Genetic Diversity and Population Structure of Siamese Rosewood (Dalbergia cochinchinensis Pierre) in Thailand Using matK and Internal Transcribed Spacer Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Molecular Analysis
2.3. Data Analysis
3. Results
3.1. Genetic Diversity of D. cochinchinensis
3.2. Haplotype Network
3.3. Phylogenetic Tree
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vatanparast, M.; Klitgård, B.B.; Adema, F.A.; Pennington, R.T.; Yahara, T.; Kajita, T. First molecular phylogeny of the pantropical genus Dalbergia: Implications for infrageneric circumscription and biogeography. S. Afr. J. Bot. 2013, 89, 143–149. [Google Scholar]
- Niyomdham, C. An accont of Dalbergia (Leguminosae-Papilionoideae) in Thailand. Thai For. Bull. 2002, 30, 124–166. [Google Scholar]
- Department of National Parks, Wildlife and Plant Conservation. Report Summarizing Statistics of Illegal Cases Related to Forestry. 2018. Available online: https://portal.dnp.go.th/Content?contentId=2134 (accessed on 20 December 2023).
- CITES. Appendices I, II and III: Valid from 4 October 2017. In Convention on International Trade in Endangered Species of Wild Fauna and Flora; CITES: Geneva, Switzerland, 2018. [Google Scholar]
- National Council for Peace and Order. Announcement of the National Council for Peace and Order No. 106/2014 Regarding Amendments to the Forest Law; National Council for Peace and Order: Bangkok, Thailand, 2014. [Google Scholar]
- Soonhuae, P.; Piewluang, C.; Boyle, T. Population Genetics of Dalbergia cochinchinensis Pierre and Implications for Genetic Conservation; Technical Publication No. 18; ASEAN Forest Tree Seed Centre Project: Saraburi, Thailand, 1994. [Google Scholar]
- Yooyuen, R.; Duangjai, S.; Changtragoon, S. Chloroplast DNA variation of Dalbergia cochinchinensis Pierre in Thailand and Laos. In Proceedings of the IUFRO World Series Volume 30: Asia and the Pacific Workshop Multinational and Transboundary Conservation of Valuable and Endangered Forest Tree Species, Guangzhou, China, 5–7 December 2011. [Google Scholar]
- Hien, V.T.T.; Phong, D.T. Genetic diversity among endangered rare Dalbergia cochinchinensis (Fabaceae) genotypes in Vietnam revealed by random amplified polymorphic DNA (RAPD) and inter simple sequence repeats (ISSR) markers. Afr. J. Biotechnol. 2012, 11, 8632–8644. [Google Scholar]
- Bal, P.; Panda, P.C. Molecular characterization and phylogenetic relationships of Dalbergia species of eastern India based on RAPD and ISSR analysis. Int. J. Innov. Science Res. Technol. 2018, 3, 417–422. [Google Scholar]
- Hartvig, I.; So, T.; Changtragoon, S.; Tran, H.T.; Bouamanivong, S.; Theilade, I.; Kjaer, E.D.; Nielsen, L.R. Population genetic structure of the endemic rosewoods Dalbergia cochinchinensis and D. oliveri at a regional scale reflects the Indochinese landscape and life-history traits. Ecol. Evol. 2018, 8, 530–545. [Google Scholar] [PubMed]
- Hartvig, I.; Czako, M.; Kjaer, E.D.; Nielsen, L.R.; Theilade, I. The use of DNA barcoding in identification and conservation of rosewood (Dalbergia spp.). PLoS ONE 2015, 10, e0138231. [Google Scholar]
- Pérez-Luna, A.; Hernández-Díaz, J.C.; Wehenkel, C.; Simental-Rodríguez, S.L.; Hernández-Velasco, J.; Prieto-Ruíz, J.A. Graft survival of Pinus engelmannii Carr. in relation to two grafting techniques with dormant and sprouting buds. PeerJ 2021, 9, e12182. [Google Scholar]
- Dunning, L.T.; Savolainen, V. Broad-scale amplification of matK for DNA barcoding plants a technical note. Bot. J. Linn. Soc. 2010, 164, 1–9. [Google Scholar]
- Sotuyo, S.; Pedraza-Ortega, E.; Martinez-Salas, E.; Linares, J.; Cabrera, L. Insights into phylogenetic divergence of Dalbergia (Leguminosae: Dalbergiae) from Mexico and Central America. Front. Ecol. Evol. 2022, 10, 910250. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acid Ser. 1999, 41, 95–98. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 18, 3022–3027. [Google Scholar]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Andrianoelina, O.; Rakotondraoelina, H.; Ramamonjisoa, L.; Maley, J.; Danthu, P.; Bouvet, J.M. Genetic diversity of Dalbergia monticola (Fabaceae) an endangered tree species in the fragmented oriental forest of Madagascar. Biodivers. Conserv. 2006, 15, 1109–1128. [Google Scholar]
- Phong, D.T.; Tang, D.V.; Hien, V.T.T.; Ton, N.D.; Hai, N.V. Nucleotide diversity of a nuclear and four chloroplast DNA regions in rare tropical wood species of Dalbergia in Vietnam: A DNA barcode identifying utility. Asian J. Appl. Sci. 2014, 2, 116–125. [Google Scholar]
- Liu, F.M.; Zhang, N.N.; Liu, X.J.; Yang, Z.J.; Jia, H.Y.; Xu, D.P. Genetic diversity and population structure analysis of Dalbergia odorifera germplasm and development of a core collection using microsatellite markers. Genes 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Niyomdham, C.; Hô, P.H.; Dy Phon, P.; Vidal, E. Leguminoseae-Papilionoideae Dalbergieae. In Flore du Cambodge du Laos et du Viêtnam; Morat, P., Ed.; Muséum National d’Histoire Naturelle: Paris, France, 1997. [Google Scholar]
- CTSP. Cambodian Tree Species. Monographs. Cambodia Tree Seed Project, FA, Cambodia, and DANIDA, Denmark. 2004. Available online: https://www.scribd.com/document/125785024/Cambodian-Tree-Species (accessed on 20 December 2023).
