Catalytic Pyrolysis Characteristics of Potassium Chloride on Ash Branch Wood and Its Kinetic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Material
2.2. Experiment Method
2.2.1. Pretreatment
2.2.2. Thermogravimetric Analysis
2.2.3. Kinetic Analysis
2.2.4. Pyrolysis Experiment
2.2.5. Characterization of Biochar
3. Results and Discussion
3.1. Effect of KCl on the Thermal Degradation
3.2. Kinetic Analysis of Pyrolysis of KCl Impregnated Materials
3.3. Pyrolysis Product Distribution
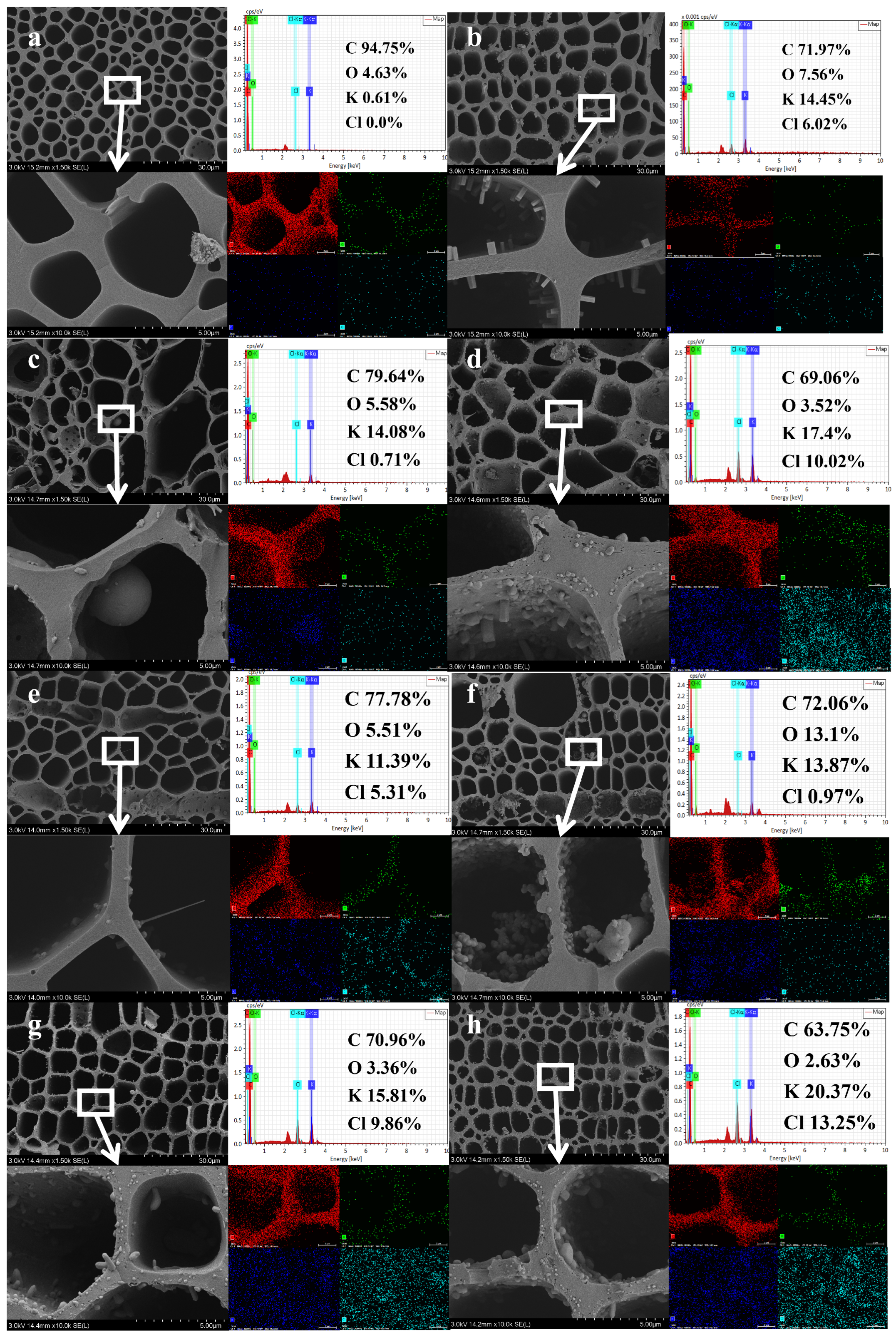
3.4. Morphology and Potassium Element Surface Distribution on Biochar
3.5. Pore Structure Analysis of Biochar
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Li, X.; Wang, B.; Chang, F.; Wei, W.; Zhang, G.; Wan, K. Simultaneous activation treatment of garden waste pyrolysis and adsorption performance of derived charcoal on SO2. Environ. Eng. 2024. Available online: https://link.cnki.net/urlid/11.2097.X.20240815.1547.010 (accessed on 28 December 2024).
