Abstract
Forests support a broad range of ecosystem services. These services cannot simply be traced back to the individual biotic and abiotic components of the forest ecosystems. Rather, they stem from complex interactions between these components. CON.ECO.FOR, the Italian branch of the ICP-Forests (International Co-operative Program on Assessment and Monitoring of Air Pollution Effects on Forests) network, established in 1994, has shown to be a unique data source for understanding forest interactions and processes. This contribution aims to draw a comprehensive picture of the Italian forest soil conditions by reviewing the main issues that have arisen and the results obtained over the last 30 years of forest soil monitoring. Forest health is often controlled by soil-mediated processes. Thus, we have evidenced how including soil parameters in environmental studies has proven to be of high value for a better understanding of forest ecosystem conditions and trends. Here, we are reviewing all of the results obtained concerning soils from the analyses of the impacts of atmospheric deposition on forest soils to the study of the relationships between organism diversity and the soil.
1. Introduction
In 1979, the United Nations Economic Commission for Europe (UNECE) took a significant step by adopting and promoting the Convention on Long-range Transboundary Air Pollution (CLRTAP). This pivotal Convention has played a crucial role in fostering collaboration between researchers and policymakers, with the shared goal of promoting clean air and mitigating the adverse effects of pollutants across geographical borders. To facilitate these collaborative efforts, the Convention has established scientific and technical programs that serve as the scientific foundation for decision-making by deepening our understanding of pollutant transport and its environmental impact. As part of this framework, seven specialist International Cooperative Programs (ICPs) were set up, each focusing on a specific aspect of environmental research. CON.ECO.FOR was established in Italy in 1994 [1] as the Italian section of the ICP-Forests (International Co-operative Program on Assessment and Monitoring of Air Pollution Effects on Forests) network [2] to monitor the atmospheric deposition of chemical species considered capable of inducing soil acidification, forest damage, and pollution of surface and underground waters. The ICP-Forests network provides one of the most comprehensive and harmonized soil databases. It includes plots representative of the most important Italian forest types, making this soil inventory essential for data synthesis and modeling. The ICP-Forests program is structured into two distinct monitoring levels, each serving a specific purpose. Level I was constructed as a dense and spatially representative grid of forest points based on a systematic transnational grid of 16 × 16 km (15 × 18 km for Italy) throughout Europe to obtain insight into the geographic and temporal variations of forest conditions. These assessments are conducted at irregular intervals. Level II was implemented as an in-depth monitoring network to monitor the cause–effect relationships of various stress factors, such as air pollutants, drought, and acidification, on forest ecosystems. It has included a number of sites, varying from 20 to 30, according to logistical and financial constraints and selected on the basis of at least one site for each administrative region, plus additional sites that were selected as relevant for monitoring. The sites of the Level II network where the soil and soil solution monitoring were carried out are presented in Table 1.
The overall objective of the ICP-Forests Program is to provide data on the implementation of emission reduction policies [3] to the Executive Body of the Air Convention (UNECE-WGE), thus enabling the development of legally binding protocols on international air pollution abatement. The Program Co-ordinating Centre (PCC), the Program Co-ordinating Group (PCG), and the Expert Panels (EPs) are responsible for data evaluation. Today, the Italian network is managed by the Carabinieri Forestry Environmental and Agrifood Command (CUFAA)—Studies and Projects office.
Recently, the National Emission Ceiling (NEC) Directive [4] was issued. This directive establishes commitments to reduce the air emissions of pollutants associated with human activities. Member states are required to monitor the adverse impacts of air pollution on terrestrial and aquatic ecosystems. The Italian Act No 81/2018 ratified the NEC Directive [4]. A new NEC network was created, and part of the terrestrial sites were selected among the CON.ECO.FOR sites (Figure 1).
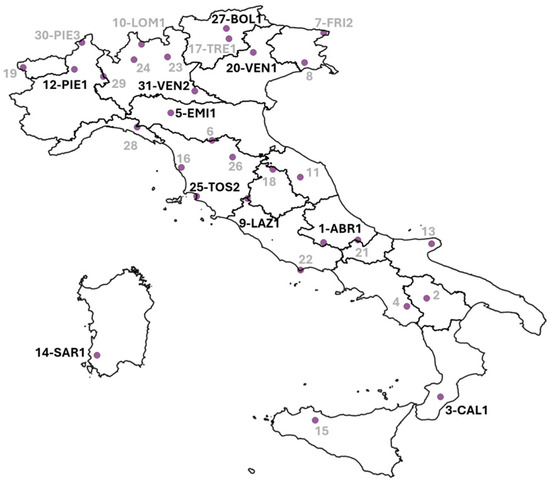
Figure 1.
Sites of the Italian Level II ICP-Forests network (CON.ECO.FOR). In sites presented with an alphanumeric code, soil solution monitoring was carried out (see Table 1). Sites in black belong to the new NEC network.
Soil and soil solution monitoring is carried out within this context. Soil, forest floor, and soil solutions were sampled and analyzed according to the standard procedures outlined in the ICP-Forests manual [5], which is regularly updated with new laboratory and field protocols. Data quality is ensured by specific quality control/quality assurance (QA/QC) protocols [6] and by regular laboratory ring tests. All of the data submitted to the database are provided with QA/QC information.
At the national level, there existed a gap in comprehensive assessments regarding several critical issues related to forest soils discussed in the present review. Specifically, topics such as the monitoring of pollutant fluxes through soil solutions, humus forms, and soil organic carbon sequestration in forest ecosystems were mostly focused on localized or regional evaluations that, despite being relevant, limit the broader understanding of the main forest ecosystem processes. The CON.ECO.FOR network structure allows for covering different forest conditions across Italy with a collaborative approach between several disciplines. By expanding to a national level, the scope of the assessments, and the understanding of forest ecosystem responses to several environmental factors, we can develop a more integrated understanding of forest status, which is critical for effective environmental management. This review aims to provide an overview by bringing together the various studies and findings on forest soils carried out within the CON.ECO.FOR network, encompassing 30 years of soil monitoring and investigations in the Italian forests.
2. Soil Solution Monitoring
In addition to the direct effects of stress factors on the canopy, the state of the forest is influenced by effects mediated by the soil through the root systems. Therefore, the soil solution is an important indicator for monitoring the impacts of pollution and other possible stress factors on forest ecosystems. The chemical composition of the soil solution is determined by complex balances and is influenced by atmospheric deposition, exchanges of matter between the soil and plants, exchanges with the atmosphere and air in the soil, and the activity of microorganisms. Therefore, monitoring the soil solution provides real-time control of nutrient availability, the presence of chemical species harmful to the root systems, and flows of polluting substances that reach the soil with atmospheric deposition.
In the Level II ICP-Forests network (Figure 1), soil solutions are sampled continuously and non-destructively and always from the same volume of soil, which remains in its natural condition. Two types of samplers are used: gravity samplers and tension samplers. In the CON.ECO.FOR, gravity samplers, collecting gravitational water, are only used to sample soil solution from the organic horizons (forest floor), while tension samplers are inserted in the mineral soil and act through a vacuum application to collect both gravitational water and water retained with a certain tension. The deepest samplers are located at 60/70 cm depth.
