Weed Control and Physiological Responses in Poplar Plantations: Assessing Glyphosate’s Impact
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Herbicide Application
2.2. Leaf Gas Exchange Assessment
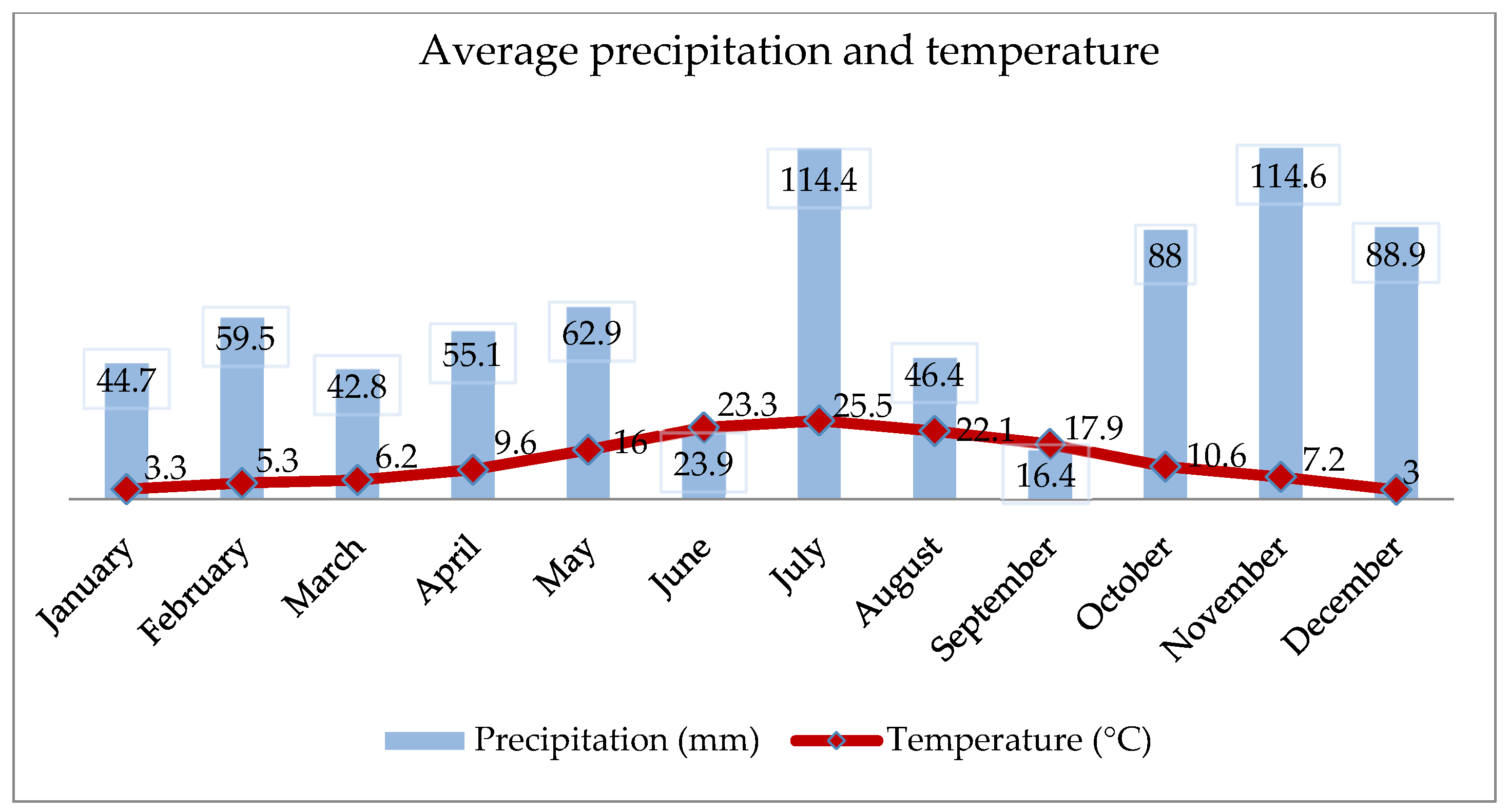
2.3. Meteorological Data (Temperature and Precipitation)
2.4. Statistical Analysis
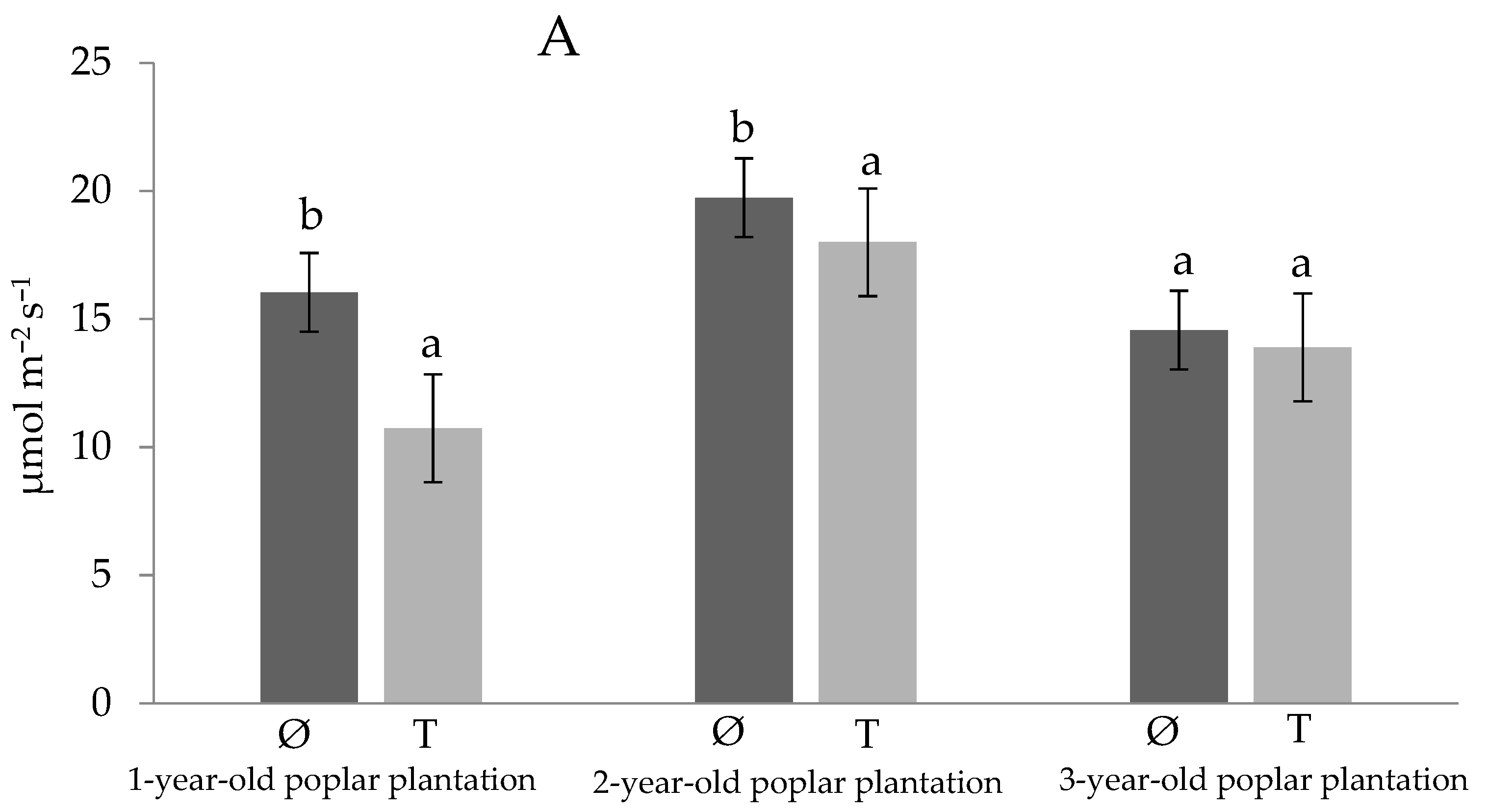
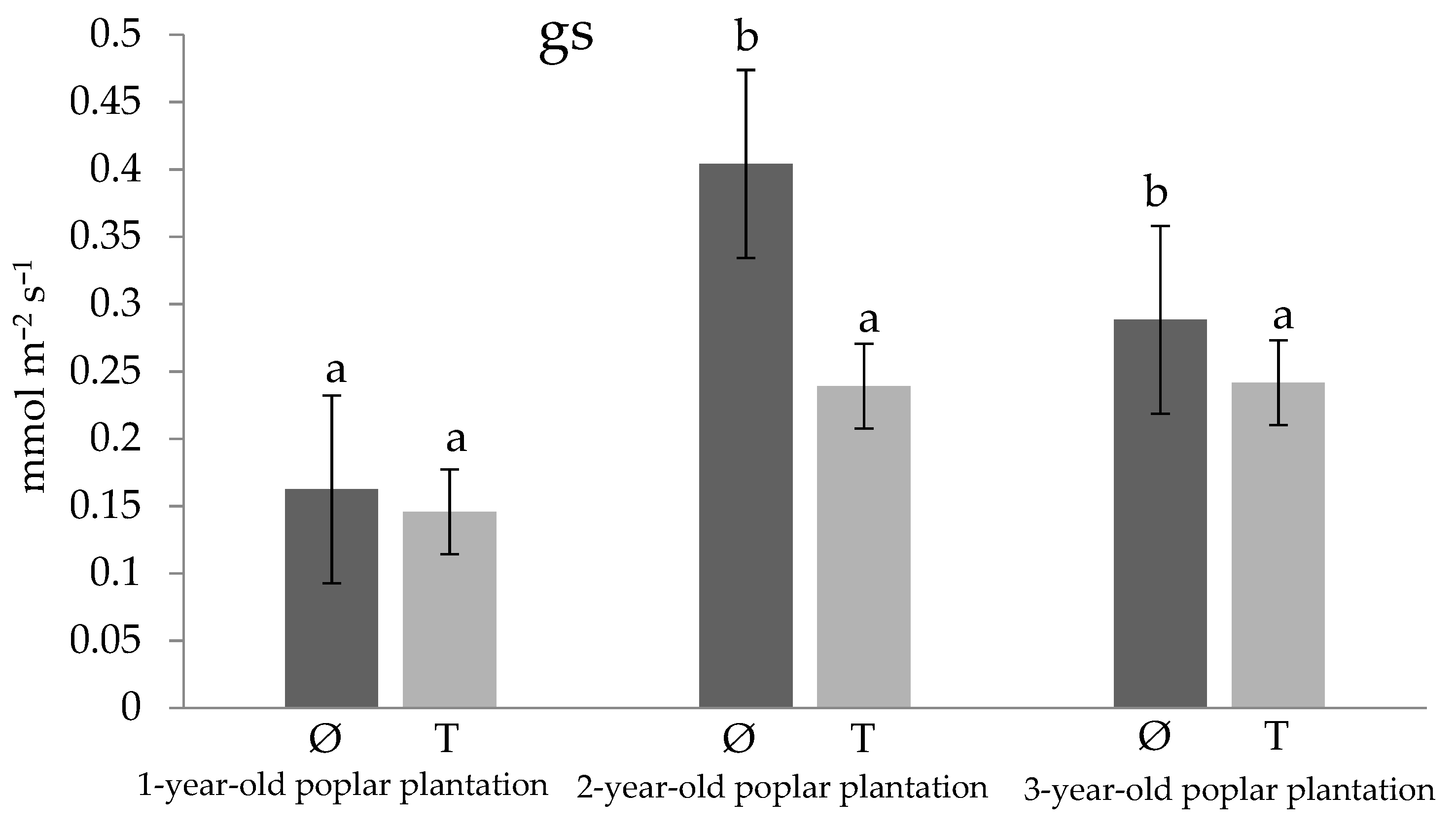
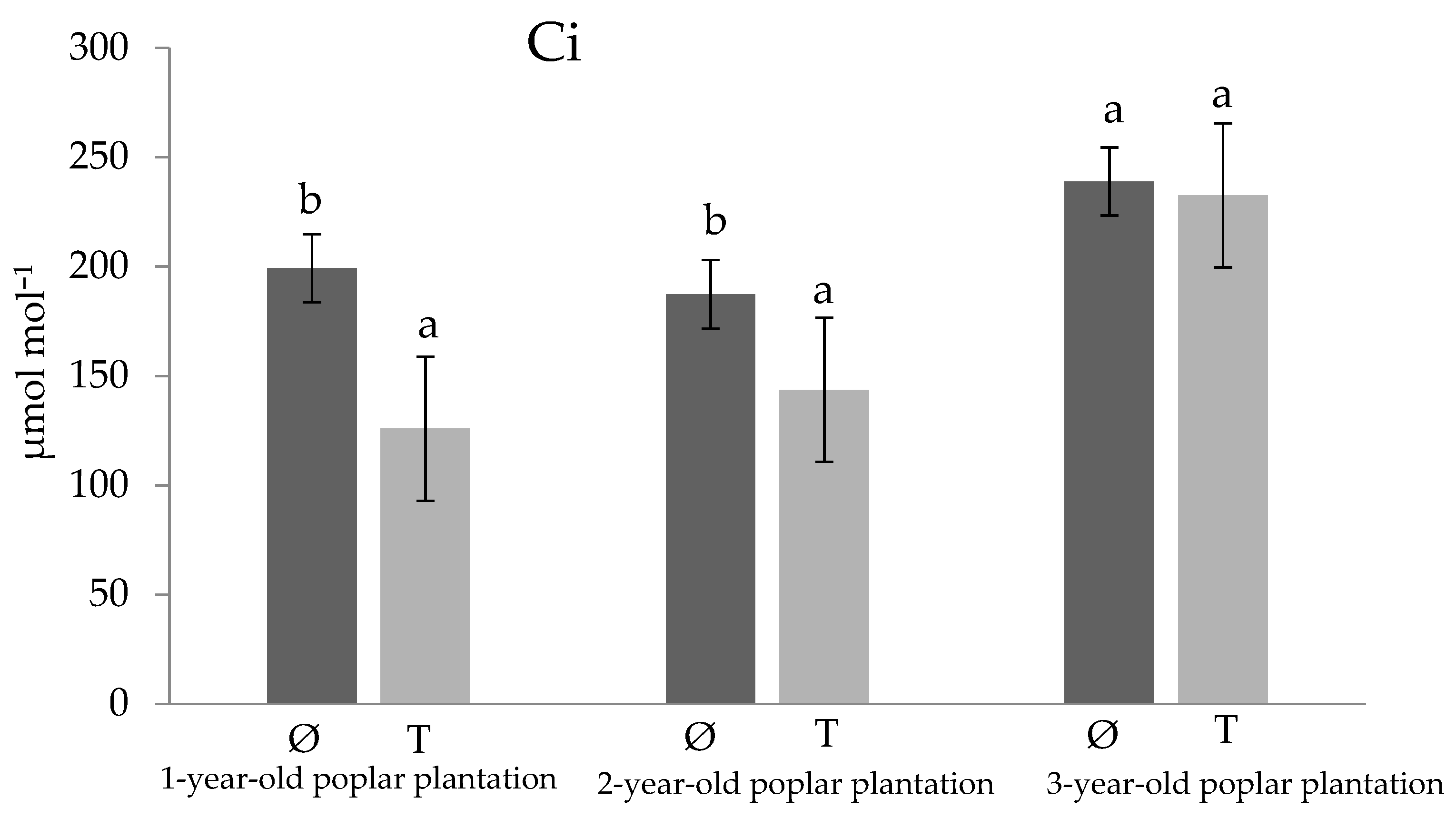
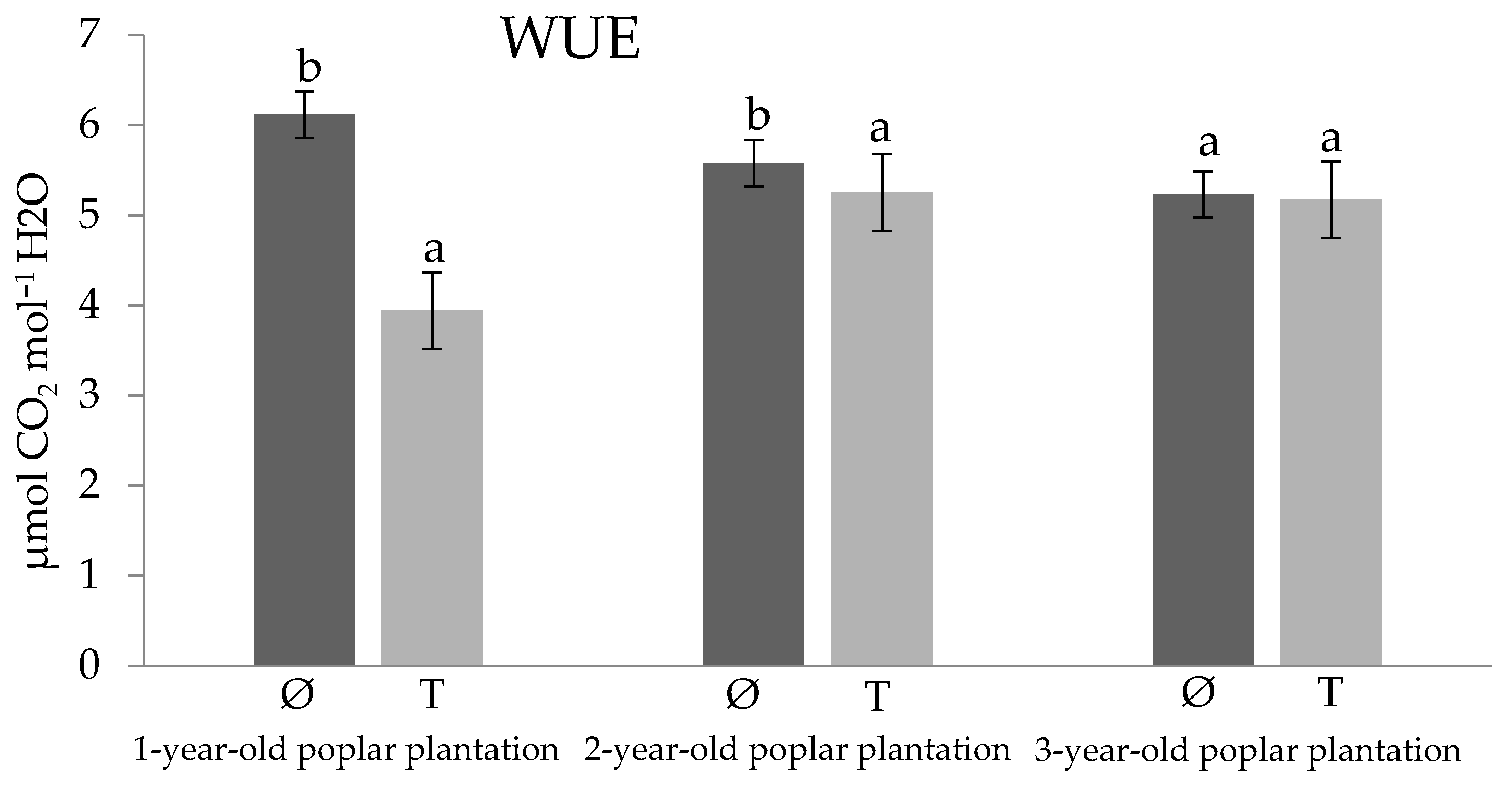
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanzo, E.; Jud, W.; Li, Z.; Albert, A.; Domagalska, M.A.; Ghirardo, A.; Niederbacher, B.; Frenzel, J.; Beemster, G.T.S.; Asard, H.; et al. Facing the future: Effects of short-term climate extremes on isoprene-emitting and nonemitting poplar. Plant Physiol. 2015, 169, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Rosso, L.; Cantamessa, S.; Bergante, S.; Biselli, C.; Fricano, A.; Chiarabaglio, P.M.; Gennaro, M.; Nervo, G.; Secchi, F.; Carra, A. Responses to drought stress in Poplar: What do we know and what can we learn? Life 2023, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- Koç, İ.; Nzokou, P. Combined effects of water stress and fertilization on the morphology and gas exchange parameters of 3-year-old Abies fraseri (pursh) Poir. Acta Physiol. Plant. 2023, 45, 49. [Google Scholar] [CrossRef]
- Cantürk, U.; Çobanoğlu, H.; Beyazyüz, F.; Koç, İ. Investigation of leaf gas exchange parameters of several chestnut population seedlings at the end of the growing season. Turk. J. Agric. Food Sci. Technol. 2023, 11, 1839–1846. [Google Scholar] [CrossRef]
- United Nations. Take Action for the Sustainable Development Goals. 17 Goals to Transform Our World. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 3 June 2024).