- Yang, H.-T.; Qi, W.-J.; Gao, C.-Q.; Yu, L.-X.; Peng, Z.-D. Nutrient release characteristics of landscaping waste organic fertilizer and the effects on Pinus tabulaeformis growth and soil fertility. J. Plant Nutr. Fertil. 2024, 30, 1173–1184. [Google Scholar]
- Nuryawan, A.; Syahputra, R.; Azhar, I.; Risnasari, I. Basic properties of the mangrove tree branches as a raw material of wood pellets and briquettes. IOP Conf. Ser. Earth Environ. Sci. 2021, 891, 012005. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Wojciechowska, N.; Seliwiak, M.; Dobrzański, T.K. Properties of Forest Tree Branches as an Energy Feedstock in North-Eastern Poland. Energies 2024, 17, 1975. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Lee, C.L.; Tan, W.P.; Peng, W.; Sonne, C.; Tsang, Y.F.; Lam, S.S. Strategic hazard mitigation of waste furniture boards via pyrolysis: Pyrolysis behavior, mechanisms, and value-added products. J. Hazard. Mater. 2022, 421, 126774. [Google Scholar] [CrossRef]
- Yıldız, Z.; Ceylan, S. Pyrolysis of tobacco factory waste biomass: TG-FTIR analysis, kinetic study and bio-oil characterization. J. Therm. Anal. Calorim. 2019, 136, 783–794. [Google Scholar] [CrossRef]
- Cerda-Barrera, C.; Fernández-Andrade, K.J.; Alejandro-Martín, S. Pyrolysis of Chilean Southern Lignocellulosic Biomasses: Isoconversional Kinetics Analysis and Pyrolytic Products Distribution. Polymers 2023, 15, 2698. [Google Scholar] [CrossRef]
- Memon, T.A.; Ku, X.; Vasudev, V. Co-Pyrolysis of Peanut Shells and Tea Plant Branches: Physicochemical Properties, Synergistic Effect and Thermo-Kinetic Analyses. BioEnergy Res. 2024, 17, 1805–1815. [Google Scholar] [CrossRef]
- Rollag, S.A.; Jeong, K.; Peterson, C.A.; Kim, K.H.; Brown, R.C. An experimental and modeling study on the catalytic effects of select metals on the fast pyrolysis of hardwood and softwood lignin. Green Chem. 2022, 24, 6189–6199. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Thermogravimetric Analysis and Kinetic Modeling of the AAEM-Catalyzed Pyrolysis of Woody Biomass. Molecules 2022, 27, 7662. [Google Scholar] [CrossRef]
- Wang, W.L.; Ren, X.Y.; Li, L.F.; Chang, J.M.; Cai, L.P.; Geng, J. Catalytic effect of metal chlorides on analytical pyrolysis of alkali lignin. Fuel Process. Technol. 2015, 134, 345–351. [Google Scholar] [CrossRef]
- Müller-Hagedorn, M.; Bockhorn, H.; Krebs, L.; Müller, U. A comparative kinetic study on the pyrolysis of three different wood species. J. Anal. Appl. Pyrolysis 2003, 68, 231–249. [Google Scholar] [CrossRef]
- Wei, J.; Wang, M.; Zhang, L.; Li, B.; Jiang, Y.; Song, X.; Yu, G.; Zhang, J.; Zhang, S.; Xu, D. Investigation on the correlation between AAEMs transformation and reactivity synergy during the co-combustion of biochar and coal char. Renew. Energy 2024, 223, 120104. [Google Scholar] [CrossRef]
