Seasonal Variations in the Growth and Physiology of Acer miaotaiense subsp. yangjuechi Fang et P. L. Chiu Seedlings Under Shading Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Shading Treatments
2.3. Measurement Indicators and Methods
2.3.1. Measurement of Growth and Morphological Indicators
- (1)
- Leaf length, width, and area were measured using a YMJ-CHA3 leaf area meter (Zhejiang Top Cloud-Agri Technology Co., Ltd., Nanjing, China).
- (2)
- To determine leaf moisture content, fresh material was collected and heated in an oven at 96 °C for 30 min. The temperature was then reduced to 60 °C, and the material was baked until a constant weight was achieved. Dry weight was measured using an electronic balance with an accuracy of 0.001 g. Subsequently, leaf water content (%) was calculated using the following formula: (leaf fresh weight-leaf dry weight)/leaf fresh weight × 100.
- (3)
- The trunk height growth was measured using a tape measure with an accuracy of 0.1 cm to assess both the spring pre-treatment and post-treatment heights. The change in trunk height (∆trunk height) was calculated as the difference between post-treatment and pre-treatment heights.
2.3.2. Measurements of Physiological Indicators
2.3.3. Determination of Photosynthetic Pigment Levels
2.3.4. Determination of Photosynthetic Light Response Curves
2.3.5. Determination of Chl Fluorescence Kinetic Parameters
2.3.6. Comprehensive Evaluation
2.4. Data Processing
3. Results
3.1. Effects of Shading on the Growth and Leaf Morphology of A. miaotaiense subsp. yangjuechi Seedlings Under Different Seasons
3.2. Effects of Shading on Osmoregulatory Substances in Leaves of A. miaotaiense subsp. yangjuechi Seedlings Under Different Seasons
3.3. Effects of Shading on MDA and Antioxidant Enzymes in Leaves of A. miaotaiense subsp. yangjuechi Seedlings Under Different Seasons
3.4. Effects of Shading on Photosynthetic Pigment Contents in the Leaves of A. miaotaiense subsp. yangjuechi Seedlings Under Different Seasons
3.5. Effects of Shading on Light Response Curves of A. miaotaiense subsp. yangjuechi Seedlings Under Different Seasons
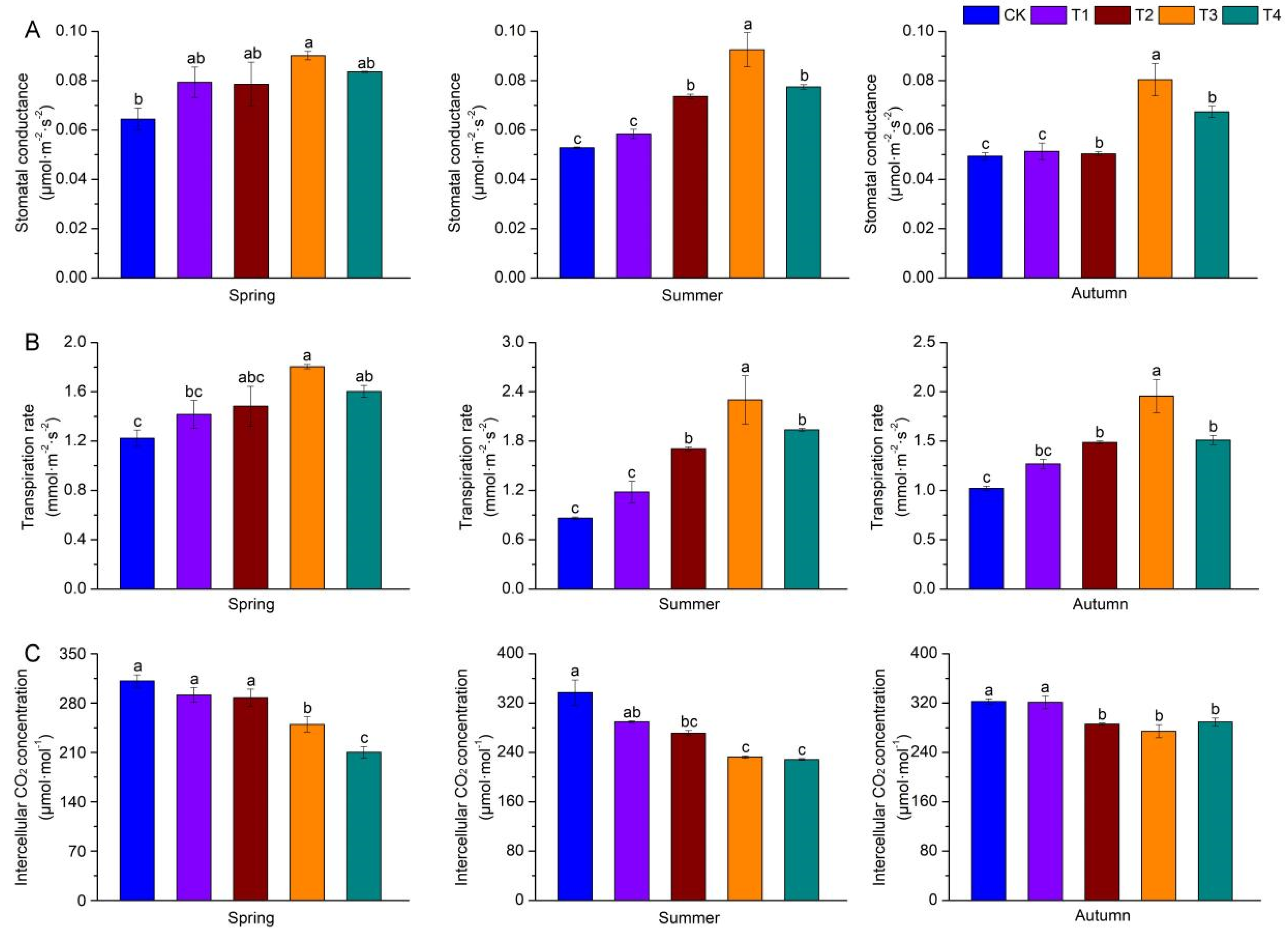
3.6. Effects of Shading on Gas Exchange Parameters of A. miaotaiense subsp. yangjuechi Seedlings Under Different Seasons
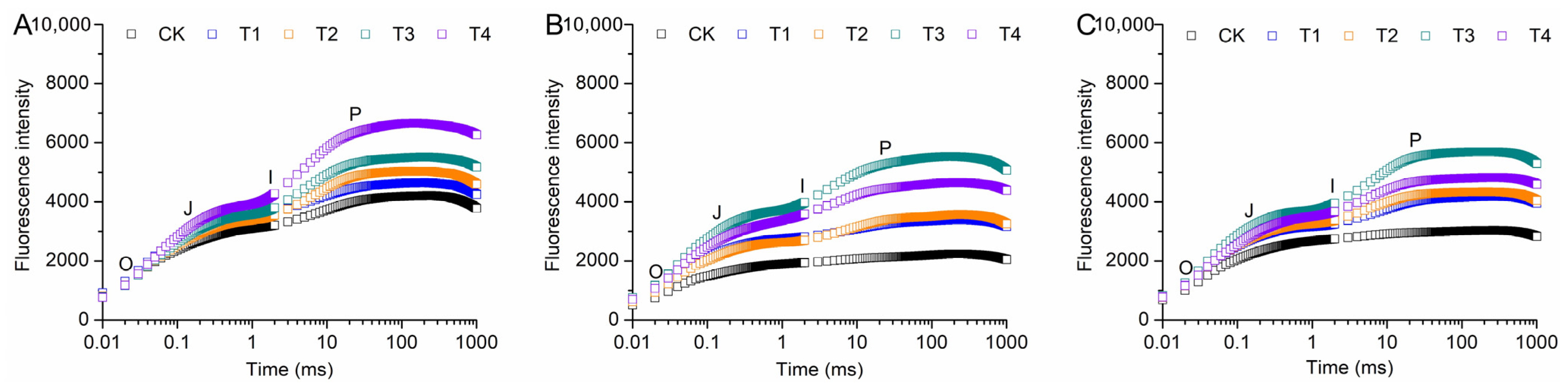
3.7. Effects of Shading on Chl Fluorescence of A. miaotaiense subsp. yangjuechi Seedlings Under Different Seasons
3.8. PCA of Shadings on A. miaotaiense subsp. yangjuechi Seedlings Under Different Seasons
3.9. Comprehensive Evaluation of Shadings on the Growth and Physiological Characteristics of A. miaotaiense subsp. yangjuechi Seedlings Under Different Seasons
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDA | Malondialdehyde |
| PRO | Proline |
| SS | Soluble sugar |
| SP | Soluble protein |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| NBT | Nitrogen blue tetrazolium |
| OD | Optical density |
| Chl | Chlorophyll |
| Cx+c | Carotenoid |
| PAR | Photosynthetically active radiation |
| Pn | Photosynthetic rate |
| Gs | Stomatal conductance |
| Ci | Intercellular carbon dioxide concentration |
| Pn max | The maximum net photosynthetic rate |
| LCP | Light compensation point |
| LSP | Light saturation point |
| Rd | Dark respiration rate |
| PSII | Photosystem II |