- Liu, J.; Li, D.; Fernández, J.E.; Coleman, M.; Hu, W.; Di, N.; Zou, S.; Liu, Y.; Xi, B.; Clothier, B. Variations in water-balance components and carbon stocks in poplar plantations with differing water inputs over a whole rotation: Implications for sustainable forest management under climate change. Agric. For. Meteorol. 2022, 320, 108958. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Atzori, G.; Fabbri, A.; Daccache, A.; Killi, D.; Carli, A.; Montesano, V.; Conte, A.; Balestrini, R.; et al. Plant physiological analysis to overcome limitations to plant phenotyping. Plants 2023, 12, 4015. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, X.; Zhu, C. Comparative apparent hydraulic conductance, leaf gas exchange, and water resource partitioning of Populus euphratica trees and saplings. Forests 2022, 13, 1982. [Google Scholar] [CrossRef]
- Pangle, R.; Kavanagh, K.; Duursma, R. Decline in canopy gas exchange with increasing tree height, atmospheric evaporative demand, and seasonal drought in co-occurring inland Pacific Northwest Conifer species. Can. J. For. Res. 2015, 45, 1086–1101. [Google Scholar] [CrossRef]
- Zhang, H.; Morison, J.I.; Simmonds, L.P. Transpiration and water relations of poplar trees growing close to the water table. Tree Physiol. 1999, 19, 563–573. [Google Scholar] [CrossRef]
- Topić, M.; Borišev, M.; Župunski, M.; Tomičić, M.; Nikolić, N.; Pajević, S.; Krstić, B.; Pilipović, A. Odgovori klonova crne topole na oporavak posle vodnog deficita u kontekstu fotosinteze, transpiracije i efikasnosti korišćenja vode. Topola 2012, 189–190, 29–38. [Google Scholar]
- Silim, S.; Nash, R.; Reynard, D.; White, B.; Schroeder, W. Leaf gas exchange and water potential responses to drought in nine poplar (Populus spp.) clones with contrasting drought tolerance. Trees 2009, 23, 959–969. [Google Scholar] [CrossRef]
- Tang, L.; Cao, P.; Zhang, S.; Liu, X.; Ge, X.; Tang, L. Two male poplar clones (Populus × euramericana ‘Siyang-1’ and Populus deltoides ‘Nanlin 3804’) exhibit distinctly different physiological responses to soil water deficit. Forests 2024, 15, 1142. [Google Scholar] [CrossRef]
- Behnke, K.; Grote, R.; Brüggemann, N.; Zimmer, I.; Zhou, G.; Elobeid, M.; Janz, D.; Polle, A.; Schnitzler, J. Isoprene emission-free poplars—A chance to reduce the impact from poplar plantations on the atmosphere. New Phytol. 2011, 194, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Jaafari, A. Mapping high poplar growth areas for bioenergy cultivation: A swarm-optimized approach. Renew. Sustain. Energy Rev. 2023, 187, 113748. [Google Scholar] [CrossRef]
- Thiffault, N.; Elferjani, R.; Hébert, F.; Paré, D.; Gagné, P. Intensive mechanical site preparation to establish short rotation hybrid poplar plantations—a case-study in Québec, Canada. Forests 2020, 11, 785. [Google Scholar] [CrossRef]
- Keča, L.; Keča, N. Commercial profitability of Poplar plantation with reference to the damages caused by fungi. South-East Eur. For. 2012, 3, 23–31. [Google Scholar] [CrossRef][Green Version]
- Lin, X.Y.; Wang, X.X.; Zeng, Q.Y.; Yang, Q. Leaf structure and photosynthesis in populus alba under naturally fluctuating environments. Photosynthetica 2022, 60, 240–250. [Google Scholar] [CrossRef]
- Nash, R.M. Drought Adaptations of Hybrid Poplar Clones Commonly Grown on the Canadian Prairies. Master’s Thesis, Department of Soil Science, University of Saskatchewan Saskatoon, Saskatoon, SK, Canada, 2009. [Google Scholar]
- Monclus, R.; Villar, M.; Barbaroux, C.; Bastien, C.; Fichot, R.; Delmotte, F.M.; Delay, D.; Petit, J.M.; Brechet, C.; Dreyer, E.; et al. Productivity, water-use efficiency and tolerance to moderate water deficit correlate in 33 poplar genotypes from a Populus deltoides x Populus trichocarpa F1 progeny. Tree Physiol. 2009, 29, 1329–1339. [Google Scholar] [CrossRef]
- Marron, N.; Delay, D.; Petit, J.M.; Dreyer, E.; Kahlem, G.; Delmotte, F.M.; Brignolas, F. Physiological traits of two Populus x euramericana clones, Luisa Avanzo and Dorskamp, during a water stress and re-watering cycle. Tree Physiol. 2002, 22, 849–858. [Google Scholar] [CrossRef]
- Singh, M.; Kukal, M.S.; Irmak, S.; Jhala, A.J. Water use characteristics of weeds: A global review, Best Practices, and Future Directions. Front. Plant Sci. 2002, 12, 794090. [Google Scholar] [CrossRef]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The problem of weed infestation of agricultural plantations vs. the assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Kogan, M.; Alister, C. Glyphosate use in forest plantations. Chil. J. Agric. Res. 2010, 70, 652–666. [Google Scholar] [CrossRef]