- Mašek, O.; Buss, W.; Brownsort, P.; Rovere, M.; Tagliaferro, A.; Zhao, L.; Cao, X.; Xu, G. Potassium doping increases biochar carbon sequestration potential by 45%, facilitating decoupling of carbon sequestration from soil improvement. Sci. Rep. 2019, 9, 5514. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Z.; Zhang, Y.; Liu, B.; Yang, Y.; Tang, Z.; Chen, Y.; Chen, H. Catalytic effect of K and Na with different anions on lignocellulosic biomass pyrolysis. Front. Chem. Sci. Eng. 2024, 18, 141. [Google Scholar] [CrossRef]
- Chen, W.; Tao, X.; Shi, X.; Guo, W.; Wang, Y.; Liu, B.; Yang, H. Insight into catalytic effects of alkali metal salts addition on bamboo and cellulose pyrolysis. npj Mater. Sustain. 2024, 2, 25. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Z.; Xia, S.; Lu, Q.; Walters, K.B. Catalytic Pyrolysis of Biomass and Polymer Wastes. Catalysts 2018, 8, 659. [Google Scholar] [CrossRef]
- Nuryawan, A.; Syahputra, R.S.; Risnasari, I. Physical properties of five species of twigs from mangrove forest. IOP Conf. Ser. Earth Environ. Sci. 2023, 1241, 012113. [Google Scholar] [CrossRef]
- Mandapati, R.N.; Ghodke, P.K. Kinetics of pyrolysis of cotton stalk using model-fitting and model-free methods. Fuel 2021, 303, 121285. [Google Scholar] [CrossRef]
- Qi, C.; Yadama, V.; Guo, K.; Wolcott, M.P. Thermal stability evaluation of sweet sorghum fiber and degradation simulation during hot pressing of sweet sorghum–thermoplastic composite panels. Ind. Crops Prod. 2015, 69, 335–343. [Google Scholar] [CrossRef]
- Qi, C. Fabrication of Orinted Biomass-High Density Polyethytlene Composites Using Hot Pressing Process and Its Molding Mechanism. Ph.D. Thesis, Northwest A&F University, Xianyang, China, 2013. [Google Scholar]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dou, B.; Zhang, H.; Zhang, H.; Chen, H.; Xu, Y.; Wu, C. Pyrolysis characteristics and non-isothermal kinetics of waste wood biomass. Energy 2021, 226, 120358. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Chen, K.; Wang, T.; Mei, M.; Li, J. Preparation of biochar from food waste digestate: Pyrolysis behavior and product properties. Bioresour. Technol. 2020, 302, 122841. [Google Scholar] [CrossRef]
- Shen, T.; Zhang, F.; Yang, S.; Wang, Y.; Liu, H.; Wang, H.; Hu, J. Comprehensive study on the pyrolysis process of chestnut processing waste (chestnut shells): Kinetic triplet, thermodynamic, in-situ monitoring of evolved gasses and analysis biochar. Fuel 2023, 331, 125944. [Google Scholar] [CrossRef]
