Abstract
Old trees are irreplaceable conservation resources with numerous ecological and socio-cultural values. While many forests have experienced significant declines under recent climate warming, the risk of growth declines in old trees remains unknown. Here, we tackle this problem by dendrochronological studies of 30 old trees in a Platycladus orientalis forest at the northern boundary of the Taihang Mountain of China. We examined annual growth trajectories of trees at individual level and discovered four severe growth decline events over the last 150 years, including the periods of 1894–1899, 1913–1919, 1964–1967 and 2004–2018. The most recent growth decline event lasted for 15-year and involced 50% to 75% of the old trees. This decline was unprecedented in both its extent and duration. Furthermore, the growth–climate relationship of these old trees has changed since 1990. Before 1990, tree growth was significantly correlated with minimum winter; after 1990, tree growth became significantly correlated with the self-calibrating Palmer Drought Index. These results suggest that warming-induced droughts after 1990 could be the primary driver of the recent growth decline. If climate warming continues and drought stresses intensify, the old trees may face an increased risk of growth decline and even mortality.
1. Introduction
Old trees are irreplaceable conservation resources that provide various ecological functions in maintaining biodiversity and ecosystem stability, offering social and cultural benefits to communities [1,2]. Growth declines in populations of old trees have been reported across many ecosystems worldwide, and these declines were associated with global climate change as well as increased human activities [3,4]. According to climate prediction models, the frequency and intensity of severe climate extremes, such as droughts caused by global warming, are expected to increase in the 21st century [5,6]. Whether old trees can withstand extreme conditions under continued global warming has become a growing concern for both the public and governments [2,7].
To address the above question, researchers have conducted studies assessing the health of large old trees [8], examining growth–climate relationships [9,10], and modeling future trends in tree growth [11,12]. The decline and death of old trees are predicted to increase with the increasing frequency and intensity of stress events [13,14]. Other studies have reported that old trees exhibit high resilience to withstand extreme environmental changes [15]. Despite the recognition of risks associated with growth declines in old trees, the causes of these declines are difficult to identify based solely on current tree growth observations. Understanding past occurrences of tree-growth declines can elucidate the ways through which old trees respond to climate change and enhance our ability to predict the risk of tree-growth decline under future climate conditions [16,17].
Tree-ring data have been employed in detecting historical growth declines in old-growth forests [18,19]. Studies have reconstructed the historical growth decline of old Chinese pine forests in northern China and found that the recent decline fell outside the historic range of variability by analyzing the tree-ring data [20].
Here, we aimed to reconstruct the history of growth decline events in an old Platycladus orientalis forest at the northern edge of the Taihang Mountain in Beijing. As the most abundant old tree species in the capital city of China [21], P. orientalis plays a crucial role in providing socio-cultural services and promoting ecological civilization [22]. To date, few studies have been conducted regarding past decline events in old trees of P. orientalis forest. By comparing the radial growth trajectories of individual trees, rather than using the conventional approach of examining overall growth trends averaged across all trees at a site, we seek to address three questions: (1) How many events of growth declines occurred in P. orientalis forest in the past? (2) Is the recent decline event intensified compared to the past? (3) What are the causes of the growth declines, and how severe will be the risks of future growth declines in old trees?
2. Materials and Methods
2.1. Study Site and Tree-Ring Data
The sampling site is located in Shangfanshan Park at the northern boundary of the Taihang Mountain in China (39°65′–39°70′ N, 115°81′–115°91′ E, Figure 1). This park is an important sacred site of Buddhism in northern China, serving as a refuge for large old trees. Thus, the natural forests and numerous ancient trees in the parks are well preserved, with the predominant tree species being P. orientalis. Meteorological data show that the study area experienced year-round synchronization of water and heat, with an annual mean temperature of 10.0 °C and an annual total precipitation of 500 mm, with approximately 79% occurring from June to September since 1950 (Figure 1). The monthly mean temperature reaches 24.4 °C in July and decreases to −6.5 °C in January. Annual mean temperatures show a rising trend whereas annual total precipitation remains unchanged (Figure 2).
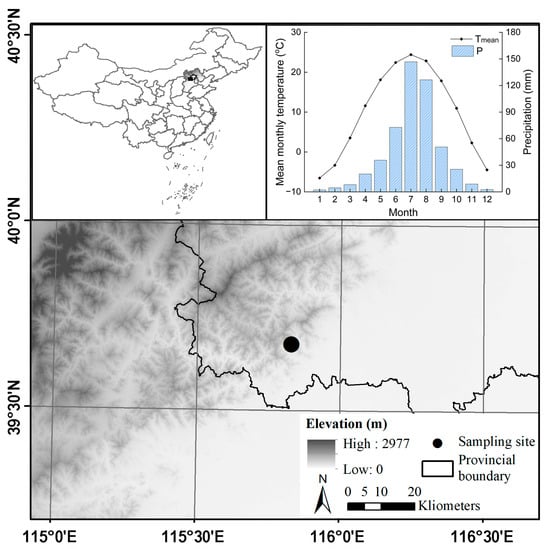
Figure 1.
