Longitudinal Anatomical Variation of Wood in Stem and Branch of Six Forest Species from the Amazon Region and Its Relationship with Wood Specific Gravity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Species Selection
2.3. Collection of Plant Material by Species
2.4. Sample Processing in the Lab
2.4.1. Determination of Wood Specific Gravity in Branch and Core Samples
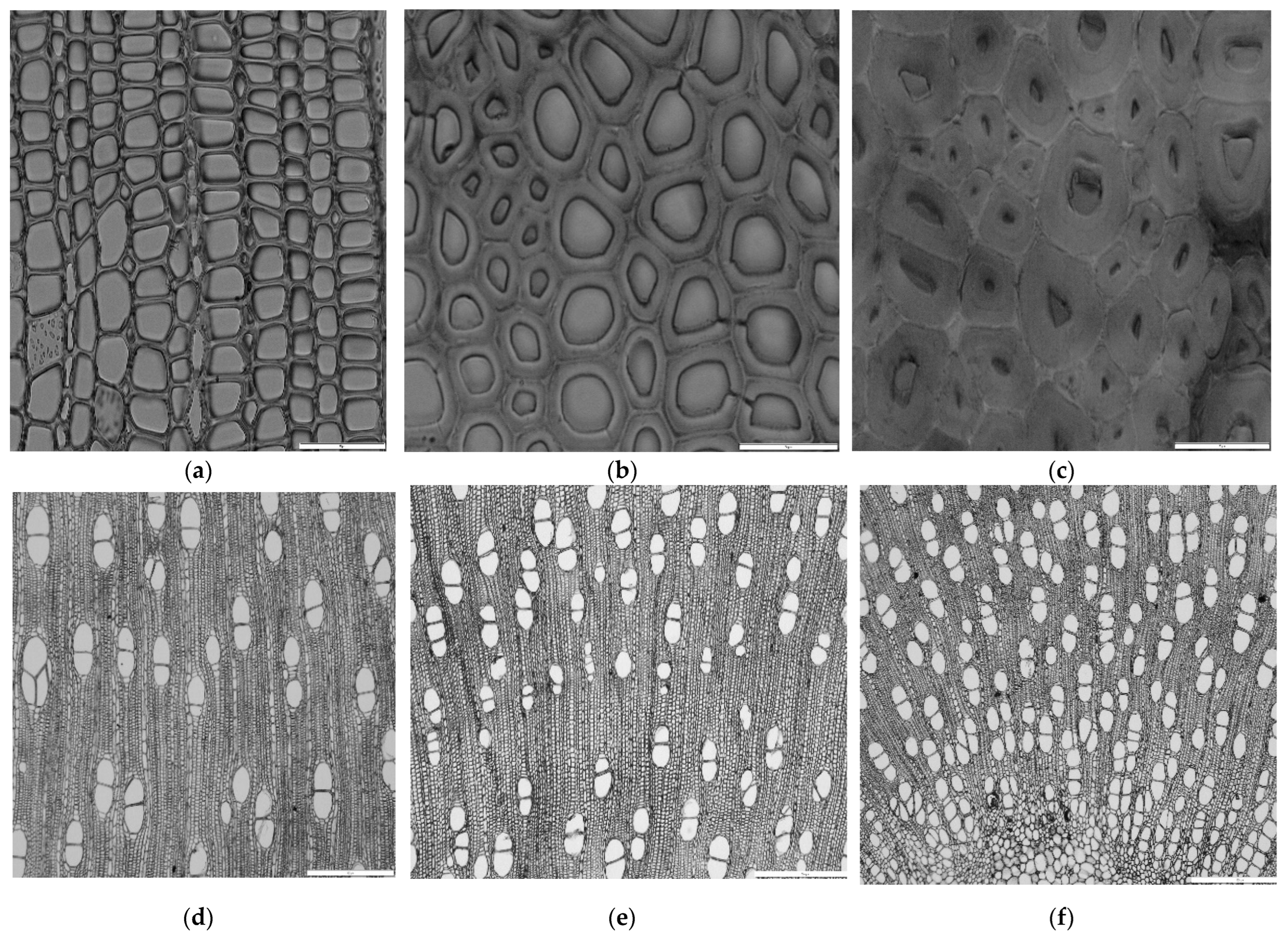
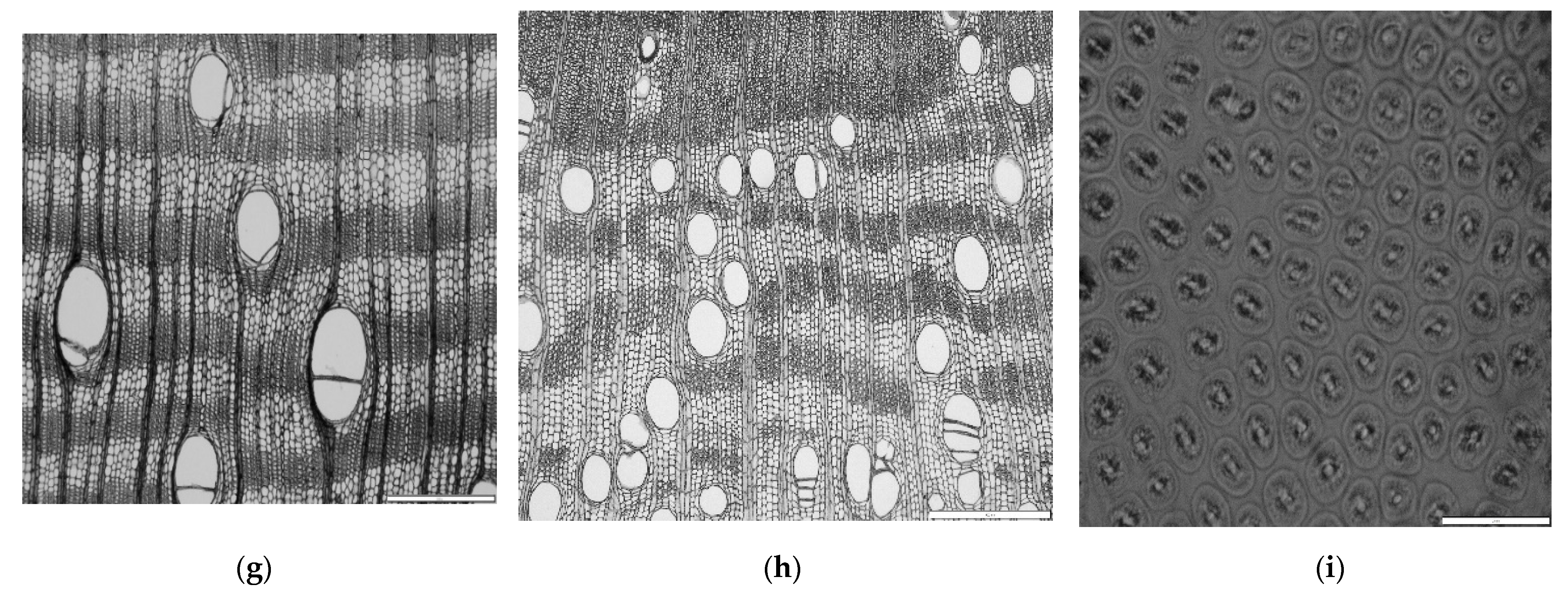
2.4.2. Anatomical Analysis
2.5. Parameter Selection
2.6. Data Analysis
3. Results
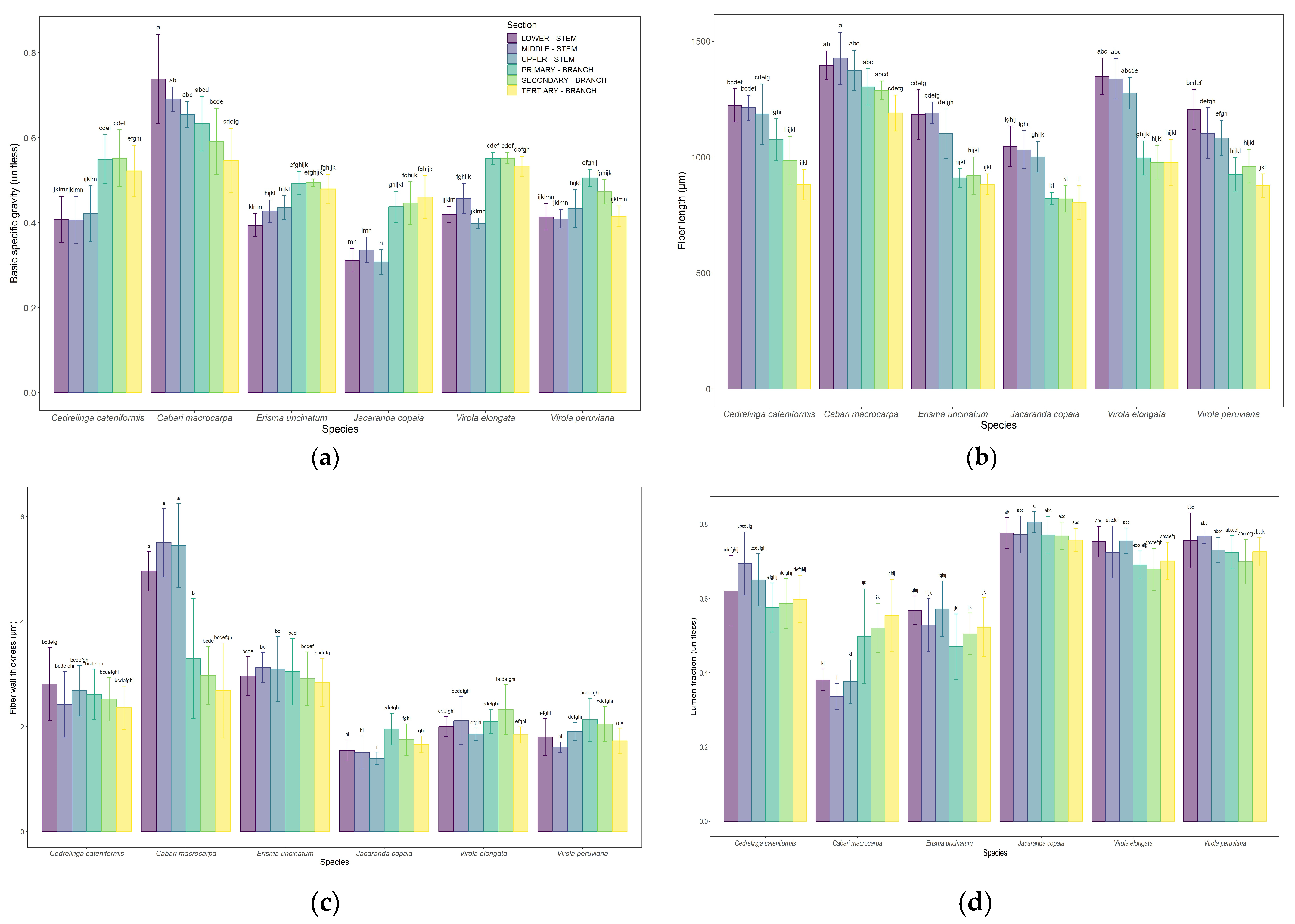
3.1. Longitudinal Variation of the Wood Between the Stem and the Branches
3.2. Stepwise Regression Between Anatomical Variables and Wood Specific Gravity
4. Discussion
4.1. Biomechanical Support: Its Functional Relationship and Use of Forest Species
4.2. Relationship Structure—Hydraulic Function of the Xylem
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Palacios-Juárez, H.; Lomelí-Ramírez, M.G.; Zamora-Natera, J.F. La estructura interna de la madera: Herramienta para manejo y aprovechamiento de los recursos forestales. In Los Recursos Forestales del Occidente de México: Biodiversidad, Manejo, Producción, Aprovechamiento y Conservación. Tomo II; Amaya Ediciones: Guadalajara, México, 2013; pp. 4–15. [Google Scholar]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.; Swenson, N.; Zanne, A. Towards a Worldwide Wood Economics, Spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Ziemińska, K.; Butler, D.W.; Gleason, S.M.; Wright, I.J.; Westoby, M. Fibre wall and lumen fractions drive wood density variation across 24 Australian angiosperms. AoB Plants 2013, 5, plt046. [Google Scholar] [CrossRef]
- Dadzie, P.K.; Amoah, M.; Frimpong-Mensah, K.; Shi, S.Q. Comparison of density and selected microscopic characteristics of stem and branch wood of two commercial trees in Ghana. Wood Sci. Technol. 2016, 50, 91–104. [Google Scholar] [CrossRef]
- Dadzie, P.K.; Amoah, M.; Ebanyenle, E.; Frimpong-Mensah, K. Characterization of density and selected anatomical features of stemwood and branchwood of E. cylindricum, E. angolense and K. ivorensis from natural forests in Ghana. Eur. J. Wood Wood Prod. 2018, 76, 655–667. [Google Scholar] [CrossRef]
- Sarmiento, C.; Patiño, S.; Paine, C.T.; Beauchêne, J.; Thibaut, A.; Baraloto, C. Within-individual variation of trunk and branch xylem density in tropical trees. Am. J. Bot. 2011, 98, 140–149. [Google Scholar] [CrossRef]
- Pulido-Rodríguez, E.; López-Camacho, R.; Torres, J.; Velasco, E.; Salgado-Negret, B. Traits and trade- offs of wood anatomy between trunks and branches in tropical dry forest species. Trees 2020, 34, 497–505. [Google Scholar] [CrossRef]