- Selvarajoo, A.; Wong, Y.L.; Khoo, K.S.; Chen, W.H.; Show, P.L. Biochar production via pyrolysis of citrus peel fruit waste as a potential usage as solid biofuel. Chemosphere 2022, 294, 133671. [Google Scholar] [CrossRef]
- Chen, D.; Liu, R. Study on the pyrolysis kinetics of pre-treated cotton stalk. Trans. CSAE 2007, 38, 95–99. [Google Scholar]
- Jiang, H.; Song, L.; Cheng, Z.; Chen, J.; Zhang, L.; Zhang, M.; Hu, M.; Li, J.; Li, J. Influence of pyrolysis condition and transition metal salt on the product yield and characterization via Huadian oil shale pyrolysis. J. Anal. Appl. Pyrolysis 2015, 112, 230–236. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Analysis of the Catalytic Effects Induced by Alkali and Alkaline Earth Metals (AAEMs) on the Pyrolysis of Beech Wood and Corncob. Catalysts 2022, 12, 1505. [Google Scholar] [CrossRef]
- Mallick, D.; Sharma, P.; Bora, B.J.; Baruah, D.; Bhowmik, R.; Barbhuiya, S.A.; Balakrishnan, D. Mechanistic investigation of pyrolysis kinetics of water hyacinth for biofuel employing isoconversional method. Sustain. Energy Technol. Assess. 2023, 57, 103175. [Google Scholar] [CrossRef]
- Wang, X. Study on Pyrolysis Kinetics of Biomass. Master’s Thesis, Anhui University of Science & Technology, Huainan, China, 2006. [Google Scholar]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Fan, H.; Gu, J.; Wang, Y.; Yuan, H.; Chen, Y.; Luo, B. Effect of potassium on the pyrolysis of biomass components: Pyrolysis behaviors, product distribution and kinetic characteristics. Waste Manag. 2021, 121, 255–264. [Google Scholar] [CrossRef]
- Li, A.; Deng, H.; Jiang, Y.; Ye, C. High-efficiency removal of Cr (VI) from wastewater by Mg-loaded biochars: Adsorption process and removal mechanism. Materials 2020, 13, 947. [Google Scholar] [CrossRef]
- Yu, Z. Experimental Study on Occurrence Forms and Transformation of Alkali Metal K During Biomass Pyrolysis. Master’s Thesis, Huazhong University of Science & Technology, Wuhan, China, 2015. [Google Scholar]
- Xie, L.; Wang, L.; Zhou, J.; Ma, H. Co-Pyrolysis for pine sawdust with potassium chloride: Insight into interactions and assisting biochar graphitization. Materials 2023, 16, 3667. [Google Scholar] [CrossRef]
- Broström, M.; Enestam, S.; Backman, R.; Mäkelä, K. Condensation in the KCl–NaCl system. Fuel Process. Technol. 2013, 105, 142–148. [Google Scholar] [CrossRef]
- Jensen, P.A.; Frandsen, F.J.; Dam-Johansen, K.; Sander, B. Experimental Investigation of the Transformation and Release to Gas Phase of Potassium and Chlorine during Straw Pyrolysis. Energy Fuels 2000, 14, 1280–1285. [Google Scholar] [CrossRef]
- Raymundo-Pinero, E.; Azaïs, P.; Cacciaguerra, T.; Cazorla-Amorós, D.; Linares-Solano, A.; Béguin, F. KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation. Carbon 2005, 43, 786–795. [Google Scholar] [CrossRef]