| φPo | Photosystem II maximum quantum yield of primary photochemistry |
| Ψo | Quantum yield for excitation energy transfer by the reaction center |
| φEo | Quantum yield for electron transfer |
| φDo | Non-photochemical deexcitation |
| PCA | Principal component analysis |
| AFA | Affiliation function analysis |
| ANOVA | Analysis of variance |
| ZAFU | Zhejiang Agriculture and Forestry University |
| CSCZ | Central Subtropical Monsoon Climate Zone |
References
- Fang, W.-P. Praecursores florae Aceracearum Sinensium. J. Syst. Evol. 1979, 17, 60–86. [Google Scholar]
- Fan, X.K.; Wu, J.; Comes, H.P.; Feng, Y.; Wang, T.; Iwasaki, T.; Yang, S.Z.; Zhu, H.; Jiang, Y.; Lee, J. Phylogenomic, morphological, and niche differentiation analyses unveil species delimitation and evolutionary history of endangered maples in Acer series Campestria (Sapindaceae). J. Syst. Evol. 2023, 61, 284–298. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, M.; Xiao, D.; Jin, G.; Pang, C.; Qi, X.; Zhou, Z. Analysis on genetic diversity of first filial generation and second filial generation of Acer yangjuechi based on SSR markers. J. Plant Resour. Environ. 2022, 31, 66–73. [Google Scholar]
- Chen, X.; Lyu, X.; Liu, Y.; Zhao, M.; Cui, X.; Zhang, D. Floral Morphology and Flowering Process of Acer yangjuechi, the Extremely Endangered Plant. Bull. Bot. Res. 2019, 39, 329–337. [Google Scholar]
- Xu, X.; Jin, H.; Chen, X.; Tian, Q.; Zhu, M.; Chen, X. Studies on the Formation of Microspores and Development of Male Gametophyte in Acer yanjuechi (Aceraceae). Plant Divers. 2012, 34, 339–346. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X. Induction, proliferation, and differentiation of Acer yangjuechi. J. Zhejiang A F Univ. 2018, 35, 975–980. (In Chinese) [Google Scholar]
- Roberts, M.; Paul, N. Seduced by the dark side: Integrating molecular and ecological perspectives on the influence of light on plant defense against pests and pathogens. New Phytol. 2006, 170, 677–699. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K. Light and temperature signal crosstalk in plant development. Curr. Opin. Plant Biol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Aleric, K.M.; Kirkman, L.K. Growth and photosynthetic responses of the federally endangered shrub, Lindera melissifolia (Lauraceae), to varied light environments. Am. J. Bot. 2005, 92, 682–689. [Google Scholar] [CrossRef]
- Groninger, J.; Seiler, J.; Peterson, J.; Kreh, R. Growth and photosynthetic responses of four Virginia Piedmont tree species to shade. Tree Physiol. 1996, 16, 773–778. [Google Scholar] [CrossRef]
- Senevirathna, A.; Stirling, C.; Rodrigo, V. Growth, photosynthetic performance and shade adaptation of rubber (Hevea brasiliensis) grown in natural shade. Tree Physiol. 2003, 23, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xie, Z.; Jiang, X. The seasonal photosynthetic responses of seedlings of the endangered plant Cathaya argyrophylla to different growth light environments. Biodivers. Sci. 2005, 13, 387–397. [Google Scholar] [CrossRef][Green Version]
- Yuan, J.; Yu, Z.; Lan, X.; Li, C.; Tian, N.; Du, F. Effects of shading treatments on photosynthetic characteristics of endangered plant Thuja koraiensis. J. Nanjing For. Univ. Nat. Sci. Ed. 2022, 46, 58–66. (In Chinese) [Google Scholar]
