Response of Non-Structural Carbohydrates and Carbon, Nitrogen and Phosphorus Stoichiometric Characteristics of Ochroma lagopus Leaves to Nitrogen Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Sample Collection and Measurements
2.4. Data Processing
3. Results and Analyses
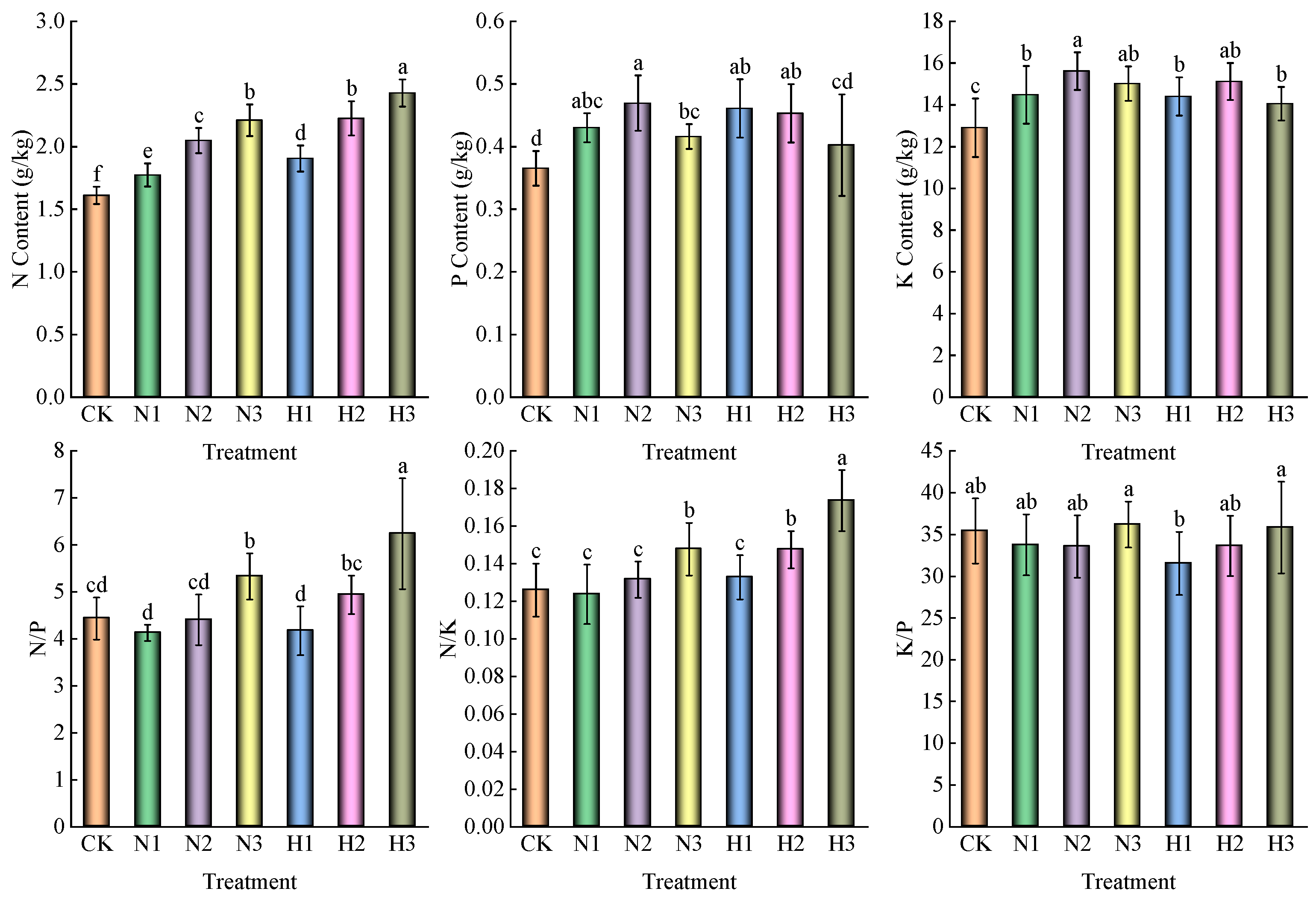
3.1. Effect of Nitrogen Addition on Soil Nutrient and Stoichiometric Characteristics
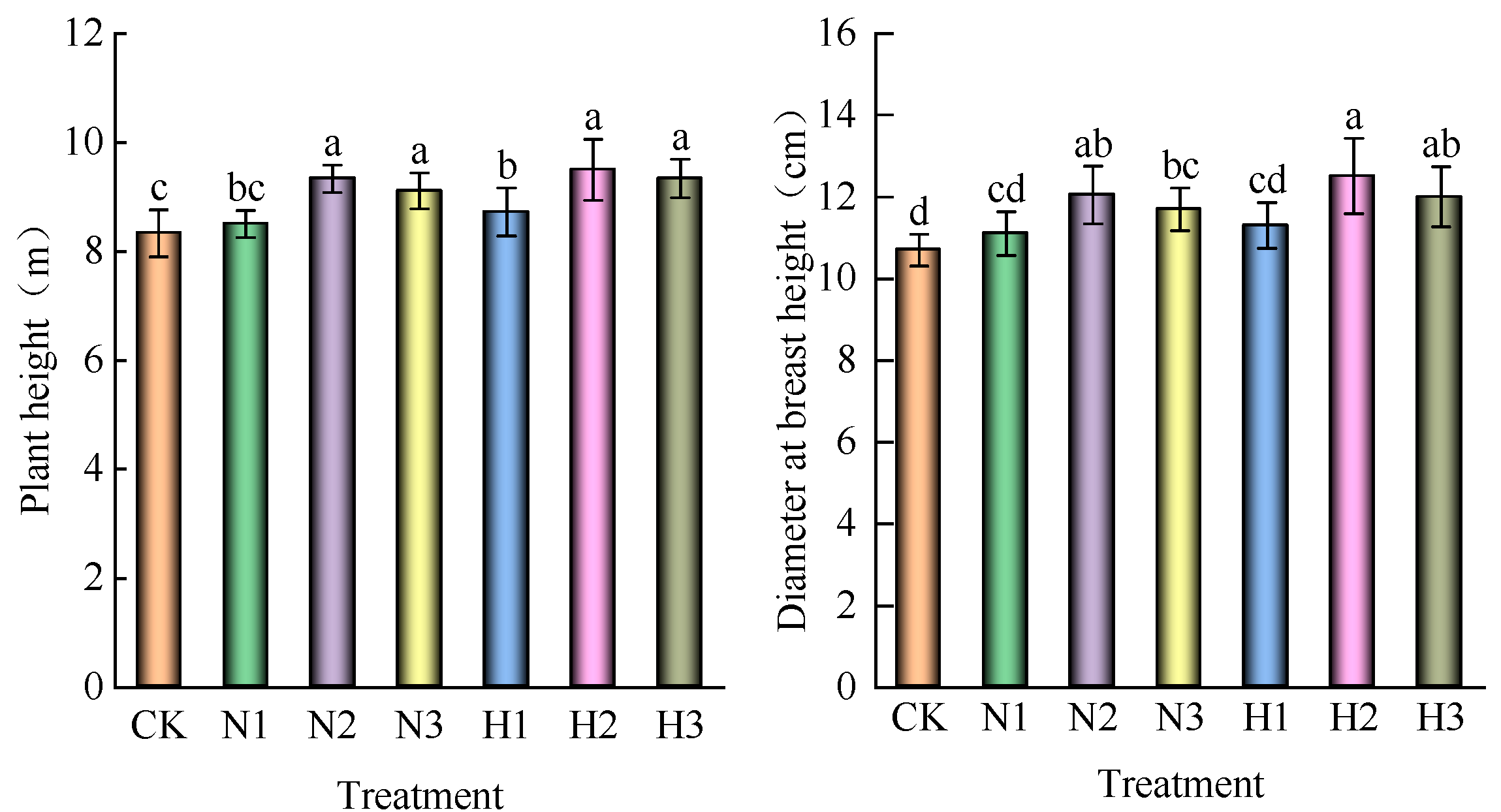
3.2. Effect of Nitrogen Addition on Height and Diameter at Breast Height of O. lagopus
3.3. Effect of Nitrogen Addition on NSC Content of O. lagopus Leaves
3.4. Effect of Nitrogen Addition on Carbon, Nitrogen, and Phosphorus Contents of O. lagopus Leaves
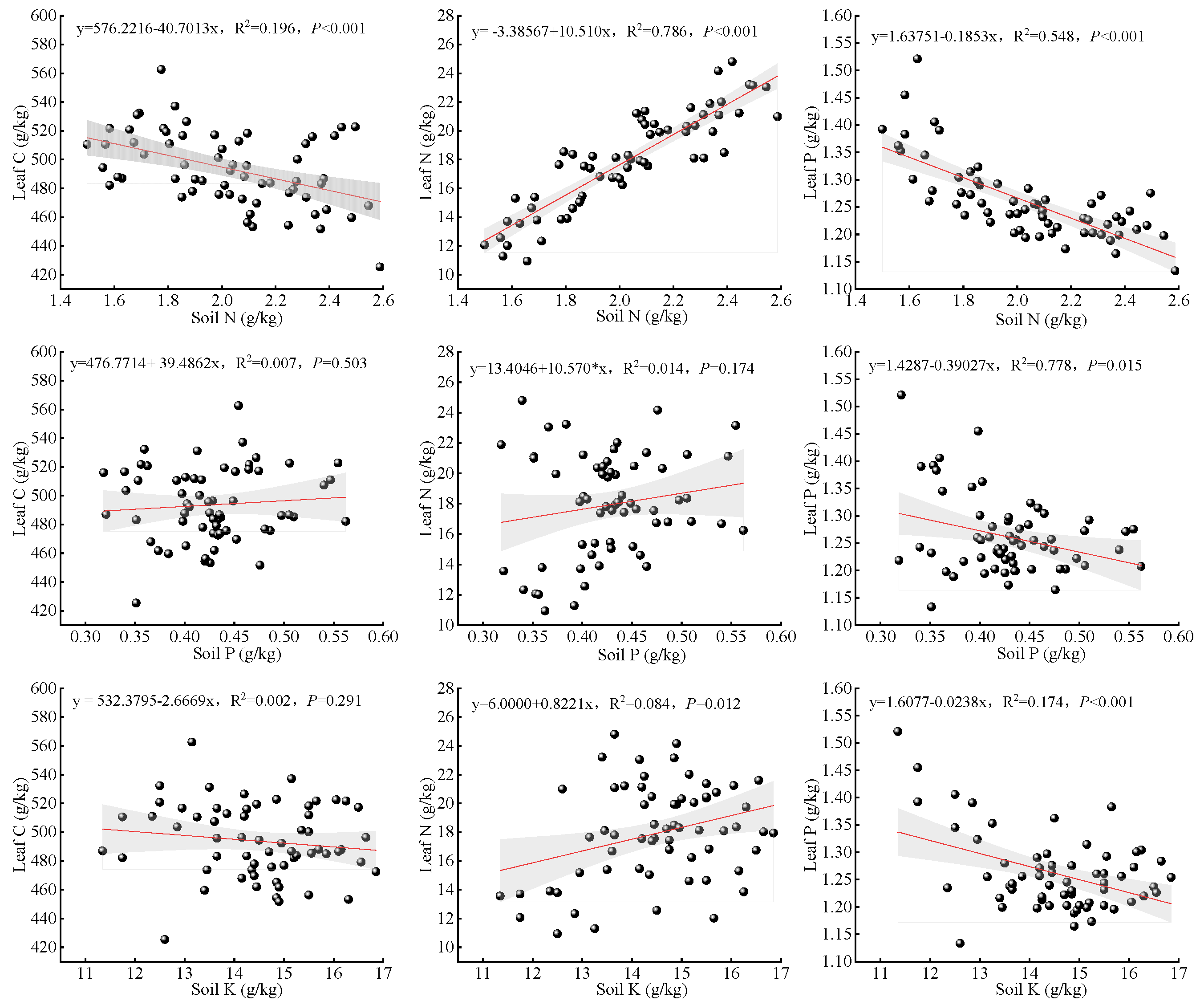
3.5. Correlation Between Soil Nutrients and O. lagopus Leaf NSC and Carbon, Nitrogen, and Phosphorus Contents
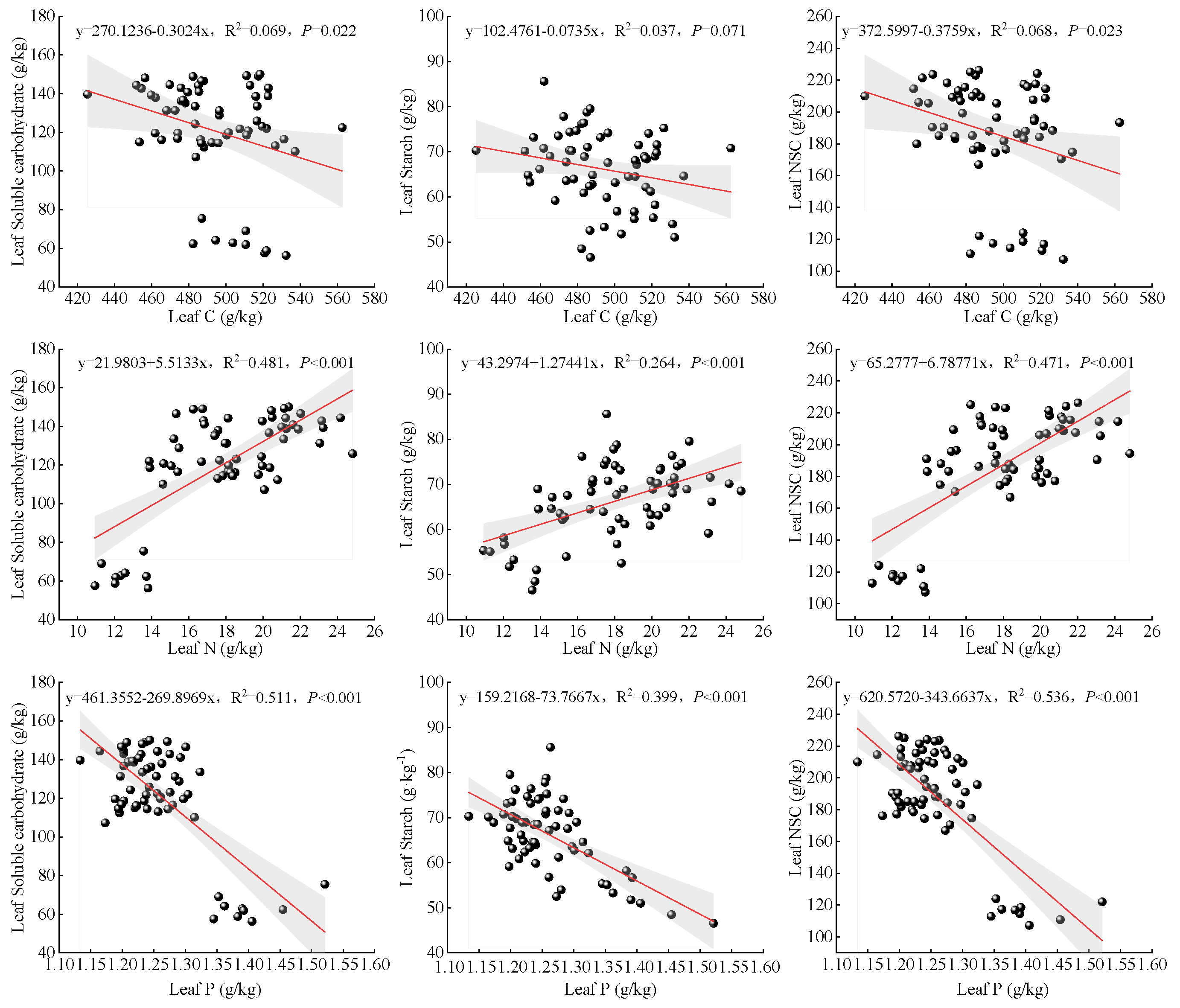
3.6. Correlation Between O. lagopus Leaf NSC Content and Carbon, Nitrogen, and Phosphorus Contents
3.7. Integrated Evaluation
4. Discussion
4.1. O. lagopus Growth Response to Nitrogen Addition
4.2. Response of Leaf NSC to Nitrogen Addition
4.3. Effect of Nitrogen Addition on O. lagopus Leaf C, N, and P Contents and Stoichiometric Ratio
4.4. Relationship Between O. lagopus Leaf NSC Content and C, N, and P Contents
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, G.N.; Xing, Y.J.; Han, S.J.; Zhang, J.H.; Wang, Q.G.; Mu, C.C. Long-time precipitation reduction and nitrogen deposition increase alter soil nitrogen dynamic by influencing soil bacterial communities and functional groups. Pedosphere 2020, 30, 363–377. [Google Scholar] [CrossRef]