- Castillo-Figueroa, D.; González-Melo, A.; Posada, J.M. Wood density is related to aboveground biomass and productivity along a successional gradient in upper Andean tropical forests. Front. Plant Sci. 2023, 14, 1276424. [Google Scholar] [CrossRef]
- González-Melo, A. Radial variations in wood density, and their implications for above-ground biomass estimations, in a tropical high-Andean forest. Dendrobiology 2021, 86, 19–28. [Google Scholar] [CrossRef]
- León, W. Anatomía xilemática de fuste y ramas de Theobroma cacao L. (Malvaceae: Byttnerioideae). Ernstia 2015, 25, 1–17. [Google Scholar]
- Tyree, M.T.; Zimmermann, M.H. Xylem Structure and The Ascent of Sap; Springer: Berlin, Germany, 2002. [Google Scholar] [CrossRef]
- Pratt, R.B.; Jacobsen, A.L. Conflicting demands on angiosperm xylem: Tradeoffs among storage, transport and biomechanics. Plant Cell Environ. 2017, 40, 897–913. [Google Scholar] [CrossRef]
- Carlquist, S. Comparative Wood Anatomy: Systematic, Ecological, and Evolutionary Aspects of Dicotyledon Wood; Springer: New York, NY, USA, 2001. [Google Scholar]
- Zimmermann, M.H. Hydraulic architecture of some diffuse-porous trees. Can. J. Bot. 1978, 56, 2286–2295. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Niklas, K.J. Plant Biomechanics: An Engineering Approach to Plant Form and Function; University of Chicago Press: Chicago, IL, USA, 1992. [Google Scholar]
- Sperry, J.S.; Meinzer, F.C.; McCulloh, K.A. Safety and efficiency conflicts in hydraulic architecture: Scaling from tissues to trees. Plant Cell Environ. 2008, 31, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Fournier, M.; Stokes, A.; Coutand, C.; Fourcaud, T.; Moulia, B. Tree biomechanics and growth strategies in the context of forest functional ecology. In Ecology and Biomechanics: A Mechanical Approach to the Ecology of Animals and Plant; Herrel, A., Speck, T., Rowe, N., Eds.; Taylor and Francis Group: Boca Raton, FL, USA, 2006; pp. 2–25. [Google Scholar]
- Williamson, G.B.; Wiemann, M.C. Measuring wood specific gravity… correctly. Am. J. Bot. 2010, 97, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Swenson, N.G.; Enquist, B.J. The relationship between stem and branch wood specific gravity and the ability of each measure to predict leaf are. Am. J. Bot. 2008, 95, 516–519. [Google Scholar] [CrossRef]
- Velásquez, J.; Toro, M.E.; Gómez, L.; Terzo, F.M.; Márquez, A. Patrón de variación axial y radial del peso específico en la madera de Erisma uncinatum Warm. Interciencia 2009, 34, 873–879. [Google Scholar]
- Longui, E.L.; Rajput, K.; Galvão de Melo, A.C.; de Araújo Alves, L.; do Nascimento, C.B. Root to branch wood anatomical variation and its influence on hydraulic conductivity in five Brazilian Cerrado species. Bosque (Valdivia) 2017, 38, 183–193. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the structure and allometry of plant vascular systems. Nature 1999, 400, 664–667. [Google Scholar] [CrossRef]
