Sodium Alginate as a Green Consolidant for Waterlogged Wood—A Preliminary Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Waterlogged Wood Treatment
2.2.2. Assessment of the Effectiveness of the Applied Wood Treatment
2.2.3. Assessment of the Stabilising Effectiveness
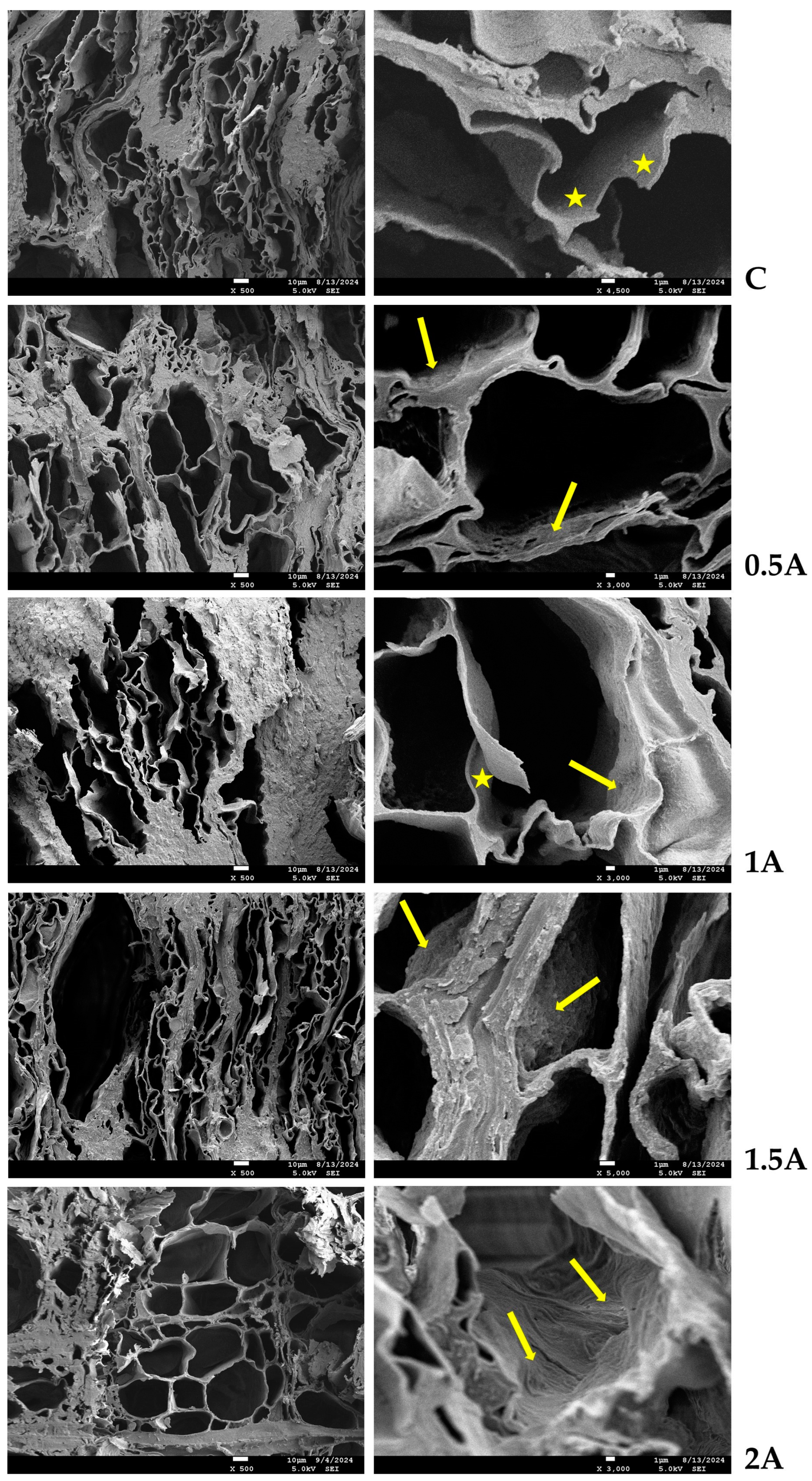
2.2.4. Scanning Electron Microscopy Imaging
2.2.5. Infrared Spectroscopy
3. Results and Discussion
3.1. Effectiveness of the Applied Alginate Treatment
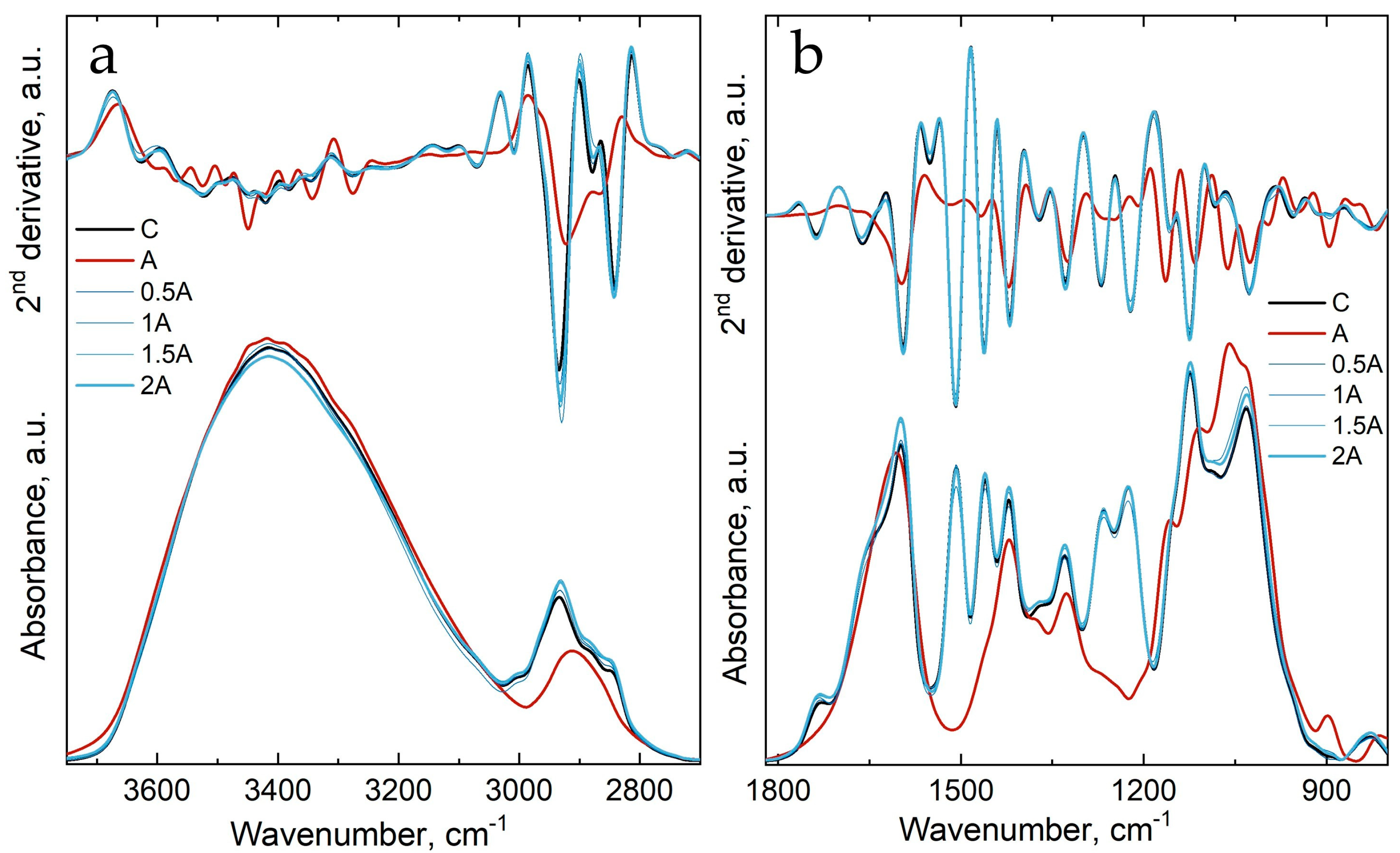
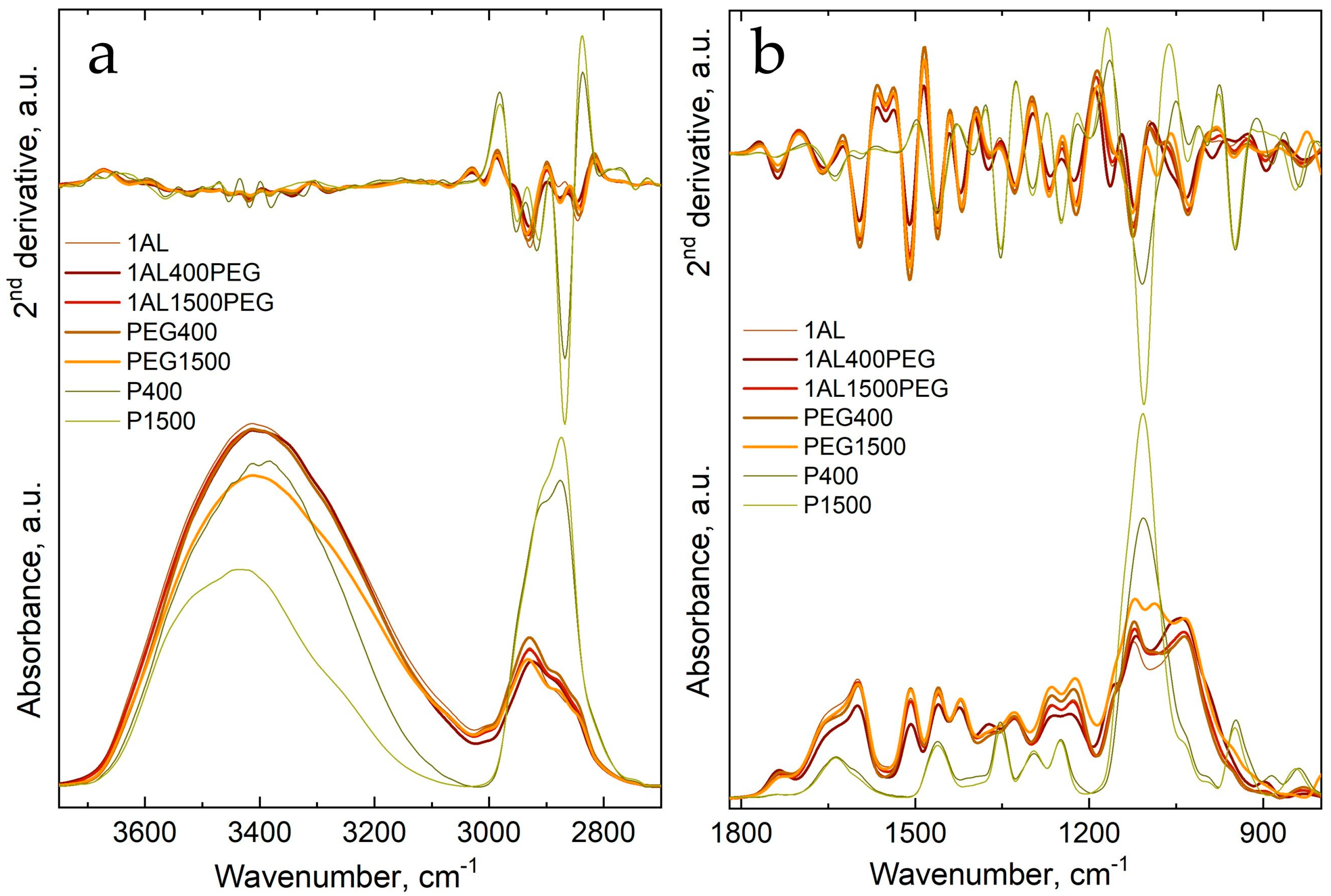
3.2. Fourier Transform Infrared Spectroscopy of Untreated and Treated Wood
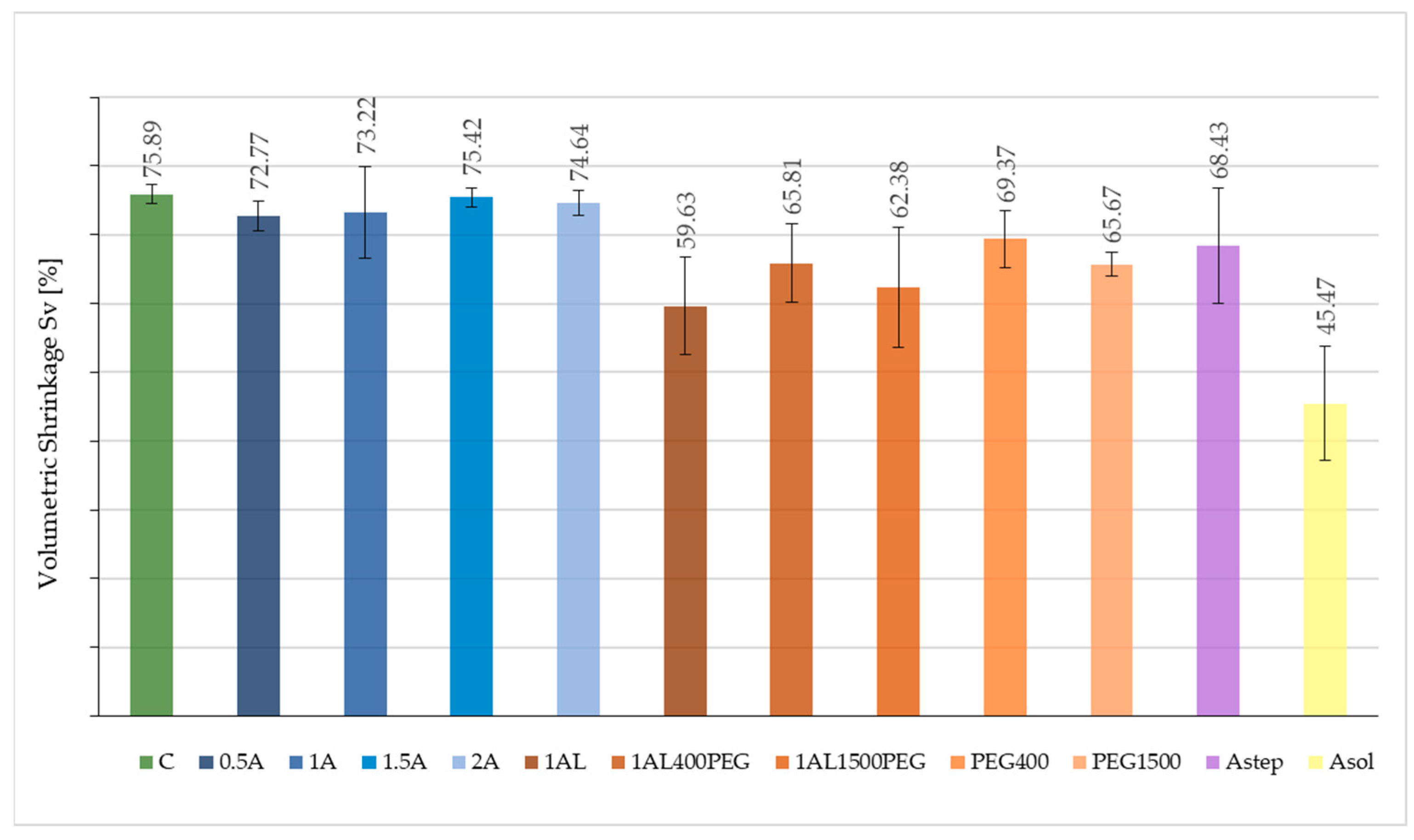
3.3. Stabilising Effectiveness of Alginate-Based Treatments
3.4. Microscopic Observations of Untreated and Treated Wood
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jordan, B.A. Site characteristics impacting the survival of historic waterlogged wood: A review. Int. Biodeterior. Biodegrad. 2001, 47, 47–54. [Google Scholar] [CrossRef]
- Broda, M.; Hill, C.A.S. Conservation of Waterlogged Wood—Past, Present and Future Perspectives. Forests 2021, 12, 1193. [Google Scholar] [CrossRef]
- Björdal, C.G. Microbial degradation of waterlogged archaeological wood. J. Cult. Herit. 2012, 13, S118–S122. [Google Scholar] [CrossRef]
- Capretti, C.; Macchioni, N.; Pizzo, B.; Galotta, G.; Giachi, G.; Giampaola, D. The characterization of waterlogged archaeological wood: The three roman ships found in naples (Italy). Archaeometry 2008, 50, 855–876. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Chavan, R.R. Advances in Understanding Microbial Deterioration of Buried and Waterlogged Archaeological Woods: A Review. Forests 2022, 13, 394. [Google Scholar] [CrossRef]
- Björdal, C.; Nilsson, T.; Daniel, G. Microbial decay of waterlogged archaeological wood found in Sweden Applicable to archaeology and conservation. Int. Biodeterior. Biodegrad. 1999, 43, 63–73. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Singh, T. Bacterial Degradation of Wood. In Secondary Xylem Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 169–190. [Google Scholar]
- Hocker, E.; Almkvist, G.; Sahlstedt, M. The Vasa experience with polyethylene glycol: A conservator’s perspective. J. Cult. Herit. 2012, 13, S175–S182. [Google Scholar] [CrossRef]
- Collett, H.; Bouville, F.; Giuliani, F.; Schofield, E. Structural Monitoring of a Large Archaeological Wooden Structure in Real Time, Post PEG Treatment. Forests 2021, 12, 1788. [Google Scholar] [CrossRef]
- Mortensen, M.N.; Egsgaard, H.; Hvilsted, S.; Shashoua, Y.; Glastrup, J. Characterisation of the polyethylene glycol impregnation of the Swedish warship Vasa and one of the Danish Skuldelev Viking ships. J. Archaeol. Sci. 2007, 34, 1211–1218. [Google Scholar] [CrossRef]
- Piva, E. Conservation of a Tudor Warship: Investigating the Timbers of the Mary Rose. Ph.D. Thesis, University of Portsmouth, Portsmouth, UK, 2017. [Google Scholar]
- Hoffmann, P.; Singh, A.; Kim, Y.; Wi, S.; Kim, I.-J.; Schmitt, U. The Bremen Cog of 1380—An electron microscopic study of its degraded wood before and after stabilization with PEG. Holzforschung 2004, 58, 211–218. [Google Scholar] [CrossRef]
- Hoffmann, P. To be and to continue being a Cog: The conservation of the Bremen Cog of 1380. Int. J. Naut. Archaeol. 2001, 30, 129–140. [Google Scholar] [CrossRef]
- Ossowski, W. (Ed.) The Copper Ship: A Medieval Shipwreck and Its Cargo; Narodowe Muzeum Morskie: Gdańsk, Poland, 2014. [Google Scholar]