- Lin, J.X.; Wang, Y.N.; Sun, S.N.; Mu, C.S.; Yan, X.F. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Peng, C.H.; Zhu, G.Y.; Chen, L.; Liu, Y.L.; Shangguan, Z.P. Positive responses of belowground C dynamics to nitrogen enrichment in China. Sci. Total Environ. 2018, 616, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; Van Grinsven, H.; Winiwarter, W. Too much of a good thing. Nature 2011, 472, 159–161. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, W.; Chen, H.; Mo, J. Impacts of nitrogen deposition on soil nitrogen cycle in forest ecosystems: A review. Acta Ecol. Sin. 2015, 35, 35–43. [Google Scholar] [CrossRef]
- Shen, Y.M.; Wang, H.; Liu, Z.J.; Li, W.; Liu, Y.H.; Li, J.J.; Wei, H.L.; Han, H. Fabrication of a water-retaining, slow-release fertilizer based on nanocomposite double-network hydrogels via ion-crosslinking and free radical polymerization. J. Ind. Eng. Chem. 2020, 93, 375–382. [Google Scholar] [CrossRef]
- Song, C.; Guan, Y.; Wang, D.; Zewudie, D.; Li, F.M. Palygorskite-coated fertilizers with a timely release of nutrients increase potato productivity in a rain-fed cropland. Field Crop Res. 2014, 166, 10–17. [Google Scholar] [CrossRef]
- Körner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- Li, M.H.; Cherubini, P.; Dobbertin, M.; Arend, M.; Xiao, W.F.; Rigling, A. Responses of leaf nitrogen and mobile carbohydrates in different Quercus species/provenances to moderate climate changes. Plant Biol. 2013, 15, 177–184. [Google Scholar] [CrossRef] [PubMed]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Du, Y.; Lu, R.L.; Xia, J.Y. Impacts of global environmental change drivers on non-structural carbohydrates in terrestrial plants. Funct. Ecol. 2020, 34, 1525–1536. [Google Scholar] [CrossRef]