- Schuldt, B.; Leuschner, C.; Brock, N.; Horna, V. Changes in wood density, wood anatomy and hydraulic properties of the xylem along the root-to-shoot flow path in tropical rainforest trees. Tree Physiol. 2013, 33, 161–174. [Google Scholar] [CrossRef]
- Kotowska, M.M.; Hertel, D.; Rajab, Y.A.; Barus, H.; Schuldt, B. Patterns in hydraulic architecture from roots to branches in six tropical tree species from cacao agroforestry and their relation to wood density and stem growth. Front. Plant Sci. 2015, 6, 191. [Google Scholar] [CrossRef]
- Fortunel, C.; Ruelle, J.; Beauchêne, J.; Fine, P.V.A.; Baraloto, C. Wood specific gravity and anatomy of branches and roots in 113 Amazonian rainforest tree species across environmental gradients. New Phytol. 2014, 202, 79–94. [Google Scholar] [CrossRef] [PubMed]
- González-Melo, A.; Salgado-Negret, B.; Norden, N.; González-M, R.; Benavides, J.P.; Cely, J.M.; Abad Ferrer, J.; Idárraga, Á.; Moreno, E.; Pizano, C.; et al. Linking seedling wood anatomical trade-offs with drought and seedling growth and survival in tropical dry forests. New Phytol. 2024, 245, 117–129. [Google Scholar] [CrossRef] [PubMed]
- López-Camacho, R.; Quintero-Gómez, A.; Amado-Ariza, S. Rasgos funcionales de la madera de tres bosques en Colombia: Bosque Seco, Andino y Alto-Andino. Cienc. Florest. 2020, 30, 856–872. [Google Scholar] [CrossRef]
- Ventanilla Única de Trámites Ambientales (VITAL). MINAMBIENTE (2008–2013). Available online: https://vital-publico.minambiente.gov.co/inicio (accessed on 2 December 2024).
- Blanco, J. Caracterización de las 30 Especies Forestales Maderables más Movilizadas en Colombia Provenientes del Bosque Natural; Organización de las Naciones Unidas para la Alimentación y la Agricultura, Ministerio de Ambiente y Desarrollo Sostenible y Unión Europea: Bogotá, Colombia, 2020. [Google Scholar]
- Montero-González, M.I.; Barrera-García, J.A.; Giraldo Benavides, B.; Lucena-Mancera, A.A. Fichas Técnicas de Especies de uso Forestal y Agroforestal de la Amazonia Colombiana; Instituto Amazónico de Investigaciones Científicas SINCHI: Bogotá, Colombia, 2016. [Google Scholar]
- Moret, A.; Ruiz, P. Determinación de ecuaciones de volumen para Mureillo (Erisma uncinatum) en la unidad C4 de la Reserva Forestal Imataca, Bolívar-Venezuela. Rev. Forest. Venez 1998, 42, 187–197. [Google Scholar]
- Justiniano, M.; Fredericksen, T.S. Ecología y Silvicultura de Especies Menos Conocidas—Cambará Hembra Erisma Uncinatum Warm. Vochysiaceae; Proyecto de Manejo Forestal Sostenible (BOLFOR): Santa Cruz, Bolivia, 1999. [Google Scholar]