- Xu, Q.; Bi, H.; Cui, G.; Guo, X.; Zhou, R.; Shu, W.; Ouyang, Z.; Zhang, G. Response of photosynthetic fluorescence of the endangered plant Manglietia ventii seedlings to shade treatment. J. Nanjing For. Univ. Nat. Sci. Ed. 2019, 43, 46–52. (In Chinese) [Google Scholar]
- Li, X.; Zhang, F.; Yang, T.; Zhe, G.; Mao, C.; Wu, Y. Effect of shading on leaf morphology and photosynthetic parameters in endangered Horsfieldia glabra seedlings. Plant Physiol. J. 2019, 55, 80–90. [Google Scholar]
- Jiang, R.; Liu, Y. Effects of light intensity on the photosynthesis and growth characteristics of Davidia involucrata seedlings. Ecol. Sci. 2017, 36, 114–120. [Google Scholar]
- Chen, C.; Jin, Z.; Yuan, M.; Luo, G.; Li, Y.; Shan, F. Seasonal changes of photosynthetic characteristics of seedlings of Magnolia sinostellata under different light intensities. J. Zhejiang Agric. For. Univ. 2022, 39, 950–959. (In Chinese) [Google Scholar]
- Xu, X.; Jin, H.; Chen, X.; Jiang, S.; Wang, D.; Guo, Y. Biological characteristics of the seed in Acer yanjuechi, an endangered species. J. For. Eng. 2012, 26, 46–49. (In Chinese) [Google Scholar]
- Chen, X.; Liu, Y.; Zhao, M.; Lu, X.; Tu, S. Influence of Environmental Factors on the Growth and Seed Setting of Acer yanjuechi. J. Zhejiang For. Sci. Technol. 2021, 41, 90–94. (In Chinese) [Google Scholar]
- Wang, X.; Huang, J. Principles and Techniques of Plant Physiology and Biochemistry Experiments, 3rd ed.; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Song, Q.; Yang, J.; Lu, Y.; Wang, T.; Di, X.; Song, H.; Zhang, H. High-throughput method for determining soluble sugar based on sulfuric acid-phenol method and its application. J. Food Saf. Qual. 2022, 13, 1480–1487. [Google Scholar]
- Jiao, J. Determination of soluble protein content in alfalfa by Coomassie brilliant blue G-250 staining. Agric. Eng. Technol. 2016, 36, 33–34. [Google Scholar]
- Li, H. Principles and Techniques of Plant Physiology and Biochemistry Experiments; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Lichtenthaler, H.; Wellburn, A. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Luo, Q.; Xu, C.; Zheng, T.; Ma, Y.; Li, Y.; Zuo, Z. Leaf morphological and photosynthetic differences among four chemotypes of Cinnamomum camphora in different seasons. Ind. Crops Prod. 2021, 169, 113651. [Google Scholar] [CrossRef]
- Gao, P.; Zuo, Z.; Wu, X.; Gao, Y.; Gao, R.; Zhang, R. Effects of cycloheximide on photosynthetic abilities, reflectance spectra and fluorescence emission spectra in Phyllostachys edulis. Trees 2016, 30, 719–732. [Google Scholar] [CrossRef]
- Strasserf, R.; Srivastava, A. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Pang, W.; Zhang, M.; Liu, M.; Wu, Q.; Huang, M.; Zhang, D.; Li, D. Effects of water stress on the growth, physiological and biochemical characteristics of Sapium discolor seedlings. J. Cent. South Univ. For. Technol. 2023, 43, 62–72. (In Chinese) [Google Scholar]
- Li, J.; Lei, X.; Wang, X.; Mu, Q.; Yang, C.; Wu, J. Effects of drought stress on the physiological characteristics of new lines of Festuca arundinacea induced by spaceflight and their comprehensive evaluation. Acta Prataculturae Sin. 2017, 26, 87–98. (In Chinese) [Google Scholar]