- Davies, R.J. TREES and Weeds: Weed Control for Successful Tree Establishment; Her Majesty’s Stationery Office: London, UK, 1987. [Google Scholar]
- Willoughby, I.H.; Forster, J. The herbicide cycloxydim is an effective alternative to propyzamide or glyphosate for the control of the forest grass weeds Molinia caerulea, Calamagrostis epigejos, Deschampsia flexuosa and Holcus lanatus. For. Int. J. For. Res. 2021, 95, 274–286. [Google Scholar] [CrossRef]
- McCarthy, N.; Bentsen, N.S.; Willoughby, I.; Balandier, P. The state of forest vegetation management in Europe in the 21st century. Eur. J. Forest Res. 2011, 130, 7–16. [Google Scholar] [CrossRef]
- Vasic, V.; Konstantinovic, B.; Orlovic, S. Application of post-emergence herbicides in the regeneration of pedunculate oak (Quercus robur L.) forests. Forestry 2014, 87, 407–416. [Google Scholar] [CrossRef]
- Vasic, V.; Orlovic, S.; Pap, P.; Kovacevic, B.; Drekic, M.; Pajnik, L.P.; Galic, Z. Application of pre-emergence herbicides in Poplar Nursery production. J. For. Res. 2015, 26, 143–151. [Google Scholar] [CrossRef]
- Tabaković-Tošić, M. The application of pesticides in forestry. In Proceedings of the Scientific Meetings Book CLXXXI in Use of Pesticides in Plant Production and Environmental Protection; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 2019. [Google Scholar]
- Editorial Team. Pesticides in Agriculture and Forestry in Serbia; Plant Protection Society of Serbia: Belgrade, Serbia, 2022; p. 465. [Google Scholar]
- Rolando, C.; Baillie, B.; Thompson, D.; Little, K. The risks associated with glyphosate-based herbicide use in planted forests. Forests 2017, 8, 208. [Google Scholar] [CrossRef]
- Fogliatto, S.; Ferrero, A.; Vidotto, F. Current and future scenarios of glyphosate use in Europe: Are there alternatives? Adv. Agron. 2020, 163, 219–278. [Google Scholar] [CrossRef]
- Neve, P.; Matzrafi, M.; Ulber, L.; Baraibar, B.; Beffa, R.; Belvaux, X.; Farré, J.T.; Mennan, H.; Ringselle, B.; Salonen, J.; et al. Current and future glyphosate use in European agriculture. Weed Res. 2024, 64, 181–196. [Google Scholar] [CrossRef]
- Richmond, M.E. Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. J. Environ. Stud. Sci. 2018, 8, 416–434. [Google Scholar] [CrossRef]
- The International Agency for Research on cancer (IARC). 2016. Q&A on Glyphosate. IARC, 150 Cours Albert Thomas, 69372 Lyon CEDEX 08, France. Available online: https://www.iarc.who.int/wp-content/uploads/2018/11/QA_Glyphosate.pdf (accessed on 9 September 2024).
- Vasseur, C.; Serra, L.; El Balkhi, S.; Lefort, G.; Ramé, C.; Froment, P.; Dupont, J. Glyphosate presence in human sperm: First report and positive correlation with oxidative stress in an infertile French population. Ecotoxicol. Environ. Saf. 2024, 278, 116410. [Google Scholar] [CrossRef] [PubMed]
- Casassus, B. EU Allows Use of Controversial Weedkiller Glyphosate for 10 More Years. Nature. 2023. Available online: https://www.nature.com/articles/d41586-023-03589-z (accessed on 19 July 2024).
- Pekeč, S. Pedološke i Hidrološke Karakteristike Zaštićenog Dela Aluvijalne Ravni u Srednjem Podunavlju. Ph.D. Thesis, Poljoprivredni Fakultet, Univerzitet u Novom Sadu, Novi Sad, Serbia, 2010. [Google Scholar]
- Pekeč, S.; Ivanišević, P.; Stojanović, D.; Marković, M.; Katanić, M.; Galović, V. Osobine različitih formi zemljišta tipa fluvisol u zaštićenom delu inundacije reke Dunav na području južne Bačke. Topola/Poplar 2012, 189/190, 19–28. [Google Scholar]
- Josifović, M. (Ed.) Flora SR Serbia I–IX; Serbian Academy of Sciences and Arts (SANU): Belgrade, Serbia, 1970–1977. [Google Scholar]
- Sarić, M. (Ed.) Flora of Serbia I; Serbian Academy of Sciences and Arts (SANU), Department of Natural and Mathematical Sciences: Belgrade, Serbia, 1992. [Google Scholar]
- Tutin, G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea I; Cambridge University Press: Cambridge, UK, 1964. [Google Scholar]
- Javorka, S.; Csapody, V. Ikonographie der Flora des Südöstlichen Mitteleuropa; Akademiai Kiado: Budapest, UK, 1975. [Google Scholar]
- Janjić, V. Herbicidi; Naučna Knjiga: Beograd, Serbia, 1985; pp. 1–589. [Google Scholar]
- Haworth, M.; Marino, G.; Centritto, M. An introductory guide to gas exchange analysis of photosynthesis and its application to plant phenotyping and precision irrigation to enhance water use efficiency. J. Water Clim. Chang. 2018, 9, 786–808. [Google Scholar] [CrossRef]
- Zhang, X.; Zang, R.; Li, S. Population differences in physiological and morphological adaptations of Populus davidiana seedlings in response to progressive drought stress. Plant Sci. 2004, 166, 791–797. [Google Scholar] [CrossRef]