- Grattan, D. A practical comparative study of several treatments for waterlogged wood. Stud. Conserv. 1982, 27, 124–136. [Google Scholar] [CrossRef]
- Jensen, P.; Schnell, U. The Implications of Using Low Molecular Weight PEG for Impregnation of Waterlogged Archaeological Wood Prior to Freeze Drying. In Proceedings of the 9th ICOM Group on Wet Organic Archaeological Materials Conference, Copenhagen, Denmark, 7–11 June 2004; pp. 279–308. [Google Scholar]
- Hoffmann, P. On the stabilization of waterlogged oakwood with PEG. II. Designing a two-step treatment for multi-quality timbers. Stud. Conserv. 1986, 31, 103–113. [Google Scholar] [CrossRef]
- Albright, A.B. The Preservation of Small Water-Logged Wood Specimens with Polyethylene Glycol. Curator Mus. J. 1966, 9, 228–234. [Google Scholar] [CrossRef]
- Smith, C.W. Archaeological Conservation Using Polymers: Practical Applications for Organic Artifact Stabilization; Texas A&M University Press: College Station, TX, USA, 2006. [Google Scholar]
- Lechner, T.; Bjurhager, I.; Kliger, R.I. Strategy for developing a future support system for the Vasawarship and evaluating its mechanical properties. Herit. Sci. 2013, 1, 35. [Google Scholar] [CrossRef]
- Bjurhager, I.; Ljungdahl, J.; Wallström, L.; Gamstedt, E.K.; Berglund, L.A. Towards improved understanding of PEG-impregnated waterlogged archaeological wood: A model study on recent oak. Holzforschung 2010, 64, 243–250. [Google Scholar] [CrossRef]
- Vorobyev, A.; van Dijk, N.P.; Gamstedt, E.K. Orthotropic creep in polyethylene glycol impregnated archaeological oak from the Vasa ship. Mech. Time Depend. Mater. 2018, 23, 35–52. [Google Scholar] [CrossRef]
- Olek, W.; Majka, J.; Stempin, A.; Sikora, M.; Zborowska, M. Hygroscopic properties of PEG treated archaeological wood from the rampart of the 10th century stronghold as exposed in the Archaeological Reserve Genius loci in Poznań (Poland). J. Cult. Herit. 2016, 18, 299–305. [Google Scholar] [CrossRef]
- Almkvist, G.; Hocker, E.; Sahlstedt, M. Iron Removal from Waterlogged Wood; SLU Repro: Uppsala, Sweden, 2013. [Google Scholar]
- Almkvist, G.; Persson, I. Degradation of polyethylene glycol and hemicellulose in the Vasa. Holzforschung 2007, 62, 64–70. [Google Scholar] [CrossRef]
- Glastrup, J.; Shashoua, Y.; Egsgaard, H.; Mortensen, M.N. Degradation of PEG in the Warship Vasa. Macromol. Symp. 2006, 238, 22–29. [Google Scholar] [CrossRef]
- Kaye, B. Conservation of waterlogged archaeological wood. Chem. Soc. Rev. 1995, 24, 35–43. [Google Scholar] [CrossRef]
- Grattan, D.W.; Clarke, R.W. 9—Conservation of Waterlogged Wood. In Conservation of Marine Archaeological Objects; Pearson, C., Ed.; Butterworth-Heinemann: Oxford, UK, 1987; pp. 164–206. ISBN 978-0-408-10668-9. [Google Scholar]
- Liu, L.; Zhang, L.; Zhang, B.; Hu, Y. A comparative study of reinforcement materials for waterlogged wood relics in laboratory. J. Cult. Herit. 2018, 36, 94–102. [Google Scholar] [CrossRef]
- Kennedy, A.; Pennington, E.R. Conservation of chemically degraded waterlogged wood with sugars. Stud. Conserv. 2014, 59, 194–201. [Google Scholar] [CrossRef]
- Babiński, L. Dimensional changes of waterlogged archaeological hardwoods pre-treated with aqueous mixtures of lactitol/trehalose and mannitol/trehalose before freeze-drying. J. Cult. Herit. 2015, 16, 876–882. [Google Scholar] [CrossRef]