Location of the old P. orientalis forest at the northern edge of the Taihang Mountain in China and the climate conditions of the study area during 1950–2020.
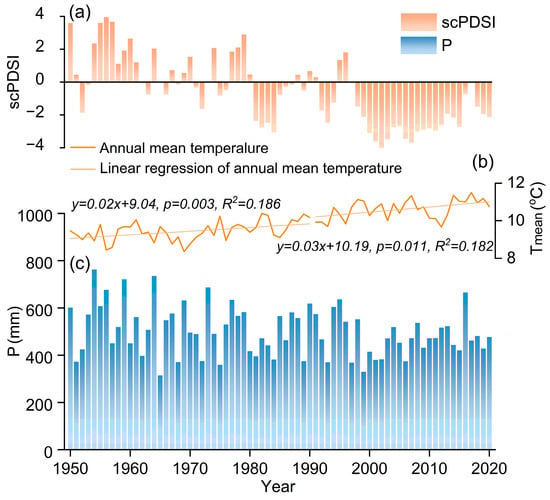
Figure 2.
Variation in the annual mean self-calibrating Palmer Drought Severity Index (scPDSI) (a), annual mean temperature (Tmean) (b), and annual total precipitation (P) (c) of the study area from previous October to current September during 1950–2020.
Sampling was conducted by collecting increment cores from the oldest P. orientalis trees, identified based on stem size, old-growth characteristics, and local traditional knowledge. These trees were selected from the hillside within the park, situated at an elevation of 570 m, away from visitor areas to minimize human disturbance and ensure the presence of well-established trees. Each tree was sampled with one core collected at breast height (1.3 m from the ground) using an increment borer with a diameter of 5.5 mm. In total, we collected 30 samples. The increment cores were air-dried, mounted, and carefully sanded in the laboratory until the borders of the annual rings were clearly defined. Tree-ring widths were measured using a LINTAB 6 system (Rinntech, Heidelberg, Germany) with an accuracy of 0.001 mm. Tree rings were crossdated, and the quality of crossdating was verified using the COFECHA program. Age-related growth trends in each sample were eliminated using the ARSTAN program by employing a negative exponential function method. The standard chronology (STD) was constructed using the bi-weight robust mean method to analyze climate-growth responses. The resultant ring-width index (RWI) series of each tree formed the database for analyzing the growth trajectories of the sampled trees.
2.2. Detection of Tree Growth Decline
The RWI fluctuates around the mean value for normal growth and consistently decreases when trees experience a growth decline. To indicate tree decline at the individual level, we applied the following criteria [20,23]: (1) a sustained period of at least 6 years with a mean RWI value of less than 0.8; (2) a minimal RWI value of less than 0.75 in at least 1 year; (3) presence of at least one tree that exhibited no decline during the same period. We calculated the percentage of trees with growth decline (PTD) by years using the Expressed Population Signal (EPS) above 0.85. When the PTD was greater than 50%, we considered that a forest decline event had occurred.
2.3. Climate Response Analysis
The Mann–Kendall test was used to determine the mutation point of the temperature series. To investigate the climate-growth relationship, we performed Pearson correlation analyses between monthly climatic factors and tree-ring chronologies before and after the abrupt temperature rise by using SPSS 26.0. Climate data were obtained from the global data set (http://climexp.knmi.nl (accessed on 28 January 2024)) at a spatial resolution of 0.5° × 0.5° (longitude × latitude). The meteorological index data included monthly mean temperature (Tmean), monthly mean minimum temperature (Tmin), monthly mean maximum temperature (Tmax), monthly total precipitation (P), and the Self-calibrating Palmer Drought Severity Index (scPDSI). The meteorological indices data selected for correlation analysis were acquired from October of the previous year to September of the current year. To explore the temporal changes and stability of climatic factors limiting the growth of old trees since the 1950s, we analyzed the correlation between the STD and seasonal climatic factors. This analysis involved sliding backward year by year with a sliding window of 30 years over the common period from 1950 to 2020.