- Pajevic, S.; Borisev, M.; Nikolic, N.; Krstic, B.; Pilipovic, A.; Orlovic, S. Phytoremediation capacity of poplar (Populus spp.) and willow (Salix spp.) clonesin relation to photosynthesis. Arch. Biol. Sci. 2009, 61, 239–247. [Google Scholar] [CrossRef]
- Emami, A.S.; Tabari Kouchaksaraei, M.; Bahramifar, N.; Salehi, A. Gas exchange responses of two poplar clones (Populus euramericana (Dode) guinier 561/41 and Populus nigra Linnaeus 63/135) to lead toxicity. J. For. Sci. 2016, 62, 422–428. [Google Scholar] [CrossRef]
- Stojnić, S.; Orlović, S.; Pilipović, A.; Vilotić, D.; Šijačić-Nikolić, M.; Miljković, D. Variation in leaf physiology among three provenances of European beech (Fagus sylvatica L.) in provenance trial in Serbia. Genetika 2012, 44, 341–353. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). State of the Global Climate; CH-1211; WMO: Geneva, Switzerland, 2021; No. 1290; ISBN 978-92-63-11290-3. [Google Scholar]
- Vasic, V.; Konstantinovic, B.; Orlovic, S. Weeds in forestry and possibilities of their control. Weed Control 2012, 147–170. [Google Scholar] [CrossRef]
- Vasić, V.; Galić, Z.; Drekić, M. Efficiency and selectivity of some herbicides in nursery production of poplar seedlings. Šumarski List. 2010, 134, 401. [Google Scholar]
- Clay, D.V.; Dixon, F.L.; Willoughby, I. Efficacy of graminicides on grass weed species of Forestry. Crop Prot. 2006, 25, 1039–1050. [Google Scholar] [CrossRef]
- Orlovic, S.; Pajevic, S.; Klasnja, B.; Galic, Z.; Markovic, M. Variability of physiological and growth characteristics of white willow (Salix alba L.) clones. Genetika 2006, 38, 145–152. [Google Scholar] [CrossRef]
- Krstić, J.; Orlović, S.; Galić, Z.; Pilipović, A.; Stojnić, S. Seasonal changes in leaf gas exchange parameters in Platanus acerifolia Willd. and Acer pseudoplatanus L. seedlings on undeveloped alluvial soil (fluvisol). Šumarstvo 2014, 1–2, 163–178. [Google Scholar]
- Kumar, P.; Singh, K.; Mishra, A.K.; Kumar, A.; Singh, R.; Rai, P.; Chaudhari, S.K.; Sharma, D.K. Effect oftemporal changes and plantation age on gas exchange characteristics of Populus deltoidesin the Indo-Gangetic plains of India. Indian J. Agrofor. 2015, 17, 22–26. [Google Scholar]
- Gomes, M.P.; Smedbol, E.; Chalifour, A.; Hénault-Ethier, L.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: An overview. J. Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef]
- Meseldžija, M.; Lazić, S.; Dudić, M.; Šunjka, D.; Rajković, M.; Marković, T.; Vukotić, J.; Ljevnaić-Mašić, B.; Jurišić, A.; Ivanović, I. Is There a Possibility to Involve the Hormesis Effect on the Soybean with Glyphosate Sub-Lethal Amounts Used to Control Weed Species Amaranthus retroflexus L.? Agronomy 2020, 10, 850. [Google Scholar] [CrossRef]
- Wysocki, K.; Banaszkiewicz, T.; Olszewski, J. Evaluation of selected parameters of photosynthesis as herbicide stress indicators on the example of glyphosate. J. Plant Prot. Res. 2018, 58, 241–245. [Google Scholar] [CrossRef]
- Krenchinski, F.H.; Albrecht, L.P.; Albrecht, A.J.; Cesco, V.J.; Rodrigues, D.M.; Portz, R.L.; Zobiole, L.H. Glyphosate affects chlorophyll, photosynthesis and water use of four intacta RR2 soybean cultivars. Acta Physiol. Plant. 2017, 39. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac’h, S.G.; Hénault-Ethier, L.; Labrecque, M.; Lucotte, M.; Juneau, P. Glyphosate-Dependent Inhibition of Photosynthesis in Willow. Front. Plant Sci. 2017, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, A.; Sixto, H.; González, I.; Pérez-Cruzado, C.; Cañellas, I.; Rodríguez-Soalleiro, R.; Oliveira, N. Time-course foliar dynamics of Poplar short rotation plantations under Mediterranean conditions. responses to different water scenarios. Biomass Bioenergy 2022, 159, 106391. [Google Scholar] [CrossRef]
- Joyeeta, D. Nitrogen Fixing Efficiency of the Leguminous Weed Plants Found in Tripura Certain Studies on Carbon and Nitrogen Metabolites of the Plants and Assessment of Soil Fertility; Tripura University: Agartala, Tripura, 2018; Available online: http://hdl.handle.net/10603/306688 (accessed on 22 August 2024).
- Lawson, T.; von Caemmerer, S.; Baroli, I. Photosynthesis and Stomatal Behaviour. In Progress in Botany 72; Lüttge, U., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 72. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; de Oliveira, R.S.; Kremer, R.J.; Constantin, J.; Bonato, C.M.; Muniz, A.S. Water use efficiency and photosynthesis of glyphosateresistant soybean as affected by glyphosate. Pestic. Biochem. Physiol. 2010, 97, 182–193. [Google Scholar] [CrossRef]
- Ferrell, J.A.; Earl, H.J.; Vencill, W.K. Duration of yellow nutsedge (Cyperus esculentus) competitiveness after herbicide treatment. Weed Sci. 2004, 52, 24–27. [Google Scholar] [CrossRef]