- Thomas, V.F.D.; Braun, S.; Flückiger, W. Effects of simultaneous ozone exposure and nitrogen loads on carbohydrate concentrations, biomass, and growth of young spruce trees (Picea abies). Environ. Pollut. 2005, 137, 507–516. [Google Scholar] [CrossRef]
- Qiu, X.C.; Wang, H.B.; Peng, D.L.; Liu, X.; Yang, F.; Li, Z.; Cheng, S. Thinning drives C:N:P stoichiometry and nutrient resorption in Larix principis-rupprechtii plantations in North China. For. Ecol. Manag. 2020, 462, 117984. [Google Scholar] [CrossRef]
- Hessen, D.O.; Ågren, G.I.; Anderson, T.R.; Elser, J.J.; De Ruiter, P.C. Carbon sequestration in ecosystems: The role of stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar] [CrossRef]
- Qin, J.; Shangguan, Z.P.; Xi, W.M. Seasonal variations of leaf traits and drought adaptation strategies of four common woody species in South Texas, USA. J. For. Res. 2019, 30, 1715–1725. [Google Scholar] [CrossRef]
- Bai, Y.F.; Chen, S.Y.; Shi, S.R.; Qi, M.J.; Liu, X.H.; Wang, H.; Wang, Y.X.; Jiang, C.Q. Effects of different management approaches on the stoichiometric characteristics of soil C, N, and P in a mature Chinese fir plantation. Sci. Total Environ. 2020, 723, 137868. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Yan, Z.B.; Niklas, K.J.; Han, W.X.; Kattge, J.; Reich, P.B.; Luo, Y.K.; Chen, Y.H.; Tang, Z.Y.; Hu, H.F.; et al. Global leaf nitrogen and phosphorus stoichiometry and their scaling exponent. Natl. Sci. Rev. 2018, 5, 728–739. [Google Scholar] [CrossRef]
- Miyajima, R.H.; Barreto, V.C.S.; de Oliveira, P.A.; Batistela, G.C.; Simões, D. Risk Analysis of the Economic Benefits of Ochroma pyramidale: A Case Study of Forest Planting in Brazil. J. Agric. Sci. Tech.-Iran. B 2018, 8, 444–453. [Google Scholar]