- Barreto, M.A.; Mucherino-Muňoz, J.J.; Menezes, I.P.P.; Tarazi, R.; Silva, J.G.; Gaiotto, F.A.; Corrêa, R. Genetic structure and diversity of Dalbergia nigra from Brazilian Atlantic forest fragments. Forests 2023, 14, 2165. [Google Scholar] [CrossRef]
- Huang, J.F.; Li, S.Q.; Xu, R.; Peng, Y.Q. East-West genetic differentiation across the Indo-Burma hotspot: Evidence from two closely related dioecious figs. BMC Plant Biol. 2023, 23, 321. [Google Scholar]

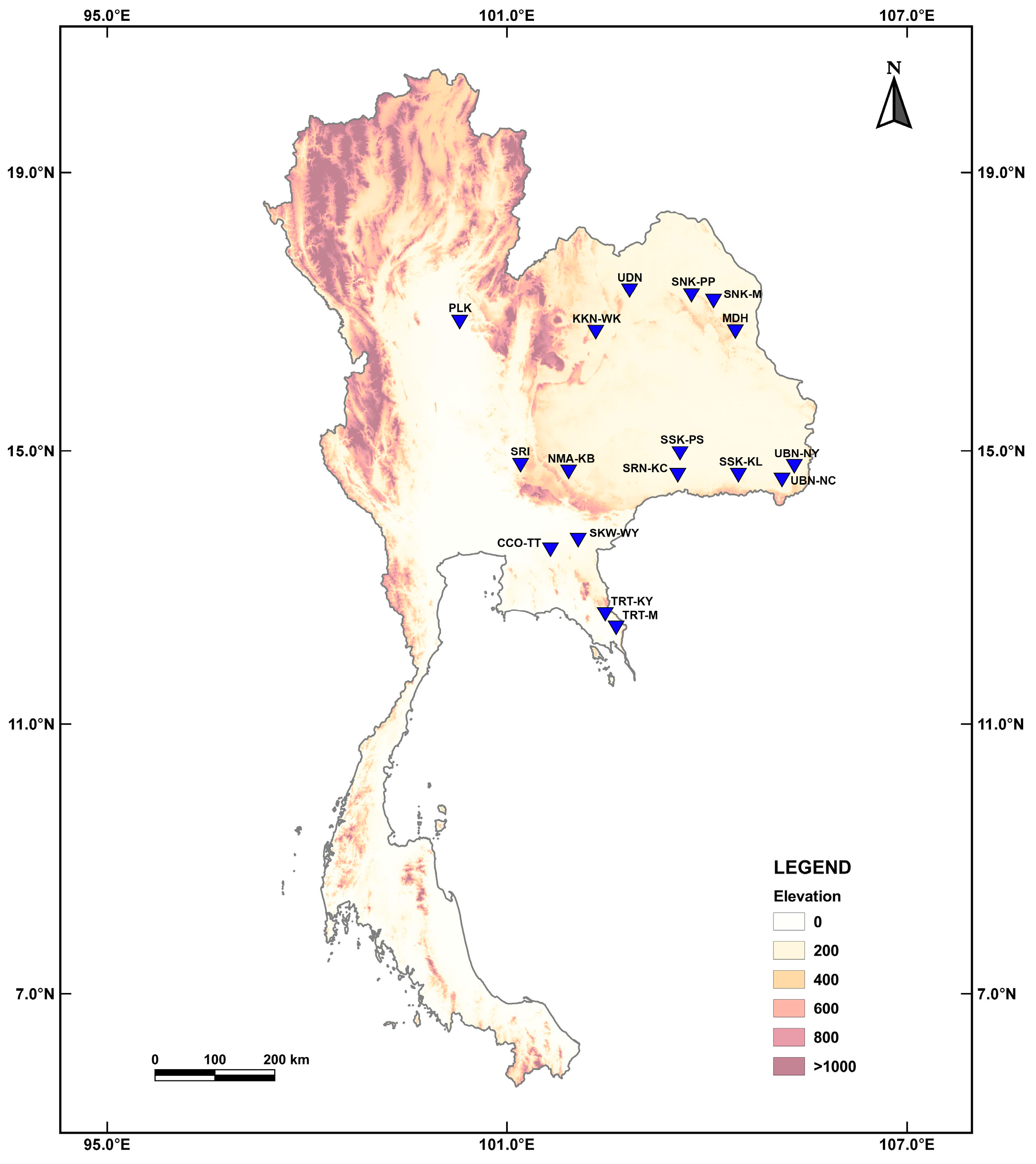
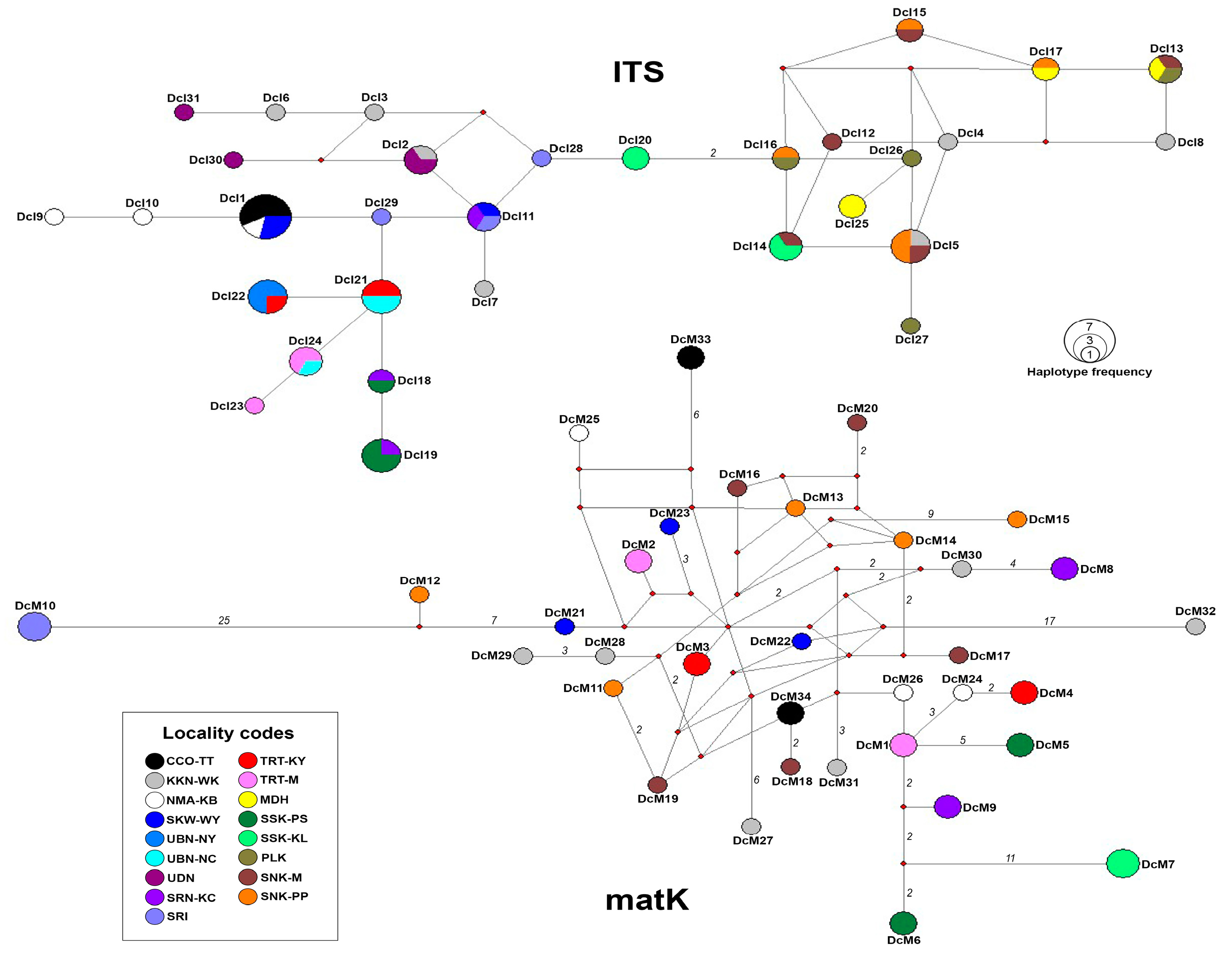
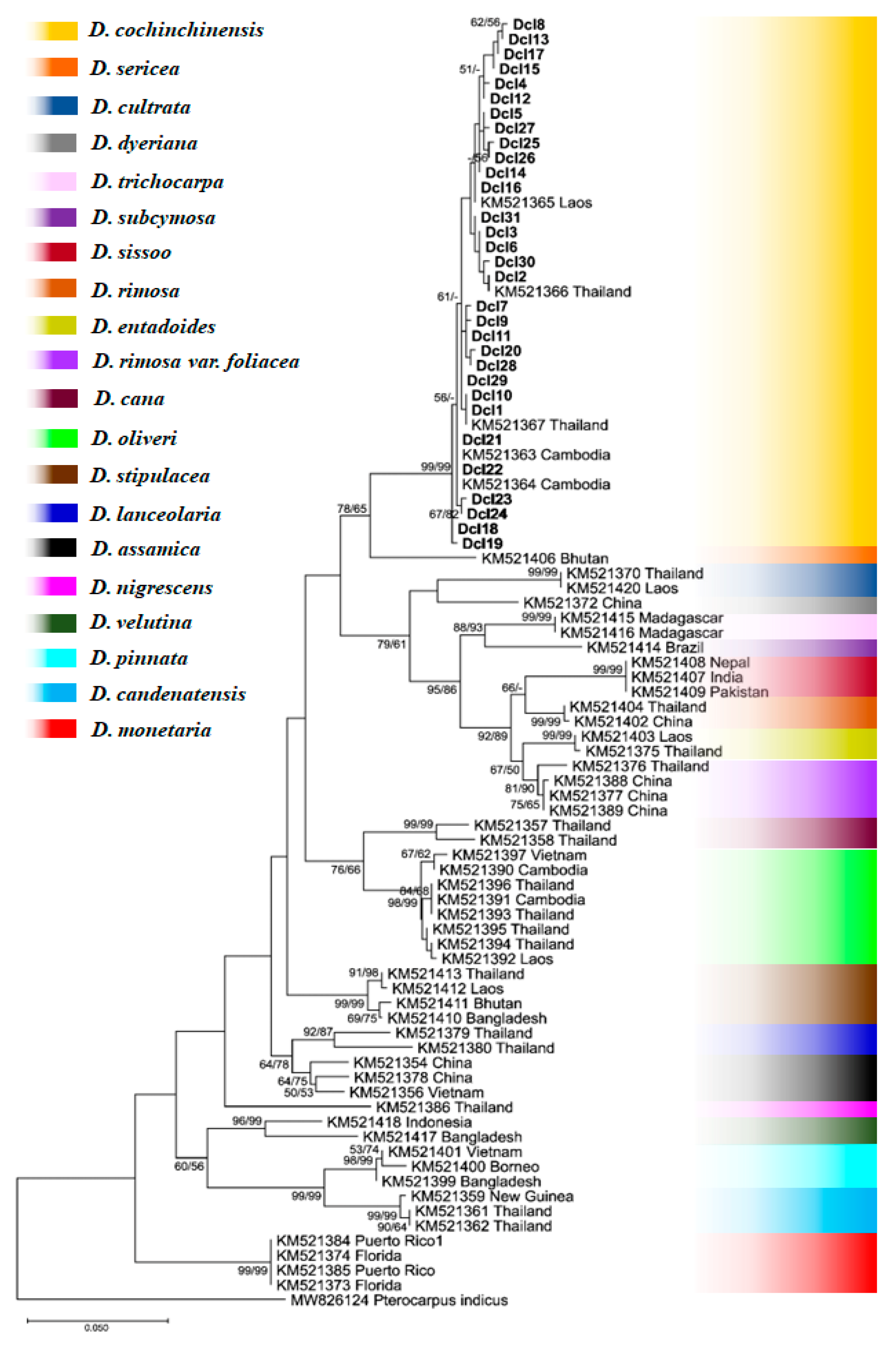
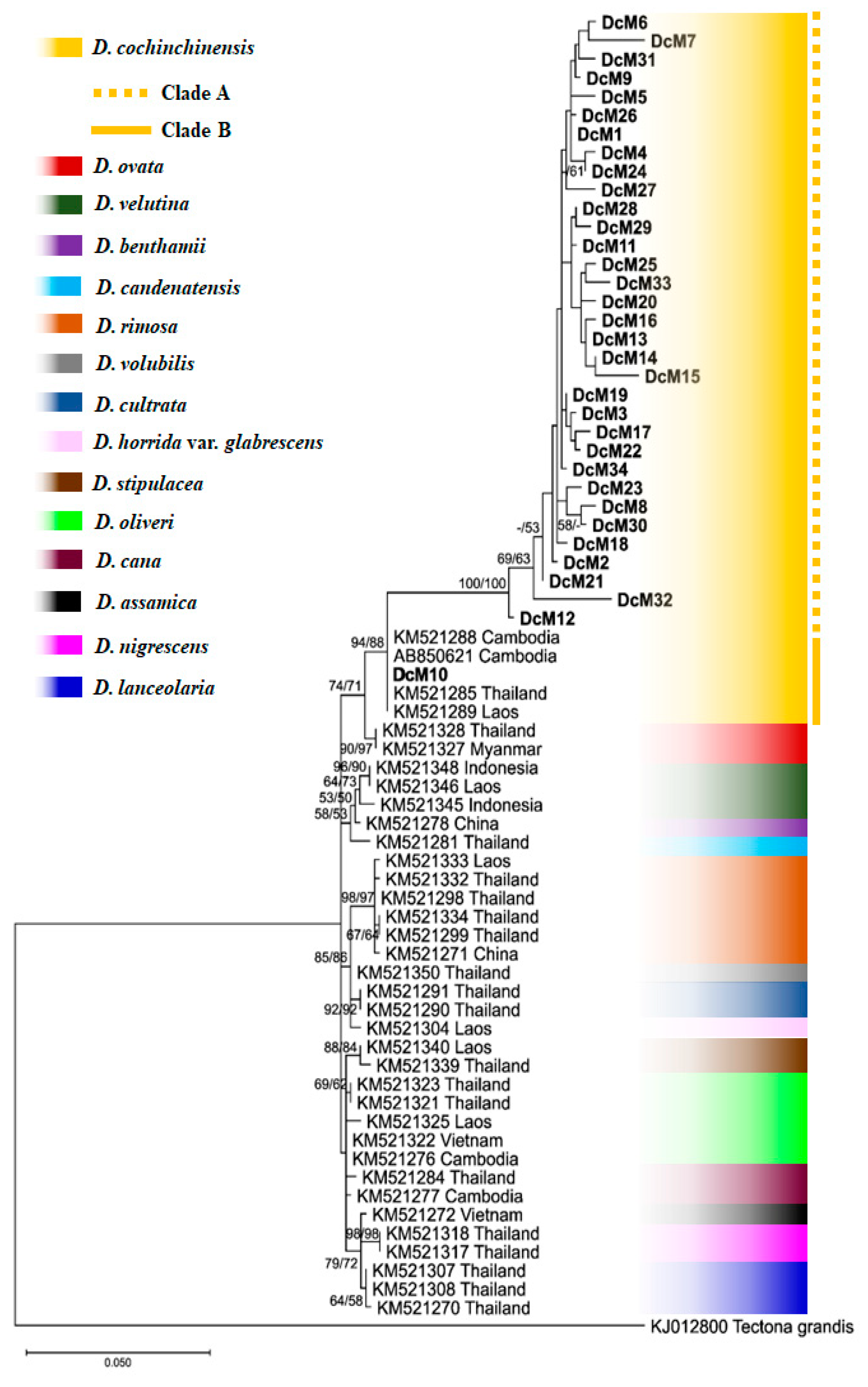
| Sample No. | Sample Code | Molecular Marker | n *** | Sample Collection Site (Habitat Characteristics) | Elevation Above Sea Level (m) | |
|---|---|---|---|---|---|---|
| matK * | ITS ** | |||||
| 1 | TRT-M1 | DcM1 | DcI23 | 4 | Mueang District, Trat Province (mixed deciduous forest, silty and sandy clay loam) | 11 |
| 2 | TRT-M2 | DcM2 | DcI24 | 4 | ||
| 3 | TRT-M3 | DcM2 | DcI24 | 12 | ||
| 4 | TRT-M4 | DcM1 | n/a | 10 | ||
| 5 | TRT-KY1 | DcM4 | DcI21 | 4 | Khlong Yai District, Trat Province (mixed deciduous forest, silty and sandy clay loam) | 16 |
| 6 | TRT-KY2 | DcM3 | DcI21 | 11 | ||
| 7 | TRT-KY3 | DcM4 | DcI22 | 22 | ||
| 8 | TRT-KY4 | DcM3 | n/a | 20 | ||
| 9 | SSK-PS1 | DcM6 | n/a | 5 | Phu Sing District, Sisaket Province (mixed deciduous forest, sandy clay loam) | 480 |
| 10 | SSK-PS2 | DcM6 | DcI19 | 530 | ||
| 11 | SSK-PS3 | DcM5 | DcI19 | 520 | ||
| 12 | SSK-PS4 | n/a | DcI19 | 521 | ||
| 13 | SSK-PS5 | DcM5 | DcI18 | 524 | ||
| 14 | SSK-KL1 | DcM7 | DcI20 | 4 | Kantaralak District, Sisaket Province (mixed deciduous forest, sandy clay loam) | 270 |
| 15 | SSK-KL2 | DcM7 | DcI14 | 265 | ||
| 16 | SSK-KL3 | DcM7 | DcI20 | 268 | ||
| 17 | SSK-KL4 | n/a | DcI14 | 271 | ||