- Cao, Y.; Li, J.; Li, S.; Zhou, B. The Effects of Long-Term Precipitation Exclusion on Leaf Photosynthetic Traits, Stomatal Conductance, and Water Use Efficiency in Phyllostachys edulis. Forests 2024, 15, 849. [Google Scholar] [CrossRef]
- Pilipović, A.; Headlee, W.L.; Zalesny, R.S.; Pekeč, S.; Bauer, E.O. Water use efficiency of poplars grown for biomass production in the Midwestern United States. GCB Bioenergy 2022, 14, 287–306. [Google Scholar] [CrossRef]
- Kesić, L.; Vuksanović, V.; Karaklić, V.; Vaštag, E. Variation of leaf water potential and leaf gas exchange parameters of seven Silver Linden (Tilia tomentosa Moench) genotypes in urban environment. Topola 2020, 206, 15–24. [Google Scholar] [CrossRef]
- Van Oorschot, J.L.P. Influence of herbicides on photosynthetic activity and transpiration rate of intact plants. Pestic. Sci. 1970, 1, 33–37. [Google Scholar] [CrossRef]
- Brant, V.; Pivec, J.; Hamouzová, K.; Zábranský, P.; Satrapová, J.; Škeříková, M. Determination of the influence of herbicides on dicotyledons plant transpiration using the sap flow method. Plant Soil Environ. 2014, 60, 562–568. [Google Scholar] [CrossRef]
- Jones, G.H. Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 1998, 49, 387–398. [Google Scholar] [CrossRef]
- Renninger, H.J.; Pitts, J.J.; Rousseau, R.J. Comparisons of biomass, water use efficiency and water use strategies acrossfive genomic groups of Populus and its hybrids. GCB Bioenergy 2023, 15, 99–112. [Google Scholar] [CrossRef]
- Franceschetti, M.B.; Galon, L.; Toso, J.O.; Melo, T.S.; Menegatti, A.D.; Concenco, G.; Bagnara, M.A.; Perin, G.F. Morpho-physiological responses of potato cultivars under weed competition. Comun. Sci. 2024, 15, e4088. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Sun, G.; Fang, X.; Zha, T.; Chen, J.; Noormets, A.; Guo, J.; McNulty, S. Water-use efficiency of a Poplar Plantation in northern China. J. For. Res. 2014, 19, 483–492. [Google Scholar] [CrossRef]


| EWRC Score | Crop Tolerance | Damaged Plants (%) |
|---|---|---|
| 1 | No effect | 0 |
| 2 | Very slight effects; some stunting and yellowing; just visible | 1 |
| 3 | Slight effects; stunting and yellowing; effects reversible | 2 |
| 4 | Substantial chlorosis and/or stunting; most effects probably reversible | 5 |
| 5 | Strong chlorosis/stunting; thinning of stand | 10 |
| 6 | Increasing severity of damage | 25 |
| 7 | Increasing severity of damage | 50 |
| 8 | Increasing severity of damage | 75 |
| 9 | Total loss of plants and yield | 100 |
| First Assessment | Second Assessment | ||||||
|---|---|---|---|---|---|---|---|
| Weed Species | Control | Glyphosate | Control | Glyphosate | |||
| No/m2 | No/m2 | Ce (%) | No/m2 | No/m2 | Ce (%) | ||
| Monocotyledons | Avena fatua L. | 5.50 ± 0.58 | 0 | 100 | 2.00 ± 0 | 0 | 100 |
| Dactylis glomerata L. | 2.75 ± 0.96 | 0 | 100 | 2.50 ± 0.58 | 0 | 100 | |
| Lolium spp. L. | 3.75 ± 1.71 | 0 | 100 | 2.75 ± 1.71 | 0 | 100 | |
| Poa pratensis L. | 4.00 ± 2.16 | 0 | 100 | 2.25 ± 1.26 | 0 | 100 | |
| Dicotyledons | Amorpha fruticosa L. | 2.00 ± 0.82 | 0.25 ± 0.5 | 87.5 | 1.50 ± 0.58 | 0.25 ± 0.5 | 83.33 |
| Aristolochia clematitis L. | 4.00 ± 2.45 | 0.25 ± 0.5 | 93.75 | 3.00 ± 1.41 | 0 | 100 | |
| Asclepias syriaca L. | 5.50 ± 1 | 0.50 ± 0.58 | 90.9 | 3.25 ± 0.96 | 0.50 ± 0.58 | 84.61 | |
| Clematis vitalba L. | 0.25 ± 0.5 | 0 | 100 | 0.25 ± 0.5 | 0 | 100 | |
| Erigeron canadensis L. | 3.50 ± 1.91 | 0 | 100 | 2.00 ± 1.15 | 0 | 100 | |
| Euphorbia cyparissias L. | 4.50 ± 1.91 | 0.75 ± 0.96 | 83.33 | 1.75 ± 1.71 | 0 | 100 | |
| Euphorbia helioscopia L. | 2.00 ± 0 | 0 | 100 | 1.25 ± 0.5 | 0 | 100 | |
| Galium aparine L. | 1.25 ± 0.96 | 0 | 100 | 2.00 ± 1.41 | 0 | 100 | |
| Rubus caesius L. | 0.25 ± 0.5 | 0 | 100 | 0.50 ± 0.58 | 0 | 100 | |
| Solidago gigantea L. | 10.00 ± 2.45 | 0 | 100 | 8.00 ± 2 | 0 | 100 | |
| Solidago speciosa L. | 4.75 ± 3.4 | 0 | 100 | 2.75 ± 1.89 | 0 | 100 | |
| Symphytum officinale L. | 0.50 ± 0.58 | 0 | 100 | 0.25 ± 0.5 | 0 | 100 | |
| Vicia villosa Roth. | 0.75 ± 0.96 | 0 | 100 | 0.75 ± 0.96 | 0 | 100 | |
| Viola arvensis Murr. | 5.75 ± 1.5 | 0 | 100 | 5.25 ± 1.89 | 0 | 100 | |
| Horsetail | Equisetum arvense L. | 1.50 ± 1.29 | 0.50 ± 0.58 | 66.66 | 1.00 ± 0.82 | 0.25 ± 0.5 | 75.00 |
| Total number of weeds | 62.50 | 2.25 | 43.00 | 1.00 | |||
| Total efficacy | - | 96.40% | - | 97.67% | |||
| Phytotoxicity | - | 1 | - | 1 | |||
| First Assessment | Second Assessment | ||||||
|---|---|---|---|---|---|---|---|
| Weed Species | Control | Glyphosate | Control | Glyphosate | |||