Sample treatment, preservation, analysis, and quality control follow the ICP-Forests protocols. Cations and anions are directly determined by ion chromatography. Time series of open field bulk deposition, throughfall deposition, and soil solution of Ca2+, Mg2+, K+, NO3−, NH4+, SO42−, and dissolved organic carbon (DOC), covering several years Figure 1 were detected. For each site at each depth, temporal changes were tested using the non-parametric Seasonal Mann–Kendall (SMK) test for monthly concentrations of soil solutions.
Monitoring the soil solution provides insights into the evolution of the soil–forest system over time. This allows for making predictions based on current trends and verifying the possible impacts of short-term events.
Thus, monitoring soil solution composition was selected within ICP-Forests as a tool to address the following questions:
- 1.
- Are soils exporting pollutants towards other environmental subsystems?
- a.
- If yes, which environmental subsystems are affected?
- b.
- If yes, what are the reasons behind this?
- 2.
- Are soils acidifying?
- a.
- Which parameters can address this issue?
- b.
- If the answer is yes, what causes acidification?
- 3.
- Can atmospheric deposition influence forest health and productivity through the soil?
- a.
- Through plant nutrition?
- b.
- What other mechanisms are involved?
2.1. Export of Pollutants from Soil
The first question can be answered directly by soil solution monitoring. The question, “Which pollutants are exported from the soil to other environmental systems?” was initially intended to be rather rhetorical, as monomeric Al3+ was identified as the critical pollutant. Sulfate anion (SO42−), recognized as the main incoming pollutant, was not expected to be particularly damaging within other subsystems, such as freshwater. Reactive nitrogen, in the form of either nitrate (NO3−) or ammonium (NH4+), was initially considered to be a minor issue. The cause of aluminum export was seen as the introduction of excess anions (sulfate) that had to be balanced by equivalent cations. This process was usually called “buffering”, and it was supposed that Al buffering could be the primary process in forest soils [7,8].
The occurrence of pollutant export can be assessed directly from soil solution monitoring results by analyzing pollutant concentrations and their changes with depth. If pollutants are found in the deepest samples, this indicates that export occurs.
A more accurate and quantitative assessment of pollutant export can be obtained by estimating water fluxes and then actual cation or anion budgets. This was originally thought to require an extensive installation of meteorological and soil water sensor arrays, soil physical analyses, and the application of soil water budget models. A first, ostensibly network-wide, assessment based on such an approach was published by de Vries et al. [9]. However, this study did not include Italian sites, as the necessary data were not, and are presently not, supplied by the CON.ECO.FOR network. The assessment was somewhat Germano-centric and biased towards Northern Europe. A significant change of perspective was the recognition of nitrate as an anion capable of producing effects, similar to sulfate. However, nitrate and ammonium, were seen as relatively ineffective for pollutant export, as they were found to be strongly retained in the soil. Implicitly, this implies that they are not significant “exported” pollutants. Aluminum export was identified as the main problem, as expected.
Later, Hruška et al. [10] introduced a simpler approach based on the use of chloride (Cl−) anion as a tracer, assuming that this anion, which is present in significant amounts in aerosols derived from seawater, has very limited interactions with the forest–soil system. Using this last approach, CON.ECO.FOR data were analyzed to estimate the actual fluxes of pollutants out of the soil rooting zone. The results differed from those obtained by de Vries et al. [9]. Aluminum fluxes could not be estimated. The total Al concentrations in the solutions of the monitored Italian forest soils are below the limit of quantification in more than 90% of the cases.
Even in sites such as PIE1, PIE3, EMI1, and ABR1 (Table 1) with low soil solid-phase pH, soil solution pH is significantly higher than in the solid-phase one, thus lying above the range for monomeric Al solubility. In contrast, significant nitrate–N fluxes were found in sites with medium (around 10 kg·ha·y−1) to high (up to 25 kg·ha·y−1) total N deposition [11]. Around the same time, Paul et al. [12] found nitrate–N to represent the main, almost the only, pollutant exported from Douglas fir plots in France, with Al flowing in trace amounts. This occurred even though all of the soils in the study were fairly acidic, with most also having low C/N ratios in the organic layer, in contrast with the findings of Dise et al. [13].

Table 1.
Characteristics of the sites within the Level II CON.ECO.FOR network where soil solution monitoring was carried out.
Table 1.
Characteristics of the sites within the Level II CON.ECO.FOR network where soil solution monitoring was carried out.
| Code | Site | Altitude (m a.s.l.) | Forest Dominant Species | Soil Solution Sampling | Research |
|---|---|---|---|---|---|
| 1-ABR1 | Selva Piana (AQ) | 1500 | Fagus sylvatica | 1999–Ongoing | Nitrogen Fluxes and Trends [11] |
| 3-CAL1 | Piano Limina (RC) | 915 | Fagus sylvatica | 2009–Ongoing | Nitrogen Fluxes and Trends [11] |
| 5-EMI1 | Boschi Carrega (PR) | 200 | Quercus petraea, Quercus cerris | 2010–Ongoing | Nitrogen Fluxes and Trends [11] and BCE Budget [14] |
| 7-FRI2 | Tarvisio (UD) | 820 | Picea abies | 2007–2012 | |
| 9-LAZ1 | Monte Rufeno (VT) | 690 | Quercus cerris | 1999–Ongoing | Nitrogen Fluxes and Trends [11] and BCE Budget [14] |
| 10-LOM1 | Val Masino (SO) | 1190 | Picea abies | 2005–2012 | |
| 12-PIE1 | Val Sessera (BI) | 1150 | Fagus sylvatica | 2006–Ongoing | Nitrogen Fluxes and Trends [11] and BCE Budget [14] |
| 14-SAR1 | Marganai (CA) | 700 | Quercus ilex | 2024–Ongoing | |
| 16-TOS1 * | Colognole (LI) | 150 | Quercus ilex | Biomarker [15] | |
| 17-TRE1 | Passo Lavazè (TN) | 1800 | Picea abies | 2006–2012 | |
| 20-VEN1 | Pian di Cansiglio (TV) | 1100 | Fagus sylvatica | 2010–Ongoing | Nitrogen Fluxes and Trends [11] and BCE Budget [14] |
| 25-TOS2 | Cala Violina (GR) | 30 | Quercus ilex | 2023–Ongoing | Biomarker [15] |
| 27-BOL1 | Renon (BZ) | 1740 | Picea abies | 2005–20122023–Ongoing | |
| 30-PIE3 | Alpe Devero (VB) | 1860 | Larix decidua | 2009–2011 | |
| 31-VEN2 | Bosco Fontana (MN) | 25 | Quercus cerris, Quercus robur | 2023–Ongoing |
(*) Sites where only the soil solid phase was sampled.