- Tahira, A.; Howard, W.; Pennington, E.R.; Kennedy, A. Mechanical strength studies on degraded waterlogged wood treated with sugars. Stud. Conserv. 2016, 62, 223–228. [Google Scholar] [CrossRef]
- Han, L.; Guo, J.; Tian, X.; Jiang, X.; Yin, Y. Evaluation of PEG and sugars consolidated fragile waterlogged archaeological wood using nanoindentation and ATR-FTIR imaging. Int. Biodeterior. Biodegrad. 2022, 170, 105390. [Google Scholar] [CrossRef]
- Parrent, J.M. The conservation of waterlogged wood using sucrose. Stud. Conserv. 1985, 30, 63–72. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Liu, L.; Zhang, B.; Jiang, L. Preparation of bacterial cellulose for xylitol-reinforced waterlogged wood. Archaeometry 2024, 66, 618–632. [Google Scholar] [CrossRef]
- Endo, R.; Kamei, K.; Iida, I.; Yokoyama, M.; Kawahara, Y. Physical and mechanical properties of waterlogged wood treated with hydrolyzed feather keratin. J. Archaeol. Sci. 2010, 37, 1311–1316. [Google Scholar] [CrossRef]
- Endo, R.; Kamei, K.; Iida, I.; Kawahara, Y. Dimensional stability of waterlogged wood treated with hydrolyzed feather keratin. J. Archaeol. Sci. 2008, 35, 1240–1246. [Google Scholar] [CrossRef]
- Endo, R.; Sugiyama, J. New attempts with the keratin-metal/magnesium process for the conservation of archaeological waterlogged wood. J. Cult. Herit. 2022, 54, 53–58. [Google Scholar] [CrossRef]
- Endo, R.; Sugiyama, J. Evaluation of cell wall reinforcement in feather keratin-treated waterlogged wood as imaged by synchrotron X-ray microtomography (μXRT) and TEM. Holzforschung 2013, 67, 795–803. [Google Scholar] [CrossRef]
- Giachi, G.; Capretti, C.; Donato, I.D.; Macchioni, N.; Pizzo, B. New trials in the consolidation of waterlogged archaeological wood with different acetone-carried products. J. Archaeol. Sci. 2011, 38, 2957–2967. [Google Scholar] [CrossRef]
- Christensen, M.; Hansen, F.K.; Kutzke, H. Phenol Formaldehyde Revisited-Novolac Resins for the Treatment of Degraded Archaeological Wood. Archaeometry 2014, 57, 536–559. [Google Scholar] [CrossRef]
- Broda, M.; Dąbek, I.; Dutkiewicz, A.; Dutkiewicz, M.; Popescu, C.-M.; Mazela, B.; Maciejewski, H. Organosilicons of different molecular size and chemical structure as consolidants for waterlogged archaeological wood—A new reversible and retreatable method. Sci. Rep. 2020, 10, 2188. [Google Scholar] [CrossRef] [PubMed]
- Broda, M.; Jakes, J.E.; Li, L.; Antipova, O.A.; Maxey, E.R.; Jin, Q. Conservation of model degraded pine wood with selected organosilicons studied by XFM and nanoindentation. Wood Sci. Technol. 2024, 58, 649–675. [Google Scholar] [CrossRef]
- Parisi, F.; Bernardini, F.; Cavallaro, G.; Mancini, L.; Milioto, S.; Prokop, D.; Lazzara, G. Halloysite nanotubes/pluronic nanocomposites for waterlogged archeological wood: Thermal stability and X-ray microtomography. J. Therm. Anal. Calorim. 2020, 141, 981–989. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Ruisi, F. Nanocomposites based on esterified colophony and halloysite clay nanotubes as consolidants for waterlogged archaeological woods. Cellulose 2017, 24, 3367–3376. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Parisi, F.; Lazzara, G. Halloysite Nanotubes Loaded with Calcium Hydroxide: Alkaline Fillers for the Deacidification of Waterlogged Archeological Woods. ACS Appl. Mater. Interfaces 2018, 10, 27355–27364. [Google Scholar] [CrossRef]
- Walsh, Z.; Janeček, E.-R.; Jones, M.; Scherman, O.A. Natural polymers as alternative consolidants for the preservation of waterlogged archaeological wood. Stud. Conserv. 2016, 62, 173–183. [Google Scholar] [CrossRef]