| No/m2 | No/m2 | Ce (%) | No/m2 | No/m2 | Ce (%) | ||
| Monocotyledons | Apera spica-venti L. P. Beauv. | 2.50 ± 1.73 | 0 | 100 | 1.50 ± 1.29 | 0 | 100 |
| Avena fatua L. | 2.75 ± 0.96 | 0 | 100 | 2.00 ± 0.82 | 0 | 100 | |
| Lolium perenne L. | 3.25 ± 1.71 | 0 | 100 | 2.25 ± 0.96 | 0 | 100 | |
| Poa pratensis L. | 4.25 ± 1.82 | 0 | 100 | 2.00 ± 0 | 0 | 100 | |
| Dicotyledons | Amorpha fruticosa L. | 2.00 ± 0.82 | 0.50 ± 0.58 | 75.00 | 1.00 ± 0.82 | 0.25 ± 0.58 | 75.00 |
| Asclepias syriaca L. | 1.75 ± 0.5 | 0 | 100 | 1.00 ± 0.82 | 0.25 ± 0.5 | 75.00 | |
| Cirsium arvense L. | 0.75 ± 0.5 | 0 | 100 | 0.50 ± 0.58 | 0 | 100 | |
| Clematis vitalba L. | 0.50 ± 0.58 | 0 | 100 | 1.00 ± 1.15 | 0 | 100 | |
| Cornus sanguinea L. | 1.25 ± 0.5 | 0.50 ± 1 | 60.00 | 0.75 ± 0.5 | 0.25 ± 0.5 | 66.66 | |
| Erigeron annus L. | 3.50 ± 1.29 | 0 | 100 | 3.50 ± 1.29 | 0 | 100 | |
| Erigeron canadensis L. | 2.00 ± 0.82 | 0 | 100 | 1.50 ± 0.58 | 0 | 100 | |
| Galium aparine L. | 2.25 ± 0.5 | 0 | 100 | 2.50 ± 0.57 | 0 | 100 | |
| Robinia pseudoacacia L. | 2.25 ± 1.29 | 0.25 ± 0.5 | 88.88 | 1.00 ± 0.82 | 0.25 ± 0.5 | 75.00 | |
| Rubus caesius L. | 0.75 ± 0.96 | 0 | 100 | 0.25 ± 0.5 | 0 | 100 | |
| Solidago gigantea L. | 6.50 ± 1.73 | 0.75 | 88.5 ± 0.96 | 7.50 ± 1.91 | 0 | 100 | |
| Solidago speciosa L. | 6.25 ± 0.5 | 0 | 100 | 3.25 ± 1.26 | 0 | 100 | |
| Symphytum officinale L. | 0.50 ± 0.58 | 0 | 100 | 0.25 ± 0.5 | 0 | 100 | |
| Vitis vinifera L. | 0.75 ± 0.5 | 0.50 | 33.33 ± 0.58 | 0.50 ± 0.58 | 0 | 100 | |
| Total number of weeds | 43.75 | 2.50 | 32.25 | 1.00 | |||
| Total efficacy | - | 94.29% | - | 96.90% | |||
| Phytotoxicity | - | 1 | - | 1 | |||
| First Assessment | Second Assessment | ||||||
|---|---|---|---|---|---|---|---|
| Weed Species | Control | Glyphosate | Control | Glyphosate | |||
| No/m2 | No/m2 | Ce (%) | No/m2 | No/m2 | Ce (%) | ||
| Monocotyledons | Apera spica-venti L. P. Beauv | 3.00 ± 1.41 | 0 | 100 | 1.75 ± 0.96 | 0 | 100 |
| Dactylis glomerata L. | 7.00 ± 1.41 | 0 | 100 | 3.75 ± 0.95 | 0 | 100 | |
| Poa pratensis L. | 4.50 ± 1 | 0 | 100 | 2.75 ± 0.96 | 0 | 100 | |
| Dicotyledons | Achillea millefolium L. | 1.50 ± 1.29 | 0 | 100 | 1.25 ± 1.50 | 0 | 100 |
| Amorpha fruticosa L. | 1.75 ± 0.95 | 0 | 100 | 1.25 ± 0.5 | 0 | 100 | |
| Asclepias syriaca L. | 1.75 ± 0.5 | 0.25 ± 0.5 | 85.71 | 1.25 ± 0.5 | 0.25 ± 0.5 | 80.00 | |
| Clematis vitalba L. | 0.25 ± 0.5 | 0 | 100 | 0.25 ± 0.5 | 0 | 100 | |
| Cornus sanguinea L. | 0.50 ± 0.58 | 0.25 ± 0.5 | 50.00 | 0.50 ± 0.5 | 0.25 ± 0.5 | 50.00 | |
| Daucus carota L. | 1.00 ± 0.82 | 0.25 ± 0.5 | 75.00 | 0.50 ± 0.57 | 0 | 100 | |
| Erigeron annus L. | 2.75 ± 1.26 | 0.75 ± 0.5 | 72.72 | 2.50 ± 1.73 | 0 | 100 | |
| Erigeron canadensis L. | 2.50 ± 0.58 | 0 | 100 | 1.50 ± 0.58 | 0 | 100 | |
| Galium aparine L. | 2.75 ± 0.5 | 0 | 100 | 2.00 ± 0.82 | 0 | 100 | |
| Geranium disectum L. | 2.50 ± 0.57 | 0 | 100 | 1.75 ± 0.96 | 0 | 100 | |
| Myosotis arvensis L. | 0.75 ± 0.5 | 0 | 100 | 1.00 ± 0.96 | 0 | 100 | |
| Solidago gigantea L. | 9.25 ± 1.89 | 0.50 ± 0.58 | 94.59 | 8.25 ± 1.71 | 0 | 100 | |
| Solidago speciosa L. | 6.50 ± 1 | 0 | 100 | 4.25 ± 0.96 | 0 | 100 | |
| Trifolium campestre Shreb. | 2.00 ± 0 | 0 | 100 | 1.25 ± 0.5 | 0 | 100 | |
| Vicia villosa Roth. | 0.50 ± 0.58 | 0 | 100 | 0.50 ± 0.58 | 0 | 100 | |
| Vitis vinifera L. | 0.50 ± 1 | 0.25 ± 0.5 | 50.00 | 0.50 ± 1 | 0.25 ± 0.5 | 50.00 | |
| Horsetail | Equisetum arvense L. | 1.25 ± 0.5 | 0.50 ± 0.58 | 60.00 | 1.25 ± 0.5 | 0.50 ± 0.58 | 60.00 |
| Total number of weeds | 52.50 | 2.75 | 38.00 | 1.25 | |||
| Total efficacy | - | 94.76% | - | 96.71% | |||
| Phytotoxicity | - | 1 | - | 1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudić, M.; Meseldžija, M.; Vasić, V.; Vranešević, M.; Kesić, L.; Orlović, S. Weed Control and Physiological Responses in Poplar Plantations: Assessing Glyphosate’s Impact. Forests 2024, 15, 1663. https://doi.org/10.3390/f15091663
Dudić M, Meseldžija M, Vasić V, Vranešević M, Kesić L, Orlović S. Weed Control and Physiological Responses in Poplar Plantations: Assessing Glyphosate’s Impact. Forests. 2024; 15(9):1663. https://doi.org/10.3390/f15091663
Chicago/Turabian StyleDudić, Milica, Maja Meseldžija, Verica Vasić, Milica Vranešević, Lazar Kesić, and Saša Orlović. 2024. "Weed Control and Physiological Responses in Poplar Plantations: Assessing Glyphosate’s Impact" Forests 15, no. 9: 1663. https://doi.org/10.3390/f15091663
APA StyleDudić, M., Meseldžija, M., Vasić, V., Vranešević, M., Kesić, L., & Orlović, S. (2024). Weed Control and Physiological Responses in Poplar Plantations: Assessing Glyphosate’s Impact. Forests, 15(9), 1663. https://doi.org/10.3390/f15091663