2.2. Acidification of Forest Soils
Concerning soil acidification, three different approaches can be used to evaluate soil solution data. The simplest method is to observe the soil solution pH. A second, still quite direct, approach is the use of some ratio between soil solution analytes; it should be noted that such ratios were essentially adapted from freshwater chemistry. The most used ratio is the acid neutralizing capacity (ANC, as per Eq. 4 in Reuss [16]). The ratio of total base cations (Bc = Ca2+ + Mg2+ + K+ + Na+) to Al3+ (Bc/Al; Cronan et al. [17]) is also commonly used and is considered an indicator of the potential toxic effects of aluminum on plant roots. A more soil-specific and complex indicator is the depletion of available base cations in the soil. Notably, basic works [7] defined this pool as consisting of cations that could be released through mineral weathering. More soil-oriented views [18,19] emphasize the importance of exchangeable base cations as the main source of buffering chemical species, which is in line with established concepts in soil science.
The issues of both soil solution pH, ANC, and Bc/Al ratio have been examined with respect to time trends and include data from Italy from Johnson et al. [20]. This study evidenced contrasting results, primarily as a consequence of a general decrease in base cation deposition across Europe, due to reductions in such industrial activities as quarrying, opencast mining, etc. An attempt at building base cation budgets on a continental scale is found in de Vries et al. [9], showing that about half of the 121 plots considered were retaining cations, while the other half were releasing them. This suggests that variability across the whole range of ICP-Forests Level II plots includes so many components that obtaining network-wide significant results is difficult, as it can also be gauged by Johnson et al. [20].
Forest soils in Italy tend to be not so strongly acidic and to have rather high cation exchange capacity (CEC) due to the presence of clay and also have lower reserves of easily weatherable minerals. For these reasons, parameters such as ANC and Bc/Al ratio were not found to be of much use. Base cation budgets [14] and soil solution pH trends [11] in CON.ECO.FOR Level II sites (Figure 1 and Table 1) were analyzed, offering results that differ significantly from established trends at the European level. As first evidence, the deposition of base cations in Italy did not show any sign of decrease, as it was found to be primarily caused by such geomorphological and climatic factors as dust and aerosol transport and not by industrial activities. As a result, the base cation budget of Italian monitored sites is mostly positive, or at least very close to neutral, and can be (slightly) negative only at sites with very high N deposition. Coherently, soil solution pH was seen as decreasing only in such highly polluted sites (Figure 2; [11]). These results supported the values of the N critical load concept, as discussed by Etzold et al. [21].
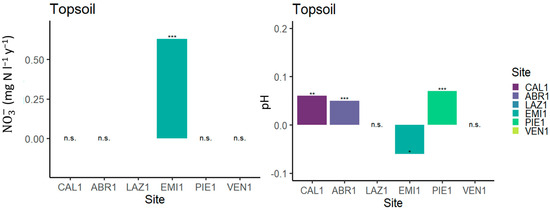
Figure 2.
Results of the trend analysis by Seasonal Kendall Test (SKT) applied to monthly data of nitrate concentration and pH at the topsoil (20 cm) for each site (Figure modified from Cecchini et al. [11]). Significance of Sen’s slope: * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; n.s.: not significant. For characteristics of the sites refer to Table 1.
A major factor contributing to soil acidification was found to be the high proportion of reduced nitrogen (ammonium, NH4+) in deposition (see Table 4 in [11]). Ammonium was found not to be exported from the soil, but rather to be regularly transformed into nitrate, a reaction chain that produces high amounts of protons (H+, Figure 3).
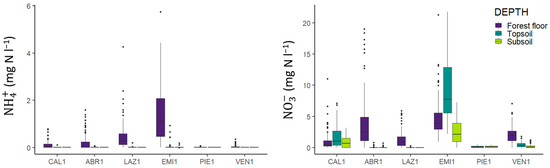
Figure 3.
Ammonium and nitrate concentrations for the forest floor (0 cm), topsoil (20 cm), and subsoil (60 cm) soil solution samples (Figure modified from Cecchini et al. [11]). For characteristics of the sites, refer to Table 1.
Then, in Italy, very few soils are actually acidifying, and the cause of acidification is the high level of reactive N deposition, including high levels of reduced nitrogen. Nevertheless, the CON.ECO.FOR data did confirm that the largest proportion of reactive N atmospheric deposition is retained by the soil–forest system [11,22]. These findings are aligned with the results obtained by calculating the hydrochemical budget based on throughfall and soil solution N fluxes, which revealed ~80% N soil retention in a remote forested site in the Italian Alps [23]. Such retention implies that plant N availability is increased by deposition within the soil–forest systems that are either N-limited or very close to such a condition.
2.3. Deposition Effects on Vegetation Through Soils
Coming to the third question, the results above suggest that the most likely effects on vegetation should be connected to the excess nitrogen availability. Ferretti et al. [22] evidenced marked effects of N deposition on forest growth. Later, Etzold et al. [21] proposed a potential maximum limit for a positive N deposition effect on forest growth, lying in the region of 30 kg·ha·y−1, a level that is not found in any Italian site. On the other hand, a damaging effect of N deposition on defoliation was suggested by Ferretti et al. [24] in an analysis that included Italian sites. As imbalances in the foliar N/P and N/Bc ratios were found to be the most likely mechanisms, soil plant nutrient availability appears to be a controlling process. Here is a clear issue for further research.
The effects of high N deposition on understory composition were documented by Dirnböck et al. [25] in a study that included many CON.ECO.FOR sites. They assessed a significant decrease in the diffusion of those ground vegetation species classified as oligotrophic. This is a kind of effect that is clearly linked with increased N availability in the soil. These authors maintained that the absence of more marked effects was due to the limited time span (2000–2009) covered by their study. Therefore, the approaches widening the time span are welcome and can potentially elucidate the long-term effects of depositions on vegetation.
A negative effect of high soil N content on the understory has been evidenced in more recent studies. Chelli et al. [26], analyzing the functional diversity for different plant traits with a space-for-time substitution approach, showed a negative effect of total nitrogen content on belowground bud bank size. Thus, high reactive N deposition may impact, through the soil, the diversity of regeneration strategies through the buds, potentially affecting understory plant sprouting after disturbance. This agrees with other studies based on long-term monitoring that evidenced a biotic homogenization of the understory after nitrogen addition [27] and a decrease in plant diversity due to excess N related to the response of the herb layer to soil N [28].
3. Soil–Ecosystem Interactions
Data from the Level I ICP-Forests network (Figure 4) allowed us to investigate environmental factors and changes in relation to the properties of forest soils. Within the BioSoil project from 2006 to 2008, 238 Italian forest sites were surveyed. For each site, five sampling points were located within a circle of 25 m in diameter. The mineral soil was sampled by horizons and by fixed depth (0–20 cm; 20–40 cm; 40–80 cm) according to standard ICP-Forests procedures [5]. A detailed soil profile description and soil classification were carried out following the World Reference Base for Soil Resources [29,30]. Organic horizons were sampled and described, considering also the zoogenic activities that are visible in the field. Dominant humus forms were described and classified according to Zanella et al. [31]. The soil-related field and laboratory parameters were harmonized, and the physical and chemical soil parameters were analyzed following the guidelines of ICP-Forests [5]. The SOC storage assessment was based on soil-measured data.