- García-Pérez, J.L.; Oliet, J.A.; Villar-Salvador, P.; Guzmán, J.E. Root Growth Dynamics and Structure in Seedlings of Four Shade Tolerant Mediterranean Species Grown under Moderate and Low Light. J. For. 2021, 12, 1540. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Chemical Diversity and Defense Metabolism: How Plants Cope with Pathogens and Ozone Pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Islam, M.; Hoque, M.; Okuma, E.; Banu, M.; Shimoishi, Y.; Nakamura, Y.; Murata, Y. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J. Plant Physiol. 2009, 166, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Matysik, J.; Alia, A.; Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Lu, Q.; Luo, W. Effects of Different Shading Conditions on Growth, Physiological and Biochemical of Firmiana kwangsiensis Seedlings. J. West China For. Sci. 2023, 52, 70–76+82. (In Chinese) [Google Scholar]
- Tang, G.; Li, X.; Lin, L.; Li, L.; Lu, J. Change of different shading on moisture conditions and the physiological response in Alhagi sparsifolia. Chin. J. Plant Ecol. 2013, 37, 354–364. (In Chinese) [Google Scholar] [CrossRef]
- Tong, L.; Zhang, L.; Gao, Y.; Chen, L.; Geng, Y.; Li, L. Growth and Physiological Characteristics of Polygonatum cyrtonema Under Different Shading Treatment. J. Southwest For. Univ. Nat. Sci. Ed. 2020, 40, 68–75. [Google Scholar]
- Alscher, R.; Erturk, N.; Heath, L. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Yang, L.; Hu, R.; Qi, W.; Xing, P.; Fu, H. Chemical constituents of Rhodiola kirilowii Maxim. J. Chin. Pharm. Sci. 2011, 20, 154–158. (In Chinese) [Google Scholar] [CrossRef]
- Yuan, X.; Yang, Z.; Li, Y.; Liu, Q.; Han, W. Effects of different levels of water stress on leaf photosynthetic characteristics and antioxidant enzyme activities of greenhouse tomato. Photosynthetica 2016, 54, 28–39. [Google Scholar] [CrossRef]
- Chai, S.; Tang, J.; Mallik, A.; Shi, Y.; Zou, R.; Li, J.; Wei, X. Eco-physiological basis of shade adaptation of Camellia nitidissima, a rare and endangered forest understory plant of Southeast Asia. BMC Ecol. 2018, 18, 5. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Bai, Y.; Xu, L.; Ding, H.; Wu, X.; Xu, H. Effects of different shading treatments on physiology, biochemistry and total flavonoids of Tetrastigma hemsleyanum in Zhejiang Province. Guihaia 2019, 39, 925–932. [Google Scholar]
- Baig, M.; Anand, A.; Mandal, P.; Bhatt, R. Irradiance influences contents of photosynthetic pigments and proteins in tropical grasses and legumes. Photosynthetica 2005, 43, 47–53. [Google Scholar] [CrossRef]
- Hashemi-Dezfouli, A.; Herrbert, S. Intensifying plant density response of corn with artificial shade. Agron. J. 1992, 84, 547–551. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Wang, Y.; Liu, Y. Effects of shades on the photosynthetic characteristics and chlorophyll fluorescence parameters of urtica dioica. Acta Ecol. Sin. 2007, 27, 3457–3464. [Google Scholar]
- Lichtenthaler, H.; Ač, A.; Marek, M.; Kalina, J.; Urban, O. Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef]
- Portsmuth, A.; Niinemets, Ü. Structural and physiological plasticity in response to light and nutrients in five temperate deciduous woody species of contrasting shade tolerance. Funct. Ecol. 2007, 21, 61–77. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, Z.; Yan, C.; Dong, Z.; Xu, W.; Wei, N.; An, S. The photosynthesis response to different light intensity for the endangered plant Parrotia subaequalis. J. Nanjing For. Univ. Nat. Sci. Ed. 2010, 34, 83–88. (In Chinese) [Google Scholar]