| 18 | SRN-KC1 | DcM9 | DcI18 | 4 | Kap Choeng District, Surin Province (mixed deciduous forest, sandy clay loam) | 242 |
| 19 | SRN-KC2 | DcM8 | DcI19 | 241 | ||
| 20 | SRN-KC3 | DcM9 | DcI11 | 222 | ||
| 21 | SRN-KC4 | DcM8 | n/a | 220 | ||
| 22 | SRI76 | DcM10 | n/a | 6 | Muak Lek District, Saraburi Province (mixed deciduous forest, sandy clay loam) | 455 |
| 23 | SRI80 | DcM10 | n/a | 370 | ||
| 24 | SRI | DcM10 | n/a | 330 | ||
| 25 | SRI6 | n/a | DcI28 | 310 | ||
| 26 | SRI27 | n/a | DcI29 | 380 | ||
| 27 | SRI34 | n/a | DcI11 | 380 | ||
| 28 | SNK-PP1 | DcM15 | DcI15 | 5 | Phu Phan District, Sakon Nakhon Province (mixed deciduous forest, sandy clay loam) | 472 |
| 29 | SNK-PP2 | DcM14 | DcI16 | 490 | ||
| 30 | SNK-PP3 | DcM13 | DcI5 | 493 | ||
| 31 | SNK-PP4 | DcM12 | DcI17 | 488 | ||
| 32 | SNK-PP5 | DcM11 | DcI5 | 469 | ||
| 33 | SNK-M1 | DcM20 | DcI12 | 5 | Mueang District, Sakon Nakhon Province (mixed deciduous forest, silty and sandy clay loam) | 324 |
| 34 | SNK-M2 | DcM19 | DcI13 | 339 | ||
| 35 | SNK-M3 | DcM18 | DcI5 | 321 | ||
| 36 | SNK-M4 | DcM17 | DcI14 | 540 | ||
| 37 | SNK-M5 | DcM16 | DcI15 | 535 | ||
| 38 | SKW-WY1 | DcM23 | DcI1 | 3 | Wang Nam Yen District, Sa Kaeo Province (mixed deciduous forest, sandy clay loam) | 143 |
| 39 | SKW-WY2 | DcM22 | DcI1 | 183 | ||
| 40 | SKW-WY3 | DcM21 | DcI11 | 163 | ||
| 41 | NMA-KB1 | DcM26 | DcI1 | 3 | Khonburi District, Nakhon Ratchasima Province (mixed deciduous forest, sandy clay loam) | 307 |
| 42 | NMA-KB2 | DcM25 | DcI9 | 306 | ||
| 43 | NMA-KB3 | DcM24 | DcI10 | 309 | ||
| 44 | KKN-WK1 | DcM32 | DcI2 | 7 | Wiang Kao District, Khon Kaen Province (mixed deciduous forest, sandy clay loam) | 568 |
| 45 | KKN-WK2 | DcM31 | DcI3 | 564 | ||
| 46 | KKN-WK3 | DcM30 | DcI4 | 668 | ||
| 47 | KKN-WK4 | DcM29 | DcI5 | 602 | ||
| 48 | KKN-WK5 | DcM28 | DcI6 | 612 | ||