- Borrega, M.; Ahvenainen, P.; Serimaa, R.; Gibson, L. Composition and structure of balsa (Ochroma pyramidale) wood. Wood Sci. Technol. 2015, 49, 403–420. [Google Scholar] [CrossRef]
- Malagat, B.; Iamba, K. Seed viability trial of two balsa varieties through direct sowing in East New Britain. Int. J. Agric. For. 2021, 11, 25–31. [Google Scholar]
- Wang, X.K.; Huang, J.L. Principles and Techniques of Plant Physiological and Biochemical Experiments, 3rd ed.; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Guo, D.L.; Mitchell, R.J.; Hendricks, J.J. Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 2004, 140, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Nakashizuka, T. Species coexistence in temperate, mixed deciduous forests. Trends Ecol. Evol. 2001, 16, 205–210. [Google Scholar] [CrossRef]
- Wyckoff, P.H.; Clark, J.S. The relationship between growth and mortality for seven cooccurring tree species in the southern Appalachian Mountains. J. Ecol. 2002, 90, 604–615. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Ourcival, J.M.; Limousin, J.M.; Rambal, S. Mast seeding under increasing drought: Results from a long-term data set and from a rainfall exclusion experiment. Ecology 2010, 91, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Lan, Z.P.; Mi, H.L.; Peng, J.J.; Ma, X.; Qin, X.Y. The growth and photosynthetic characteristics of Cinnamomum longepaniculatum ‘Longnao Xiangzhang’ steckling under different N, P and K level by drip fertilization. J. Nanjing For. Univ. 2019, 43, 39–45. [Google Scholar]