- Sharp, R.; Matthews, M.; Boyer, J. Kok effect and the quantum yield of photosynthesis: Light partially inhibits dark respiration. Plant Physiol. 1984, 75, 95–101. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhang, S. Photosynthetic physiological characteristics of four medicinal plants in the Taibai Mountain. J. Northwest For. Univ. 2013, 28, 6–10+79. (In Chinese) [Google Scholar]
- Wang, X.; Peng, Y.; Singer, J.; Fessehaie, A.; Krebs, S.; Arora, R. Seasonal changes in photosynthesis, antioxidant systems and ELIP expression in a thermonastic and non-thermonastic Rhododendron species: A comparison of photoprotective strategies in overwintering plants. Plant Sci. 2009, 177, 607–617. [Google Scholar] [CrossRef]
- Liang, W.; Nie, D.; Wu, S.; Bai, W.; Shen, S. Effects of shading on the growth and photosynthesis of Macropanax rosthornii seedlings. Chin. J. Ecol. 2015, 34, 413–419. (In Chinese) [Google Scholar]
- Jiang, Y.; Huang, Z.; Hao, H. Seasonal Variations in Chlorophyll Fluorescence of Castanopsis hystrix Seedlings Under Different Light Intensities. J. Southwest For. Univ. Nat. Sci. Ed. 2014, 34, 8–12. (In Chinese) [Google Scholar]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Gan, T.; Zhao, N.; Yin, G.; Chen, M.; Wang, X.; Liu, J.; Liu, W. Optimal chlorophyll fluorescence parameter selection for rapid and sensitive detection of lead toxicity to marine microalgae Nitzschia closterium based on chlorophyll fluorescence technology. J. Photochem. Photobiol. B Biol. 2019, 197, 111551. [Google Scholar] [CrossRef]


| Shading Treatments | Leaf Length (cm) | Leaf Width (cm) | Leaf Area (cm2) | Leaf Moisture Content (%) | Growth of Trunk Height (cm) |
|---|---|---|---|---|---|
| CK | 9.17 ± 0.26 b | 8.07 ± 0.65 ab | 41.01 ± 1.27 b | 67.77 ± 0.62 a | 7.60 ± 0.94 b |
| T1 | 8.56 ± 0.04 b | 6.93 ± 0.19 b | 38.71 ± 0.88 b | 69.57 ± 1.70 a | 13.90 ± 2.08 ab |
| T2 | 9.01 ± 0.23 b | 7.17 ± 0.15 b | 41.56 ± 1.74 b | 68.27 ± 0.63 a | 13.73 ± 3.90 ab |
| T3 | 10.64 ± 0.12 a | 8.95 ± 0.07 a | 51.95 ± 0.65 a | 71.09 ± 0.78 a | 17.33 ± 1.86 a |
| T4 | 10.57 ± 0.48 a | 8.87 ± 0.29 a | 49.32 ± 0.70 a | 69.25 ± 1.03 a | 15.40 ± 3.54 a |
| Physiological Indicators | Shading Treatments | Shading Time (d) | ||
|---|---|---|---|---|
| Spring | Summer | Autumn | ||
| Pro (μmol·g−1) | CK | 40.27 ± 0.81 Ba | 56.19 ± 2.49 Aa | 30.20 ± 1.21 Ca |
| T1 | 37.58 ± 1.58 Bab | 46.97 ± 1.54 Aab | 24.56 ± 1.43 Cb | |
| T2 | 29.96 ± 1.96 Bab | 41.91 ± 3.42 Ab | 19.33 ± 1.21 Cc | |
| T3 | 24.90 ± 1.17 Bb | 30.91 ± 2.10 Ab | 15.91 ± 1.07 Cc | |
| T4 | 25.92 ± 0.74 Bb | 36.79 ± 0.52 Ab | 17.75 ± 0.63 Cc | |
| SS (U·g−1) | CK | 19.96 ± 0.19 Bd | 22.31 ± 0.62 Ac | 17.20 ± 0.18 Cd |
| T1 | 21.14 ± 0.96 Acd | 22.69 ± 1.01 Ac | 22.54 ± 0.16 Ac | |
| T2 | 21.85 ± 0.39 Bbc | 30.63 ± 1.25 Ab | 23.19 ± 1.54 Bc | |
| T3 | 24.14 ± 0.46 Ca | 36.32 ± 0.11 Aa | 29.56 ± 0.59 Ba | |
| T4 | 22.64 ± 0.51 Cb | 34.23 ± 0.79 Aa | 26.92 ± 0.26 Bb | |
| SP (U·g−1·min−1) | CK | 7.90 ± 0.14 Cd | 12.36 ± 0.31 Ac | 11.44 ± 0.36 Bd |
| T1 | 9.09 ± 0.22 Bc | 11.81 ± 0.07 Ad | 12.02 ± 0.35 Ad | |
| T2 | 8.94 ± 0.22 Cc | 14.86 ± 0.26 Ab | 13.08 ± 0.23 Bc | |
| T3 | 11.73 ± 0.12 Bb | 16.81 ± 0.33 Aa | 16.66 ± 0.36 Aa | |
| T4 | 12.91 ± 0.21 Ba | 13.18 ± 0.49 ABc | 14.85 ± 0.18 Ab | |