| 49 | KKN-WK6 | DcM27 | DcI7 | 606 | ||
| 50 | KKN-WK7 | n/a | DcI8 | 577 | ||
| 51 | CCO-TT1 | DcM34 | DcI1 | 4 | Tha Takiap District, Chachoengsao Province (mixed deciduous forest, sandy clay loam/laterite) | 88 |
| 52 | CCO-TT2 | DcM33 | DcI1 | 101 | ||
| 53 | CCO-TT3 | DcM34 | DcI1 | 100 | ||
| 54 | CCO-TT4 | DcM33 | DcI1 | 108 | ||
| 55 | UBN-NC1 | n/a | DcI24 | 3 | Na Chaluai District, Ubon Ratchathani Province (mixed deciduous forest, sandy clay loam) | 300 |
| 56 | UBN-NC2 | n/a | DcI21 | 348 | ||
| 57 | UBN-NC4 | n/a | DcI21 | 302 | ||
| 58 | UBN-NY1 | n/a | DcI22 | 3 | Nam Yuen District, Ubon Ratchathani Province (mixed deciduous forest, sandy clay loam) | 384 |
| 59 | UBN-NY2 | n/a | DcI22 | 371 | ||
| 60 | UBN-NY3 | n/a | DcI22 | 388 | ||
| 61 | MDH8 | n/a | DcI25 | 4 | Kham Chai District, Mukdahan Province (mixed deciduous forest, sandy clay loam) | 390 |
| 62 | MDH12 | n/a | DcI13 | 396 | ||
| 63 | MDH41 | n/a | DcI25 | 401 | ||
| 64 | MDH67 | n/a | DcI17 | 416 | ||
| 65 | UDN36 | n/a | DcI30 | 4 | Nong Wua So District, Udon Thani Province (mixed deciduous forest, sandy clay loam) | 377 |
| 66 | UDN37 | n/a | DcI2 | 383 | ||
| 67 | UDN43 | n/a | DcI2 | 238 | ||
| 68 | UDN45 | n/a | DcI31 | 243 | ||
| 69 | PLK5 | n/a | DcI16 | 4 | Nakhon Thai District, Phisanulok Province (mixed deciduous forest, silty and sandy clay loam) | 622 |
| 70 | PLK9 | n/a | DcI26 | 615 | ||
| 71 | PLK21 | n/a | DcI27 | 630 | ||
| 72 | PLK32 | n/a | DcI13 | 640 | ||
| Populations | ITS | matK | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | S | H | Uh | Hd ± SD | Nd ± SD | n | S | H | Uh | Hd ± SD | Nd ± SD | |
| TRT-M | 3 | 1 | 2 | 1 | 0.667 ± 0.314 | 0.0010 ± 0.0005 | 4 | 5 | 2 | 2 | 0.667 ± 0.204 | 0.0041 ± 0.0012 |
| TRT-KY | 3 | 1 | 2 | 0 | 0.667 ± 0.314 | 0.0010 ± 0.0005 | 4 | 4 | 2 | 2 | 0.667 ± 0.204 | 0.0033 ± 0.0001 |