- Richardson, A.D.; Carbone, M.S.; Huggett, B.A.; Furze, M.E.; Czimczik, C.I.; Walker, J.C.; Xu, X.M.; Schaberg, P.G.; Murakami, P. Distribution and mixing of old and new nonstructural carbon in two temperate trees. New Phytol. 2015, 206, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.B.; Yadava, P.S. Seasonal dynamics in soil microbial biomass C, N and P in a mixed-oak forest ecosystem of Manipur, Northeast India. Appl. Soil Ecol. 2006, 31, 220–227. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhao, N.; Liu, C.C.; Yang, H.; Li, M.L. C:N:P stoichiometry in China’s forests: From organs to ecosystems. Funct. Ecol. 2018, 32, 50–60. [Google Scholar] [CrossRef]
- Domingues, T.F.; Meir, P.; Feldpausch, T.R.; Saiz, G.; Veenendaal, E.M.; Schrodt, F.; Bird, M.; Djagbletey, G.; Hien, F.; Compaore, H.; et al. Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant Cell Environ. 2010, 33, 959–980. [Google Scholar] [CrossRef]
- Rowe, E.C.; Smart, S.M.; Kennedy, V.H.; Emmett, B.A.; Evans, C.D. Nitrogen deposition increases the acquisition of phosphorus and potassium by heather Calluna vulgaris. Environ. Pollut. 2008, 155, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from thesoil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Nakaji, T.; Fukami, M.; Dokiya, Y.; Izuta, T. Effects of high nitrogen load on growth, photosynthesis and nutrient status of Cryptomeria japonica and Pinus densiflora seedlings. Trees 2001, 15, 453–461. [Google Scholar] [CrossRef]
- McNulty, S.G.; Boggs, J.; Aber, J.D.; Rustad, L.; Magill, A. Red spruce ecosystem level changes following 14 years of chronic N fertilization. Forest Ecol. Manag. 2005, 219, 279–291. [Google Scholar] [CrossRef]
- Wang, N.; Fu, F.; Wang, B.; Wang, R. Carbon, nitrogen and phosphorus stoichiometry in Pinus tabulaeformis forest ecosystems in warm temperate Shanxi Province, north China. J. For. Res. 2018, 29, 1665–1673. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, G.; Yang, C.; Ou, Z.; Peng, C. Responses of chlorophyll fluorescence and xanthophyll cycle in leaves of Schima superba Gardn. & Champ. and Pinus massoniana Lamb. to simulated acid rain at Dinghushan Biosphere Reserve, China. Acta Physiol. Plant. 2007, 29, 33–38. [Google Scholar]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Millard, P.; Sommerkorn, M.; Grelet, G.A. Environmental change and carbon limitation in trees: A biochemical, ecophysiological and ecosystem appraisal. New Phytol. 2007, 175, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.R.; Lee Allen, H.; Albaugh, T.J.; Rubilar, R.; Carlson, C.A. Tree nutrition and forest fertilization of pine plantations in the southern United States. South. J. Appl. For. 2007, 31, 5–11. [Google Scholar] [CrossRef]
- Wan, H.W.; Yang, Y.; Bai, S.Q.; Xu, Y.H.; Bai, Y.F. Variations in leaf functional traits of six species along a nitrogen addition gradientin leymus chinensis steppeininner mongolia. J. Plant Ecol. 2008, 32, 611–621. [Google Scholar]




| Treatment | Fertiliser Amount/Per Plant/g | Fertilisation Tree/Plant |
|---|---|---|
| CK | 0 | 90 |
| N1 | Urea 300 | 90 |
| N2 | Urea 450 | 90 |
| N3 | Urea 600 | 90 |
| H1 | Slow-release fertiliser 300 | 90 |
| H2 | Slow-release fertiliser 450 | 90 |
| H3 | Slow-release fertiliser 600 | 90 |
| Index | Y1 Principal Component | Y2 Principal Component |
|---|---|---|
| Diameter at breast height | 0.92 | −0.22 |
| Plant height | 0.92 | −0.33 |
| Leaf Soluble carbohydrate | 0.93 | 0.28 |
| Leaf Starch | 0.91 | −0.02 |
| Leaf NSC | 0.94 | 0.22 |
| Leaf Soluble carbohydrate/Starch | 0.85 | 0.51 |
| Leaf C | −0.73 | 0.63 |
| Leaf N | 0.92 | −0.16 |
| Leaf P | −0.95 | −0.06 |
| Leaf C/N | −0.97 | 0.11 |
| Leaf C/P | 0.77 | 0.54 |