- Walsh-Korb, Z.; Stelzner, I.; dos Santos Gabriel, J.; Eggert, G.; Avérous, L. Morphological Study of Bio-Based Polymers in the Consolidation of Waterlogged Wooden Objects. Materials 2022, 15, 681. [Google Scholar] [CrossRef]
- Christensen, M.; Larnøy, E.; Kutzke, H.; Hansen, F.K. Treatment of waterlogged archaeological wood using chitosan- and modified chitosan solutions. part 1: Chemical compatibility and microstructure. J. Am. Inst. Conserv. 2015, 54, 3–13. [Google Scholar] [CrossRef]
- Gregory, D.J.; Shashoua, Y.; Hansen, N.B.; Jensen, P. Anyone for a Nice Cup of Tea? The Use of Bacterial Cellulose for Conservation of Waterlogged Archaeological Wood. In Proceedings of the ICOM-CC 18th Triennial Conference, Copenhagen, Denmark, 4–8 September 2017. [Google Scholar]
- Cipriani, G.; Salvini, A.; Baglioni, P.; Bucciarelli, E. Cellulose as a renewable resource for the synthesis of wood consolidants. J. Appl. Polym. Sci. 2010, 118, 2939–2950. [Google Scholar] [CrossRef]
- Cipriani, G.; Salvini, A.; Fioravanti, M.; Di Giulio, G.; Malavolti, M. Synthesis of hydroxylated oligoamides for their use in wood conservation. J. Appl. Polym. Sci. 2012, 127, 420–431. [Google Scholar] [CrossRef]
- McHale, E.; Braovac, S.; Steindal, C.C.; Gillis, R.B.; Adams, G.G.; Harding, S.E.; Benneche, T.; Kutzke, H. Synthesis and characterisation of lignin-like oligomers as a bio-inspired consolidant for waterlogged archaeological wood. Pure Appl. Chem. 2016, 88, 969–977. [Google Scholar] [CrossRef]
- McHale, E.; Steindal, C.C.; Kutzke, H.; Benneche, T.; Harding, S.E. In situ polymerisation of isoeugenol as a green consolidation method for waterlogged archaeological wood. Sci. Rep. 2017, 7, 46481. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Han, X.; Zhang, Z.; Wang, J. Effectiveness Evaluation of Silicone Oil Emulsion in Situ Polymerization for Dehydration of Waterlogged Wooden Artifacts. Molecules 2024, 29, 4971. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Wang, K.; Hu, D. Well-controlled SI-ARGET ATRP of EGDMA for maintaining the dimensions of waterlogged archaeological wood. Wood Sci. Technol. 2023, 57, 523–535. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, K.; Hu, D. Surface-initiated ARGET ATRP for maintaining the dimension of waterlogged archaeological wood (Pinus massoniana): Polymer distribution behaviors and anti-shrinkage mechanism. Wood Sci. Technol. 2021, 56, 205–218. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, K.; Hu, D. An aqueous approach to functionalize waterlogged archaeological wood followed by improved surface-initiated ARGET ATRP for maintaining dimensional stability. Cellulose 2021, 28, 2433–2443. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, K.; Hu, D. High retreatability and dimensional stability of polymer grafted waterlogged archaeological wood achieved by ARGET ATRP. Sci. Rep. 2019, 9, 9879. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.R.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes/Keratin composites for wool treatment. Appl. Clay Sci. 2023, 238, 106930. [Google Scholar] [CrossRef]
- Papacchini, A.; Dominici, S.; Di Giulio, G.; Fioravanti, M.; Salvini, A. Bio-based consolidants for waterlogged archaeological wood: Assessment of the performance and optimization of the diagnostic protocol. J. Cult. Herit. 2019, 40, 49–58. [Google Scholar] [CrossRef]