| SSK-KL | 4 | 3 | 2 | 1 | 0.667 ± 0.204 | 0.0030 ± 0.0009 | 3 | 0 | 1 | 1 | 0.000 ± 0.000 | 0.0000 ± 0.0000 |
| SSK-PS | 4 | 1 | 2 | 0 | 0.500 ± 0.265 | 0.0008 ± 0.0004 | 4 | 11 | 2 | 2 | 0.667 ± 0.204 | 0.0089 ± 0.0027 |
| SRN-KC | 3 | 3 | 3 | 0 | 1.000 ± 0.272 | 0.0035 ± 0.0012 | 4 | 10 | 2 | 2 | 0.667 ± 0.204 | 0.0081 ± 0.0025 |
| SRI | 3 | 2 | 3 | 2 | 1.000 ± 0.272 | 0.0020 ± 0.0007 | 3 | 0 | 1 | 1 | 0.000 ± 0.000 | 0.0000 ± 0.0000 |
| SNK-M | 5 | 5 | 5 | 1 | 1.000 ± 0.126 | 0.0042 ± 0.0009 | 5 | 12 | 5 | 5 | 1.000 ± 0.126 | 0.0073 ± 0.0011 |
| SNK-PP | 5 | 4 | 4 | 0 | 0.900 ± 0.161 | 0.0036 ± 0.0007 | 5 | 22 | 5 | 5 | 1.000 ± 0.126 | 0.0122 ± 0.0032 |
| SKW-WY | 3 | 2 | 2 | 0 | 0.667 ± 0.314 | 0.0020 ± 0.0009 | 3 | 10 | 3 | 3 | 1.000 ± 0.272 | 0.0081 ± 0.0023 |
| NMA-KB | 3 | 2 | 3 | 2 | 1.000 ± 0.272 | 0.0020 ± 0.0007 | 3 | 10 | 3 | 3 | 1.000 ± 0.272 | 0.0081 ± 0.0025 |
| KKN-WK | 7 | 11 | 7 | 5 | 1.000 ± 0.076 | 0.0078 ± 0.0012 | 6 | 28 | 6 | 6 | 1.000 ± 0.096 | 0.0138 ± 0.0034 |
| CCO-TT | 4 | 1 | 1 | 0 | 0.000 ± 0.000 | 0.0000 ± 0.0000 | 4 | 12 | 2 | 2 | 0.667 ± 0.204 | 0.0097 ± 0.0030 |
| UBN-NY | 3 | 1 | 1 | 0 | 0.000 ± 0.000 | 0.0000 ± 0.0000 | n/a | n/a | n/a | n/a | n/a | n/a |
| UBN-NC | 3 | 1 | 2 | 0 | 0.667 ± 0.314 | 0.0010 ± 0.0005 | n/a | n/a | n/a | n/a | n/a | n/a |
| UDN | 4 | 5 | 3 | 2 | 0.833 ± 0.222 | 0.0040 ± 0.0013 | n/a | n/a | n/a | n/a | n/a | n/a |
| MDH | 4 | 4 | 3 | 1 | 0.833 ± 0.222 | 0.0038 ± 0.0011 | n/a | n/a | n/a | n/a | n/a | n/a |
| PLK | 4 | 6 | 4 | 2 | 1.000 ± 0.177 | 0.0045 ± 0.0013 | n/a | n/a | n/a | n/a | n/a | n/a |
| Total | 65 | 17 | 31 | 17 | 0.968 ± 0.008 | 0.0069 ± 0.0003 | 48 | 66 | 34 | 25 | 0.986 ± 0.007 | 0.0161 ± 0.0022 |