- Arafat Hossain, M.; Ganesan, P.; Jewaratnam, J.; Chinna, K. Optimization of process parameters for microwave pyrolysis of oil palm fiber (OPF) for hydrogen and biochar production. Energy Convers. Manag. 2017, 133, 349–362. [Google Scholar] [CrossRef]


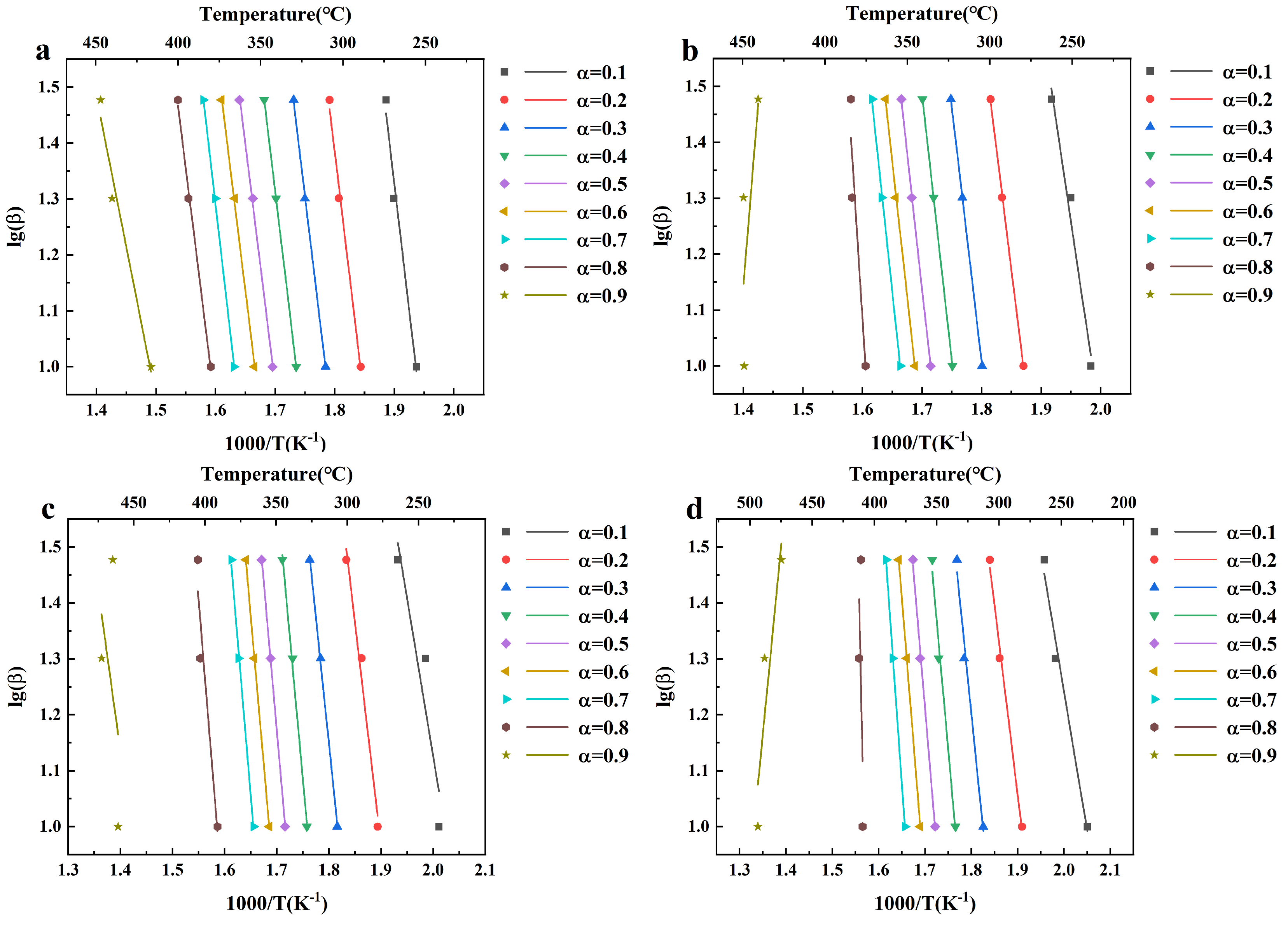


| ABW | ABW-5% | ABW-10% | ABW-15% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E (kJ·mol−1) | A (s−1) | R2 | E (kJ·mol−1) | A (s−1) | R2 | E (kJ·mol−1) | A (s−1) | R2 | E (kJ·mol−1) | A (s−1) | R2 | |
| 0.2 | 150.17 | 4.11 | 0.9997 | 143.90 | 1.64 | 0.9963 | 102.99 | 3.18 | 0.9618 | 123.24 | 2.58 | 0.9890 |
| 0.3 | 155.56 | 6.99 | 0.9998 | 151.88 | 4.34 | 0.9918 | 144.81 | 1.05 | 0.9984 | 149.02 | 3.55 | 0.9755 |
| 0.4 | 159.34 | 8.53 | 0.9996 | 158.82 | 1.06 | 0.9916 | 164.91 | 4.41 | 0.9939 | 168.83 | 1.21 | 0.9778 |
| 0.5 | 163.33 | 1.19 | 0.9997 | 162.88 | 1.62 | 0.9960 | 186.08 | 1.02 | 0.9995 | 179.49 | 6.22 | 0.9953 |
| 0.6 | 167.93 | 2.14 | 0.9999 | 160.51 | 9.74 | 0.9957 | 193.29 | 6.26 | 0.9994 | 187.12 | 1.96 | 1.0000 |
| 0.7 | 186.02 | 5.06 | 0.9924 | 162.82 | 3.26 | 0.9949 | 193.19 | 2.07 | 0.9955 | 211.95 | 1.89 | 0.9984 |
| Name of Sample | SBET (m2/g) | VBJH (cm3/g) | Dap (nm) |
|---|---|---|---|
| ABW | 0.8 | 0.00258 | 1.5 |
| Biochar 600−3 | 2.4 | 0.00677 | 12.6 |
| Biochar 600−3−5 | 4.2 | 0.00914 | 10.5 |
| Biochar 600−3−10 | 2.5 | 0.00693 | 29.5 |
| Biochar 600−3−15 | 2.3 | 0.00508 | 12.0 |
| Biochar 450−3−10 | 1.9 | 0.00569 | 23.4 |
| Biochar 750−3−10 | 5.5 | 0.00961 | 29.7 |
| Biochar 600−2−10 | 2.8 | 0.00626 | 15.6 |
| Biochar 600−4−10 | 2.7 | 0.00617 | 12.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Gao, J.; Wang, T.; Hao, T.; Lu, Y.; Hu, Y.; Wang, X.; He, Z.; Wang, Z.; Yi, S. Catalytic Pyrolysis Characteristics of Potassium Chloride on Ash Branch Wood and Its Kinetic Study. Forests 2025, 16, 57. https://doi.org/10.3390/f16010057
Zhang L, Gao J, Wang T, Hao T, Lu Y, Hu Y, Wang X, He Z, Wang Z, Yi S. Catalytic Pyrolysis Characteristics of Potassium Chloride on Ash Branch Wood and Its Kinetic Study. Forests. 2025; 16(1):57. https://doi.org/10.3390/f16010057
Chicago/Turabian StyleZhang, Lanxin, Jingjing Gao, Tinghuan Wang, Tengfei Hao, Yizhi Lu, Yurong Hu, Xiaoxu Wang, Zhengbin He, Zhenyu Wang, and Songlin Yi. 2025. "Catalytic Pyrolysis Characteristics of Potassium Chloride on Ash Branch Wood and Its Kinetic Study" Forests 16, no. 1: 57. https://doi.org/10.3390/f16010057
APA StyleZhang, L., Gao, J., Wang, T., Hao, T., Lu, Y., Hu, Y., Wang, X., He, Z., Wang, Z., & Yi, S. (2025). Catalytic Pyrolysis Characteristics of Potassium Chloride on Ash Branch Wood and Its Kinetic Study. Forests, 16(1), 57. https://doi.org/10.3390/f16010057