- Ciolacu, F.; Nicu, R.; Balan, T.; Bobu, E. Chitosan Derivatives as Bio-based Materials for Paper Heritage Conservation. BioResources 2016, 12, 735–747. [Google Scholar] [CrossRef]
- Çakmak, Y.; Çakmakçi, E.; Apohan, N.K.; Karadag, R. Isosorbide, pyrogallol, and limonene-containing thiol-ene photocured bio-based organogels for the cleaning of artworks. J. Cult. Herit. 2022, 55, 391–398. [Google Scholar] [CrossRef]
- Biro, C. Researching and Developing Bio-based Materials for the Transport, Conservation and Exhibition of Museum Collections: A Case Study from France. Mus. Int. 2023, 75, 136–147. [Google Scholar] [CrossRef]
- Irizar, P.; Pintor-Rial, A.; Martinez-Arkarazo, I.; Olazabal, M.; Ruiz-Rubio, L.; Cardiano, P.; Gomez-Laserna, O. Bio-based hybrid nanocomposites as multifunctional sustainable materials for stone conservation. Prog. Org. Coat. 2023, 185, 107899. [Google Scholar] [CrossRef]
- Paolino, B.; Sorrentino, M.C.; Pacifico, S. Greener solutions for biodeterioration of organic-media cultural heritage: Where are we? Herit. Sci. 2024, 12, 334. [Google Scholar] [CrossRef]
- Caruso, M.R.; D’agostino, G.; Milioto, S.; Cavallaro, G.; Lazzara, G. A review on biopolymer-based treatments for consolidation and surface protection of cultural heritage materials. J. Mater. Sci. 2023, 58, 12954–12975. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2015, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Akbar, M.; Yaqoob, A.; Ahmad, A.; Luque, R. Chapter 1—Sodium Alginate: An Overview. In Sodium Alginate-Based Nanomaterials for Wastewater Treatment; Ahmad, A., Ahmad, I., Kamal, T., Asiri, A.M., Tabassum, S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–17. ISBN 978-0-12-823551-5. [Google Scholar]
- Lee, C.; Volpi, F.; Fiocco, G.; Weththimuni, M.L.; Licchelli, M.; Malagodi, M. Preliminary Cleaning Approach with Alginate and Konjac Glucomannan Polysaccharide Gel for the Surfaces of East Asian and Western String Musical Instruments. Materials 2022, 15, 1100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Fiocco, G.; Vigani, B.; Recca, T.; Rossi, S.; Licchelli, M.; Malagodi, M.; Weththimuni, M.L.; Volpi, F. Retentive bio-based chemical gel for removing glues from water-sensitive wooden artworks. J. Cult. Herit. 2024, 68, 9–16. [Google Scholar] [CrossRef]
- Salim, E.; Taha, A. Evaluating the Use of Sodium Alginate Polymer for Eco-friendly Consolidation and De-acidification of Ancient Printed Papers. Egypt. J. Chem. 2023, 66, 585–596. [Google Scholar] [CrossRef]
- Smith, C.A.; Paterson, R.A.; Lowe, B.J.; Kanawa, R.T. Consolidation of Black-dyed Māori Textile Artefacts: Evaluating the Efficacy of Sodium Alginate. Stud. Conserv. 2016, 63, 139–154. [Google Scholar] [CrossRef]
- Abdel-Maksoud, G.; Ghozy, H.; Ezzat, R.; Helmy, M.; Elsaka, M.; Gamal, M.; Mohamed, W.S. Evaluation of the efficiency of sodium alginate for the consolidation of bone artifacts. Egypt. J. Chem. 2022, 66, 357–366. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Zhang, X.; Gu, Y.; Su, B.; Sun, X.; Chen, Q. Alginate–Nanosilver Hydrogels: A Self-Dissolving System for Comprehensive Preservation of Waterlogged Wooden Artifacts. ACS Sustain. Chem. Eng. 2024, 12, 16091–16103. [Google Scholar] [CrossRef]
- Broda, M.; Spear, M.J.; Curling, S.F.; Ormondroyd, G.A. The Viscoelastic Behaviour of Waterlogged Archaeological Wood Treated with Methyltrimethoxysilane. Materials 2021, 14, 5150. [Google Scholar] [CrossRef] [PubMed]