| Physiological Indicators | Shading Treatments | Shading Time (d) | ||
|---|---|---|---|---|
| Spring | Summer | Autumn | ||
| MDA (umol·g−1) | CK | 58.22 ± 2.49 Ba | 70.72 ± 2.78 Aa | 78.80 ± 6.17 Aa |
| T1 | 53.68 ± 0.25 Bab | 61.81 ± 4.44 Aab | 71.66 ± 6.29 Aa | |
| T2 | 50.11 ± 0.58 Aab | 54.20 ± 3.16 Abc | 51.72 ± 4.11 Ab | |
| T3 | 44.46 ± 5.40 Ab | 46.41 ± 1.36 Ac | 39.35 ± 7.76 Ab | |
| T4 | 47.51 ± 0.34 Ab | 51.95 ± 0.93 Ac | 46.72 ± 3.17 Ab | |
| SOD (U·g−1) | CK | 719.44 ± 6.96 Aa | 733.05 ± 39.64 Aa | 563.18 ± 27.54 Ba |
| T1 | 683.94 ± 18.39 Aa | 686.98 ± 38.11 Aa | 557.76 ± 22.73 Ba | |
| T2 | 680.33 ± 4.66 Aa | 681.21 ± 25.67 Aa | 521.49 ± 21.84 Ba | |
| T3 | 632.11 ± 9.71 Ab | 639.03 ± 21.92 Ab | 483.24 ± 6.84 Bb | |
| T4 | 567.04 ± 8.25 Ac | 572.46 ± 9.55 Ac | 511.60 ± 17.65 Bb | |
| POD (U·g−1 min−1) | CK | 545.11 ± 23.59 Ca | 746.56 ± 15.34 Aa | 652.78 ± 4.68 Ba |
| T1 | 502.78 ± 37.07 Ca | 668.84 ± 27.39 Aab | 618.89 ± 9.56 Bab | |
| T2 | 483.89 ± 16.77 Ca | 656.33 ± 7.18 Abc | 582.33 ± 16.25 Bb | |
| T3 | 422.92 ± 10.76 Bb | 548.11 ± 15.22 Ac | 388.11 ± 7.85 Cd | |
| T4 | 408.06 ± 14.95 Cb | 579.11 ± 8.34 Ac | 506.89 ± 18.22 Bc | |
| Physiological Indicators | Shading Treatments | Shading Time (d) | ||
|---|---|---|---|---|
| Spring | Summer | Autumn | ||
| Chl a (μg·mm−2) | CK | 0.26 ± 0.01 Ab | 0.28 ± 0.02 Ac | 0.23 ± 0.01 Bc |
| T1 | 0.27 ± 0.01 Bb | 0.30 ± 0.01 Abc | 0.22 ± 0.0 Cc | |
| T2 | 0.33 ± 0.01 Aa | 0.32 ± 0.02 Aabc | 0.24 ± 0.01 Bbc | |
| T3 | 0.34 ± 0.02 Aa | 0.36 ± 0.01 Aa | 0.31 ± 0.01 Ba | |
| T4 | 0.36 ± 0.02 Aa | 0.35 ± 0.02 Aab | 0.29 ± 0.02 Bab | |
| Chl b (μg·mm−2) | CK | 0.07 ± 0.01 Bb | 0.10 ± 0.03 Aa | 0.07 ± 0.01 Bb |
| T1 | 0.11 ± 0.01 Aa | 0.12 ± 0.01 Aa | 0.08 ± 0.02 Bb | |
| T2 | 0.12 ± 0.01 Aa | 0.12 ± 0.01 Aa | 0.07 ± 0.02 Bb | |
| T3 | 0.13 ± 0.01 Ba | 0.16 ± 0.03 Aa | 0.15 ± 0.01 ABa | |
| T4 | 0.13 ± 0.01 Aa | 0.14 ± 0.03 Aa | 0.14 ± 0.01 Aa | |
| Cx+c (μg·mm−2) | CK | 0.03 ± 0.01 Aa | 0.05 ± 0.01 Aa | 0.04 ± 0.01 Aa |
| T1 | 0.04 ± 0.014 Aa | 0.04 ± 0.01 Aa | 0.04 ± 0.01 Aa | |
| T2 | 0.04 ± 0.01 Aa | 0.05 ± 0.01 Aa | 0.05 ± 0.01 Aa | |
| T3 | 0.05 ± 0.01 Aa | 0.04 ± 0.02 Aa | 0.04 ± 0.01 Aa | |
| T4 | 0.04 ± 0.01 Aa | 0.05 ± 0.01 Aa | 0.04 ± 0.01 Aa | |
| Physiological Indicators | Shading Treatments | Shading Time (d) | ||
|---|---|---|---|---|
| Spring | Summer | Autumn | ||
| Pn max (μmol·m−2·s−1) | CK | 2.26 ± 0.18 Ac | 2.19 ± 0.21 Ac | 1.97 ± 0.09 Ae |
| T1 | 4.58 ± 0.51 Aab | 3.50 ± 0.43 ABb | 2.61 ± 0.03 Bd | |
| T2 | 4.93 ± 0.69 Aab | 3.78 ± 0.32 Bb | 3.48 ± 0.07 Cc | |
| T3 | 6.18 ± 0.98 Aa | 5.78 ± 0.58 Aa | 4.61 ± 0.45 Ba | |
| T4 | 6.32 ± 0.05 Aa | 5.29 ± 0.26 Ba | 4.05 ± 0.12 Cb | |
| LCP (μmol·m−2·s−1) | CK | 19.17 ± 1.39 Ba | 14.17 ± 0.43 Ca | 25.88 ± 0.33 Aa |
| T1 | 16.35 ± 1.30 Bb | 13.12 ± 0.81 Cab | 18.08 ± 0.49 Ab | |
| T2 | 13.39 ± 1.09 Abc | 10.67 ± 0.13 Bbc | 15.49 ± 1.55 Ab | |
| T3 | 12.09 ± 1.07 Bcd | 9.84 ± 2.25 Cc | 16.54 ± 0.16 Ab | |
| T4 | 9.31 ± 1.04 Bd | 7.69 ± 0.59 Bc | 16.53 ± 1.98 Ab | |
| LSP (μmol·m−2·s−1) | CK | 865.80 ± 6.00 Ac | 709.46 ± 6.00 Bb | 683.73 ± 87.25 Cc |
| T1 | 892.93 ± 12.79 Ac | 741.24 ± 86.87 Ab | 895.49 ± 56.79 Ab | |
| T2 | 990.91 ± 44.37 Aab | 981.13 ± 76.19 Aab | 1022.59 ± 13.14 Aab | |
| T3 | 915.15 ± 18.11 Bbc | 1066.66 ± 0.01 Aa | 1043.63 ± 17.53 Aa | |