3. Results
3.1. Tree-Ring Chronology
The longest sequence of sample cores spanned 224 years (1798–2022). The periods meeting the condition EPS > 0.85 were from 1864 to 2020, with 30 sample replicates. The high inter-series correlation coefficient (0.310) and signal-to-noise ratio (13.478) of the chronology indicate its high quality and the presence of strong communal climate signals among individual trees. The mean sensitivity and standard deviation were 0.567 and 0.340, respectively, indicating relatively high interannual variability in annual ring width (Table 1).

Table 1.
Statistic characteristics of the standard chronology of the old P. orientalis forest.
3.2. History of Forest Decline
Tree growth declines have been widespread among individual trees over the past 150 years. Such declines synchronized to form decline events in four periods: 1894–1898, 1913–1919, 1964–1966, and 2004–2018 (Figure 3a). The first three growth decline events lasted for 5, 7, and 3 years, and the maximum proportion of trees with growth decline reached 53%, 57% and 57%, respectively. In the most recent decline event (2004–2018), the proportion of trees with growth decline continued to exceed 50% for 15 years, with a maximum of 70%. In addition, the growth trajectory of these old trees also exhibited a significant decreasing trend after 1990 (slope = −0.024 ± 0.004, R2 = 0.55, p < 0.001), decreasing from 1.635 to 0.340 during the 1990–2020 period (Figure 3b).
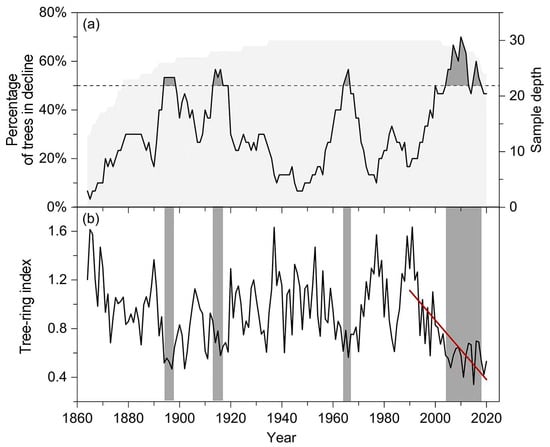
Figure 3.
Tree growth declines (a) and ring-width index chronology (b) of the P. orientalis forest over the past 150 years. The light gray shaded area in panel (a) indicates the sample depth with the scale on the right Y-axis and the gray parts refer to the interval of intense growth decline events with the proportion of trees with growth decline exceeding 50%. Gray bars in panel (b) represent the period of growth decline event and the red line shows the significant decreasing trend in tree-ring width chronology after 1990 (slope = −0.024 ± 0.004, R2 = 0.55, p < 0.001).
The mean RWI of trees with and without growth decline during the four forest decline periods distinctly varied in growth trajectories. Trees exhibiting growth decline consistently showed lower RWI values than trees without decline traits of the four growth declining events (Figure 4).
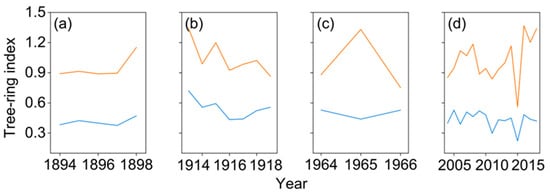
Figure 4.
Mean ring-width indices of trees with (blue line) and without (red line) growth decline during the period of 1894–1898 (a), 1913–1919 (b), 1964–1966 (c), and 2004–2018 (d) of growth declining events.
3.3. Growth–Climate Relationship
The Mann–Kendall temperature mutation results indicate that there is an abrupt warming after the year 1990, with the annual mean temperature increasing from 9.45 °C during the 1950–1990 period to 10.61 °C during the 1991–2020 period (Figure 2 and Figure 5a). We detected the climate-growth response of old trees before and after 1990, and the results showed that the tree-ring chronology of old trees exhibited a significant positive correlation with Tmin from December of the previous year to March during the 1950–1990 period (r = 0.349, p < 0.05, Figure 6). However, after the 1990s, it showed a significant positive correlation with scPDSI from October of the previous year to July of the current year (r = 0.441, p < 0.05). The radial growth of old trees exhibited a significant negative correlation with mean and maximum temperatures in May during the 1950–2020 period (p < 0.05).
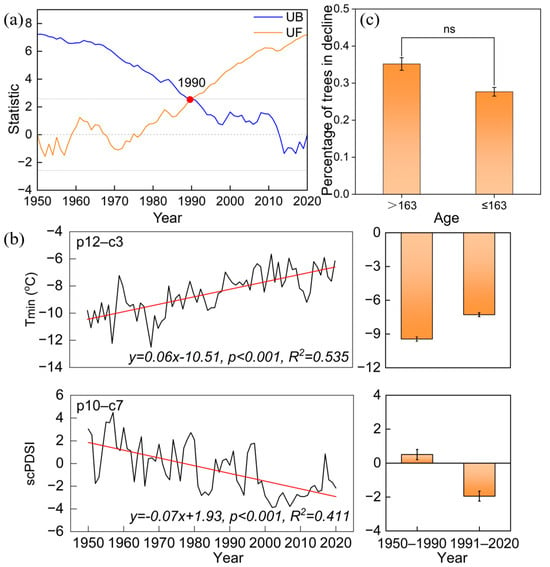
Figure 5.