- Giraldo Benavides, B.; Oidor Causaya, M.; Ochica Gaitán, P.; Ruiz Fuentes, H. Técnicas Integrales de Viverismo en la Amazonia Colombiana; Instituto Amazónico de Investigaciones Científicas SINCHI: Bogotá, Colombia, 2020. [Google Scholar]
- CONSORCIO IMA-A.M.C.A. Formulación del Plan de Ordenación Forestal del Departamento del Guaviare en el Bloque 1 Integrado por los Bloques Norte a, Norte b, Occidente a y Occidente b, en un Área de 706.846 Hectáreas; de Conformidad con las Normas, Lineamientos Vigentes y los Términos de Referencia; MinAmbiente, CDA, Visión Amazonía: Bogotá, Colombia, 2022. [Google Scholar]
- Peñuela Mora, M.C.; Jiménez Rojas, E.M. Plantas del Centro Experimental Amazónico –CEA– Mocoa, Putumayo; Corporación para el Desarrollo Sostenible del Sur de la Amazonía- Corpoamazonia; Universidad Nacional de Colombia–Sede Amazonía: Leticia, Amazonas, 2010. [Google Scholar]
- Rodríguez León, C.H.; Sterling Cuellar, A. Sucesión Ecológica y Restauración en Paisajes Fragmentados de la Amazonia Colombiana. Tomo 2. Buenas Prácticas para la Restauración de los Bosques; Instituto Amazónico de Investigaciones Científicas SINCHI: Bogotá, Colombia, 2021. [Google Scholar]
- SINCHI; CDA. Experiencia Piloto de Ordenación Forestal de Atapabó; Instituto Amazónico de Investigaciones Científicas SINCHI: Bogotá, Colombia, 2016. [Google Scholar]
- Valderrama-Freyre, H. Plantas de importancia económica y ecológica en el jardín botánico ARBORETUM el Huayo, Iquitos, Perú. Folia Amaz. 2006, 14, 159–175. [Google Scholar] [CrossRef]
- Zárate, R.; Valles-Pérez, L.A.; Maco-García, J.T. Inventario de cumalas (Myristicaceae) en la amazonía peruana. Folia Amaz. 2012, 21, 7–22. [Google Scholar] [CrossRef]
- Ureta Adrianzén, M. Aporte de biomasa aérea de las especies arbóreas de la familia Myristicaceae en los bosques Amazónicos del Perú. Rev. Biol. Trop. 2015, 63, 263–273. [Google Scholar] [CrossRef]
- Sinchi (Instituto Amazónico de Investigaciones Científicas Sinchi, San José del Guaviare, Guaviare, Colombia). Personal communication, 2023.
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Giraldo-Benavides, B.; Zubieta-Vega, M.; Vargas-Ávila, G.; Barrera-García, J.A. Bases Técnicas Para el Desarrollo Forestal en el Departamento del Guaviare, Amazonia Colombiana; Instituto Amazónico de Investigaciones Científicas—Sinchi: Bogotá, Colombia, 2013. [Google Scholar]
- Giraldo-Benavides, B.; Vargas-Ávila, G.; Zubieta-Vega, M.; Barrera-García, J.A.; Montero-González, M.I. Investigación en Sistemas Productivos Sostenibles en la Amazonia Norte Colombiana (Arreglos Agroforestales, Arreglos de Enriquecimiento Forestal); Instituto Amazónico de Investigaciones Científicas—Sinchi: Bogotá, Colombia, 2013. [Google Scholar]