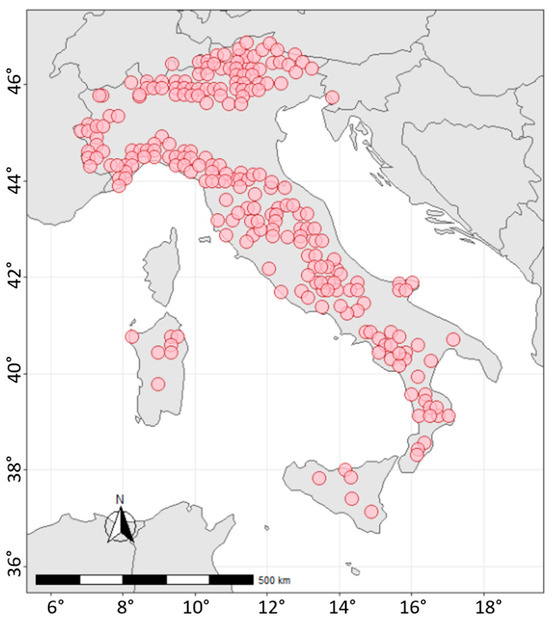
Figure 4.
Location of the Italian Level I sites (CON.ECO.FOR) within the ICP-Forests network.
The main questions arising were the following:
- (a)
- Can humus forms be an ecological indicator of OC storage and organic matter composition in Mediterranean forest soils?
- (b)
- How do environmental and pedological factors control SOC storage?
- (c)
- How do environmental drivers affect humus form distribution/occurrence?
- (d)
- What is the role of soil as a local driver in shaping abundance-weighted trait patterns of forest understories at biogeographic scales?
3.1. Humus Forms as Indicators of OC Status in Mediterranean Forest Soils
To track environmental responses to global changes, the study of humus forms appears promising due to their short-term reactivity [32]. Humus forms are defined according to morphological characteristics and topsoil architecture, including soil organic (OL-OF-OH) and organo-mineral (A, AE) horizons [31]. Humus forms were proposed as an expression of how soil organic matter (SOM) develops and interacts with soil mineral components. Particular focus is given to the pedofauna engineering activity, which shapes the morphological properties of the organic and topsoil layers. Humus forms result from both abiotic and biotic factors and from aboveground–belowground feedback [33], which regulates nutrient cycling and the microbial soil environment [34].
Research based on data from CON.ECO.FOR Level I sites (Figure 4) pointed out the potentiality of humus forms as indicators of soil organic carbon (SOC) storage in the Mediterranean environment [35] as well as in the Northwestern Alps [36]. The reasons for this potentiality were analyzed by integrating the description and classification of humus forms according to the European classification system [31], with OM compositional and enzyme activity analyses. Clear differences in soil organic matter (SOM) composition and enzymatic activity were evidenced between humus forms [37]. Mull forms, with a biomacrostructured A horizon, were found to have the highest contents in plant nutrients and highest enzyme activities, followed by amphi forms, while mull forms with a mesostructured A horizon and moder forms were characterized by opposite biochemical properties. A similar trend was observed in the qualitative changes in SOM composition. In macro-structured humus forms, the soil organic matter was more transformed, whereas the results indicated the accumulation of fresh and biodegradable material in moder forms.
To deeply investigate the source and fate of organic matter in the soil, two well-studied Mediterranean Level II sites (Table 1: TOS1 and TOS2 [38]) were selected [15]. The contribution of above- and below-ground plant-derived OC to SOC and the mechanisms driving this contribution were addressed by molecular characterization of the lipid markers [39]. The distributions of biomarkers differed between the two types of soils and humus forms, suggesting contrasting transformation, transport, and stabilization mechanisms related to soil properties and water stress. A better knowledge of these dynamics might promote soil management strategies and practices to increase the SOC pool [40].
3.2. Environmental Factors Controlling Humus Form Occurrence and SOC Storage
The research focus then moved to a national scale. Level I forest sites (Figure 4) represent the heterogeneity of Italian forests across three biogeographic regions (Alpine, Continental, and Mediterranean). CON.ECO.FOR datasets provide a wide range of ecosystem variables, offering the opportunity to integrate soil and other data to better elucidate forest processes and responses to environmental changes. Machine-learning techniques, such as boosted regression trees, were applied to the Italian dataset to evidence the environmental factors that affect humus form (moder, amphi, and mull) occurrence [41] and soil organic carbon storage [42]. On the other hand, soil parameters were used as local factors to point out trait patterns of forest understories [26,43].
The modeling approach in the Italian territory showed that, although specific plant effects were evident predictors in moder and mull distribution models, forest species are geographically located based on soil and climate conditions [41,44]. Parent material drives humus form distribution through soil nutritional status. Mull forms develop in sites where there is nutrient-rich input from plants, such as broadleaved and parent materials, favoring pedofauna engineering activity [32]. On the other hand, moder formed in sites characterized by nutrient scarcity, driven by poor inputs from litter decomposition and parent material weathering, which are synergic with low temperatures, in slowing down nutrient release and constraining fauna activity, as found in subalpine forest soils [45]. The nature of the Italian territory and climates allowed us to explore in-depth and clarify the main processes leading to amphi form development, forms that are still little known. Amphi forms, characterized by the presence of both organic horizons (OH) and a well-structured A horizon, were observed on sites with high subsoil phosphorus and litter P content that likely favor pedofauna activity. On the other hand, a reduced effective soil volume, together with climate seasonality, constrains burrowing earthworm activity during summer drought. These findings explained why amphi forms were frequently observed across Italian forests, as in Castelporziano (Central Italy; [46]), the Ligurian Alps (North–Western Italy; [47]), the Veneto region (Northeastern Italy; [48]), and Sardinia [49].
Combined national-, regional-, and local-scale results confirmed the potential of humus forms as an indicator of nutritional status, OC dynamics and stabilization, and responses to global changes in forest ecosystems. Thus, being easily and cheaply observed soil properties, humus forms can be considered a valuable tool for assessing topsoil management practices to improve forest health.
Forest soils are the largest carbon sink in the terrestrial environment [50] and are a key factor in forest health. Unraveling the conditions controlling soil carbon storage is critical for assessing current and future carbon budgets. CON.ECO.FOR soil data were thus used to investigate carbon sequestration and the environmental parameters shaping soil carbon storage variability across Italian forest sites (Figure 4; [42]). SOC storage was estimated for mineral forest soils by considering the topsoil (0–20 cm) and the 0–80 cm soil depth. Dominant predictors were soil type (RSG-WRB; [29,30]) and subsoil phosphorus content for both soil portions. The carbon content in Italian forest soils is more closely related to soil properties than to forest cover. However, when topsoil and subsoil SOC stocks are considered separately (Figure 5), significant differences were identified between forest species. In particular, SOC storage is significantly lower under C. sativa and L. decidua, compared with other species. This study, capturing the complexity of the entire territory, represents a key analysis of SOC storage for forest ecosystems in Italy, since most of the previous studies were based on local-scale research. At the national level, SOC stocks were estimated within the National Forest Inventories (NFI) only for the first 30 cm depth and related to vegetation [51]. Within the NFI, topsoil N stocks, a parameter closely linked to SOC stocks, were related to different environmental factors [52]. The applied models explained about half of the data variability, with the dominance of forest type and latitude as the driving factors and with the remaining fraction likely due to pedological variables that were not included in the models.