Mann–Kendall test of annual mean temperature series in the study area (a), variation in mean minimum temperature (Tmin) from December of the previous year to March of the current year and mean scPDSI from December of the previous year to July of the current year in the study area during 1950–2020, along with a comparison of average values before and after the warming mutation (b), and a comparison of the proportion of trees in decline across different age groups (c). UF represents the positive test value series and UB represents the reverse test value series. The red point marks the mutation point of the annual mean temperature in 1990.
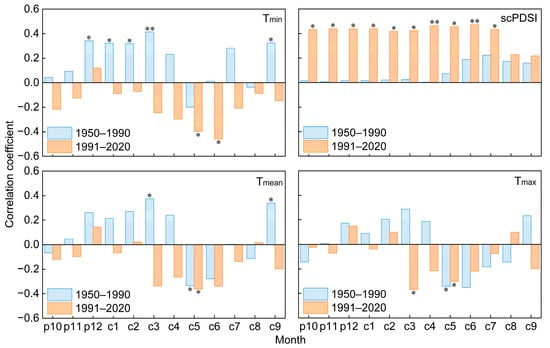
Figure 6.
Correlation between standardized tree-ring chronology and climate factors during the 1950–1990 and 1991–2020 periods. prepresents the months in the previous year, c represents the current year, * and ** represent significant correlations of p < 0.05 and p < 0.01, respectively. The same applies below.
Moving correlation analysis emphasized a distinct shift in the growth–climate response of these old trees during the 1950–2020 period (Figure 7). The significant positive correlation between tree radial growth and minimum winter temperature (December of the previous year to March of the current year) gradually weakened after the 1970s, shifting from a positive relationship with a mean correlation of 0.379 (p < 0.05) to a negative relationship with a mean correlation of −0.032 (p > 0.05) before and after 1968. By contrast, the radial growth of trees showed a significant negative correlation with the minimum temperature in the growing season (May to June), with a mean correlation of −0.528 (p < 0.05) after 1968. The positive correlation between tree radial growth and scPDSI significantly increased from a mean correlation coefficient of 0.175 (p > 0.05) to 0.519 (p < 0.05) before and after 1972.
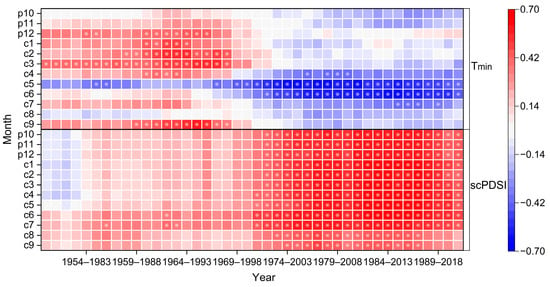
Figure 7.
Moving correlation results between tree ring width chronology from 1950 to 2020 and climate factors Tmin and scPDSI. Moving correlation analysis is conducted in windows of 30 years, offset by 1 year. Stars in the plot indicate windows with significant correlations (p < 0.05) for the given variable.
4. Discussion
4.1. Recent Unprecedented Growth Decline of Old Trees over the Last Two Centuries
A few studies often use the continued slow or even stagnant tree growth exhibited by stand tree-ring chronologies to detect forest growth decline [24]. Using average or whole growth at the stand level may overlook growth changes occurring at the individual tree level [25]. Meanwhile, an increasing number of studies suggest that the ability of old trees to withstand extreme events varies widely among individuals [26,27]. In addition, not all old trees show growth reduction during forest decline [23,28]. In the present study, we identified four intervals of forest decline in old P. orientalis trees over the last two centuries by comparing the growth trajectories of individual trees (Figure 3 and Figure 4) rather than relying on average annual tree-ring widths at the stand level [18,23,29]. By defining the long-term low radial growth of individual trees and considering the percentage of low-growth individual trees, we successfully reconstructed the historical tree-growth declines in an old P. orientalis forest at the northern boundary of the Taihang Mountain in Beijing.