- Chave, J. Measuring Wood Density for Tropical Forest Trees. A Field Manual for the CTFS Sites; Universite Paul Sabatier: Toulouse, France, 2005; pp. 1–7. [Google Scholar]
- Salgado-Negret, B.; Pulido-Rodríguez, E.N.; Cabrera, M.; Ruíz-Osorio, C.; Paz, H. Protocolo para la medición de rasgos funcionales en plantas. In La Ecología Funcional como Aproximación al Estudio, Manejo y Conservación de la Biodiversidad: Protocolos y Aplicaciones; Salgado-Negret, B., Ed.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2015; pp. 37–79. [Google Scholar]
- Nieto-Vargas, J.E. Variación de Rasgos de Madera en Bosque Seco Tropical a Través de un Gradiente de Sequía en Colombia. Master’s Thesis, Universidad Distrital Francisco José de Caldas, Bogotá, Colombia, 2020. [Google Scholar]
- Gärtner, H.; Schweingruber, F.H. Microscopic Preparation Techniques for Plant Stem Analysis; Kessel Publishing House: Remagen, Germany, 2013. [Google Scholar]
- Tardif, J.C.; Conciatori, F. Microscopic Examination of Wood: Sample Preparation and Techniques for Light Microscopy. In Plant Microtechniques and Protocols; Yeung, E., Stasolla, C., Sumner, M., Huang, B., Eds.; Springer: Cham, Switzerland, 2015; pp. 373–415. [Google Scholar] [CrossRef]
- Polanco-Tapia, C.; Grande-Pulido, D.C. Análisis ecoanatómico, evolutivo y comparativo de la madera de 40 especies de dos asociaciones del Bosques altoandino colombiano. Colomb. For. 2009, 12, 183–203. [Google Scholar] [CrossRef][Green Version]
- Jansen, S.; Kitin, P.; De Pauw, H.; Idris, M.; Beeckman, H.; Smets, E. Preparation of wood specimens for transmitted light microscopy and scanning electron microscopy. Belg. J. Bot. 1998, 131, 41–49. [Google Scholar]
- Rasband, W.S. ImageJ; National Institutes of Health: Bethesda, MD, USA, 1997–2018. Available online: https://imagej.net/ij/ (accessed on 31 October 2023).
- Scholz, A.; Klepsch, M.; Karimi, Z.; Jansen, S.; Stephan, D.; Torres-ruiz, J.M. How to quantify conduits in wood? Front. Plant Sci. 2013, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Deklerck, V.; Price, E.; Vanden Abeele, S.; Lievens, K.; Espinoza, E.; Beeckman, H. Timber identification of Autranella, Baillonella and Tieghemella in the taxonomically challenging Sapotaceae family. Plant Methods 2021, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- IAWA Committee. IAWA list of microscopic features for hardwood identification. IAWA Bull. 1989, 10, 219–332. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020; Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2020; Available online: https://www.infostat.com.ar/ (accessed on 31 March 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 31 March 2024).