- Broda, M.; Mazela, B.; Radka, K. Methyltrimethoxysilane as a stabilising agent for archaeological waterlogged wood differing in the degree of degradation. J. Cult. Herit. 2019, 35, 129–139. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J. Reactivity of Waterlogged Archeological Elm Wood with Organosilicon Compounds Applied as Wood Consolidants: 2D 1H–13C Solution-State NMR Studies. Molecules 2022, 27, 3407. [Google Scholar] [CrossRef]
- Grattan, D.W. Treatment of Waterlogged Wood. In Wet Site Archaeology; CRC Press: Boca Raton, FL, USA, 1988; ISBN 978-1-351-07775-0. [Google Scholar]
- Christensen, M.; Kutzke, H.; Hansen, F.K. New materials used for the consolidation of archaeological wood–past attempts, present struggles, and future requirements. J. Cult. Herit. 2012, 13, S183–S190. [Google Scholar] [CrossRef]
- Broda, M.; Mazela, B.; Dutkiewicz, A. Organosilicon compounds with various active groups as consolidants for the preservation of waterlogged archaeological wood. J. Cult. Herit. 2019, 35, 123–128. [Google Scholar] [CrossRef]
- Broda, M. Biological effectiveness of archaeological oak wood treated with methyltrimethoxysilane and PEG against Brown-rot fungi and moulds. Int. Biodeterior. Biodegradation 2018, 134, 110–116. [Google Scholar] [CrossRef]
- Morgós, A.; Imazu, S.; Ito, K. Sugar Conservation of Waterlogged Archaeological Finds in the Last 30 Years. In Proceedings of the Condition. 2015 Conservation and Digitalization Conference, Gdańsk, Poland, 19–22 May 2015; pp. 15–20. [Google Scholar]
- Wachsmann, S.; Raveh, K.; Cohen, O. The Kinneret boat project Part, I. The excavation and conservation of the Kinneret boat. Int. J. Naut. Archaeol. 1987, 16, 233–245. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Popescu, M.-C.; Vasile, C. Structural analysis of photodegraded lime wood by means of FT-IR and 2D IR correlation spectroscopy. Int. J. Biol. Macromol. 2011, 48, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Torniainen, P.; Popescu, C.-M.; Jones, D.; Scharf, A.; Sandberg, D. Correlation of Studies between Colour, Structure and Mechanical Properties of Commercially Produced ThermoWood® Treated Norway Spruce and Scots Pine. Forests 2021, 12, 1165. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Application of FTIR spectroscopy to the characterization of archeological wood. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 63–70. [Google Scholar] [CrossRef]
- Kondo, T. Hydrogen Bonds in Cellulose and Cellulose Derivatives. Polysacch. Struct. Divers. Funct. Versatility 2005, 69–98. [Google Scholar]
- Xiao, Q.; Gu, X.; Tan, S. Drying process of sodium alginate films studied by two-dimensional correlation ATR-FTIR spectroscopy. Food Chem. 2014, 164, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.-M.; Jones, D.; Kržišnik, D.; Humar, M. Determination of the effectiveness of a combined thermal/chemical wood modification by the use of FT–IR spectroscopy and chemometric methods. J. Mol. Struct. 2020, 1200, 127133. [Google Scholar] [CrossRef]
- Nguyen, H.; Lagarde, F.; Louarn, G.; Daniel, P. A new way to discriminate polluted wood by vibrational spectroscopies. Talanta 2017, 167, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Gelbrich, J.; Mai, C.; Militz, H. Chemical changes in wood degraded by bacteria. Int. Biodeterior. Biodegrad. 2008, 61, 24–32. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Chao, Y.; Nawawi, D.S.; Akiyama, T.; Yokoyama, T.; Matsumoto, Y. Analysis of Lignin Aromatic Structure in Wood Based on the IR Spectrum. J. Wood Chem. Technol. 2012, 32, 294–303. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Broda, M. Interactions between Different Organosilicons and Archaeological Waterlogged Wood Evaluated by Infrared Spectroscopy. Forests 2021, 12, 268. [Google Scholar] [CrossRef]