The findings of our study demonstrate that declines in tree growth of the old P. orientalis forest have been prevalent over the past 150 years, with four intense growth-decline events occurring, the most recent being the most severe and unprecedented over the period of study (Figure 3). Similar growth declines have also been identified in other regions [30]. For example, forest decline due to extreme drought events in the early 20th century was more severe than the recent decline in northeastern Australia, as determined from historical records of forest growth data from 1845 to 2017 [31]. In addition, the decline of an old Chinese pine forest in Hengshan Park has been found to fall outside the historic range of variability [20]. Severe growth decline in a spruce forest induced by stress conditions under increased cloud cover was also observed in Menyuan County, Qilian Mountains [32]. Different regions exhibit high spatial heterogeneity. For instance, tree-growth reduction in Tibetan juniper forests has been intermittent over the past 350 years and dispersed across the Tibetan Plateau [33], whereas the recent forest growth decline has been alleviated [34]. Factors such as topography, microclimate, and other environmental conditions exert differential effects on the tree growth of old forests [35]. Thus, the characteristics of historical growth declines in old forests vary among different regions [36,37]. Despite these past declines, the old P. orientalis forest at the northern edge of the Taihang Mountain exhibits high resilience to disturbances. This characteristic is evidenced by the ability of trees to recover their growth rates to levels comparable to the pre-decline period during the first three decline events in history (Figure 3).
4.2. Altered Growth–Climate Response after Rapid Warming Exacerbating Forest Decline
Compared to the first three events of growth decline, the most recent decline in the old P. orientalis forest after the 1990s lasted for 15 years, and the percentage of trees with growth decline fell outside the historic range of variability (Figure 3). This finding suggests that the forces driving this recent decline have changed. Correlation analyses between tree growth and climatic factors indicate that the growth–climate response of old trees has changed after the 1990s because of rapid warming. This transition has led to a change in the key limiting factor of radial growth in old P. orientalis, shifting from minimum winter temperature to drought caused by high temperatures in the growing season (Figure 6 and Figure 7). Since the 1950s, the average temperature in the current study area has been increasing, with a rapidly increasing trend from the 1990s, accompanied by intensifying drought conditions (Figure 2 and Figure 5b). After this period, the growth–climate response of trees has changed, with tree growth exhibiting a closely declining trend in response to increasing drought severity. This observation suggests that the altered growth–climate response following climate warming may exacerbate the forest decline of these old trees after the 1990s.
Sustained reductions in tree growth and even mortality caused by droughts under climate warming have been widely reported [38,39]. Warming climate change has led to increased evapotranspiration and atmospheric moisture demand, resulting in drought stress and a pervasive decline in tree growth [37,40]. Li et al. [29] reported a severe decline in Chinese pine growth, attributed to drought. Regional drought in the 1920s has been identified as a primary cause of tree growth decline in Qinghai spruce forests in the Qilian Mountains [31]. In contrast to these findings, other studies suggest that the minimum temperature during the cold season is the main driver of the growth decline in Tibetan juniper forests, and the increased winter minimum temperature due to climate warming substantially mitigates the growth decline in juniper forests on the Tibetan Plateau [32]. Tree growth in areas limited by the minimum winter temperature could benefit from climate warming [41]. However, moisture conditions became the primary limiting factor of growth in our study area, supported by the positive relationship between tree growth and scPDSI after 1972 (Figure 6 and Figure 7). The benefit of increasing minimum temperature due to climate warming may be offset by the increasing severity of drought, which could exacerbate the forest growth decline of old P. orientalis.
In areas characterized by high altitudes or limited by minimum winter temperatures, climate warming could reduce damages and constraints caused by low temperatures before and during the early growing season of juniper tree growth [34]. This occurrence could prolong the growing period of trees and contribute to their radial growth [42]. However, in the current study, increments in temperature were not accompanied by changes in precipitations (Figure 2 and Figure 5b). Elevated temperature is expected to intensify transpiration and atmospheric evaporative demand, thus reducing the available moisture in the soil [43,44], leading to drought stress during tree growth [39,45]. Trees under drought stress may close their stomata to prevent hydraulic failure, resulting in carbon starvation due to reduced photosynthetic uptake of carbon dioxide [46,47]. Moreover, elevated drought conditions, together with increasing temperatures, can increase respiratory demands, exacerbating the carbon-starvation process [48].
Another potential factor that could reduce the resilience of old trees might be related to aging effects. Old trees are often thought to be more sensitive to climate warming and exhibit decreased resistance to drought [12]. However, in this study, no significant difference in the percentage of trees with growth decline was found between different age groups (Figure 5c). In addition, no increasing trend in forest growth decline with tree age was observed over the past 150 years. The durations, intervals, and severities of the first three growth declines in the old P. orientalis forest were similar and did not show an increasing trend with tree age (Figure 3), suggesting that these old trees grew well and could minimize the effects of aging on growth. Thus, the effects of tree aging might not be the primary factor causing the unprecedented growth decline in recent decades.