- Commercial Timbers: Descriptions, Illustrations, Identification, and Information Retrieval. Available online: https://www.delta-intkey.com/wood/index.htm (accessed on 8 July 2023).
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Data from: Towards a Worldwide Wood Economics Spectrum [Dataset]. Dryad. 2009. Available online: https://datadryad.org/stash/dataset/doi:10.5061/dryad.234 (accessed on 28 June 2024).
- The InsideWood Database. Available online: https://insidewood.lib.ncsu.edu/search?1 (accessed on 28 June 2024).
- Okai, R.; Frimpong-Mensah, K.; Yeboah, D. Characterization of moisture content and specific gravity of branchwood and stemwood of Aningeria robusta and Terminalia ivorensis. Holz Als Roh-Werkstof 2003, 61, 155–158. [Google Scholar] [CrossRef]
- MacFarlane, D.W. Functional Relationships Between Branch and Stem Wood Density for Temperate Tree Species in North America. Front. For. Glob. Chang. 2020, 3, 63. [Google Scholar] [CrossRef]
- Bhat, K.M.; Bhat, K.V.; Dhamodaran, T.K. Fibre Length Variation in Stem and Branches of Eleven Tropical Hardwoods. IAWA Journa 1989, 10, 63–70. [Google Scholar] [CrossRef]
- Longui, E.L.; de Lima, I.L.; Florsheim, S.M.B. Root-branch anatomical investigation of Eriotheca gracilipes young trees: A biomechanical and ecological approach. Sci. For. 2012, 40, 23–33. [Google Scholar]
- Yaman, B. Anatomical differences between stem and branch wood of Ficus carica L. subsp. carica. Mod. Phytomorphology 2014, 6, 79–83. [Google Scholar]
- Longui, E.L.; Galão, A.T.D.; Rajput, K.S.; De Melo, A.C.G. Anatomical investigation of root, stem and branch wood in 10-year-old Inga laurina in the context of anatomical adaptation to hydraulic and mechanical stresses. An. Biol. 2018, 40, 31–39. [Google Scholar] [CrossRef]
- León, W. Estudio anatómico de la madera e incidencias tecnológicas en 7 especies del género Protium Burm. F. (Burseraceae). Rev. Forest. Venez. 2002, 46, 73–82. [Google Scholar]
- León, W. Anatomía y densidad o peso específico de la madera. Rev. For. Venez. 2010, 54, 67–76. [Google Scholar]
- Salgado-Negret, B.; Pérez, F.; Markesteijn, L.; Castillo, M.J.; Armesto, J. Diverging drought-tolerance strategies explain tree species distribution along a fog-dependent moisture gradient in a temperate rain forest. Oecologia 2013, 173, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Brodie, T.W.; Cobb, A.R.; Zwieniecki, M.A.; Holbrook, N.M. Direct measurements of intervessel pit membrane hydraulic resistance in two angiosperm tree species. Am. J. Bot. 2006, 93, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Choat, B.; Pletsers, A. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am. J. Bot. 2009, 96, 409–419. [Google Scholar] [CrossRef]
- Jansen, S.; Baas, P.; Gasson, P.; Smets, E. Vestured Pits: Do They Promote Safer Water Transport? Int. J. Plant Sci. 2003, 164, 405–413. [Google Scholar] [CrossRef]
- Pfautsch, S.; Hölttä, T.; Mencuccini, M. Hydraulic functioning of tree stems—Fusing ray anatomy, radial transfer and capacitance. Tree Physiol. 2015, 35, 706–722. [Google Scholar] [CrossRef]

| Species | Common Name | DBH (cm) | Total Height (m) | Commercial Height (m) | Diameter Crown Axis X (m) | Diameter Crown Axis Y (m) |
|---|---|---|---|---|---|---|
| Jacaranda copaia | Pavito | 37.6 | 21.5 | 9.9 | 8.1 | 8.1 |
| Virola elongata | Sangretoro | 29.9 | 22.3 | 11.3 | 9.8 | 9.6 |
| Virola peruviana | Sangretoro | 31.2 | 18.8 | 14.4 | 7.6 | 5.8 |
| Cedrelinga cateniformis | Achapo | 39.0 | 24.0 | 9.9 | 11.1 | 8.2 |
| Erisma uncinatum | Milpo | 39.2 | 21.6 | 13.6 | 7.2 | 5.5 |