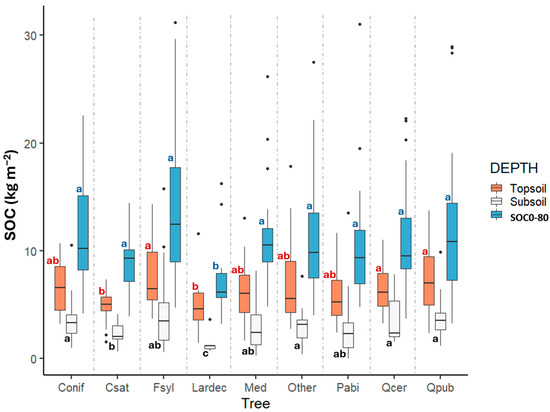
Figure 5.
Distribution of average soil organic carbon (SOC) stored in the topsoil (0–20 cm), subsoil (40–80 cm), and total soil profile (SOC0−80 cm) among dominant tree species. Species groups include Picea abies (Pabi), Fagus sylvatica (Fsyl), Castanea sativa (Csat), Quercus cerris (Qcer), and Quercus pubescens (Qpub). “Conifers” refers to Pinus nigra and Larix decidua, while “Other” includes all broadleaved species except F. sylvatica, C. sativa, and oaks. “Med” represents sclerophyll oaks, Quercus ilex, and other Mediterranean species [42]. Lowercase letters indicate significant differences between species at each soil depth based on the Kruskal–Wallis non-parametric test.
Both the models on humus forms and SOC storage within the CON.ECO.FOR network evidence the pivotal role of the interactions between different environmental factors. Another interesting common point between the models was the unexpected weight of phosphorus content in driving both SOC sequestration and humus form differentiation.
Forest ecosystem investigations should thus integrate soil types and pedological parameters to propose forestry management practices acting to mitigate climate and land use changes.
3.3. The Role of Soil Parameters in Driving Understory Plant Traits
In addition to soil measurements, the Level I CON.ECO.FOR network has implemented a comprehensive understory plant survey. The understory layer, hosting a significant portion of the vascular plant diversity in temperate forests [53], is essential for maintaining a variety of ecological functions, including productivity and nutrient cycling [54], and can be pivotal in predicting future shifts in biodiversity. Thus, understanding how plant communities respond to global environmental changes has become a critical area of research.
Several soil parameters were included in functional biogeography studies [26,43,55] to better understand understory trait–environment relationships. Modeling environmental control factors on such plant traits as specific leaf area (SLA), plant height (H), seed mass (SM), and belowground traits related to space occupancy and re-sprouting showed how climate–soil interactions explain the largest proportion of variation for most of the traits, highlighting the need to integrate soil properties as local drivers into a broad-scale functional biogeography study. In particular, the soil parameters related to resource amount and availability for plants (i.e., N, pH, N/C, available K, and soil effective volume) showed a positive relation with mean understory trait values related to resource economics and plant growth [26]. Furthermore, soil N and pH affected the functional diversity of plant regeneration via seeds and buds, with eutrophication generating homogenization in strategies of vegetative sprouting after disturbance and acidification fostering a trade-off between enhanced seed dispersal strategies and more similar vegetative sprouting [43]. Soil–climate interactions should be considered when interpreting the relationships between traits and climate because the adaptive value of traits in a given climate depends on the soil properties. Thus, including soil properties in the modeling framework on vegetation responses to global changes will likely improve the predictiveness performance [56].
4. Conclusions and Future Perspectives
In summary, research conducted over the past 30 years on forest soil monitoring has demonstrated that the results obtained within the CON.ECO.FOR network can confirm that the only atmospheric pollutant effectively exported from forest soils is nitrate.
In most cases, Italian forests function as effective filters for nitrogen pollution. However, special attention must be paid to nitrate leaching, particularly in northern regions. Even remote sites in these regions are close to the Po Plain, one of the most polluted areas in Europe, and, therefore, experience an excess of nitrogen deposition loads.
The results indicate that the limited soil acidification observed is connected to high reactive nitrogen deposition. The estimation of basic exchangeable cation budgets appears to be the indicator that allows a clearer understanding of ongoing processes. In this sense, while our results serve as a preliminary estimate, they show how atmospheric deposition of basic cations on Italian forest soils has a significant impact on both soil buffering conditions and forest nutrition. Consequently, forest soil acidification is either prevented or significantly mitigated by the current regime of BCE atmospheric deposition.
The network then enables us to understand the drivers and factors controlling both pollutant export and acidification and continues to provide essential data, particularly in relation to the European NEC directive.
Within the intensive monitoring Level I network, environment–humus modeling yields significant insights that are consistent with other reported studies. Humus forms are sensitive and responsive to changes in nutrient flows and soil conditions, as influenced by factors such as forest growth cycles, management practices, and specific soil characteristics. This highlights the potential of humus forms as indicators of ecosystem conditions and as tools for assessing the impacts of both natural and human-induced disturbances. As humus forms integrate feedback between soil biota, climate, and management, understanding such feedback mechanisms is a potential tool for developing effective forest management strategies.
Furthermore, the data provided within the CON.ECO.FOR highlight the importance of including pedogenic soil types in SOC storage models, which can improve the prediction of forest soil organic carbon sequestration. How different pathways lead to SOC accumulation and the weights of interactions between predictors point out that the variability of SOC stocks depends on the ecological context. Often, the interaction between several factors, triggering positive feedback, is decisive in favoring the high content and long persistence of soil organic carbon.
Generally, the results obtained within the CON.ECO.FOR network highlight the importance of considering the effects of multiple biotic/abiotic factors and climatic variables on forest systems to implement more efficient practices and mitigation strategies.
The consistency of our results and their alignment with Pan-European findings suggest that concerns about the representativeness of the network do not impede its relevance. However, the variability in climate, geology, and soil types across Italy necessitates careful monitoring of forest ecosystems to track changes in chemical trends and soil solution fluxes. Thus, forest soil monitoring in Northern Italy has already been strengthened.
A major task for sustainable forest management is to better estimate the water balance within the soil–vegetation–atmosphere system. This involves developing and testing improved procedures for estimating water fluxes, from precipitation to soil drainage. The soil data collected within the CON.ECO.FOR network can be utilized to estimate hydraulic parameters, which are crucial for modeling hydrological processes across various spatial scales. Models such as BROOK90 [57] and HYDRUS-1D [58] are continuously updated to meet modern software standards and enhance their relevance for recent European applications in forest ecosystems (e.g., [59,60,61]). Time–domain reflectometry (TDR) measurement of soil water content, taken at depths corresponding to soil water samplers, can be used for model calibration and validation. By improved estimation of water and nutrient fluxes, better nutrient budgets can be compiled to provide insights for effective land and water management practices.