4.3. Risk in Growth Decline of Old Forests
This study indicates that the growth–climate response has been altered, and the key factors limiting tree growth have changed to drought since the 1990s (Figure 6 and Figure 7). This occurrence, typically referred to as the “divergence problem”, has also been observed worldwide recently. Wang et al. [49] found that the radial growth of larch in northern China experienced drought stress after a sudden temperature change while being influenced by the lowest temperature before warming. Trees in high-latitude boreal forests exhibit a decreasing climate sensitivity and radial growth after climate warming [50]. Previous research also indicates that short-term limitations imposed by hydraulic damage can alter the way trees respond to climate, leading to temporal variation in growth climate sensitivity. Trees may die when they fail to return to their target growth–climate response [51].
In the current study, although the old forest survived the first three declines, the durations and intensities of growth decline have been considerably stronger in recent decades after the altered growth–climate response than in the past. There is a certain threshold of tree resilience, which may change abruptly in a gradually changing climate [52]. If changes in temperature and drought continue unabated and exceed the threshold, these old trees would face a higher risk of mortality because they may not be able to recover from severe declines. In the conservation of old-growth forests, managers should consider these potential threats to accurately assess and predict the health and survival of old trees under global warming.
5. Conclusions
In summary, we reconstructed the growth decline of an old conifer forest by comparing the individual-level growth trajectories using tree-ring data. We identified four intervals of forest decline in old P. orientalis in the study area over the past two centuries, with the most recent decline since the 1990s falling outside the historic range of variability. In addition, the high level of synchronicity between the intensification of growth decline and the altered growth–climate response in recent decades indicates that the altered growth–climate response after climate warming may exacerbate the growth decline of these old trees. Although the old P. orientalis trees at the northern edge of the Taihang Mountain survived from the first three declines in history, the unprecedented decline in recent decades following the altered growth–climate response suggests a high risk of growth decline and even mortality with continued climate warming. Our study provides information and methods for identifying the historical growth declines in old forests, facilitating the assessment of potential threats to old trees and the accurate prediction of their health and survival under future climate scenarios.
Author Contributions
Y.L. (Yan Li): Writing—review and editing, Methodology, Formal analysis, Conceptualization. T.W.: Writing—original draft, Formal analysis. Y.D.: Software, Investigation. X.H.: Investigation, Methodology. Y.L. (Yang Liu): Software, Investigation. Y.M.: Writing—review and editing. X.M. and P.L.: Supervision, Project administration. Z.H.: Writing—review and editing, Supervision, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Young Teachers Research Innovation Ability Enhancement Program of Beijing University of Agriculture (grant number: QJKC-2022058), the Classified Development of Colleges and Universities in Beijing—Construction Project of Teachers with Urban Agriculture and Forestry Characteristics (grant number: 11000024T000002961733) and the Emergency Open Competition Project of the National Forestry and Grassland Administration (grant number: 202303).
Data Availability Statement
The data will be made available on request.
Acknowledgments
We thank Qi-Bin Zhang (State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences), for his help with reviewing, editing and providing conceptual guidance for this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lindenmayer, D.B.; Laurance, W.F. The ecology, distribution, conservation and management of large old trees. Biol. Rev. 2017, 92, 1434–1458. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, G.; Cannon, C.H.; Liu, J.; Munne-Bosch, S. Ancient trees: Irreplaceable conservation resource for ecosystem restoration. Trends Ecol. Evol. 2022, 37, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Delamônica, P.; Laurance, S.G.; Vasconcelos, H.L.; Lovejoy, T.E. Rainforest fragmentation kills big trees. Nature 2000, 404, 836. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F. Global decline in large old trees. Science 2012, 338, 1305–1306. [Google Scholar] [CrossRef]