| Cabari macrocarpa | Fariñero | 34.5 | 16.9 | 7.0 | 9.4 | 9.6 |
| Functional Classification | Parameter | Abbreviation | N° of Measurements | Unit | Camera Zoom | Section |
|---|---|---|---|---|---|---|
| Biomechanical support | Wood basic specific gravity | SG | 1 | Unitless | - | - |
| Fiber length | Fl | 50 | µm | 4× | Maceration | |
| Fiber wall thickness * | Fwt | 100 | µm | 100× | Transverse | |
| Lumen fraction | Lf | 100 | Unitless | - | Transverse | |
| Conductive function | Vessel density | Vde | 5 | N°/mm2 | 4× | Transverse |
| Vessel diameter * | Vdi | 100 | µm | 4× | Transverse | |
| Theoretical hydraulic conductivity | Kh | 100 | m4/MPa−1 × s−1 | - | - | |
| Intervessel pit diameter * | Ip | 50 | µm | 100× | Tangential | |
| Storage | Ray height | Rh | 50 | µm | 4× | Tangential |
| Ray width | Rw | 50 | µm | 4× | Tangential |
| Species | Section | SG * | Fl | Fwt | Lf | Vde | Vdi | Kh | Ip | Rh | Rw |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jacaranda copaia | Lower stem | 0.31 | 1046.28 | 1.55 | 0.78 | 2.20 | 183.36 | 3.60 × 10−6 | 5.31 | 452.77 | 47.63 |
| Middle stem | 0.34 | 1031.12 | 1.51 | 0.77 | 2.84 | 192.96 | 4.70 × 10−6 | 6.50 | 382.42 | 44.97 | |
| Upper stem | 0.31 | 1001.32 | 1.40 | 0.81 | 3.84 | 185.17 | 4.20 × 10−6 | 6.42 | 345.63 | 48.84 | |
| Primary branch | 0.44 | 821.15 | 1.96 | 0.77 | 3.92 | 140.29 | 1.40 × 10−6 | 6.35 | 271.93 | 41.80 | |
| Secondary branch | 0.45 | 819.57 | 1.75 | 0.77 | 5.84 | 118.85 | 1.10 × 10−6 | 5.94 | 265.37 | 45.23 | |
| Tertiary branch | 0.46 | 803.59 | 1.66 | 0.76 | 8.60 | 109.04 | 5.90 × 10−7 | 6.35 | 241.31 | 32.03 | |
| Cedrelinga cateniformis | Lower stem | 0.41 | 1222.96 | 2.81 | 0.62 | 2.52 | 274.45 | 1.70 × 10−5 | 7.00 | 161.19 | 17.86 |
| Middle stem | 0.41 | 1212.52 | 2.43 | 0.69 | 2.08 | 292.40 | 2.30 × 10−5 | 7.04 | 164.84 | 16.26 | |
| Upper stem | 0.42 | 1185.13 | 2.69 | 0.65 | 2.56 | 297.05 | 2.40 × 10−5 | 7.17 | 171.05 | 17.89 | |
| Primary branch | 0.55 | 1074.47 | 2.62 | 0.58 | 6.04 | 175.77 | 4.00 × 10−6 | 6.17 | 127.28 | 14.59 | |
| Secondary branch | 0.55 | 984.37 | 2.52 | 0.59 | 6.64 | 152.55 | 1.90 × 10−6 | 5.49 | 112.17 | 13.15 | |
| Tertiary branch | 0.52 | 881.11 | 2.36 | 0.60 | 14.00 | 108.94 | 7.00 × 10−7 | 5.03 | 119.22 | 14.62 | |
| Virola peruviana | Lower stem | 0.41 | 1204.09 | 1.80 | 0.76 | 16.64 | 124.42 | 6.90 × 10−7 | 5.75 | 510.45 | 33.50 |
| Middle stem | 0.41 | 1102.88 | 1.61 | 0.77 | 16.52 | 129.57 | 8.20 × 10−7 | 5.52 | 422.91 | 32.28 | |
| Upper stem | 0.43 | 1082.36 | 1.91 | 0.73 | 20.40 | 122.81 | 6.40 × 10−7 | 5.65 | 383.01 | 32.77 | |
| Primary branch | 0.51 | 925.34 | 2.12 | 0.72 | 17.56 | 99.00 | 2.80 × 10−7 | 4.50 | 354.43 | 28.62 | |
| Secondary branch | 0.47 | 960.17 | 2.05 | 0.70 | 31.00 | 80.17 | 1.20 × 10−7 | 4.88 | 423.24 | 21.28 | |
| Tertiary branch | 0.42 | 876.34 | 1.73 | 0.73 | 35.24 | 63.46 | 4.80 × 10−8 | 4.64 | 351.24 | 16.11 | |
| Virola elongata | Lower stem | 0.42 | 1348.02 | 2.00 | 0.75 | 11.68 | 110.83 | 3.30 × 10−7 | 6.47 | 485.53 | 28.41 |
| Middle stem | 0.46 | 1337.62 | 2.12 | 0.72 | 14.68 | 107.60 | 4.50 × 10−7 | 5.75 | 434.32 | 27.06 | |
| Upper stem | 0.40 | 1276.09 | 1.85 | 0.75 | 12.36 | 108.95 | 4.40 × 10−7 | 6.00 | 444.01 | 27.61 | |
| Primary branch | 0.55 | 996.25 | 2.10 | 0.69 | 18.32 | 85.94 | 1.60 × 10−7 | 5.36 | 342.34 | 22.67 | |
| Secondary branch | 0.55 | 977.94 | 2.32 | 0.68 | 24.52 | 81.50 | 1.40 × 10−7 | 4.82 | 305.05 | 21.44 | |
| Tertiary branch | 0.53 | 977.16 | 1.85 | 0.70 | 28.60 | 65.16 | 5.60 × 10−8 | 4.74 | 327.56 | 17.77 | |