| Populations | TRT-M | TRT-KY | SSK-KL | SSK-PS | SRN-KC | SNK-M | SNK-PP | SKW-WY | NMA-KB | KKN-WK | CCO-TT | SRI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRT-M | − | 0.0061 | 0.0204 | 0.0086 | 0.0086 | 0.0072 | 0.0114 | 0.0071 | 0.0063 | 0.0111 | 0.0092 | 0.0448 |
| TRT-KY | 0.0061 | − | 0.0223 | 0.0117 | 0.0098 | 0.0074 | 0.0113 | 0.0065 | 0.0065 | 0.0123 | 0.0098 | 0.0429 |
| SSK-KL | 0.0201 | 0.0219 | − | 0.0179 | 0.0210 | 0.0225 | 0.0260 | 0.0227 | 0.0223 | 0.0235 | 0.0235 | 0.0597 |
| SSK-PS | 0.0085 | 0.0116 | 0.0177 | − | 0.0117 | 0.0132 | 0.0169 | 0.0137 | 0.0104 | 0.0146 | 0.0135 | 0.0513 |
| SRN-KC | 0.0085 | 0.0097 | 0.0207 | 0.0116 | − | 0.0108 | 0.0140 | 0.0094 | 0.0094 | 0.0127 | 0.0117 | 0.0467 |
| SNK-M | 0.0072 | 0.0073 | 0.0222 | 0.0130 | 0.0107 | − | 0.0095 | 0.0080 | 0.0091 | 0.0119 | 0.0093 | 0.0444 |
| SNK-PP | 0.0113 | 0.0112 | 0.0256 | 0.0167 | 0.0139 | 0.0095 | − | 0.0116 | 0.0122 | 0.0151 | 0.0126 | 0.0429 |
| SKW-WY | 0.0071 | 0.0065 | 0.0223 | 0.0136 | 0.0093 | 0.0080 | 0.0115 | − | 0.0094 | 0.0124 | 0.0111 | 0.0411 |
| NMA-KB | 0.0063 | 0.0065 | 0.0219 | 0.0104 | 0.0093 | 0.0090 | 0.0121 | 0.0093 | − | 0.0118 | 0.0102 | 0.0476 |
| KKN-WK | 0.0110 | 0.0122 | 0.0231 | 0.0144 | 0.0126 | 0.0118 | 0.0149 | 0.0123 | 0.0116 | − | 0.0129 | 0.0493 |
| CCO-TT | 0.0091 | 0.0097 | 0.0231 | 0.0134 | 0.0116 | 0.0093 | 0.0124 | 0.0110 | 0.0102 | 0.0128 | − | 0.0467 |
| SRI | 0.0432 | 0.0414 | 0.0572 | 0.0493 | 0.0451 | 0.0429 | 0.0414 | 0.0398 | 0.0459 | 0.0475 | 0.0451 | − |
| Populations | TRT-M | TRT-KY | SSK-KL | SSK-PS | SRN-KC | SNK-M | SNK-PP | SKW-WY | NMA-KB | KKN-WK | CCO-TT | UBN-NY | UBN-NC | MDH | UDN | PLK | SRI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRT-M | − | 0.0025 | 0.0088 | 0.0046 | 0.0045 | 0.0114 | 0.0108 | 0.0050 | 0.0065 | 0.0104 | 0.0050 | 0.0035 | 0.0015 | 0.0122 | 0.0080 | 0.0103 | 0.0050 |