- Khanna, L.; Verma, N.K. PEG/CaFe2O4 nanocomposite: Structural, morphological, magnetic and thermal analyses. Phys. B Condens. Matter 2013, 427, 68–75. [Google Scholar] [CrossRef]
- Grattan, D.W.; Mccawley, J.C.; Cook, C. The potential of the Canadian winter climate for the freeze-drying of degraded waterlogged wood: Part II. Stud. Conserv. 1980, 25, 118–136. [Google Scholar] [CrossRef]
- Babiński, L.; Fabisiak, E.; Dąbrowski, H.P.; Kittel, P. Study on dimensional stabilization of 12,500-year-old waterlogged subfossil Scots pine wood from the Koźmin Las site, Poland. J. Cult. Herit. 2016, 23, 119–127. [Google Scholar] [CrossRef]
- Kılıç, N.; Kılıç, A.G. Evaluation of the Polyethylene Glycol Impregnation and Vacuum Freeze-Drying Method for Waterlogged Archaeological Wood: Conservation of the Yenikapı 1 Shipwreck. Stud. Conserv. 2023, 69, 336–347. [Google Scholar] [CrossRef]
- Stelzner, J.; Stelzner, I.; Martinez-Garcia, J.; Gwerder, D.; Wittköpper, M.; Muskalla, W.; Cramer, A.; Heinz, G.; Egg, M.; Schuetz, P. Stabilisation of waterlogged archaeological wood: The application of structured-light 3D scanning and micro computed tomography for analysing dimensional changes. Herit. Sci. 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]

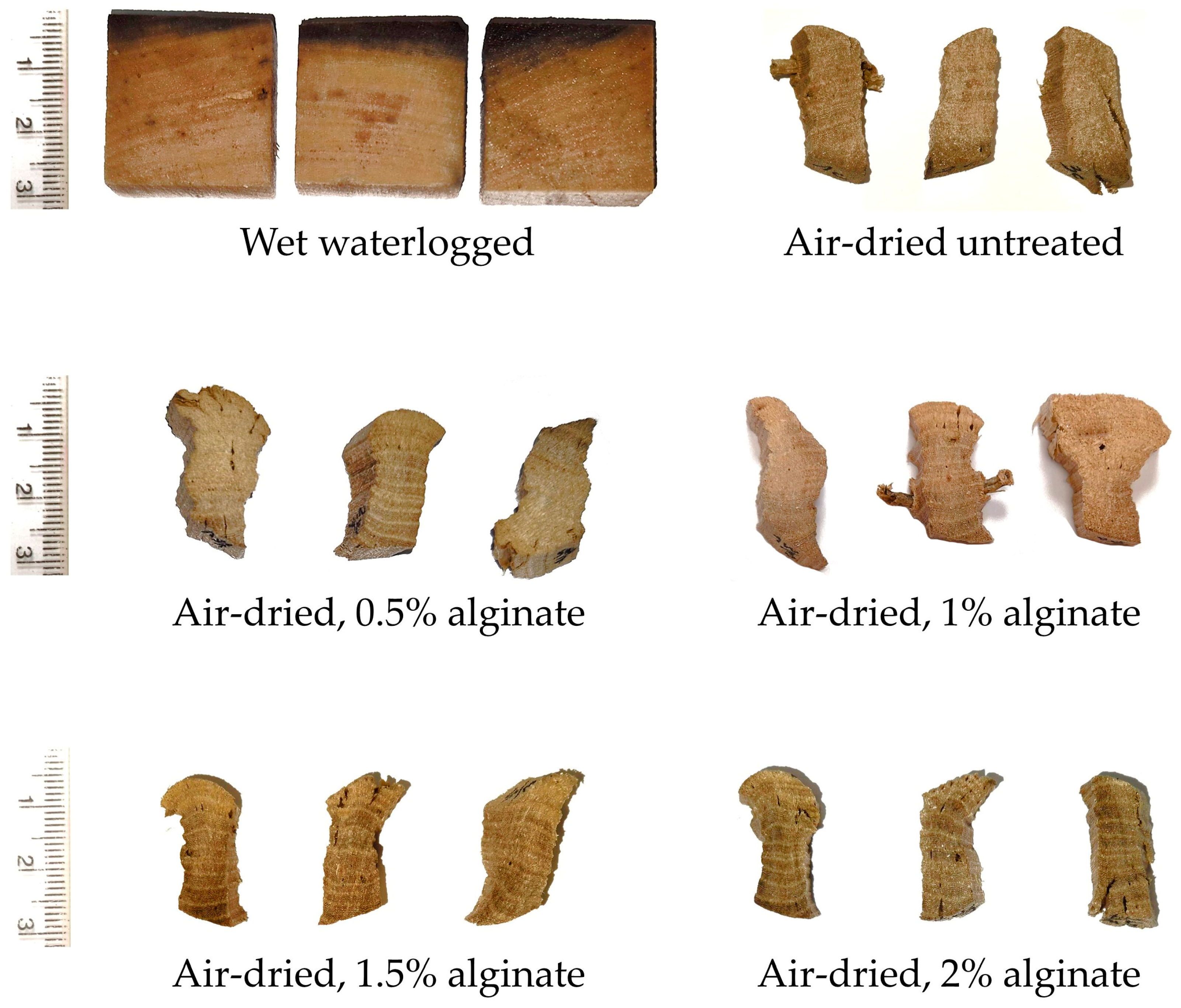
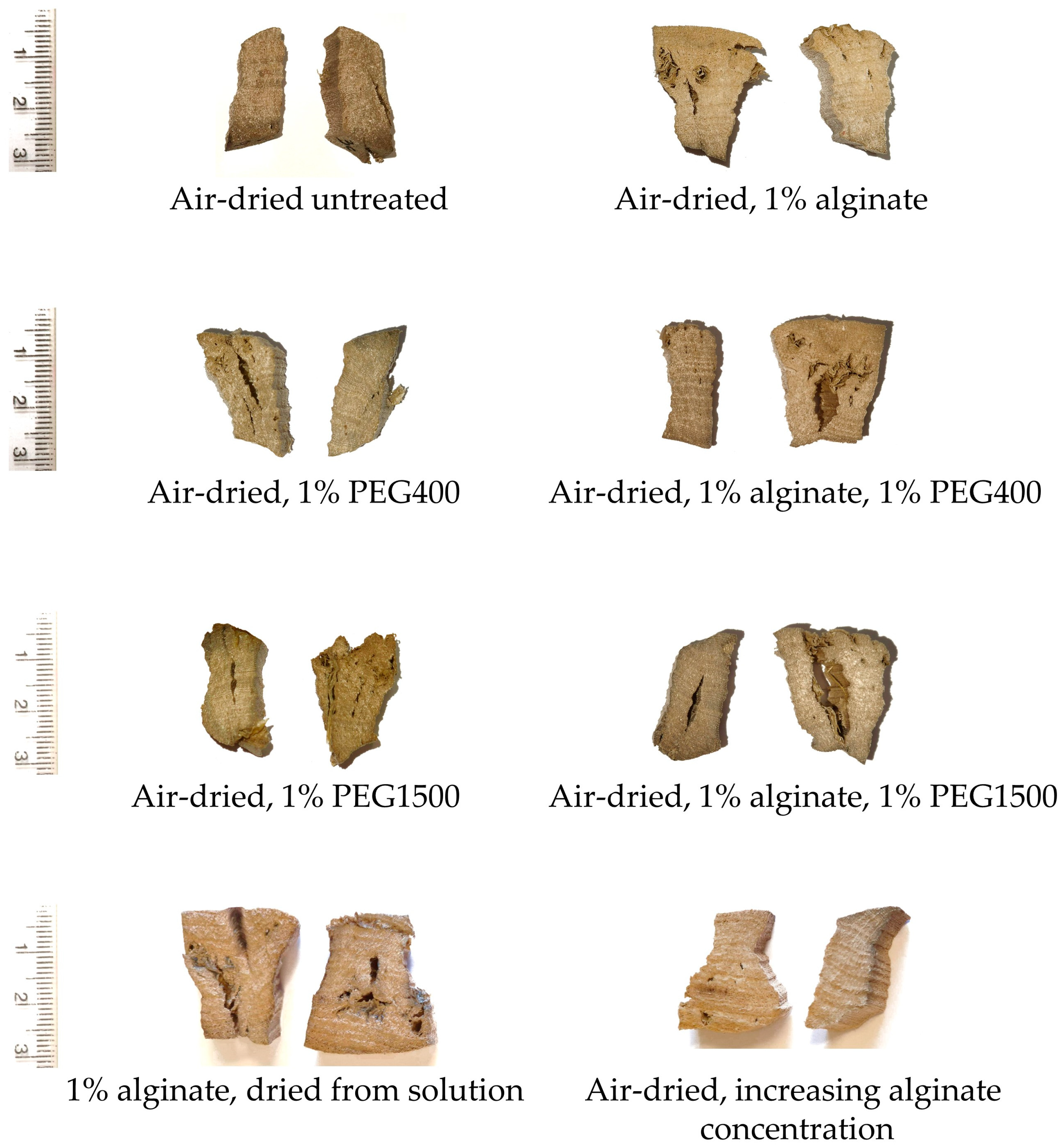

| Sample ID | Method Type | Chemical Applied | Experimental Details |
|---|---|---|---|
| 0.5A | oscillating pressure | 0.5% alginate solution | −0.9 bar vacuum for 0.5 h + 10 bars pressure for 6 h, repeated 6 times every 24 h, air-drying |
| 1A | oscillating pressure | 1% alginate solution | −0.9 bar vacuum for 0.5 h + 10 bars pressure for 6 h, repeated 6 times every 24 h, air-drying |
| 1.5A | oscillating pressure | 1.5% alginate solution | −0.9 bar vacuum for 0.5 h + 10 bars pressure for 6 h, repeated 6 times every 24 h, air-drying |
| 2A | oscillating pressure | 2% alginate solution | −0.9 bar vacuum for 0.5 h + 10 bars pressure for 6 h, repeated 6 times every 24 h, air-drying |
| 1AL | soaking | 1% alginate solution | immersion in solution for 3 weeks, air-drying |
| 1AL400PEG | soaking | 1% alginate solution + 1% PEG 400 | immersion in solution for 3 weeks, air-drying |
| 1AL1500PEG | soaking | 1% alginate solution + 1% PEG 1500 | immersion in solution for 3 weeks, air-drying |
| PEG400 | soaking | 1% PEG 400 | immersion in solution for 3 weeks, air-drying |
| PEG1500 | soaking | 1% PEG1500 | immersion in solution for 3 weeks, air-drying |
| Asol | soaking | 1% alginate solution | immersion in solution for 3 weeks, then left in solution for slow drying |
| Astep | soaking | alginate solution from 0.5% to 1.5% | immersion in solutions with gradually increasing alginate concentrations: 0.5%/1 week, 1%/1 week, 1.5%/1 week, air-drying |
| C | no treatment | - | - |
| Sample ID | ρ [g cm−3] | WPG [%] | Sv [%] | ASEv [%] |
|---|---|---|---|---|
| 0.5A | 1072.4 ± 18.0 | 1.97 ± 0.02 | 72.8 ± 2.2 | 4.1 ± 2.2 |
| 1A | 1072.1 ± 8.5 | 2.74 ± 0.03 | 73.2 ± 6.7 | 3.5 ± 6.7 |
| 1.5A | 1064.5 ± 5.8 | 3.56 ± 0.02 | 75.4 ± 1.3 | 0.6 ± 1.3 |
| 2A | 1055.7 ± 9.1 | 2.41 ± 0.03 | 74.6 ± 1.9 | 1.7 ± 1.9 |
| 1AL | 1083.6 ± 2.8 | 5.76 ± 0.01 | 59.6 ± 7.0 | 21.4 ± 7.0 |