The results obtained from the CON.ECO.FOR network can foster advancements in both fieldwork and modeling approaches concerning the relationship between soil systems and plant health. Research should primarily focus on traits that offer insights into plant functionality and strategies, thereby highlighting their connections to forest health. Factors often overlooked, such as available water-holding capacity and nutrient status, should be incorporated to explore their synergic effects as modulators of vegetation structure and distribution. The heavy influence of P availability that was often found should be analyzed in connection with leaf nutrient status, which is another kind of data provided by ICP-Forests and CON.ECO.FOR.
Author Contributions
Conceptualization, S.C. (Stefano Carnicelli) and A.A.; investigation, A.A., S.C. (Stefano Carnicelli), S.C. (Stefano Chelli), R.C., G.C. (Guia Cecchini) and G.C. (Giandiego Campetella); data curation, A.A., S.C. (Stefano Carnicelli) and S.C. (Stefano Chelli); writing—original draft preparation, A.A. and S.C. (Stefano Carnicelli); writing—review and editing, A.A., S.C. (Stefano Carnicelli) and S.C. (Stefano Chelli); visualization, A.A.; funding acquisition, S.C. (Stefano Chelli), S.C. (Stefano Carnicelli) and G.C. (Giandiego Campetella). All authors have read and agreed to the published version of the manuscript.
Funding
The research has been carried out within the project “LIFE MODERn(NEC)—new MOnitoring system to Detect the Effects of Reduced pollutants emissions resulting from NEC Directive adoption”—LIFE20 GIE/IT/000091. S.C. was supported by the MultiForDiv project (MUR, PRIN 2022, n.2022A42HL4) financed by the European Union—Next Generation EU, Missione 4 Componente 1, CUP J53D23006480001. S.C. (Stefano Chelli) and G.C. (Giandiego Campetella) were further supported by the RE.DI. (Reducing risks of natural Disasters) Consortium.
Data Availability Statement
No new data were created in this study. Data sharing is not applicable to this article; we refer to the original articles cited in the present review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mosello, R.; Brizzio, M.C.; Kotzias, D.; Marchetto, A.; Rembges, D.; Tartari, G. The chemistry of atmospheric deposition in Italy in the framework of the National Programme for Forest Ecosystems Control (CONECOFOR). J. Limnol. 2002, 61, 77. [Google Scholar] [CrossRef]
- de Vries, W.; Vel, E.; Reinds, G.J.; Deelstra, H.; Klap, J.M.; Leeters, E.E.J.M.; Hendriks, C.M.A.; Kerkvoorden, M.; Landmann, G.; Herkendell, J.; et al. Intensive monitoring of forest ecosystems in Europe. For. Ecol. Manag. 2003, 174, 77–95. [Google Scholar] [CrossRef]
- Vanguelova, E.I.; Benham, S.; Pitman, R.; Moffat, A.J.; Broadmeadow, M.; Nisbet, T.; Durrant, D.; Barsoum, N.; Wilkinson, M.; Bochereau, F.; et al. Chemical fluxes in time through forest ecosystems in the UK–Soil response to pollution recovery. Environ. Pollut. 2010, 158, 1857–1869. [Google Scholar] [CrossRef]
- EU. Directive 2016/2284/EU, DIRECTIVE (EU) 2016/2284 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL–of 14 December 2016—On the Reduction of National Emissions of Certain Atmospheric Pollutants, Amending Directive 2003/35/EC and repealing Directive 2001/81/EC. 2016. Available online: https://eur-lex.europa.eu/eli/dir/2016/2284 (accessed on 14 June 2023).
- FSCC, Expert Panel on Soil & Forest Soil Coordinating Centre. Part IIIa. Sampling and Analysis of Soil. Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests. ICP Forests, Hamburg, 2006, p. 130. Available online: https://www.icp-forests.org/pdf/manual/2000/Chapt_3a_2006(1).pdf (accessed on 15 November 2023).
- König, N.; Cools, N.; Derome, K.; Kowalska, A.; De Vos, B.; Fürst, A. Chapter 22—Data quality in laboratories: Methods and results for soil, foliar, and water chemical analyses. In Developments in Environmental Science; Ferretti, M., Fischer, R., Eds.; Elsevier: Oxford, UK, 2013; Volume 12, pp. 415–453. [Google Scholar]
- Ulrich, B. Interaction of Forest Canopies with Atmospheric Constituents: SO2, Alkali and Alkali Earth Cations and Chloride. Effects of Accumulations of Air Pollutants in Forest Ecosystems. In Effects of Air Pollutants in Forest Ecosystems; Ulrich, B., Pankrath, J., Eds.; Reidel Publishing Company: Dordrecht, The Netherlands, 1983; pp. 35–45. [Google Scholar]
- Ulrich, B. Natural and anthropogenic components of soil acidification. Zeitschrift Für Pflanzenernährung, Düngung Und Bodenkunde 1986, 149, 702–717. [Google Scholar] [CrossRef]
- de Vries, W.; van der Salm, C.; Reinds, G.J.; Erisman, J.W. Element fluxes through European forest ecosystems and their relationships with stand and site characteristics. Environ. Pollut. 2007, 148, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Hruška, J.; Oulehle, F.; Šamonil, P.; Šebesta, J.; Tahovská, K.; Hleb, R.; Houška, J.; Šikl, J. Long-term forest soil acidification, nutrient leaching and vegetation development: Linking modelling and surveys of a primeval spruce forest in the Ukrainian Transcarpathian Mts. Ecol. Model. 2012, 244, 28–37. [Google Scholar] [CrossRef]
- Cecchini, G.; Andreetta, A.; Marchetto, A.; Carnicelli, S. Soil solution fluxes and composition trends reveal risks of nitrate leaching from forest soils of Italy. CATENA 2021, 200, 105175. [Google Scholar] [CrossRef]
- Paul, A.; Legout, A.; Zeller, B.; van der Heijden, G.; Bonnaud, P.; Reichard, A.; Nourrisson, G.; Ranger, J. Soil solution chemistry in 11 monitoring plots of Douglas-fir plantations in France: Implications for soil fertility. Plant Soil 2022, 479, 207–231. [Google Scholar] [CrossRef]
- Dise, N.B.; Matzner, E.; Forsius, M. Evaluation of organic horizon C:N ratio as an indicator of nitrate leaching in conifer forests across Europe. Environ. Pollut. 1998, 102, 453–456. [Google Scholar] [CrossRef]