| TRT-KY | 0.0025 | − | 0.0073 | 0.0031 | 0.0030 | 0.0099 | 0.0093 | 0.0035 | 0.0043 | 0.0088 | 0.0035 | 0.0010 | 0.0010 | 0.0107 | 0.0063 | 0.0088 | 0.0035 |
| SSK-KL | 0.0087 | 0.0072 | − | 0.0089 | 0.0075 | 0.0047 | 0.0044 | 0.0063 | 0.0078 | 0.0060 | 0.0068 | 0.0083 | 0.0073 | 0.0064 | 0.0072 | 0.0049 | 0.0048 |
| SSK-PS | 0.0046 | 0.0031 | 0.0088 | − | 0.0020 | 0.0120 | 0.0114 | 0.0053 | 0.0068 | 0.0104 | 0.0056 | 0.0041 | 0.0031 | 0.0129 | 0.0078 | 0.0110 | 0.0049 |
| SRN-KC | 0.0045 | 0.0030 | 0.0075 | 0.0020 | − | 0.0109 | 0.0103 | 0.0040 | 0.0055 | 0.0091 | 0.0045 | 0.0040 | 0.0030 | 0.0117 | 0.0064 | 0.0098 | 0.0035 |
| SNK-M | 0.0113 | 0.0098 | 0.0046 | 0.0119 | 0.0108 | − | 0.0034 | 0.0094 | 0.0109 | 0.0069 | 0.0094 | 0.0109 | 0.0099 | 0.0046 | 0.0098 | 0.0041 | 0.0083 |
| SNK-PP | 0.0107 | 0.0092 | 0.0043 | 0.0113 | 0.0102 | 0.0034 | − | 0.0088 | 0.0103 | 0.0065 | 0.0088 | 0.0103 | 0.0093 | 0.0038 | 0.0091 | 0.0035 | 0.0077 |
| SKW-WY | 0.0050 | 0.0035 | 0.0062 | 0.0052 | 0.0040 | 0.0093 | 0.0087 | − | 0.0022 | 0.0078 | 0.0010 | 0.0045 | 0.0035 | 0.0102 | 0.0053 | 0.0083 | 0.0023 |
| NMA-KB | 0.0065 | 0.0043 | 0.0077 | 0.0067 | 0.0055 | 0.0108 | 0.0102 | 0.0022 | − | 0.0091 | 0.0015 | 0.0040 | 0.0050 | 0.0117 | 0.0063 | 0.0098 | 0.0038 |
| KKN-WK | 0.0104 | 0.0087 | 0.0060 | 0.0103 | 0.0090 | 0.0068 | 0.0065 | 0.0078 | 0.0090 | − | 0.0084 | 0.0095 | 0.0089 | 0.0077 | 0.0067 | 0.0065 | 0.0065 |
| CCO-TT | 0.0050 | 0.0035 | 0.0067 | 0.0056 | 0.0045 | 0.0093 | 0.0087 | 0.0010 | 0.0015 | 0.0084 | − | 0.0045 | 0.0035 | 0.0102 | 0.0060 | 0.0083 | 0.0030 |
| UBN-NY | 0.0035 | 0.0010 | 0.0082 | 0.0041 | 0.0040 | 0.0108 | 0.0102 | 0.0045 | 0.0040 | 0.0094 | 0.0045 | − | 0.0020 | 0.0117 | 0.0068 | 0.0098 | 0.0045 |
| UBN-NC | 0.0015 | 0.0010 | 0.0072 | 0.0031 | 0.0030 | 0.0098 | 0.0092 | 0.0035 | 0.0050 | 0.0089 | 0.0035 | 0.0020 | − | 0.0107 | 0.0065 | 0.0088 | 0.0035 |