- Jones, P.D.; Osborn, T.J.; Briffa, K.R. The evolution of climate over the last millennium. Science 2001, 292, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, Y.; Williams, A.P.; Gentine, P. Projected increases in intensity, frequency, and terrestrial carbon costs of compound drought and aridity events. Sci. Adv. 2019, 5, eaau5740. [Google Scholar] [CrossRef]
- Cannon, C.H.; Piovesan, G.; Munne-Bosch, S. Old and ancient trees are life history lottery winners and vital evolutionary resources for long-term adaptive capacity. Nat. Plants 2022, 8, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.T.; Zhang, Z.F.; Kang, T.H. The relative importance and priority of the health evaluation indicators of old-growth and giant trees. J. Korean Inst. Landsc. Arch. 2017, 45, 149–159. [Google Scholar] [CrossRef]
- Qi, X.; Fang, K.; Du, H.; He, J.; He, H.; Wu, Z. Age-related growth responses of birch to warming along an elevational gradient on Changbai Mountain. Eur. J. For. Res. 2022, 141, 293–305. [Google Scholar] [CrossRef]
- Begović, K.; Schurman, J.S.; Svitok, M.; Pavlin, J.; Langbehn, T.; Svobodová, K.; Mikoláš, M.; Janda, P.; Synek, M.; Marchand, W.; et al. Large old trees increase growth under shifting climatic constraints: Aligning tree longevity and individual growth dynamics in primary mountain spruce forests. Glob. Chang. Biol. 2023, 29, 143–164. [Google Scholar] [CrossRef]
- Bigler, C. Trade-offs between growth rate, tree size and lifespan of Mountain Pine (Pinus montana) in the Swiss National Park. PLoS ONE 2016, 11, e0150402. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, H.; Xu, C.; Shi, L.; Zhu, X.; Qi, Y.; He, W. Old-growth forests show low canopy resilience to droughts at the southern edge of the taiga. Glob. Chang. Biol. 2021, 27, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Xu, C.; Liu, H. Hydraulic adaptability promotes tree life spans under climate dryness. Glob. Ecol. Biogeogr. 2022, 31, 51–61. [Google Scholar] [CrossRef]
- Mu, Y.M.; Lyu, L.X.; Li, Y.; Fang, O.Y. Tree-ring evidence of ecological stress memory. Proc. Biol. Sci. 2022, 289, 20221850. [Google Scholar] [CrossRef] [PubMed]
- Ogle, K.; Barber, J.J.; Barron-Gafford, G.A.; Bentley, L.P.; Young, J.M.; Huxman, T.E.; Loik, M.E.; Tissue, D.T. Quantifying ecological memory in plant and ecosystem processes. Ecol. Lett. 2015, 18, 221–235. [Google Scholar] [CrossRef]
- Peltier, D.M.; Barber, J.J.; Ogle, K. Quantifying antecedent climatic drivers of tree growth in the Southwestern US. J. Ecol. 2018, 106, 613–624. [Google Scholar] [CrossRef]
- Amoroso, M.M.; Daniels, L.D.; Larson, B.C. Temporal patterns of radial growth in declining Austrocedrus chilensis forests in Northern Patagonia: The use of tree-rings as an indicator of forest decline. Forest Ecol. Manag. 2012, 265, 62–70. [Google Scholar] [CrossRef]
- Mu, Y.M.; Fang, O.Y.; Cheng, X.; Qiu, H. Recent tree growth decline unprecedented over the last four centuries in a Tibetan juniper forest. J. For. Res. 2019, 30, 1429–1436. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.B. History of tree growth declines recorded in old trees at two sacred sites in northern China. Front. Plant Sci. 2017, 8, 1779. [Google Scholar] [CrossRef]
- Fu, Q.C.; Qiu, E.; Zhang, Y.; Huang, L.H.; Wang, H.C.; Jiang, S.S. Discussion of the distribution pattern and driving factors of 2 large old tree resources in Beijing. Forests 2022, 13, 1500. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.B.; Fang, O.Y.; Mu, Y.M.; Jia, H.; Lyu, L. Recovery time of juniper trees is longer in wet than dry conditions on the Tibetan Plateau in the past two centuries. For. Ecol. Manag. 2021, 497, 119514. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Carrer, M. Individualistic and time-varying tree-ring growth to climate sensitivity. PLoS ONE 2011, 6, e22813. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Navarro-Cerrillo, R.M.; Swetnam, T.W.; Zavala, M.K. Is drought the main decline factor at the rear edge of Europe? The case of southern Iberian pine plantations. For. Ecol. Manag. 2012, 271, 158–169. [Google Scholar] [CrossRef]
- Desoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in tree. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Cantero, A.; Sánchez-Salguero, R.; Sánchez-Miranda, A.; Granda, E.; Serra-Maluquer, X.; Ibáñez, R. Forest growth responses to drought at short- and long-term scales in Spain: Squeezing the stress memory from tree rings. Front. Ecol. Evol. 2018, 6, 9. [Google Scholar] [CrossRef]