- Cecchini, G.; Andreetta, A.; Marchetto, A.; Carnicelli, S. Atmospheric deposition control of soil acidification in central Italy. CATENA 2019, 182, 104102. [Google Scholar] [CrossRef]
- Reuss, J.O. The Transfer of Acidity from Soils to Surface Waters. In Soil Acidity; Ulrich, B., Sumner, M.E., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; p. 203. [Google Scholar]
- Cronan, C.; April, R.; Bartlett, R.; Bloom, P.; Driscoll, C.; Gherini, S.; Henderson, G.; Joslin, J.D.; Kelly, J.M.; Parnell, R.; et al. Aluminum toxicity in forests exposed to acidic deposition: The ALBIOS results. Water Air Soil Pollut. 1989, 48, 181–192. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Lawrence, G.B.; Bulger, A.J.; Butler, T.J.; Cronan, C.S.; Eagar, C.; Lambert, K.F.; Likens, G.E.; Stoddard, J.L.; Weathers, K.C. Acidic Deposition in the Northeastern United States: Sources and Inputs, Ecosystem Effects, and Management Strategies. BioScience 2001, 51, 180. [Google Scholar] [CrossRef]
- Armbruster, M.; MacDonald, J.; Dise, N.B.; Matzner, E. Throughfall and output fluxes of Mg in European forest ecosystems: A regional assessment. For. Ecol. Manag. 2002, 164, 137–147. [Google Scholar] [CrossRef]
- Andreetta, A.; Dignac, M.-F.; Carnicelli, S. Biological and physico-chemical processes influence cutin and suberin biomarker distribution in two Mediterranean forest soil profiles. Biogeochemistry 2011, 112, 41–58. [Google Scholar] [CrossRef]
- Johnson, J.; Graf Pannatier, E.; Carnicelli, S.; Cecchini, G.; Clarke, N.; Cools, N.; Hansen, K.; Meesenburg, H.; Nieminen, T.M.; Pihl-Karlsson, G.; et al. The response of soil solution chemistry in European forests to decreasing acid deposition. Glob. Change Biol. 2018, 24, 3603–3619. [Google Scholar] [CrossRef] [PubMed]
- Etzold, S.; Ferretti, M.; Reinds, G.J.; Solberg, S.; Gessler, A.; Waldner, P.; Schaub, M.; Simpson, D.; Benham, S.; Hansen, K.; et al. Nitrogen deposition is the most important environmental driver of growth of pure, even-aged and managed European forests. For. Ecol. Manag. 2020, 458, 117762. [Google Scholar] [CrossRef]
- Ferretti, M.; Marchetto, A.; Arisci, S.; Bussotti, F.; Calderisi, M.; Carnicelli, S.; Cecchini, G.; Fabbio, G.; Bertini, G.; Matteucci, G.; et al. On the tracks of Nitrogen deposition effects on temperate forests at their southern European range—An observational study from Italy. Glob. Change Biol. 2014, 20, 3423–3438. [Google Scholar] [CrossRef]
- Balestrini, R.; Di Martino, N.; Van Miegroet, H. Nitrogen Cycling and Mass Balance for a Forested Catchment in the Italian Alps. Assess. Nitrogen Status. Biogeochem. 2006, 78, 97–123. [Google Scholar] [CrossRef]
- Ferretti, M.; Calderisi, M.; Marchetto, A.; Waldner, P.; Thimonier, A.; Jonard, M.; Cools, N.; Rautio, P.; Clarke, N.; Hansen, K.; et al. Variables related to nitrogen deposition improve defoliation models for European forests. Ann. For. Sci. 2015, 72, 897–906. [Google Scholar] [CrossRef]
- Dirnböck, T.; Grandin, U.; Bernhardt-Römermann, M.; Beudert, B.; Canullo, R.; Forsius, M.; Grabner, M.-T.; Holmberg, M.; Kleemola, S.; Lundin, L.; et al. Forest floor vegetation response to nitrogen deposition in Europe. Glob. Change Biol. 2013, 20, 429–440. [Google Scholar] [CrossRef]
- Chelli, S.; Simonetti, E.; Wellstein, C.; Campetella, G.; Carnicelli, S.; Andreetta, A.; Giorgini, D.; Puletti, N.; Bartha, S.; Canullo, R. Effects of climate, soil, forest structure and land use on the functional composition of the understorey in Italian forests. J. Veg. Sci. 2019, 30, 1110–1121. [Google Scholar] [CrossRef]
- Govaert, S.; Vangansbeke, P.; Blondeel, H.; De Lombaerde, E.; Verheyen, K.; De Frenne, P. Forest understorey plant responses to long-term experimental warming, light and nitrogen addition. Plant Biol. 2021, 23, 1051–1062. [Google Scholar] [CrossRef]
- Gilliam, F.S. Excess Nitrogen in Temperate Forest Ecosystems Decreases Herbaceous Layer Diversity and Shifts Control from Soil to Canopy Structure. Forests 2019, 10, 66. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, First Update 2007; World Soil Resources Reports 103; FAO: Rome, Italy, 2007; p. 128. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; p. 203. Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 22 January 2022).
- Zanella, A.; Jabiol, B.; Ponge, J.F.; Sartori, G.; De Waal, R.; Van Delft, B.; Graefe, U.; Cools, N.; Katzensteiner, K.; Hager, H.; et al. A European morpho-functional classification of humus forms. Geoderma 2011, 164, 138–145. [Google Scholar] [CrossRef]
- Ponge, J.-F. Humus forms in terrestrial ecosystems: A framework to biodiversity. Soil Biol. Biochem. 2003, 35, 935–945. [Google Scholar] [CrossRef]
- Ponge, J.F. Plant-soil feedback mediated by humus forms: A review. Soil Biol. Biochem. 2013, 57, 1048–1060. [Google Scholar] [CrossRef]
- Van Breemen, N.; Finzi, A.C. Plant-soil interactions: Ecological aspects and evolutionary implications. In Springer eBooks; Springer: Berlin/Heidelberg, Germany, 1998; pp. 1–19. [Google Scholar] [CrossRef]
- Andreetta, A.; Ciampalini, R.; Moretti, P.; Vingiani, S.; Poggio, G.; Matteucci, G.; Tescari, F.; Carnicelli, S. Forest humus forms as potential indicators of soil carbon storage in Mediterranean environments. Biol. Fertil. Soils 2010, 47, 31–40. [Google Scholar] [CrossRef]
- Bonifacio, E.; Falsone, G.; Petrillo, M. Humus forms, organic matter stocks and carbon fractions in forest soils of northwestern Italy. Biol. Fert. Soils 2011, 47, 555–566. [Google Scholar] [CrossRef]
- Andreetta, A.; Macci, C.; Giansoldati, V.; Masciandaro, G.; Carnicelli, S. Microbial activity and organic matter composition in Mediterranean humus forms. Geoderma 2013, 209–210, 198–208. [Google Scholar] [CrossRef]
- Bussotti, F.; Borghini, F.; Celesti, C.; Leonzio, C.; Cozzi, A.; Bettini, D.; Ferretti, M. Leaf shedding, crown condition and element return in two mixed holm oak forests in Tuscany, central Italy. For. Ecol. Manag. 2003, 176, 273–285. [Google Scholar] [CrossRef]