| Erisma uncinatum | Lower stem | 0.39 | 1182.86 | 2.97 | 0.57 | 3.24 | 173.26 | 3.00 × 10−6 | 8.89 | 391.72 | 33.90 |
| Middle stem | 0.43 | 1190.30 | 3.13 | 0.53 | 3.92 | 205.36 | 6.80 × 10−6 | 8.70 | 363.13 | 33.95 | |
| Upper stem | 0.44 | 1100.32 | 3.10 | 0.57 | 3.48 | 206.96 | 5.70 × 10−6 | 8.89 | 346.72 | 39.24 | |
| Primary branch | 0.49 | 910.05 | 3.05 | 0.47 | 5.16 | 151.53 | 2.00 × 10−6 | 8.46 | 273.81 | 36.30 | |
| Secondary branch | 0.49 | 919.77 | 2.91 | 0.51 | 7.64 | 119.63 | 7.40 × 10−7 | 4.94 | 317.45 | 32.16 | |
| Tertiary branch | 0.48 | 882.74 | 2.84 | 0.52 | 9.80 | 128.91 | 1.10 × 10−6 | 7.39 | 230.80 | 33.26 | |
| Cabari macrocarpa | Lower stem | 0.74 | 1395.42 | 4.96 | 0.38 | 6.00 | 127.50 | 8.30 × 10−7 | 7.42 | 466.20 | 51.81 |
| Middle stem | 0.69 | 1426.58 | 5.50 | 0.34 | 4.72 | 126.04 | 7.30 × 10−7 | 7.07 | 463.24 | 52.22 | |
| Upper stem | 0.66 | 1374.43 | 5.45 | 0.38 | 4.52 | 133.70 | 1.00 × 10−6 | 7.94 | 481.23 | 46.21 | |
| Primary branch | 0.63 | 1302.60 | 3.30 | 0.50 | 7.56 | 129.70 | 1.10 × 10−6 | 6.05 | 329.63 | 33.58 | |
| Secondary branch | 0.59 | 1288.23 | 2.98 | 0.52 | 7.27 | 117.19 | 6.80 × 10−7 | 4.95 | 347.77 | 31.34 | |
| Tertiary branch | 0.55 | 1189.80 | 2.69 | 0.55 | 11.24 | 98.54 | 3.00 × 10−7 | 5.83 | 282.16 | 37.87 | |
| Standard error | 0.02 | 35.50 | 0.21 | 0.03 | 1.62 | 9.52 | 1.10 × 10−6 | 0.33 | 23.67 | 2.69 |
| Predictor Variable | Coef. | S.E. | T | p-Value | LC (95%) | UC (95%) | N | R2 | M.S.E. |
|---|---|---|---|---|---|---|---|---|---|
| Stem | |||||||||
| Intercept | 0.0200 | 0.0600 | 0.41 | 0.6808 | −0.0900 | 0.1400 | 90 | 0.82 | 0.003 |
| Fiber length | 0.0001 | 0.0001 | 2.50 | 0.0142 | 0.0000 | 0.0002 | |||
| Fiber wall thickness | 0.0700 | 0.0100 | 11.69 | <0.0001 | 0.0600 | 0.0900 | |||
| Vessel density | 0.0045 | 0.0010 | 4.56 | <0.0001 | 0.0025 | 0.0100 | |||
| Ray width | 0.0010 | 0.0005 | 1.99 | 0.0498 | 0.0001 | 0.0021 | |||
| Branch | |||||||||
| Intercept | 0.3200 | 0.0400 | 8.24 | <0.0001 | 0.2500 | 0.4000 | 90 | 0.52 | 0.002 |
| Fiber length | 0.0003 | 0.0000 | 6.53 | <0.0001 | 0.0002 | 0.0003 | |||
| Fiber wall thickness | 0.0300 | 0.0100 | 3.35 | 0.0012 | 0.0100 | 0.0500 | |||
| Vessel diameter | −0.0006 | 0.0002 | −3.09 | 0.0027 | −0.0009 | −0.0002 | |||
| Ray height | −0.0002 | 0.0001 | −3.64 | 0.0005 | −0.0004 | −0.0001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Guevara, C.; Pulido-Rodríguez, N.; Giraldo Benavides, B.; Barrera García, J. Longitudinal Anatomical Variation of Wood in Stem and Branch of Six Forest Species from the Amazon Region and Its Relationship with Wood Specific Gravity. Forests 2025, 16, 33. https://doi.org/10.3390/f16010033
Martínez-Guevara C, Pulido-Rodríguez N, Giraldo Benavides B, Barrera García J. Longitudinal Anatomical Variation of Wood in Stem and Branch of Six Forest Species from the Amazon Region and Its Relationship with Wood Specific Gravity. Forests. 2025; 16(1):33. https://doi.org/10.3390/f16010033
Chicago/Turabian StyleMartínez-Guevara, Carolina, Nancy Pulido-Rodríguez, Bernardo Giraldo Benavides, and Jaime Barrera García. 2025. "Longitudinal Anatomical Variation of Wood in Stem and Branch of Six Forest Species from the Amazon Region and Its Relationship with Wood Specific Gravity" Forests 16, no. 1: 33. https://doi.org/10.3390/f16010033
APA StyleMartínez-Guevara, C., Pulido-Rodríguez, N., Giraldo Benavides, B., & Barrera García, J. (2025). Longitudinal Anatomical Variation of Wood in Stem and Branch of Six Forest Species from the Amazon Region and Its Relationship with Wood Specific Gravity. Forests, 16(1), 33. https://doi.org/10.3390/f16010033