| 1AL400PEG | 1078.5 ± 3.5 | 1.67 ± 0.04 | 65.8 ± 5.7 | 13.3 ± 5.7 |
| 1AL1500PEG | 1064.4 ± 9.3 | 5.35 ± 0.02 | 62.4 ± 8.8 | 17.8 ± 8.8 |
| PEG400 | 1058.7 ± 3.7 | 0.77 ± 0.02 | 69.4 ± 4.2 | 8.6 ± 4.2 |
| PEG1500 | 1040.2 ± 6.5 | 2.71 ± 0.03 | 65.7 ± 1.8 | 13.5 ± 1.8 |
| Asol | 1074.5 ± 11.1 | 6.69 ± 0.01 | 45.5 ± 8.2 | 40.1 ± 8.2 |
| Astep | 1091.8 ± 24.2 | 4.81 ± 0.03 | 68.4 ± 8.4 | 9.8 ± 11.2 |
| C | 1080.0 ± 23.0 | - | 75.9 ± 1.4 | - |
| Band Position (cm−1) | Band Assignment |
|---|---|
| 3420 (in C) | O2–H2⋯O6 intramolecular stretching vibration modes (in cellulose) |
| 3345/3343 (in C/A) | O5–H5⋯O3 intramolecular stretching vibration modes |
| 3120/3128 (in C/A) | C–H stretching vibration in methyl and methylene group |
| 3107 (in A) | C–H stretching vibration in methyl and methylene group |
| 2934/2922 (in C/A) | asymmetric stretching vibration of C–H bonds in methyl and methylene groups |
| 2878/2867 (in C/A) | symmetric stretching vibration of C–H bonds in methylene groups |
| 2841 (in C) | asymmetric stretching vibration of C–H bonds in methylene groups |
| Band Position (cm−1) | Band Assignment |
|---|---|
| 1736 (in C) | C=O stretching vibration of carbonyl, carboxyl and acetyl groups |
| 1661 (in C) | conjugated C–O in quinines coupled with C=O stretching of various groups |
| 1371/1374 (in C/A) | C–H deformation vibration in polysaccharides and alginate |
| 1328/1325 (in C/A) | C–H stretching vibration in polysaccharides and alginate |
| 1083 (in C) | glucose ring stretching vibration |
| 1027/1026 (in C/A) | C–O ester stretching vibrations in wood and OH bending vibration of alginate |
| 956/946 (in C/A) | C–O stretching vibration in wood and specific to guluronic and mannuronic acids from alginate |
| 893/896 (in C/A) | C–H deformation vibration in cellulose and specific to guluronic and mannuronic acids from alginate |
| 854 (in A) | C–H deformation vibration in cellulose and specific to guluronic and mannuronic acids from alginate |
| Band Position (cm−1) | Band Assignment |
|---|---|
| 1743 | C–O stretching vibration |
| 1636 | O–H bending vibration |
| 1461/1462 | asymmetrical stretching vibration of CH2 group |
| 1401 | in-plane bending (scissoring) vibration of CH2 group |
| 1351 | out-of-plane bending (wagging) vibration of CH2 group |
| 1108 | –CH2–O–CH2– groups |
| 995 | out-of-plane bending vibrations of CH2 |
| 886 | C–C stretching vibrations |
| 838 | C–C stretching vibrations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villani, E.; Popescu, C.-M.; Jancelewicz, M.; Stagno, V.; Capuani, S.; Broda, M. Sodium Alginate as a Green Consolidant for Waterlogged Wood—A Preliminary Study. Forests 2025, 16, 325. https://doi.org/10.3390/f16020325
Villani E, Popescu C-M, Jancelewicz M, Stagno V, Capuani S, Broda M. Sodium Alginate as a Green Consolidant for Waterlogged Wood—A Preliminary Study. Forests. 2025; 16(2):325. https://doi.org/10.3390/f16020325
Chicago/Turabian StyleVillani, Elisa, Carmen-Mihaela Popescu, Mariusz Jancelewicz, Valeria Stagno, Silvia Capuani, and Magdalena Broda. 2025. "Sodium Alginate as a Green Consolidant for Waterlogged Wood—A Preliminary Study" Forests 16, no. 2: 325. https://doi.org/10.3390/f16020325
APA StyleVillani, E., Popescu, C.-M., Jancelewicz, M., Stagno, V., Capuani, S., & Broda, M. (2025). Sodium Alginate as a Green Consolidant for Waterlogged Wood—A Preliminary Study. Forests, 16(2), 325. https://doi.org/10.3390/f16020325