- Otto, A.; Simpson, M.J. Degradation and Preservation of Vascular Plant-derived Biomarkers in Grassland and Forest Soils from Western Canada. Biogeochemistry 2005, 74, 377–409. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R.; Preston, C.M.; Nierop, K.G.J. Strengthening the soil organic carbon pool by increasing contributions from recalcitrant aliphatic bio(macro)molecules. Geoderma 2007, 142, 1–10. [Google Scholar] [CrossRef]
- Andreetta, A.; Cecchini, G.; Bonifacio, E.; Comolli, R.; Vingiani, S.; Carnicelli, S. Tree or soil? Factors influencing humus form differentiation in Italian forests. Geoderma 2016, 264, 195–204. [Google Scholar] [CrossRef]
- Andreetta, A.; Chelli, S.; Bonifacio, E.; Canullo, R.; Cecchini, G.; Carnicelli, S. Environmental and pedological factors influencing organic carbon storage in Italian forest soils. Geoderma Reg. 2023, 32, e00605. [Google Scholar] [CrossRef]
- Chelli, S.; Bricca, A.; Tsakalos, J.L.; Andreetta, A.; Bonari, G.; Campetella, G.; Carnicelli, S.; Cervellini, M.; Puletti, N.; Wellstein, C.; et al. Multiple drivers of functional diversity in temperate forest understories: Climate, soil, and forest structure effects. Sci. Total Environ. 2024, 916, 170258. [Google Scholar] [CrossRef] [PubMed]
- Andreetta, A.; Cecchini, G.; Carnicelli, S. Forest humus forms in Italy: A research approach. Appl. Soil Ecol. 2018, 123, 384–390. [Google Scholar] [CrossRef]
- Ascher, J.; Sartori, G.; Graefe, U.; Thornton, B.; Ceccherini, M.T.; Pietramellara, G.; Egli, M. Are humus forms, mesofauna and microflora in subalpine forest soils sensitive to thermal conditions? Biol. Fertil. Soils 2012, 48, 709–725. [Google Scholar] [CrossRef]
- De Nicola, C.; Zanella, A.; Testi, A.; Fanelli, G.; Pignatti, S. Humus forms in a Mediterranean area (Castelporziano Reserve, Rome, Italy): Classification, functioning and organic carbon storage. Geoderma 2014, 235–236, 90–99. [Google Scholar] [CrossRef]
- Bonifacio, E.; D’Amico, M.; Catoni, M.; Stanchi, S. Humus forms as a synthetic parameter for ecological investigations. Some examples in the Ligurian Alps (North–Western Italy). Appl. Soil Ecol. 2018, 123, 568–571. [Google Scholar] [CrossRef]
- Ponge, J.-F.; Sartori, G.; Garlato, A.; Ungaro, F.; Zanella, A.; Jabiol, B.; Obber, S. The impact of parent material, climate, soil type and vegetation on Venetian forest humus forms: A direct gradient approach. Geoderma 2014, 226–227, 290–299. [Google Scholar] [CrossRef]
- Vacca, A.; Serra, G.; Scalenghe, R. Vegetation, soils, and humus forms of Sardinian holm oak forests and approximated cross-harmonization of vegetation types, WRB Soil Groups and humus forms in selected Mediterranean ecosystems. Appl. Soil Ecol. 2018, 123, 659–663. [Google Scholar] [CrossRef]
- Lal, R.; Smith, P.; Jungkunst, H.F.; Mitsch, W.J.; Lehmann, J.; Nair, P.K.R.; McBratney, A.B.; de Moraes Sá, J.C.; Schneider, J.; Zinn, Y.L.; et al. The carbon sequestration potential of terrestrial ecosystems. J. Soil Water Conserv. 2018, 73, 145A–152A. [Google Scholar] [CrossRef]
- Gasparini, P.; Di Cosmo, L. Forest carbon in Italian forests: Stocks, inherent variability and predictability using NFI data. For. Ecol. Manag. 2015, 337, 186–195. [Google Scholar] [CrossRef]
- Rodeghiero, M.; Vesterdal, L.; Marcolla, B.; Vescovo, L.; Aertsen, W.; Martinez, C.; Di Cosmo, L.; Gasparini, P.; Gianelle, D. Soil nitrogen explanatory factors across a range of forest ecosystems and climatic conditions in Italy. For. Ecol. Manag. 2018, 408, 25–35. [Google Scholar] [CrossRef]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. BioScience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Landuyt, D.; De Lombaerde, E.; Perring, M.P.; Hertzog, L.R.; Ampoorter, E.; Maes, S.L.; De Frenne, P.; Ma, S.; Proesmans, W.; Blondeel, H.; et al. The functional role of temperate forest understorey vegetation in a changing world. Glob. Change Biol. 2019, 25, 3625–3641. [Google Scholar] [CrossRef]
- Chelli, S.; Ottaviani, G.; Simonetti, E.; Wellstein, C.; Canullo, R.; Carnicelli, S.; Andreetta, A.; Puletti, N.; Bartha, S.; Cervellini, M.; et al. Climate is the main driver of clonal and bud bank traits in Italian forest understories. Perspect. Plant Ecol. Evol. Syst. 2019, 40, 125478. [Google Scholar] [CrossRef]
- Simpson, A.H.; Richardson, S.J.; Laughlin, D.C. Soil-climate interactions explain variation in foliar, stem, root and reproductive traits across temperate forests. Glob. Ecol. Biogeogr. 2016, 25, 964–978. [Google Scholar] [CrossRef]
- Federer, C.A.; Vörösmarty, C.; Fekete, B. Sensitivity of Annual Evaporation to Soil and Root Properties in Two Models of Contrasting Complexity. J. Hydrometeorol. 2003, 4, 1276–1290. [Google Scholar] [CrossRef]
- Šimůnek, J.; van Genuchten, M.T.; Šejna, M. Recent developments and applications of the HYSDRUS computer software packages. Vadose Zone J. 2016, 15, vzj2016. [Google Scholar] [CrossRef]
- Schmidt-Walter, P.; Trotsiuk, V.; Meusburger, K.; Zacios, M.; Meesenburg, H. Advancing Simulations of Water Fluxes, Soil Moisture and Drought Stress by Using the LWF-Brook90 Hydrological Model in R. Agric. For. Meteorol. 2020, 291, 108023. [Google Scholar] [CrossRef]
- Meusburger, K.; Trotsiuk, V.; Schmidt-Walter, P.; Baltensweiler, A.; Brun, P.; Bernhard, F.; Gharun, M.; Habel, R.; Hagedorn, F.; Köchli, R.; et al. Soil–Plant Interactions Modulated Water Availability of Swiss Forests during the 2015 and 2018 Droughts. Glob. Change Biol. 2022, 28, 5928–5944. [Google Scholar] [CrossRef] [PubMed]
- Schübl, M.; Brunetti, G.; Fuchs, G.; Stumpp, C. Estimating Vadose Zone Water Fluxes from Soil Water Monitoring Data: A Comprehensive Field Study in Austria. Hydrol. Earth Syst. Sci. 2023, 27, 1431–1455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).