| T4 | 1066.67 ± 0.01 Aa | 1076.00 ± 118.43 Aa | 1033.70 ± 13.70 Aab | |
| Rd (μmol·m−2·s−1) | CK | 0.67 ± 0.04 Bab | 0.57 ± 0.05 Ba | 0.91 ± 0.05 Aa |
| T1 | 0.75 ± 0.09 Aa | 0.56 ± 0.01 Ba | 0.85 ± 0.02 Aab | |
| T2 | 0.62 ± 0.05 ABab | 0.55 ± 0.02 Bab | 0.68 ± 0.05 Ac | |
| T3 | 0.51 ± 0.06 Bbc | 0.38 ± 0.04 Cb | 0.67 ± 0.01 Ac | |
| T4 | 0.40 ± 0.03 Bc | 0.47 ± 0.06 Bab | 0.72 ± 0.04 Abc | |
| Physiological Indicators | Shading Treatments | Shading Time (d) | ||
|---|---|---|---|---|
| Spring | Summer | Autumn | ||
| φPo | CK | 0.53 ± 0.03 Ac | 0.44 ±0.02 Bc | 0.42 ± 0.02 Bd |
| T1 | 0.55 ± 0.02 Ac | 0.48 ± 0.02 Bc | 0.51 ± 0.02 Bc | |
| T2 | 0.58 ± 0.01 Ac | 0.55 ±0.02 Ab | 0.53 ±0.03 Ac | |
| T3 | 0.63 ± 0.01 Ab | 0.62 ± 0.01 Aa | 0.60 ± 0.02 Aa | |
| T4 | 0.68 ± 0.01 Aa | 0.59 ± 0.02 Bab | 0.58 ± 0.01 Bb | |
| Ψo | CK | 0.42 ± 0.04 Ab | 0.31 ± 0.02 Bb | 0.20 ± 0.03 Cc |
| T1 | 0.44 ± 0.04 Aab | 0.35 ± 0.05 Aab | 0.37 ± 0.03 Ab | |
| T2 | 0.48 ± 0.02 Aab | 0.45 ± 0.02 ABa | 0.40 ± 0.04 Bb | |
| T3 | 0.50 ± 0.02 Aab | 0.45 ± 0.03 Aa | 0.49 ± 0.03 Aa | |
| T4 | 0.56 ± 0.01 Aa | 0.42 ± 0.03 Bab | 0.40 ± 0.04 Bb | |
| φEo | CK | 0.23 ± 0.04 Ac | 0.13 ±0.01 Bc | 0.08 ± 0.02 Cc |
| T1 | 0.25 ± 0.03 Abc | 0.17 ± 0.03 Bbc | 0.22 ± 0.02 Ab | |
| T2 | 0.28 ± 0.02 Abc | 0.25 ± 0.02 ABab | 0.22 ± 0.03 Bb | |
| T3 | 0.31 ± 0.01 Ab | 0.28 ± 0.02 Aa | 0.32 ± 0.02 Aa | |
| T4 | 0.38 ± 0.01 Aa | 0.23 ± 0.02 Bab | 0.23 ± 0.03 Bb | |
| φDo | CK | 0.47 ± 0.03 Ba | 0.56 ± 0.02 Aa | 0.58 ± 0.03 Aa |
| T1 | 0.45 ± 0.01 Ba | 0.52 ± 0.02 Aa | 0.49 ± 0.02 Aab | |
| T2 | 0.42 ± 0.01 Ba | 0.45 ±0.02 Ab | 0.48 ± 0.03 Aab | |
| T3 | 0.37 ± 0.01 Ab | 0.38 ±0.01 Ac | 0.40 ±0.02 Ab | |
| T4 | 0.32 ± 0.01 Bc | 0.41 ± 0.02 Abc | 0.42 ± 0.01 Ab | |
| Subordinate Function Values | Spring Shading Treatments | Summer Shading Treatments | Autumn Shading Treatments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | T4 | CK | T1 | T2 | T3 | T4 | CK | T1 | T2 | T3 | T4 | |
| Leaf length | 0.219 | 0.064 | 0.177 | 0.613 | 0.595 | - | - | - | - | - | - | - | - | - | - |
| Leaf width | 0.400 | 0.111 | 0.173 | 0.622 | 0.604 | - | - | - | - | - | - | - | - | - | - |
| Leaf area | 0.271 | 0.141 | 0.299 | 0.888 | 0.740 | - | - | - | - | - | - | - | - | - | - |
| Leaf moisture content | 0.176 | 0.464 | 0.256 | 0.636 | 0.412 | - | - | - | - | - | - | - | - | - | - |
| Growth of trunk height | 0.141 | 0.481 | 0.510 | 0.666 | 0.562 | - | - | - | - | - | - | - | - | - | - |
| Proline | 0.086 | 0.182 | 0.612 | 0.756 | 0.818 | 0.145 | 0.475 | 0.583 | 0.667 | 0.652 | 0.099 | 0.413 | 0.704 | 0.895 | 0.792 |
| Soluble sugars | 0.072 | 0.092 | 0.352 | 0.871 | 0.584 | 0.080 | 0.104 | 0.619 | 0.987 | 0.851 | 0.019 | 0.422 | 0.472 | 0.953 | 0.754 |
| Soluble protein | 0.050 | 0.258 | 0.232 | 0.722 | 0.929 | 0.296 | 0.027 | 0.558 | 0.895 | 0.265 | 0.112 | 0.201 | 0.367 | 0.924 | 0.643 |
| Malondialdehyde | 0.148 | 0.308 | 0.433 | 0.632 | 0.525 | 0.136 | 0.421 | 0.664 | 0.914 | 0.736 | 0.139 | 0.257 | 0.584 | 0.788 | 0.666 |
| Superoxide dismutase | 0.041 | 0.241 | 0.262 | 0.534 | 0.901 | 0.300 | 0.476 | 0.498 | 0.660 | 0.915 | 0.291 | 0.331 | 0.600 | 0.883 | 0.673 |
| Peroxidase | 0.348 | 0.313 | 0.257 | 0.924 | 0.435 | 0.101 | 0.411 | 0.461 | 0.892 | 0.768 | 0.025 | 0.144 | 0.271 | 0.950 | 0.535 |
