Lv G. A Proteomic Study on Seed Germination of Nitraria roborowskii Kom. Forests. 2024; 15(9):1661. https://doi.org/10.3390/f15091661
Chicago/Turabian StyleRen, Shangfu, and Guanghui Lv. 2024. "A Proteomic Study on Seed Germination of Nitraria roborowskii Kom." Forests 15, no. 9: 1661. https://doi.org/10.3390/f15091661