| MDH | 0.0121 | 0.0106 | 0.0064 | 0.0127 | 0.0116 | 0.0046 | 0.0038 | 0.0101 | 0.0116 | 0.0077 | 0.0101 | 0.0116 | 0.0106 | − | 0.0102 | 0.0039 | 0.0092 |
| UDN | 0.0080 | 0.0062 | 0.0071 | 0.0078 | 0.0064 | 0.0097 | 0.0090 | 0.0052 | 0.0062 | 0.0066 | 0.0060 | 0.0067 | 0.0065 | 0.0101 | − | 0.0085 | 0.0043 |
| PLK | 0.0102 | 0.0087 | 0.0049 | 0.0109 | 0.0097 | 0.0040 | 0.0034 | 0.0082 | 0.0097 | 0.0064 | 0.0082 | 0.0097 | 0.0087 | 0.0039 | 0.0084 | − | 0.0073 |
| SRI | 0.0050 | 0.0035 | 0.0047 | 0.0049 | 0.0035 | 0.0083 | 0.0077 | 0.0023 | 0.0038 | 0.0065 | 0.0030 | 0.0045 | 0.0035 | 0.0091 | 0.0042 | 0.0072 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prompen, B.; Saijuntha, W.; Pilap, W.; Thanonkeo, S. Genetic Diversity and Population Structure of Siamese Rosewood (Dalbergia cochinchinensis Pierre) in Thailand Using matK and Internal Transcribed Spacer Markers. Forests 2025, 16, 332. https://doi.org/10.3390/f16020332
Prompen B, Saijuntha W, Pilap W, Thanonkeo S. Genetic Diversity and Population Structure of Siamese Rosewood (Dalbergia cochinchinensis Pierre) in Thailand Using matK and Internal Transcribed Spacer Markers. Forests. 2025; 16(2):332. https://doi.org/10.3390/f16020332
Chicago/Turabian StylePrompen, Benjarat, Weerachai Saijuntha, Warayutt Pilap, and Sudarat Thanonkeo. 2025. "Genetic Diversity and Population Structure of Siamese Rosewood (Dalbergia cochinchinensis Pierre) in Thailand Using matK and Internal Transcribed Spacer Markers" Forests 16, no. 2: 332. https://doi.org/10.3390/f16020332
APA StylePrompen, B., Saijuntha, W., Pilap, W., & Thanonkeo, S. (2025). Genetic Diversity and Population Structure of Siamese Rosewood (Dalbergia cochinchinensis Pierre) in Thailand Using matK and Internal Transcribed Spacer Markers. Forests, 16(2), 332. https://doi.org/10.3390/f16020332