| Chlorophyll a | 0.075 | 0.075 | 0.550 | 0.582 | 0.697 | 0.257 | 0.419 | 0.536 | 0.756 | 0.706 | 0.211 | 0.146 | 0.280 | 0.747 | 0.599 |
| Chlorophyll b | 0.043 | 0.043 | 0.561 | 0.578 | 0.732 | 0.247 | 0.374 | 0.374 | 0.534 | 0.468 | 0.311 | 0.330 | 0.304 | 0.862 | 0.737 |
| Carotenoid | 0.236 | 0.236 | 0.614 | 0.603 | 0.530 | 0.508 | 0.418 | 0.557 | 0.494 | 0.559 | 0.517 | 0.505 | 0.685 | 0.642 | 0.431 |
| Maximum net photosynthetic rate | 0.063 | 0.506 | 0.573 | 0.825 | 0.840 | 0.109 | 0.389 | 0.449 | 0.875 | 0.770 | 0.049 | 0.262 | 0.552 | 0.926 | 0.741 |
| Light Saturation Point | 0.323 | 0.554 | 0.651 | 0.819 | 0.895 | 0.145 | 0.272 | 0.568 | 0.668 | 0.928 | 0.027 | 0.541 | 0.712 | 0.643 | 0.643 |
| Light Compensation Point | 0.150 | 0.333 | 0.671 | 0.561 | 1.000 | 0.187 | 0.241 | 0.643 | 0.786 | 0.802 | 0.253 | 0.669 | 0.918 | 0.960 | 0.935 |
| Dark respiration rate | 0.313 | 0.398 | 0.431 | 0.766 | 0.885 | 0.260 | 0.294 | 0.328 | 0.881 | 0.567 | 0.166 | 0.905 | 0.591 | 0.603 | 0.518 |
| Stomatal conductance | 0.177 | 0.590 | 0.568 | 0.888 | 0.888 | 0.011 | 0.120 | 0.416 | 0.786 | 0.492 | 0.085 | 0.133 | 0.283 | 0.825 | 0.514 |
| Transpiration rate | 0.120 | 0.401 | 0.496 | 0.961 | 0.671 | 0.015 | 0.191 | 0.483 | 0.812 | 0.610 | 0.039 | 0.252 | 0.439 | 0.843 | 0.460 |
| Intercellular carbon dioxide concentration | 0.231 | 0.358 | 0.384 | 0.627 | 0.882 | 0.356 | 0.633 | 0.742 | 0.969 | 0.992 | 0.162 | 0.179 | 0.669 | 0.828 | 0.621 |
| φPo | 0.361 | 0.449 | 0.559 | 0.742 | 0.931 | 0.195 | 0.340 | 0.585 | 0.814 | 0.702 | 0.354 | 0.497 | 0.641 | 0.839 | 0.774 |
| Ψo | 0.465 | 0.517 | 0.633 | 0.698 | 0.768 | 0.274 | 0.381 | 0.630 | 0.623 | 0.459 | 0.235 | 0.548 | 0.666 | 0.831 | 0.664 |
| φEo | 0.415 | 0.475 | 0.597 | 0.721 | 0.871 | 0.112 | 0.235 | 0.517 | 0.611 | 0.432 | 0.181 | 0.428 | 0.550 | 0.777 | 0.600 |
| φDo | 0.361 | 0.449 | 0.559 | 0.742 | 0.931 | 0.195 | 0.340 | 0.585 | 0.814 | 0.702 | 0.354 | 0.497 | 0.641 | 0.839 | 0.774 |
| Mean value | 0.211 | 0.322 | 0.456 | 0.719 | 0.745 | 0.197 | 0.328 | 0.540 | 0.772 | 0.669 | 0.181 | 0.383 | 0.546 | 0.828 | 0.654 |
| Response evaluation | 5 | 4 | 3 | 2 | 1 | 5 | 4 | 3 | 1 | 2 | 5 | 4 | 3 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Wang, Z.; Guan, J.; Zhao, M.; Yu, L.; Zhou, X.; Xia, G. Seasonal Variations in the Growth and Physiology of Acer miaotaiense subsp. yangjuechi Fang et P. L. Chiu Seedlings Under Shading Treatments. Forests 2025, 16, 296. https://doi.org/10.3390/f16020296
Chen T, Wang Z, Guan J, Zhao M, Yu L, Zhou X, Xia G. Seasonal Variations in the Growth and Physiology of Acer miaotaiense subsp. yangjuechi Fang et P. L. Chiu Seedlings Under Shading Treatments. Forests. 2025; 16(2):296. https://doi.org/10.3390/f16020296
Chicago/Turabian StyleChen, Taomei, Zhiping Wang, Jingwen Guan, Mingshui Zhao, Lin Yu, Xinyang Zhou, and Guohua Xia. 2025. "Seasonal Variations in the Growth and Physiology of Acer miaotaiense subsp. yangjuechi Fang et P. L. Chiu Seedlings Under Shading Treatments" Forests 16, no. 2: 296. https://doi.org/10.3390/f16020296
APA StyleChen, T., Wang, Z., Guan, J., Zhao, M., Yu, L., Zhou, X., & Xia, G. (2025). Seasonal Variations in the Growth and Physiology of Acer miaotaiense subsp. yangjuechi Fang et P. L. Chiu Seedlings Under Shading Treatments. Forests, 16(2), 296. https://doi.org/10.3390/f16020296