| Leaf N/P | 0.93 | −0.15 |
| Eigenvalue variance | 9.66 | 1.29 |
| Contribution rate (%) | 80.51 | 10.71 |
| Cumulative contribution rate (%) | 80.51 | 91.22 |
| Index | Principal Component Y1 Score | Principal Component Y2 Score | Aggregate Score | Ranking |
|---|---|---|---|---|
| CK | −5.36 | −13.77 | −5.79 | 7 |
| N1 | −1.51 | −4.92 | −1.74 | 6 |
| N2 | 1.37 | 2.59 | 1.38 | 3 |
| N3 | 0.78 | 3.47 | 1.00 | 4 |
| H1 | −0.42 | −1.17 | −0.47 | 5 |
| H2 | 2.78 | 6.48 | 2.93 | 1 |
| H3 | 2.36 | 7.33 | 2.69 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Zhao, Z.; Li, Z.; Duan, G.; Wen, C.; Du, G.; Wu, J. Response of Non-Structural Carbohydrates and Carbon, Nitrogen and Phosphorus Stoichiometric Characteristics of Ochroma lagopus Leaves to Nitrogen Addition. Forests 2025, 16, 117. https://doi.org/10.3390/f16010117
Chen G, Zhao Z, Li Z, Duan G, Wen C, Du G, Wu J. Response of Non-Structural Carbohydrates and Carbon, Nitrogen and Phosphorus Stoichiometric Characteristics of Ochroma lagopus Leaves to Nitrogen Addition. Forests. 2025; 16(1):117. https://doi.org/10.3390/f16010117
Chicago/Turabian StyleChen, Gang, Zhijuan Zhao, Zhiqi Li, Guihe Duan, Chengjing Wen, Guanben Du, and Junwen Wu. 2025. "Response of Non-Structural Carbohydrates and Carbon, Nitrogen and Phosphorus Stoichiometric Characteristics of Ochroma lagopus Leaves to Nitrogen Addition" Forests 16, no. 1: 117. https://doi.org/10.3390/f16010117
APA StyleChen, G., Zhao, Z., Li, Z., Duan, G., Wen, C., Du, G., & Wu, J. (2025). Response of Non-Structural Carbohydrates and Carbon, Nitrogen and Phosphorus Stoichiometric Characteristics of Ochroma lagopus Leaves to Nitrogen Addition. Forests, 16(1), 117. https://doi.org/10.3390/f16010117