- Li, Z.; Liu, G.; Fu, B.; Zhang, Q.; Hu, C.; Luo, S. Evaluation of temporal stability in tree growth-climate response in Wolong National Natural Reserve, western Sichuan, China. Chin. J. Ecol. 2010, 34, 1045–1057. [Google Scholar]
- Liu, H.Y.; Williams, A.P.; Allen, C.D.; Guo, D.L.; Wu, X.C.; Anenkhonov, O.A.; Liang, E.Y.; Sandanov, D.V.; Yin, Y.; Qi, Z.H.; et al. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Chang. Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef]
- Liang, E.Y.; Leuschner, C.; Dulamsuren, C.; Wagner, B.; Hauck, M. Global warming-related tree growth decline and mortality on the north-eastern Tibetan plateau. Clim. Chang. 2016, 134, 163–176. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, B.; Zheng, J.; Zhang, Q.; Li, Y.; Ma, F.; Fang, O.Y. Linkage between spruce forest decline and cloud cover increase in the Qilian Mountains of the northeastern Tibetan Plateau. Trees 2023, 37, 1097–1106. [Google Scholar] [CrossRef]
- Mu, Y.M.; Zhang, Q.B.; Fang, O.Y.; Lyu, L.; Cherubini, P. Pervasive tree-growth reduction in Tibetan juniper forests. For. Ecol. Manag. 2021, 480, 118642. [Google Scholar] [CrossRef]
- Mu, Y.M.; Fang, O.Y.; Lyu, L. Nighttime warming alleviates the incidence of juniper forest growth decline on the Tibetan Plateau. Sci. Total Environ. 2021, 782, 146924. [Google Scholar] [CrossRef] [PubMed]
- Fang, O.Y.; Zhang, Q.B.; Vitasse, Y.; Zweifel, R.; Cherubini, P. The frequency and severity of past droughts shape the drought sensitivity of juniper trees on the tibetan plateau. For. Ecol. Manag. 2021, 486, 118968. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Y.; Zhu, C.; Li, Z.; Fang, G.; Li, Y.; Fu, A. Climate change may accelerate the decline of desert riparian forest in the lower Tarim River, Northwestern China: Evidence from tree-rings of Populus euphratica. Ecol. Indic. 2020, 111, 105997. [Google Scholar] [CrossRef]
- Jing, M.; Zhu, L.; Liu, S.; Cao, Y.; Zhu, Y.; Yan, W. Warming-induced drought leads to tree growth decline in subtropics: Evidence from tree rings in central China. Front. Plant Sci. 2022, 13, 964400. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Field, C.B. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. USA 2012, 109, 233–237. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.G.; Alam, S.; Zhai, L.H.; Dawson, A.; Stadt, K. Drought causes reduced growth of trembling aspen in western Canada. Glob. Chang. Biol. 2017, 23, 2887–2902. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Yang, B.; He, M.; Shishov, V.; Tychkov, I.; Vaganov, E.; Rossi, S.; Ljungqvist, F.C.; Bräuning, A.; Griebbinger, J. New perspective on spring vegetation phenology and global climate change based on Tibetan Plateau tree-ring data. Proc. Natl. Acad. Sci. USA 2017, 114, 6966–6971. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cook, E.R.; Li, J.; Lu, H. Unprecedented January–July warming recorded in a 178-year tree-ring width chronology in the Dabie Mountains, southeastern China. Palaeogeogr. Palaeocl. 2013, 381, 92–97. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Corti, T.; Davin, E.L.; Hirschi, M.; Jaeger, E.B.; Lehner, I.; Orlowsky, B.; Teuling, A.J. Investigating soil moisture-climate interactions in a changing climate: A review. Earth-Sci. Rev. 2010, 99, 125–161. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Z.Y. Increasing compound drought and hot event over the Tibetan Plateau and its effects on soil water. Ecol. Indic. 2023, 153, 110413. [Google Scholar] [CrossRef]
- Restaino, C.M.; Peterson, D.L.; Littell, J. Increased water deficit decreases Douglas fir growth throughout western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 9557–9562. [Google Scholar] [CrossRef]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Adams, H.D.; Germino, M.J.; Breshears, D.D.; Barron-Gafford, G.A.; Guardiola-Claramonte, M.; Zou, C.B.; Huxman, T.E. Non-structural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol. 2013, 197, 1142–1151. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.X.; Jia, J.H.; Zhang, Z.H.; Guo, M.M. Responses of radial growth of Larix principis-rupprechtii to abrupt warming. Chin. J. Appl. Ecol. 2023, 34, 2629–2636. [Google Scholar] [CrossRef]
- Darrigo, R.D.; Jacoby, G.C.; Free, R.M. Tree-ring width and maximum latewood density at the North American tree line: Parameters of climatic change. Can. J. For. Res. 1992, 22, 1290–1296. [Google Scholar] [CrossRef]
- Peltier, D.M.; Ogle, K. Tree growth sensitivity to climate is temporally variable. Ecol. Lett. 2020, 23, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Holling, C.S. Resilience and stability of ecological systems. Ann. Rev. Ecol. System. 1973, 4, 1–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).